Abstract
Background
Two 8-week, randomized, double-blind, controlled studies previously evaluated the efficacy and tolerability of single-pill combinations of telmisartan 40–80 mg/amlodipine 5–10 mg (T40–80/A5–10) in patients with hypertension not at diastolic blood pressure (DBP) goal (DBP <90 mm Hg) after 6 weeks of amlodipine 5 mg monotherapy (A5) (TEAMSTA-5) or amlodipine 10 mg monotherapy (A10) (TEAMSTA-10). The long-term (≥6 months) tolerability and efficacy of single-pill combinations of T40–T80/A5–A10 have now been evaluated in 2 open-label studies in patients who had successfully completed either TEAMSTA-5 or TEAMSTA-10 (TEAMSTA-5 and TEAMSTA-10 Follow-Ups).
Methods
In the TEAMSTA-5 Follow-Up, 976 patients whose blood pressure was not initially controlled by taking A5 received T40/A5 for 4 or 8 weeks, with consecutive uptitration to T80/A5 if DBP was ≥90 mm Hg. In TEAMSTA-10 Follow-Up, 838 patients not initially achieving blood pressure control using A10 received T40/A10 for 4 weeks before randomization to T40/A10 or T80/A10; after 4 weeks, patients randomized to T40/A10 with DBP ≥90 mm Hg were uptitrated to T80/A10. In both studies, add-on antihypertensive medication was allowed if DBP was not at goal.
Results
Treatment compliance in both follow-up studies was ≥98.4%. Single-pill combinations of T40–T80/A5–A10 resulted in additional clinically relevant blood pressure reductions and 67% to 93% of patients achieved DBP goal (<90 mm Hg); only 1% to 19% of patients received additional medication for hypertension, of whom 29% to 76% achieved DBP goal. Long-term treatment with T40–T80/A5–A10 was well tolerated, with comparable adverse event profiles for all telmisartan/amlodipine combinations. The most common drug-related adverse events were peripheral edema (1.9%–3.9%) and dizziness (1.5% in the T80/A5 group only); these were consistent with the known tolerability profiles of telmisartan/amlodipine combinations. Overall treatment discontinuation rates due to adverse events were low (0.7%–1.5%).
Conclusions
In patients not achieving DBP goal with either A5 or A10 monotherapy, the vast majority achieved DBP goal with single-pill combinations of T40–T80/A5–A10. Long-term treatment was well tolerated with high compliance, promoting treatment adherence regardless of telmisartan/amlodipine dose. ClinicalTrials.gov identifiers: NCT00614380 (TEAMSTA-5 Follow-up) and NCT00624052 (TEAMSTA-10 Follow-up).
Key words: amlodipine, angiotensin II receptor blocker, calcium channel blocker, essential hypertension, single-pill combination, telmisartan
Introduction
To avoid increased risk of cardiovascular (CV) morbidity and mortality, adequate and sustained control of blood pressure (BP) is essential, even in the absence of evidence of target organ damage (TOD).1 Major guidelines consistently recommend the initiation of antihypertensive treatment for all patients with systolic blood pressure (SBP)/diastolic blood pressure (DBP) >140/90 mm Hg, with adjustment of treatment to achieve a BP goal of ≤140/90 mm Hg.2–8 For patients with diabetes or renal disease, guidelines recommend even lower targets of <130/80 mm Hg. Although recent evidence from outcome studies like the Action to Control CardiOvascular Risk in Diabetes (ACCORD) trial questions this paradigm, at least for patients with diabetes and established TOD,9 there is still a common understanding that these patients should be treated toward the target of 130/80 mm Hg. Thus, most patients will require combination therapy to achieve their goal BP.10 In patients with SBP >20 mm Hg and/or DBP >10 mm Hg above goal, combination therapy is even regarded as initial therapy by guidelines worldwide.1
The dihydropyridine calcium channel blocker (CCB) amlodipine is well established, effective, and the longest-acting antihypertensive agent of its class, and there is extensive evidence of its efficacy in CV outcome studies, including the Avoiding Cardiovascular events through COMbination therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) study that showed a significant reduction of CV events and death in patients with hypertension at high CV risk.11–15 Nevertheless, clinical studies have consistently found that >50% of patients may fail to achieve adequate BP control when treated with amlodipine 5 mg (A5) monotherapy.10,16 Uptitration from A5 to the highest marketed dose of amlodipine (10 mg [A10]) may result in DBP goal being achieved in 60% to 87% of patients; however, a substantial proportion of patients (approximately 20%) discontinue treatment as a result of side effects, such as peripheral edema.17–21 The angiotensin II receptor blocker (ARB) telmisartan and the CCB amlodipine have complementary and synergistic modes of action. Therefore, combination treatment results in superior BP reductions than either monotherapy in Stage 1 and 2 hypertension across a range of different patient types, including those with added risk factors such as diabetes, obesity, or metabolic syndrome.22 Furthermore, T40–T80/A5–A10 provides a BP goal (<140/90 mm Hg) achievement rate of up to 87% after 8 weeks.22 In an 8-week ambulatory BP monitoring study with T40–T80/A5–A10, 82.7% of patients achieved ambulatory BP control as defined by the American Heart Association guidelines (ie, <130/80 mm Hg)6 for the full 24 hours.23 In patients with severe hypertension (SBP ≥180), T80/A10 resulted in superior SBP reductions of up to 49.5 mm Hg after 8 weeks, with 80% of the maximum effect being achieved after 2 weeks.24 Furthermore, the tolerability profile of the telmisartan/amlodipine combination was similar to placebo, and the incidence of peripheral edema was up to 90% lower with the combination compared with A10 monotherapy. CCB-induced peripheral edema is caused by capillary hypertension during upright posture,25 and renin angiotensin system blockade with ARBs is known to reduce the effect by venous dilation.25–27 The unique pharmacologic profile of telmisartan,28,29 with the longest half-life of approximately 24 hours, the longest receptor binding, and the highest lipophilicity, contribute to its effective 24-hour BP control and beyond that sets it apart from other ARBs, such as valsartan.30 The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial Program (ONTARGET) has shown that telmisartan provides CV protection in high-risk patients with and without hypertension.31,32 Telmisartan has a placebo-like safety and tolerability profile,33 and based on the ONTARGET it is the only ARB indicated for CV prevention (ie, reduction of CV morbidity in patients with manifest atherothrombotic disease and CV disease, such as myocardial infarction and stroke, and in patients with type 2 diabetes and with TOD).34
In an 8-week, randomized, double-blind, controlled study of 1046 patients with hypertension not at BP goal (DBP <90 mm Hg) after 6 weeks of A5 monotherapy (ie, TElmisartan plus AMlodipine Study-Amlodipine 5 mg [TEAMSTA-5]), single-pill combinations (SPCs) of T40/A5 and T80/A5 were superior to A5 and to A10 in BP reduction as well as in the rate of achievement of BP goal, and with significantly (up to 87%) less peripheral edema with the telmisartan/amlodipine combination compared with A10 (4.3% vs 27.2%; P<0.0001).35 In another 8-week, randomized, double-blind, controlled study of 947 patients not at DBP goal after 6 weeks of A10 monotherapy (ie, TElmisartan plus AMlodipine Study-Amlodipine 10 mg [TEAMSTA-10]), T40/A10 and T80/A10 SPCs were superior to A10 in BP reduction as well as the rate of achievement of BP goal.36
Hypertension and associated CV risk is a chronic condition, so antihypertensive treatments are typically administered for long periods of time. With chronic treatments, tolerability is a critical factor for patient adherence, and effective treatments must combine sustained efficacy with good tolerability. Open-label follow-up studies (TEAMSTA-5 and TEAMSTA-10 Follow-Ups) have now evaluated the long-term (≥6 months) tolerability and efficacy of T40–T80/A5–A10 SPCs in patients who successfully completed either TEAMSTA-5 or TEAMSTA-10. The studies were designed in accordance with effective hypertension treatment guidelines,1–8 in that uptitration and additional antihypertensive medication was introduced at the discretion of the physician if patients were not achieving adequate control. This article reports the results from these open-label, follow-up trials—the first to report the long-term tolerability and efficacy of telmisartan/amlodipine SPC therapy.
Methods
Study Designs
Both of the 34-week, open-label, multicenter, multinational studies conformed to the principles of the Declaration of Helsinki (1996 version) and were approved by the local institutional review boards or independent ethics committees, plus the Competent Authority for European countries (or local laws and regulations in the other countries) of all participating centers before the start of the studies. From the TEAMSTA-5 study, 976 patients were enrolled in the TEAMSTA-5 Follow-Up study and were treated according to the design outlined in Figure 1A. In the TEAMSTA-10 Follow-Up study, all 838 patients enrolled upon completion of the TEAMSTA-10 study received T40/A10 at Visit 1 for 4 weeks, and were subsequently treated according to the design outlined in Figure 1B. Patients in both studies were advised to take their medication in the morning, at the same time each day. On the morning of clinic visit days, medication was taken after BP measurement. The duration of treatment in both studies was a minimum of 6 months with 6 visits. The rationale and primary aim of both follow-up studies were to collect long-term tolerability and efficacy data in a daily routine setting for all relevant telmisartan/amlodipine SPCs (with or without additional antihypertensive treatment) marketed: T40/A5, T80/A5, T40/A10, and T80/A10.
Figure 1.
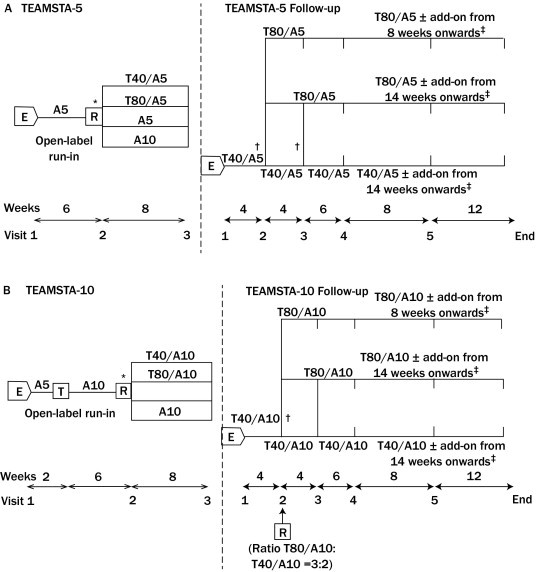
Design of (A) TEAMSTA-5 and TEAMSTA-5 Follow-Up and (B) TEAMSTA-10 and TEAMSTA-10 Follow-Up studies evaluating the safety and efficacy of telmisartan 40 mg/amlodipine 5 mg (T40/A5), telmisartan 80 mg/amlodipine 5 mg (T80/A5), telmisartan 40 mg/amlodipine 10 mg (T40/A10), and telmisartan 80 mg/amlodipine 10 mg (T80/A10) single-pill combinations with or without additional antihypertensive medication (add-on). E= enrollment; R = randomization; T = treatment. *Patients could only be randomized if diastolic blood pressure (DBP) was not adequately controlled after 6 weeks of A5 or A10 monotherapy (ie, DBP ≥90 mm Hg); †At these visits, patients were uptitrated to T80/A5 or T80/A10 if DBP ≥90 mm Hg (ie, response-driven titration); ‡Add-on therapy could be given if trough DBP ≥90 mm Hg.
Patients
Both follow-up studies included adult (≥18 years) patients of either gender with essential hypertension before they entered the preceding double-blind studies (ie, TEAMSTA-5 and TEAMSTA-10) (mean seated cuff DBP ≥95 mm Hg if receiving antihypertensive treatment before, or mean seated cuff DBP ≥100 mm Hg in treatment-naïve patients). Patients had to be enrolled to the open-label follow-up study within 14 days of completing the respective double-blind TEAMSTA-5 or TEAMSTA-10 study and were required to provide additional written informed consent. The following exclusion criteria applied to both TEAMSTA-5 and TEAMSTA-10 Follow-Up studies: mean seated cuff SBP ≥180 mm Hg and/or mean seated cuff DBP ≥120 mm Hg; history of poor compliance during the double-blind respective TEAMSTA study (ie, <80% or >120% of the specified medication intake during the course of the study); any medical condition during the preceding TEAMSTA-5 or TEAMSTA-10 study that could worsen if treated with the study SPCs; pregnant, nursing, or premenopausal women not using adequate birth control; clinically significant hepatic impairment, severe renal impairment, congestive heart failure, obstructive cardiomyopathy, or aortic or mitral valve stenosis, and hypersensitivity to any component of the study medication.
Tolerability Evaluations
Reported adverse events (coded according to the Medical Dictionary for Regulatory Activities version 11.0), including reported or diagnosed peripheral edema, laboratory parameters, and vital signs were recorded at each visit throughout the follow-up studies. The intensity, seriousness, and relationship to study drug (in the opinion of the investigator) of all adverse events were documented. Compliance was measured by counting returned medications at each visit.
Efficacy Evaluations
Seated in-clinic trough (24 hours postdose) cuff BP was measured using a sphygmomanometer or other validated device at all visits. The primary efficacy end point was the proportion of patients at DBP goal (mean seated trough cuff DBP <90 mm Hg at end of study (Week 34, Visit 6). Secondary efficacy end points included: mean change in seated trough cuff SBP and DBP from Visit 1 at study end, proportions of patients achieving BP goal (mean seated trough cuff SBP and DBP <140/90 mm Hg) at study end, and proportions of patients requiring uptitration and additional antihypertensive medication at study end.
Statistical Analysis
Tolerability was assessed for all patients who took any dose of a T40–T80/A5 or T40–T80/A10 SPC. Efficacy analysis was performed in patients who took any of the T40–T80/A5 or T40–T80/A10 SPCs and for whom at least 1 on-treatment BP efficacy measurement was available (with last observation carried forward). Due to this being an open-label extension study, there were no hypotheses tested and no formal statistics conducted. Descriptive statistics comprised mean, standard deviation, minimum, median, and maximum for the analysis of continuous end points. For the analysis of categorical end points the number in each category and percentage were presented.
Results
Demographics and Patient Characteristics
Patients in the TEAMSTA-5 Follow-Up study were enrolled at 120 centers in Europe (ie, Belgium, Denmark, Finland, France, the Netherlands, Norway, and Sweden), Asia (ie, Korea, Philippines, and Taiwan), Canada, and South Africa. Those in the TEAMSTA-10 Follow-Up study were enrolled at 66 centers in Europe (ie, Austria, Bulgaria, Czech Republic, Ireland, Italy, Russia, Slovakia, Spain, UK, and Ukraine), Australia, and New Zealand. The demographics and characteristics of patients who successfully completed the preceding double-blind TEAMSTA-5 and TEAMSTA-10 studies and who were enrolled to the TEAMSTA-5 and TEAMSTA-10 Follow-Up studies are summarized in Table I and are also published elsewhere.35,36
Table I.
Demographics and characteristics of patients enrolled to TElmisartan plus AMlodipine STudy (TEAMSTA) Follow-Up studies.
| Variable | TEAMSTA-5 Follow-Up |
TEAMSTA-10 Follow-Up |
|---|---|---|
| Overall (N = 976) | Overall (N = 838) | |
| ← - - - - - - - - - - - - - - Mean (SD) - - - - - - - - - - - - - - → | ||
| SBP, mm Hg | 137.9 (13.9) | 138.2 (10.9) |
| DBP, mm Hg | 88.1 (8.7)† | 87.7 (6.8)⁎ |
| Age‡, y | 53.9 (10.6) | 56.4 (9.7) |
| BMI, kg/m2‡ | 29.2 (5.3) | 30.2 (4.6) |
| ← - - - - - - - - - - - - - - - - n (%) - - - - - - - - - - - - - - - - → | ||
| DBP at goal§ | 522 (54.1)† | 497 (59.5)⁎ |
| SBP/DBP at goal∥ | 381 (39.5)† | 372 (44.6)⁎ |
| Age ≥65‡, y | 162 (16.6) | 172 (20.5) |
| Males | 611 (62.6) | 471 (56.2) |
| Raceঠ| ||
| White | 746 (76.4) | 833 (99.4) |
| Asian | 211 (21.6) | 1 (0.1) |
| Black | 15 (1.5) | 1 (0.1) |
| Other | 4 (0.4) | 3 (0.3) |
| Duration of hypertension‡ | ||
| <1 y | 277 (28.4) | 105 (12.5) |
| <1–5 y | 340 (34.8) | 269 (32.1) |
| 6–10 y | 186 (19.1) | 230 (27.4) |
| >10 y | 173 (17.7) | 234 (27.9) |
| Diabetes‡ | 56 (5.7) | 77 (9.2) |
Add-on = additional antihypertensive medication; BMI = body mass index; DBP = diastolic blood pressure; SBP = systolic blood pressure; T40/A5 = telmisartan 40 mg plus amlodipine 5 mg; T40/A10 = telmisartan 40 mg plus amlodipine 10 mg; T80/A5 = telmisartan 80 mg plus amlodipine 5 mg; T80/A10 = telmisartan 80 mg plus amlodipine 10 mg.
n = 835.
n = 965.
Recorded at baseline of respective TEAMSTA study.
<90 mm Hg.
<140/90 mm Hg.
Not recorded in 2 patients in TEAMSTA-10.
Patient Disposition
In the preceding TEAMSTA-5 and TEAMSTA-10 studies, 1097 and 947 patients were randomized and treated, respectively, of whom 51 and 42 patients prematurely discontinued. Of those who completed the studies, 976 and 838 patients were enrolled in the TEAMSTA-5 and TEAMSTA-10 Follow-Up studies, respectively.35,36 Of the original trial study centers, 18 (out of 226) did not participate in this follow-up. The dispositions of patients enrolled to the TEAMSTA-5 and TEAMSTA-10 Follow-Up studies are shown in Table II. Treatment compliance in both follow-up studies was ≥98.4%. Of 976 enrolled patients in the TEAMSTA-5 Follow-Up study, 589 (60.3%) patients remained on T40/A5 throughout the study, with 564 (57.8%) of these patients receiving T40/A5 only and 25 (2.6%) receiving T40/A5 plus additional antihypertensive medication. The remaining 387 (39.7%) patients were uptitrated to T80/A5—206 (21.1%) of these patients receiving T80/A5 only and 181 (18.5%) receiving T80/A5 plus additional antihypertensive medication. Of 838 patients enrolled in the TEAMSTA-10 Follow-Up study, 219 (26.1%) were randomized to T40/A10 and 527 (62.9%) were either randomized to T80/A10 (n = 436; 52.0%) at Visit 2 or underwent response-driven uptitration from T40/A10 to T80/A10 (n = 91; 10.9%) at Visit 3. In addition, 92 (11.0%) patients received either T40/A10 (n = 8; 1.0%) or T80/A10 (n = 84; 10.1%) plus additional antihypertensive medication. In both follow-up studies, diuretics were the most commonly prescribed additional antihypertensive medication (Table III).
Table II.
Dispositions of patients enrolled to the TElmisartan plus AMlodipine STudy (TEAMSTA) Follow-Up studies.
| Visit | TEAMSTA-5 Follow-Up |
TEAMSTA-10 Follow-Up |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall⁎ | T40/A5⁎ | Uptitrated to T80/A5 | Uptitrated to T80/A5 + add-on | T40/A5 + add-on | Overall† | T40/A10† | T40/A10 + add-on | T80/A10 | T80/A10 + add-on | |
| ← - - - - - - - - - - - - - - n (%) - - - - - - - - - - - - - - → | ||||||||||
| 1 (week 0) | 976 (100) | 976 (100) | 0 | 0 | 0 | 838 (100) | 838 (100) | 0 | 0 | 0 |
| 2 (week 4) | 959 (100) | 959 (100) | 0 | 0 | 0 | 831 (100) | 831 (100) | 0 | 0 | 0 |
| 3 (week 8) | 952 (100) | 738 (67.0) | 313 (32.9) | 0 | 1 (0.1) | 822 (100) | 336 (40.9) | 0 | 486 (50.1) | 0 |
| 4 (week 14) | 949 (100) | 575 (60.5) | 257 (27.1) | 116 (12.2) | 1 (0.1) | 818 (100) | 217 (26.5) | 3 (0.4) | 554 (67.7) | 44 (5.4) |
| 5 (week 22) | 939 (100) | 550 (58.6) | 210 (22.4) | 164 (17.5) | 15 (1.5) | 811 (100) | 208 (25.6) | 8 (1.0) | 518 (63.9) | 77 (9.5) |
| End of study | 976 (100) | 564 (57.6) | 206 (21.1) | 181 (18.5) | 25 (2.6) | 838 (100) | 219 (26.1) | 8 (1.0) | 527 (62.9) | 84 (10.0) |
Add-on = additional antihypertensive medication; T40/A5 = telmisartan 40 mg plus amlodipine 5 mg; T80/A5 = telmisartan 80 mg plus amlodipine 5 mg; T40/A10 = telmisartan 40 mg plus amlodipine 10 mg; T80/A10 = telmisartan 80 mg plus amlodipine 10 mg.
At each visit, the number of patients is reflecting the treatment that the patient was allocated to at the preceding visit. Eleven patients were excluded from the efficacy analysis due to no blood pressure measurement at Visits 2–5 or end of study.
At each visit, the number of patients is reflecting the treatment that the patient was allocated to at the preceding visit. Three patients were excluded from the efficacy analysis due to no blood pressure measurement at Visits 2–5 or end of study.
Table III.
Additional antihypertensive medication received by patients during TElmisartan plus AMlodipine STudy (TEAMSTA) Follow-Up studies.
| Class of Antihypertensive Medication | T40–80/A5 (n = 206) | T40–80/A10 (n = 92) |
|---|---|---|
| ← - - - - - - - - - No. of patients (%) - - - - - - - - - → | ||
| Diuretics | 161 (78.2) | 59 (64.1) |
| β-blockers | 59 (28.4) | 23 (25.0) |
| ACE inhibitors | 2 (1.0) | 3 (3.3) |
| Other ARBs | 1 (0.5) | 1 (1.1) |
| Other CCBs | 2 (1.0) | 0 |
| Other | 20 (9.7) | 21 (22.8) |
ACE = angiotensin-converting enzyme; ARBs = angiotensin II receptor blockers; CCBs = calcium channel blockers; T40–80/A5 = telmisartan 40–80 mg plus amlodipine 5 mg; T40–80/A10 = telmisartan 40–80 mg plus amlodipine 10 mg.
Tolerability
In the TEAMSTA-5 Follow-Up study, 381 (39.0%) and 201 (50.6%) patients, respectively, experienced adverse events during treatment with T40/A5 and T80/A5 (Table IV). As a consequence of the design of the study, mean duration of exposure to T40/A5 was shorter. When adjusted for duration of treatment, incidences per 100 patient-years were comparable for T40/A5 and T80/A5: 94.5 and 96.7, respectively. In the TEAMSTA-10 Follow-Up study, 102 (12.2%) and 157 (25.7%) patients, respectively, experienced adverse events during treatment with T40/A10 and T80/A10. Again, mean duration of exposure to T40/A10 was shorter. Adjustment for the duration of treatment resulted in comparable incidences per 100 patient-years for T40/A10 and T80/A10: 50.2 and 46.3, respectively. In both open-label studies, adverse events were generally mild or moderate in intensity: incidences of severe adverse events with T40/A5, T80/A5, T40/A10 and T80/A10, respectively, were 1.7% (incidence per 100 patient-years: 4.2), 2.3% (incidence per 100 patient-years: 4.3), 0.6% (incidence per 100 patient-years: 2.5), and 1.0% (incidence per 100 patient-years: 1.8).
Table IV.
Summary of reported adverse events after long-term, open-label treatment with telmisartan plus amlodipine single-pill combinations.
| Adverse Event | Combined |
TEAMSTA-5 Follow-Up |
TEAMSTA-10 Follow-Up |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T40/A5-10 (n = 1814) |
T80/A5-10 (n = 1008) |
T40/A5 (n = 976) |
T80/A5 (n = 397) |
T40/A10 (n = 838) |
T80/A10 (n = 611) |
|||||
| Incidence Per 100 Patient-Years | Incidence Per 100 Patient-Years | n (%) | Incidence Per 100 Patient-Years | n (%) | Incidence Per 100 Patient-Years | n (%) | Incidence Per 100 Patient-Years | n (%) | Incidence Per 100 Patient-Years | |
| Patients with any adverse event | 72.4 | 71.5 | 381 (39.0) | 94.5 | 201 (50.6) | 96.7 | 102 (12.2) | 50.2 | 157 (25.7) | 46.3 |
| Patients with severe adverse event | 17 (1.7) | 4.2 | 9 (2.3) | 4.3 | 5 (0.6) | 2.5 | 6 (1.0) | 1.8 | ||
| Patients with study-drug related adverse event | 13.2 | 12.8 | 51 (5.2) | 12.6 | 30 (7.6) | 14.4 | 28 (3.3) | 13.8 | 38 (6.2) | 11.2 |
| Patients with study-drug related adverse event | 13.2 | 12.8 | 51 (5.2) | 12.6 | 30 (7.6) | 14.4 | 28 (3.3) | 13.8 | 38 (6.2) | 11.2 |
| Treatment-related adverse events with incidence >1% | ||||||||||
| Peripheral edema | 6.8 | 6.2 | 23 (2.4) | 5.7 | 11 (2.8) | 5.3 | 16 (1.9) | 7.9 | 24 (3.9) | 7.1 |
| Dizziness | – | 4.1 | – | – | 6 (1.5) | 2.9 | – | – | – | – |
| Patients with adverse events leading to discontinuation | 3.0 | 2.3 | 12 (1.2) | 3.0 | 4 (1.0) | 1.9 | 6 (0.7) | 3.0 | 9 (1.5) | 2.7 |
| Patients with serious adverse events | 3.8 | 3.4 | 22 (2.3) | 5.5 | 6 (1.5) | 2.9 | 4 (0.5) | 2.0 | 13 (2.1) | 3.8 |
T40/A5 = telmisartan 40 mg plus amlodipine 5 mg; T80/A5 = telmisartan 80 mg plus amlodipine 5 mg; T40/A10 = telmisartan 40 mg plus amlodipine 10 mg; T80/A10 = telmisartan 80 mg plus amlodipine 10 mg.
In total, 51 (5.2%) and 30 (7.6%) patients, respectively, experienced study drug-related adverse events during treatment with T40/A5 and T80/A5 (Table IV). After adjustment for the duration of treatment, incidences were 12.6 and 14.4 patients per 100 patient-years, respectively, for T40/A5 and T80/A5. There were 28 (3.3%) and 38 (6.2%) patients, respectively, who experienced drug-related adverse events during treatment with T40/A10 and T80/A10, which amounted to duration-adjusted incidences of 13.8 and 11.2 patients per 100 patient-years, respectively. Discontinuation due to adverse events occurred in 12 (1.2%), 4 (1.0%), 6 (0.7%), and 9 (1.5%) patients, respectively, while in receipt of T40/A5, T80/A5, T40/A10, and T80/A10; duration-adjusted incidences were 3.0, 1.9, 3.0, and 2.7 patients per 100 patient-years, respectively. Serious adverse events occurred in 22 (2.3%), 6 (1.5%), 4 (0.5%), and 13 (2.1%) patients, respectively, while being treated with T40/A5, T80/A5, T40/A10, and T80/A10; respective duration-adjusted incidences were 5.5, 2.9, 2.0, and 3.8 patients per 100 patient-years. With the exception of 1 case of hypotonia (decreased muscle tone) in a patient receiving T80/A5, none of the serious adverse events were considered related to study drug treatment.
The most common treatment-related adverse event was peripheral edema (Table IV), which was reported in 2.4%, 2.8%, 1.9%, and 3.9% of patients, respectively, while receiving T40/A5, T80/A5, T40/A10, and T80/A10. Adjustment for the duration of treatment yielded incidences of 5.7, 5.3, 7.9, and 7.1 patients per 100 patient-years, respectively. The peripheral edema in 1 patient receiving T40/A10 and in 2 receiving T80/A10 resulted in treatment discontinuation. It should be noted that all patients in TEAMSTA-10 Follow-Up study had previously received A5 monotherapy for 2 weeks plus A10 monotherapy for 6 weeks in the run-in of TEAMSTA-10, during which 7.0% of patients discontinued due to this treatment-related adverse event.35 Also in TEAMSTA-5, 1.3% had discontinued A5 run-in treatment due to peripheral edema.36 There were no reports of treatment-related syncope in the long-term follow-up studies and only 2 patients experienced orthostatic hypotension, which in both instances was considered to be related to T40/A5 treatment.
Combining tolerability data from TEAMSTA-5 and -10 Follow-Up studies suggested that the duration-adjusted incidences of drug-related adverse events, drug-related peripheral edema, adverse events leading to premature discontinuations, and serious adverse events were comparable in patients treated with T40/A5–A10 or T80/A5–A10 (Figure 2).
Figure 2.
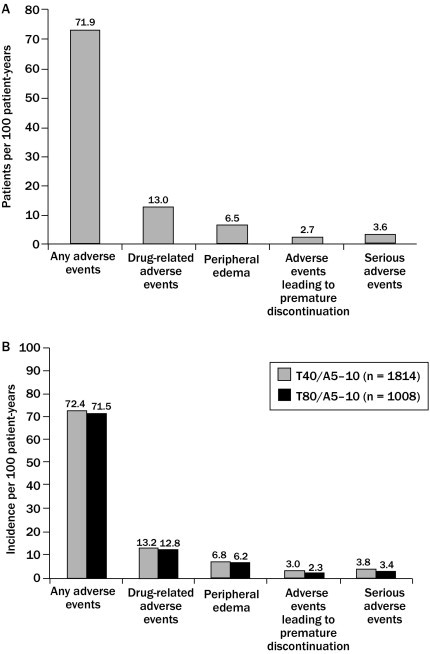
Comparison of the duration-adjusted (A) patients per 100 patient years and (B) incidence per 100 patient years of drug-related adverse events, peripheral edema, adverse events leading to premature discontinuation, and serious adverse events in patients receiving long-term, open-label treatment with telmisartan 40 mg/amlodipine 5–10 mg (T40/A5–10) and patients receiving telmisartan 80 mg/amlodipine 5–10 mg (T80/A5–10) (data for TEAMSTA-5 [n = 1814] and −10 [n = 1008] Follow-Up; pooled post-hoc analysis).
No clinically relevant changes were reported in laboratory parameters or vital signs in patients enrolled to either of the open-label TEAMSTA-5 and -10 Follow-Up studies.
Efficacy
Overall, 767 (79.5%) of 965 patients evaluated for efficacy of T40–T80/A5-based regimens in the TEAMSTA-5 Follow-Up study achieved the mean seated trough cuff goal DBP of <90 mm Hg at study end (Visit 6) (Figure 3A). In 553 patients treated with T40/A5 only, 504 (91.1%) achieved DBP goal. In 206 patients uptitrated to T80/A5, 160 (77.7%) achieved DBP goal. In 181 patients uptitrated to T80/A5 and who needed additional antihypertensive medication, and in 25 receiving T40/A5 plus additional antihypertensive medication, respectively, 84 (46.4%) and 19 (76.0%) achieved DBP goal. Overall, 745 (89.2%) of 835 patients evaluated for efficacy of T40–T80/A10-based regimens in the TEAMSTA-10 Follow-Up study achieved the mean seated trough cuff goal DBP of <90 mm Hg at study end (Visit 6) (Figure 3C). In 216 patients treated with T40/A10 only and 436 randomized to T80/A10 without receipt of additional antihypertensive medication, respectively, 201 (93.1%) and 402 (92.2%) achieved DBP goal. In 91 patients who were uptitrated to T80/A10, 72 (79.1%) achieved DBP goal at study end. In 92 patients receiving T40–T80/A10 who needed additional antihypertensive medication, 70 (76.1%) achieved DBP goal at study end.
Figure 3.
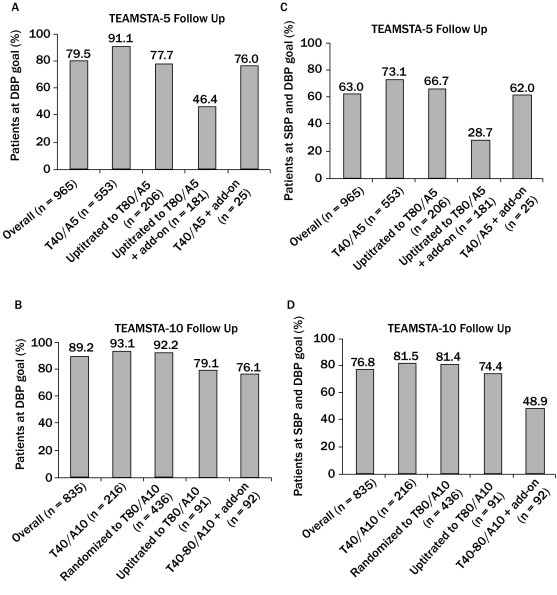
Proportions of patients achieving (A and B) diastolic blood pressure (DBP) goal (mean seated trough cuff DBP <90 mm Hg) and (C and D) systolic blood pressure (SBP) goal (mean seated trough cuff SBP/DBP <140/90 mm Hg) after long-term, open-label treatment with telmisartan 40–80 mg/amlodipine 5 mg (T40–T80/A5) single-pill combinations with or without additional antihypertensive medication (add-on), and telmisartan 40–80 mg/amlodipine 10 mg (T40–T80/A10) single-pill combinations with or without add-on.
Overall, 608 (63.0%) of 965 evaluable patients treated with T40–T80/A5-based treatment regimens achieved mean seated trough cuff BP goal of <140/90 mm Hg at study end. In 553 patients receiving T40/A5 only, 418 (73.1%) achieved the BP goal (Figure 3B). In 206 patients uptitrated to T80/A5 but not receiving additional antihypertensive medication, 130 (66.7%) achieved BP goal. Among 835 patients treated with T40–T80/A10-based treatment regimen, overall 641 (76.8%) achieved BP goal at study end (Figure 3D). In 216 patients receiving T40/A10 and the 436 randomized to T80/A10, respectively, 176 (81.5%) and 355 (81.4%) achieved BP goal. In 91 patients initially treated with T40/A10 and subsequently uptitrated to T80/A10, 65 (71.4%) achieved BP goal. In 92 patients in whom additional antihypertensive medication was also prescribed, 55 (59.8%) achieved BP goal.
At the start of TEAMSTA-5 Follow-Up (Visit 1), in 965 patients evaluable for efficacy (the full analysis set); mean seated trough cuff SBP/DBP was 137.9/88.1 mm Hg (Figure 4A). Mean seated trough cuff SBP/DBP was further decreased by –5.0/–4.4 mm Hg to 132.9/83.7 mm Hg at study end (Visit 6) in the overall population. At the start of TEAMSTA-10 Follow-Up, in the 835 patients evaluable for efficacy, mean seated trough cuff SBP/DBP was 138.2/87.7 mm Hg. Mean seated trough cuff SBP/DBP was further decreased by –5.7/–4.9 mm Hg to 132.5/82.8 mm Hg at study end (Visit 6) in the overall population (Figure 4B).
Figure 4.
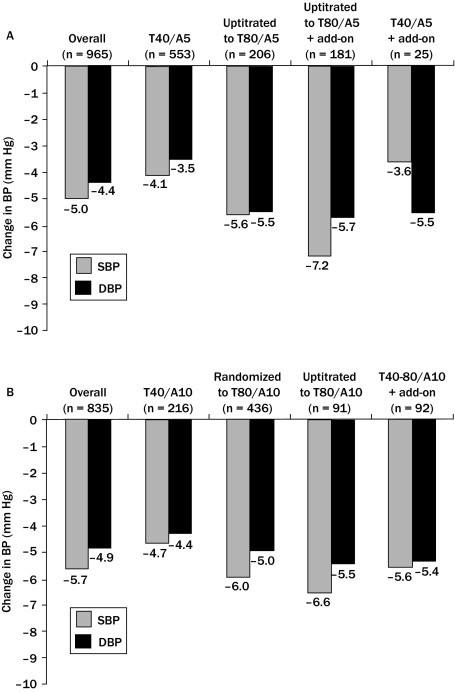
Changes in mean seated trough cuff blood pressure (BP) in patients receiving long-term, open-label treatment with (A) telmisartan 40–80 mg/amlodipine 5 mg (T40–T80/A5) single–pill combinations with or without additional antihypertensive medication (add-on), and (B) telmisartan 40–80 mg/amlodipine 10 mg (T40–T80/A10) single-pill combinations with or without add-on. DBP = Diastolic blood pressure; SBP = systolic blood pressure.
Discussion
Treatment compliance to antihypertensive medication is crucial if long-term benefit, in terms of reduced CV morbidity and mortality, is to be gained. Tolerability is another important contributory factor to patients' compliance with their medication.37 Hence, physicians select therapy with proven efficacy from the findings of short-term, well-monitored clinical studies and proven long-term safety, tolerability and efficacy, demonstrated in long-term studies in a real-life, office-based setting. Two 8-week, double-blind randomized studies (ie, TEAMSTA-5 and TEAMSTA-10) showed that SPCs of T40–T80/A5 and T40–T80/A10, in addition to providing superior BP reductions compared with A5 or A10 monotherapy (in patients who previously did not respond to A5 or A10 monotherapy), were also better tolerated.35,36 The subsequent ≥6 months, open-label, follow-up studies evaluating the tolerability and efficacy of T40–T80/A5 and T40–T80/A10 SPC treatments reflect the situation occurring in routine clinical practice, requiring the investigators to perform response-driven uptitration and also allowing them the option of prescribing additional antihypertensive medication if a patient's condition proved more difficult to treat.
Both the TEAMSTA-5 and TEAMSTA-10 Follow-Up studies reported on here suggested that T40–T80/A5 and T40–T80/A10 SPCs were well tolerated during long-term treatment. The majority of adverse events were mild or moderate in intensity, and when adjusted for duration of exposure the frequencies of events were very similar for all 4 of the telmisartan/amlodipine SPCs evaluated. The adverse event profiles were consistent with the known tolerability profile of telmisartan plus amlodipine reported in recent studies and very similar between TEAMSTA-5 and TEAMSTA-10. In a placebo-controlled, factorial-design study,38 the combination exhibited a safety and tolerability profile similar to placebo, with the most common adverse events being peripheral edema and headache. Peripheral edema, particularly of the lower limbs, is a common adverse event associated with CCBs—including amlodipine—that may lead to poor treatment compliance or even treatment discontinuation.39 The proportion of patients who experienced peripheral edema considered to be drug related during the long-term, open-label treatment with T40–T80/A5 and T40–T80/A10 SPCs in our studies was small, and peripheral edema was only rarely cited as a reason for premature discontinuation of treatment. The incidence of severe adverse events was low but slightly more frequent in the TEAMSTA-5 Follow-Up compared with TEAMSTA-10 Follow-Up. One serious adverse event (hypotonia in 1 patient treated with T40/A5) was reported as related to treatment; all other serious adverse events were reported as not related to treatment. The difference in adverse events incidences between the studies might be due to the different populations as a result of the run-in filter phases of the respective preceding short-term studies. There was no evidence that the antihypertensive efficacy of telmisartan/amlodipine SPCs could result in syncope or serious orthostatic hypertension. No clinically relevant changes in laboratory findings and vital signs were reported during either follow-up study. These observations suggest that there are no particular tolerability concerns associated with long-term treatment with T40–T80/A5 and T40–T80/A10 SPCs and that, in routine clinical practice, prescribing telmisartan/amlodipine SPCs may facilitate treatment compliance and result in improved control of BP and the consequent reduction of CV risk. Although the true extent of treatment compliance is difficult to establish, it has been suggested that noncompliance may be the cause of about half of the so-called failures to respond to antihypertensive therapy in day-to-day clinical practice.40 It must be acknowledged that some patients with amlodipine-related adverse events were excluded from the TEAMSTA-5 and TEAMSTA-10 Follow-Up studies because of their inability to tolerate amlodipine monotherapy and consequent premature discontinuation during the run-in period of the preceding TEAMSTA-5 and TEAMSTA-10 studies.
These follow-up studies also suggested that the long-term use of T40–T80/A5 and T40–T80/A10 SPCs resulted in the majority of patients achieving DBP and BP goals (mean seated trough cuff <90 mm Hg and <140/90 mm Hg, respectively). In addition, we found that substantial additional reductions in SBP/DBP were achieved by the end of these long-term, open-label, follow-up studies. Approximately 21% of patients required an uptitration from T40/A5 to T80/A5 and approximately 11% from T40/A10 to T80/A10 because of inadequate response to the lower dose. At the end of the study, only a minority of patients (21.1% in TEAMSTA-5 Follow-Up and 10.9% in TEAMSTA-10 Follow-Up) were receiving additional antihypertensive medication (mainly diuretics, 64.1%–78.5%). These findings may reflect the long-term efficacy of the T/A SPCs to treat patients to BP goal. It appears that the tolerability profile is comparable, regardless of the telmisartan dose of 40 or 80 mg. Importantly, the reduced need for add-on antihypertensive medication with a telmisartan/amlodipine SPC may reduce the risk of additional drug-related adverse events and, consequently, improve treatment compliance.
One condition of these studies was that, due to their objectives to collect sufficient long-term tolerability and efficacy data on all relevant telmisartan/amlodipine SPCs (ie, T40/A5, T40/A10, T80/A5, and T80/A10), an uptitration was not always performed before additional antihypertensive medication was introduced. The overall BP goal rate achieved might have been even higher if uptitration to T80/A10 had been performed in all patients in the TEAMSTA-10 study who had not reached their BP goal. In patients receiving T80/A10 in the TEAMSTA-10 Follow-Up study, only 10% received additional antihypertensive medication by study end. By contrast, in the TEAMSTA-5 Follow-Up study, 18.5% of patients uptitrated to T80/A5 received additional antihypertensive medication.
These studies have potential limitations. Unlike the 2 preceding trials, the follow-up studies each had an open-label design. Data from open-label trials should be interpreted with caution. However, the follow-up studies do provide important long-term tolerability and efficacy data, which are consistent with previous observations in short-term studies with this combination.
Conclusions
In open-label follow-up studies, the telmisartan/amlodipine SPCs exhibited good long-term tolerability profiles that were comparable, regardless of the telmisartan dose (40 or 80 mg), and that were similar to the tolerability profiles observed in recent double-blind, placebo-controlled studies. The most common drug-related adverse event was peripheral edema, which occurred in <4% of patients, of predominantly mild or moderate intensity. In patients initially not at DBP goal with A10 monotherapy (ie, those participating in TEAMSTA-10), the majority of patients were at DBP goal with a T40/A10 or T80/A10 SPC, with only approximately 10% receiving additional antihypertensive medication by the end of the follow-up study. In those patients who were initially not at DBP goal with A5 (ie, those participating in TEAMSTA-5), the majority of patients achieved DBP goal with a T40/A5 or T80/A5 SPC by the end of the follow-up study. Compliance was good throughout the study, which promotes treatment adherence and persistence. The overall conclusion is that, in patients not at BP goal with either A5 or A10, long-term treatment with SPCs of T40–80/A5–10 is well tolerated and most patients achieve sustained reductions in BP and BP goal.
Conflicts of Interest
The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgements
Writing and editorial assistance was provided by Ann Ralph, PhD, of PAREXEL, who was contracted by Boehringer Ingelheim International GmbH for these services. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors and were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development. The authors received no compensation related to the development of the manuscript. The studies reported were sponsored by Boehringer Ingelheim. Dr. Neldam received funding in his role as a trial investigator and has no other conflicts of interest. Drs. Lang and Edwards are employees of Boehringer Ingelheim and were the responsible managers for these studies. Dr. Jones is an employee of Boehringer Ingelheim and was the study statistician.
References
- 1.Mancia G., Laurent S., Agabiti-Rosei E. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization, International Society of Hypertension Writing Group 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian A.V., Bakris G.L., Black H.R. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 4.Graham I., Atar D., Borch-Johnsen K. European guidelines on cardiovascular disease prevention in clinical practice. Eur J Cardiovasc Prev Rehabil. 2007;14(Suppl 2):S1–S113. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G., De Backer G., Dominiczak A. 2007 ESH–ESC practice guidelines for the management of arterial hypertension. J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3282f0580f. [DOI] [PubMed] [Google Scholar]
- 6.Rosendorff C., Black H.R., Cannon C.P. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–2788. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 7.Ogihara T., Kikuchi K., Matsuoka H. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2009) Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- 8.Sanchez R.A., Ayala M., Baglivo H. Latin American guidelines on hypertension. J Hypertens. 2009;27:905–922. doi: 10.1097/HJH.0b013e32832aa6d2. [DOI] [PubMed] [Google Scholar]
- 9.ACCORD Study Group. Cushman W.C., Evans G.W. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tedesco M.A., Natale F., Calabrò R. Effects of monotherapy and combination therapy on blood pressure control and target organ damage: a randomized prospective intervention study in a large population of hypertensive patients. J Clin Hypertens. 2006;8:634–641. doi: 10.1111/j.1524-6175.2006.05504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitt B., Byington R.P., Furberg C.D. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. Circulation. 2000;102 doi: 10.1161/01.cir.102.13.1503. 1503–1110. [DOI] [PubMed] [Google Scholar]
- 12.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 13.Nissen S.E., Tuzcu E.M., Libby P. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292:2217–2225. doi: 10.1001/jama.292.18.2217. [DOI] [PubMed] [Google Scholar]
- 14.Dahlöf B., Sever P.S., Poulter N.R. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 15.Jamerson K., Weber M.A., Bakris G.L. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. New Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 16.Oparil S., Weber M. Angiotensin receptor blocker and dihydropyridine calcium channel blocker combinations: an emerging strategy in hypertension therapy. Postgrad Med. 2009;121:25–39. doi: 10.3810/pgm.2009.03.1974. [DOI] [PubMed] [Google Scholar]
- 17.Gaudio C., Ferri F.M., Giovannini M. Comparative effects of irbesartan versus amlodipine on left ventricular mass index in hypertensive patients with left ventricular hypertrophy. J Cardiovasc Pharmacol. 2003;42:622–628. doi: 10.1097/00005344-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Pepine C.J., Cooper-DeHoff R.M., Weiss R.J. Comparison of effects of nisoldipine-extended release and amlodipine in patients with systemic hypertension and chronic stable angina pectoris. Am J Cardiol. 2003;91:274–279. doi: 10.1016/s0002-9149(02)03154-5. [DOI] [PubMed] [Google Scholar]
- 19.Koenig W. Efficacy and tolerability of felodipine and amlodipine in the treatment of mild to moderate hypertension: a randomised double-blind multicentre trial. Drug Investig. 1993;5:200–205. [Google Scholar]
- 20.Kloner R.A., Weinberger M., Pool J.L. Comparative effects of candesartan cilexetil and amlodipine in patients with mild systemic hypertension: Comparison of Candesartan and Amlodipine for Safety, Tolerability and Efficacy (CASTLE) Study Investigators. Am J Cardiol. 2001;87:727–731. doi: 10.1016/s0002-9149(00)01491-0. [DOI] [PubMed] [Google Scholar]
- 21.Dahlöf B., Lindholm L.H., Carney S. Main results of the losartan versus amlodipine (LOA) study on drug tolerability and psychological general well-being. J Hypertens. 1997;15:1327–1335. doi: 10.1097/00004872-199715110-00018. [DOI] [PubMed] [Google Scholar]
- 22.Littlejohn T.W., 3rd, Majul C.R., Olvera R. Telmisartan plus amlodipine in patients with moderate or severe hypertension: results from a subgroup analysis of a randomized, placebo-controlled, parallel-group, 4 x 4 factorial study. Postgrad Med. 2009;121:5–14. doi: 10.3810/pgm.2009.03.1972. [DOI] [PubMed] [Google Scholar]
- 23.White W.B., Littlejohn T.W., Majul C.R. Effects of telmisartan and amlodipine in combination on ambulatory blood pressure in stages 1–2 hypertension. Blood Press Monit. 2010;15:205–212. doi: 10.1097/MBP.0b013e32833c5722. [DOI] [PubMed] [Google Scholar]
- 24.Neutel J.M., Mancia G., Black H.R. Single-pill combination of telmisartan 80 mg/amlodipine 10 mg provides superior blood pressure reductions in patients with severe hypertension: TEAMSTA Severe HTN Study [abstract # LB-PO-10] J Clin Hypertens. 2010;12:537. [Google Scholar]
- 25.Makani H., Bangalore S., Romero J. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med. 2011;124:128–135. doi: 10.1016/j.amjmed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 26.White W.B., Viadero J.J., Lane T.J., Podesla S. Effects of combination therapy with captopril and nifedipine in severe or resistant hypertension. Clin Pharmacol Ther. 1986;39:43–48. doi: 10.1038/clpt.1986.8. [DOI] [PubMed] [Google Scholar]
- 27.Gustafsson D. Microvascular mechanisms involved in calcium antagonist edema formation. J Cardiovasc Pharmacol. 1987;10(Suppl 1):S121–S131. doi: 10.1097/00005344-198710001-00023. [DOI] [PubMed] [Google Scholar]
- 28.Kakuta H., Sudoh K., Sasamata M., Yamagishi S. Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockers. Int J Clin Pharmacol Res. 2005;25:41–46. [PubMed] [Google Scholar]
- 29.Stangier J., Su C.A., Roth W. Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J Int Med Res. 2000;28:149–167. doi: 10.1177/147323000002800401. [DOI] [PubMed] [Google Scholar]
- 30.Lacourcière Y., Krzesinski J.M., White W.B. Sustained antihypertensive activity of telmisartan compared with valsartan. Blood Press Monit. 2004;9:203–210. doi: 10.1097/00126097-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf S., Teo K.K., ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 32.Yusuf S., Teo K., Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher H., Mancia G. The safety profile of telmisartan as monotherapy or combined with hydrochlorothiazide: a retrospective analysis of 50 studies. Blood Press. 2008;17(Suppl 1):32–40. doi: 10.1080/08038020802144383. [DOI] [PubMed] [Google Scholar]
- 34.European Medicines Agency MICARDIS summary of product characteristics. http://www.emea.europa.eu Accessed February 13, 2012.
- 35.Neldam S., Lang M., Jones R. Telmisartan and amlodipine single-pill combinations vs amlodipine monotherapy for superior blood pressure lowering and improved tolerability in patients with uncontrolled hypertension: results of the TEAMSTA-5 study. J Clin Hypertens. 2011;13:459–466. doi: 10.1111/j.1751-7176.2011.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neldam S., Edwards C., Jones R. Switching patients with uncontrolled hypertension on amlodipine 10 mg to single-pill combinations of telmisartan and amlodipine: results of the TEAMSTA-10 study. Curr Med Res Opin. 2011;27:2145–2153. doi: 10.1185/03007995.2011.624089. [DOI] [PubMed] [Google Scholar]
- 37.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2004;19:1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Littlejohn T.W., 3rd, Majul C.R., Olvera R. Results of treatment with telmisartan-amlodipine in hypertensive patients. J Clin Hypertens. 2009;11:207–213. doi: 10.1111/j.1751-7176.2009.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weir M.R. Incidence of pedal edema formation with dihydropyridine calcium channel blockers: issues and practical significance. J Clin Hypertens. 2003;5:330–335. doi: 10.1111/j.1524-6175.2003.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephenson J. Noncompliance may cause half of antihypertensive drug ‘failures’. JAMA. 1999;282:313–314. doi: 10.1001/jama.282.4.313. [DOI] [PubMed] [Google Scholar]


