Abstract
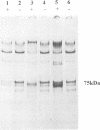
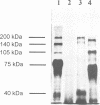
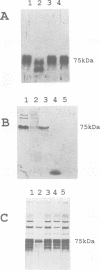
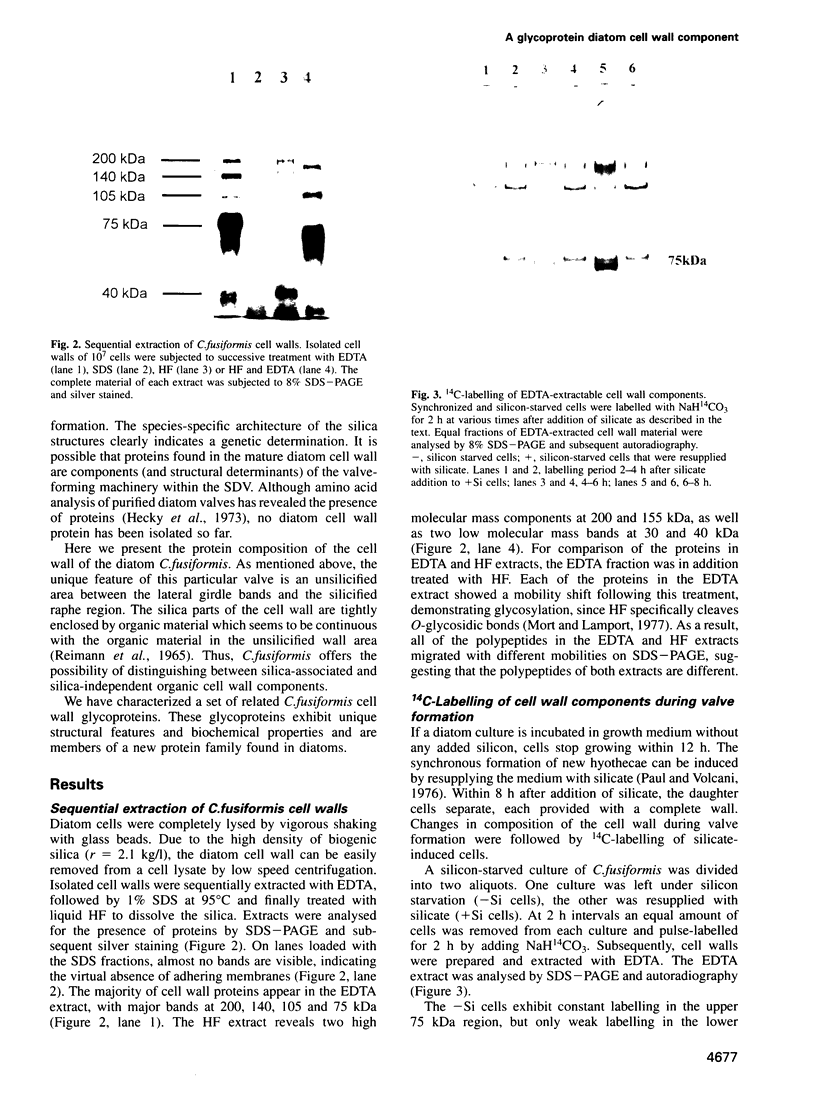
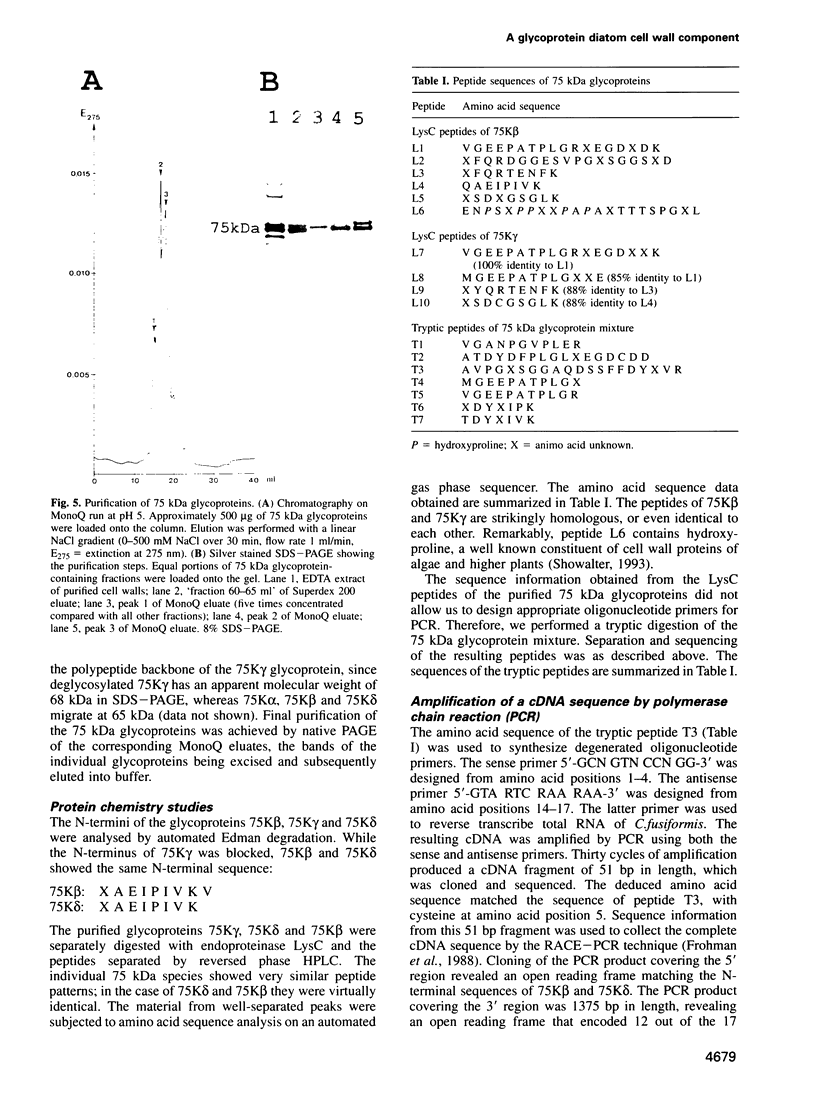
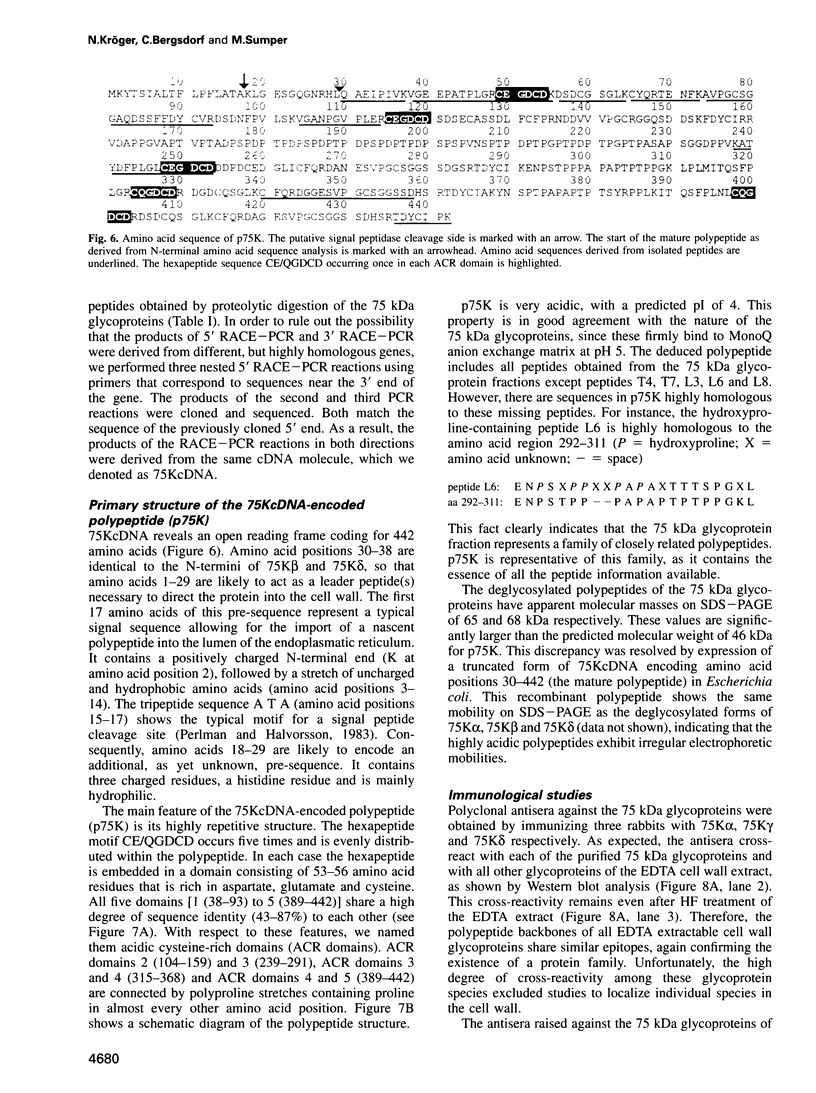
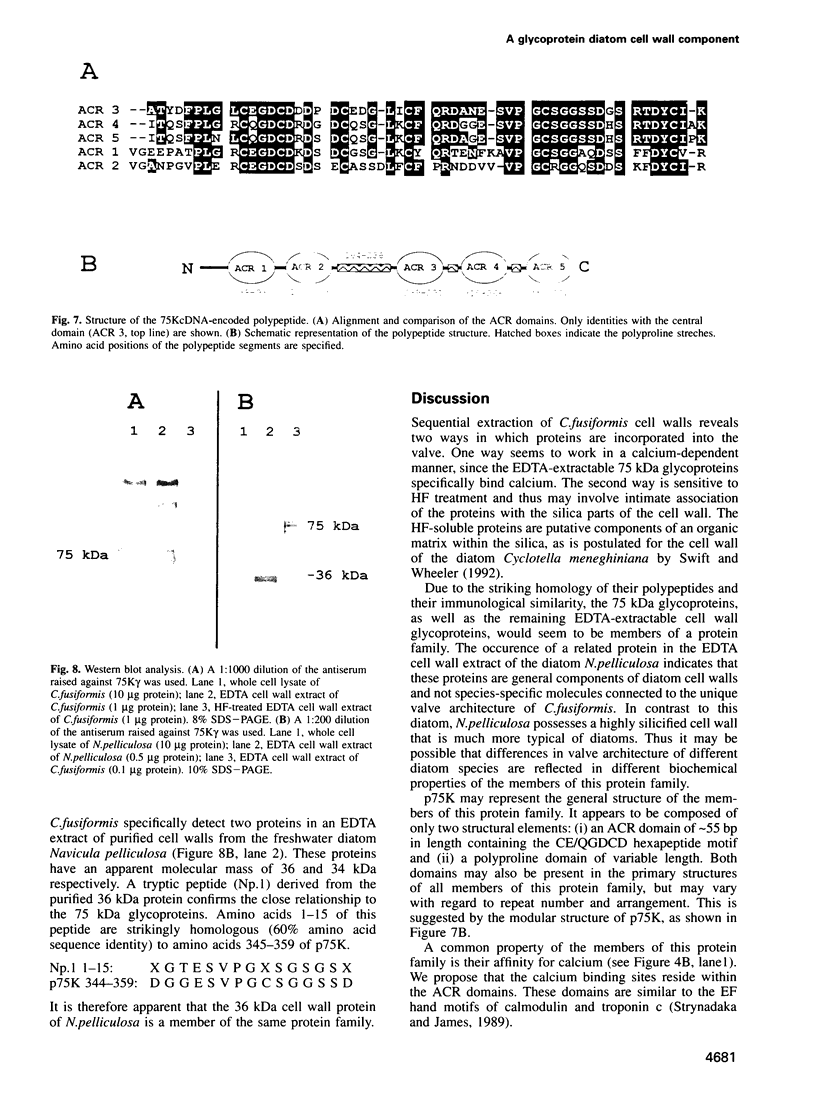
Diatoms possess silica-based cell walls with species-specific structures and ornamentations. Silica deposition in diatoms offers a model to study the processes involved in biomineralization. A new wall is produced in a specialized vesicle (silica deposition vesicle, SDV) and secreted. Thus proteins involved in wall biogenesis may remain associated with the mature cell wall. Here it is demonstrated that EDTA treatment removes most of the proteins present in mature cell walls of the marine diatom Cylindrotheca fusiformis. A main fraction consists of four related glycoproteins with a molecular mass of approximately 75 kDa. These glycoproteins were purified to homogeneity. They consist of repeats of Ca2+ binding domains separated by polypeptide stretches containing hydroxyproline. The proteins in the EDTA extract aggregate and precipitate in the presence of Ca2+. Immunological studies detected related proteins in the cell wall of the freshwater diatom Navicula pelliculosa, indicating that these proteins represent a new family of proteins that are involved in the biogenesis of diatom cell walls.
Full text
PDF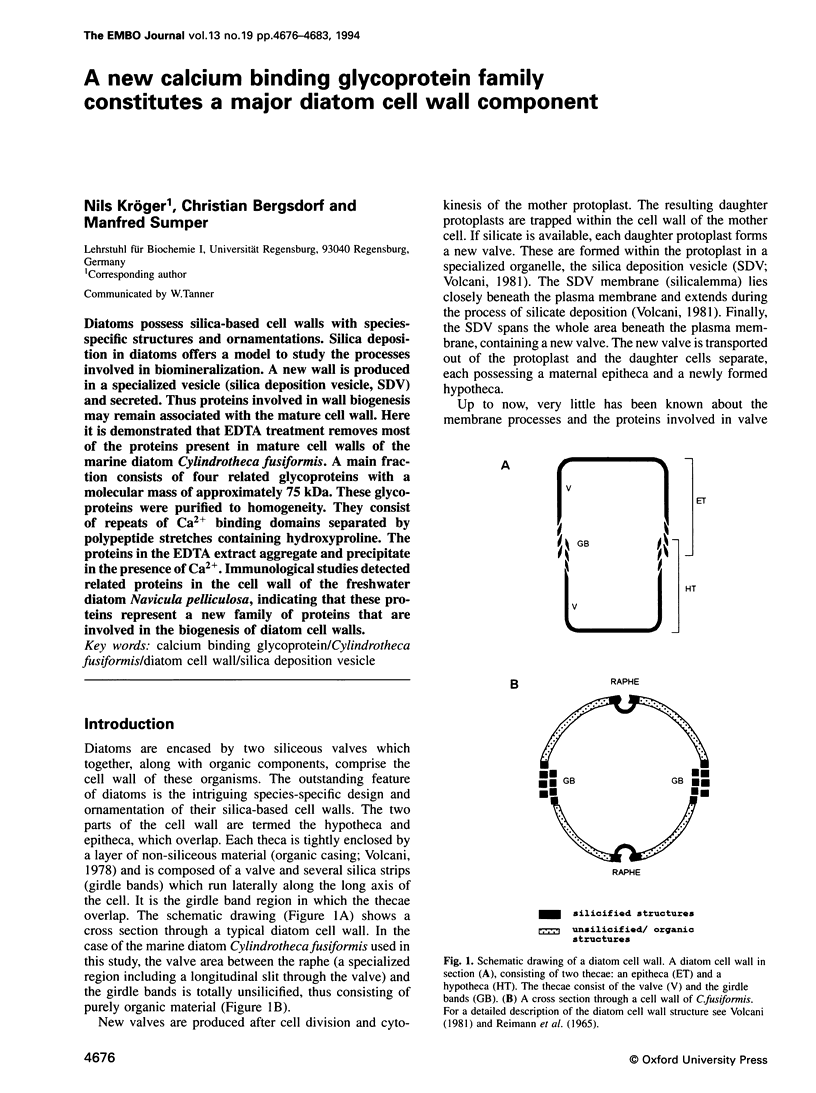



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartaud A., Ozon R., Walsh M. P., Haiech J., Demaille J. G. Xenopus laevis oocyte calmodulin in the process of meiotic maturation. J Biol Chem. 1980 Oct 10;255(19):9404–9408. [PubMed] [Google Scholar]
- Darley W. M., Volcani B. E. Role of silicon in diatom metabolism. A silicon requirement for deoxyribonucleic acid synthesis in the diatom Cylindrotheca fusiformis Reimann and Lewin. Exp Cell Res. 1969 Dec;58(2):334–342. doi: 10.1016/0014-4827(69)90514-x. [DOI] [PubMed] [Google Scholar]
- Ertl H., Mengele R., Wenzl S., Engel J., Sumper M. The extracellular matrix of Volvox carteri: molecular structure of the cellular compartment. J Cell Biol. 1989 Dec;109(6 Pt 2):3493–3501. doi: 10.1083/jcb.109.6.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M. M., Kirk D. L. Translational regulation of protein synthesis, in response to light, at a critical stage of Volvox development. Cell. 1985 Jun;41(2):419–428. doi: 10.1016/s0092-8674(85)80015-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- Paul J. S., Volcani B. E. Photorespiration in diatoms. IV. Two pathways of glycolate metabolism in synchronized cultures of Cylindrotheca fusiformis. Arch Microbiol. 1976 Nov 2;110(23):247–252. doi: 10.1007/BF00690234. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- REIMANN B. E., LEWIN J. C., VOLCANI B. E. STUDIES ON THE BIOCHEMISTRY AND FINE STRUCTURE OF SILICA SHELL FORMATION IN DIATOMS. I. THE STRUCTURE OF THE CELL WALL OF CYLINDROTHECA FUSIFORMIS REIMANN AND LEWIN. J Cell Biol. 1965 Jan;24:39–55. doi: 10.1083/jcb.24.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. M. Structure and function of plant cell wall proteins. Plant Cell. 1993 Jan;5(1):9–23. doi: 10.1105/tpc.5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Weiner S., Addadi L. Acidic macromolecules of mineralized tissues: the controllers of crystal formation. Trends Biochem Sci. 1991 Jul;16(7):252–256. doi: 10.1016/0968-0004(91)90098-g. [DOI] [PubMed] [Google Scholar]
- Weiner S. Organization of extracellularly mineralized tissues: a comparative study of biological crystal growth. CRC Crit Rev Biochem. 1986;20(4):365–408. doi: 10.3109/10409238609081998. [DOI] [PubMed] [Google Scholar]
- Wenzl S., Sumper M. Sulfation of a cell surface glycoprotein correlates with the developmental program during embryogenesis of Volvox carteri. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3716–3720. doi: 10.1073/pnas.78.6.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]