Abstract
Vinculin is an essential cell adhesion protein, found at both focal adhesions and adherens junctions, where it couples transmembrane proteins to the actin cytoskeleton. Vinculin is involved in controlling cell shape, motility and cell survival, and has more recently been shown to play a role in force transduction. The tail domain of vinculin (Vt) has the ability to both bind and bundle actin filaments. Binding to actin induces a conformational change in Vt believed to promote formation of a Vt dimer that is able to crosslink actin filaments. We have recently provided additional evidence for the actin-induced Vt dimer and have shown that the vinculin carboxyl (C)-terminal hairpin is critical for both the formation of the Vt dimer and for bundling F-actin. We have also demonstrated the importance of the C-terminal hairpin in cells as deletion of this region impacts both adhesion properties and force transduction. Intriguingly, we have identified bundling deficient variants of vinculin that show different cellular phenotypes. These results suggest additional role(s) for the C-terminal hairpin, distinct from its bundling function. In this commentary, we will expand on our previous findings and further investigate these actin bundling deficient vinculin variants.
Keywords: vinculin, dimerization, F-actin bundling, focal adhesion, scaffold
Introduction
Vinculin is a ubiquitously expressed scaffolding protein that links transmembrane receptors with the actin cytoskeleton at focal adhesions (FAs) and adherens junctions.1 Vinculin has also been shown to regulate cell adhesion formation, adhesion strength and motility.2 The importance of vinculin has been demonstrated in mouse knockout studies where embryos die by day 103 and isolated fibroblasts have a number of defects including: decreased adhesion, decreased spreading, increased motility, increased FAK and paxillin signaling, decreased stiffness and resistance to apoptosis and anoikis.4-10
Vinculin is maintained in its inactive, autoinhibited state through multiple interactions between its large helical head and smaller helical tail domain. A flexible protein-rich linker region connects the head and tail domains.11 Each domain is able to recognize multiple binding partners: the head domain (Vh) binds to α/β-catenin, α-actinin, talin and IpaA;12-16 the linker region interacts with CAP/ponsin, nArgBP2, vinexin α/β, vasodilator-stimulated phosphoprotein and Arp2/3 complex;17-21 the tail domain (Vt) recognizes paxillin, PKCα, raver1, filamentous actin (F-actin) and phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2].22-26 Through the interaction of multiple binding partners, head-tail interactions are released and vinculin is able to bind and recruit other interactors.27 When vinculin is localized at FAs, these binding partners include talin and F-actin. Coordinate binding of talin and F-actin promotes vinculin activation, enabling it to serve as a mechanotransducer by mediating the linkage between integrins and the actin cytoskeleton.28 Additionally, the interaction between F-actin and vinculin is believed to play a role in many cellular processes including cell motility, cell structure and survival. In this commentary, we will further investigate the importance of the interaction between vinculin and F-actin.
The F-Actin/Vinculin Interaction Results in Vinculin Self-Association and F-Actin Bundles
Vt contains an F-actin binding site that is partially masked due to autoinhibitory interactions with Vh in the inactive full length protein.29 Upon binding F-actin, Vt has been shown to self-associate through a cryptic dimerization site that enables actin filaments to be crosslinked.30,31 Moreover, Janssen et al. combined electron microscopy, computational docking and mutagenesis approaches and proposed a structural model for the Vt/F-actin complex (Janssen model).32 In the Janssen model, F-actin interacts with Vt at two sites; the top of helix 2 and 3 (upper site) as well as the bottom of helix 3 and helix 5 (lower site) leading into the C-terminal hairpin (Fig. 1B).32 They also proposed that deletion of the amino (N)-terminal strap impaired actin bundling while deletion of the C-terminus enhanced the bundling efficiency of Vt.32 However as our group has shown previously, N-terminal strap deletions do not impair actin bundling.31 Additionally, we have shown that deletions larger than five amino acids from the C-terminus affect the structural integrity and stability of Vt,31,33 making it difficult to interpret data from larger C-terminal hairpin deletion variants. By introducing smaller deletions to the C-terminal hairpin that do not to alter the structural integrity of Vt (Vt ΔC2, 1,064–1,066; Vt ΔC5, 1,061–1,066),31,33 we have shown that the C-terminus and in particular, the last five residues, are essential for F-actin bundling, in contrast to previously published results.31,32
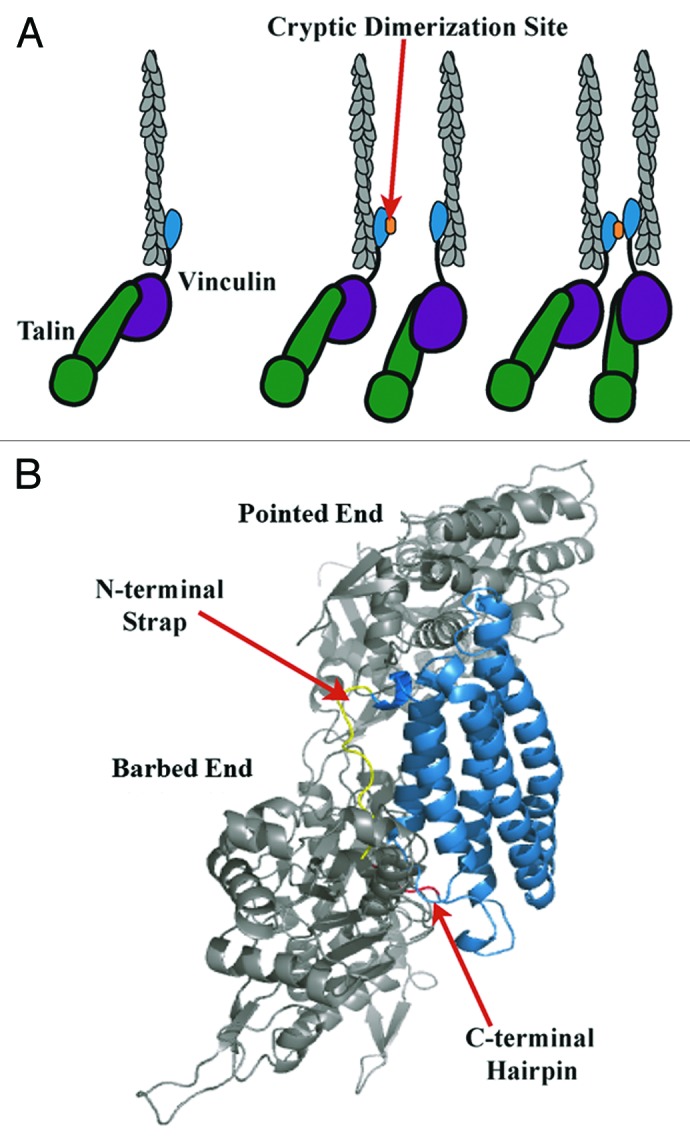
Figure 1. Model of vinculin activation, F-actin binding and bundling. (A) As modified from Janssen et al.,32 release of autoinhibitory interactions within full length vinculin via binding of talin (green) to Vh (purple) and F-actin (gray) to Vt (blue), leads to vinculin activation.32 F-actin binding causes a conformational change in Vt that exposes a cryptic dimerization site (orange) that enables Vt self-association and F-actin bundling.30,32 (B) According to the Janssen model, the C-terminal hairpin (red) and N-terminal strap (yellow) point into the F-actin interface.32 However, the Janssen model may require further validation given the resolution of the micrograph, multiple conformational clashes between Vt and F-actin, and contrasting data that has arisen indicating that the C-terminal hairpin is necessary for bundle formation. Hence, given our findings, a refined or alternative model for this critical interaction should lead to more specific tools to study the function of the vinculin/F-actin interaction.
To further explore the Vt dimer induced upon association with F-actin, we conducted crosslinking studies at a range of actin concentrations on WT Vt and our actin bundling deficient mutants.31 Whereas both a dimer and trimer species are formed for WT Vt and Vt ΔC5 in the presence of F-actin, the Vt ΔC5 variant dimer band runs at a lower molecular weight and displays a significant decrease in intensity at higher F-actin concentrations. These results suggest that the actin bundling defect associated with deletion of the C-terminal hairpin may result from the inability of Vt ΔC5 to form an actin-induced dimer. To see if similar results are observed with a smaller deletion mutant that is also deficient in actin bundling,31 we performed crosslinking on Vt ΔC2 in the presence and absence of F-actin. As shown in Figure 2, a similar decrease in band intensity of the actin-induced dimer band is observed for Vt ΔC2 as for Vt ΔC5.31 These findings suggest that the C-terminal hairpin, especially residues Y1065 and Q1066, are critical for formation of a functional actin-induced Vt dimer. In addition, we have also recently demonstrated that Vt ΔC5 is critical for FA morphology and the ability of cells to stiffen in response to force.31 In this commentary, we will examine how these bundling-deficient vinculin C-terminal deletion variants impact other cellular functions, and also address whether deficiencies in actin bundling also impact the ability of vinculin to function as a scaffold at FAs.
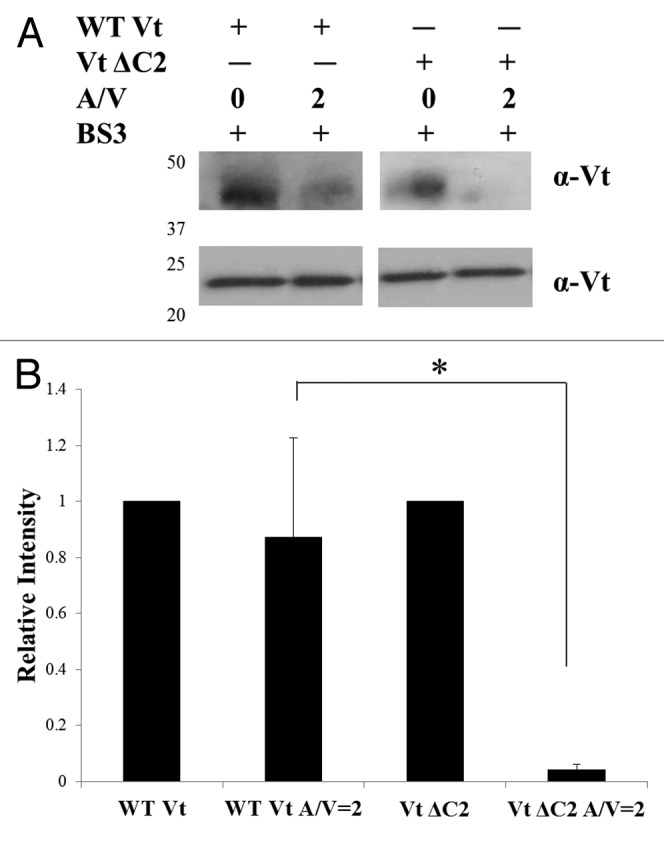
Figure 2. Detection of the actin-induced Vt dimer. Crosslinking experiments were conducted as previously described.31 (A) Western blots of WT Vt and Vt ΔC2 in the absence and presence of actin (A/V = 2) and probed against Vt. Crosslinked samples were run on SDS-PAGE gels (lower panel) to observe the Vt monomer band (21 kDa) or NuPAGE Bis-tris 10% gradient gels (Invitrogen) (upper panel) to observe dimer species and blotted with a rabbit anti-chicken Vt antibody [a gift from Dr. Susan Craig (John Hopkins University)]. (B) Quantification of crosslinked bands by densitometry. In the presence of actin, a significant decrease in the amount of the actin-induced dimer is observed for Vt ΔC2 relative to WT Vt. Similar results were previously obtained for the larger bundling deficient variant, Vt ΔC5.31 Densitometry is the average ± SEM combined from three independent experiments. Statistical significance was determined using the Student’s t-test. *p ≤ 0.05.
The Mechanical Role of the F-Actin/Vinculin Interaction at Focal Adhesions
Many cellular processes are dependent on locally generated forces in their environment, including migration as evidenced by durotaxis, cell survival or death.34-36 The ability of cells to sense changes in stiffness is believed to be primarily mediated through proteins at FAs, including vinculin. In addition its ability to sense external forces, vinculin has been reported to regulate how applied mechanical stress alters adhesion composition in order to induce cell morphological changes.37-39 Recent evidence indicates that vinculin is crucial for transmission of mechanical forces resulting from either actomyosin or cell-generated forces, since the recruitment of vinculin to FAs corresponds to the amount of force applied to the extracellular matrix (ECM).40,41 Furthermore, the F-actin/vinculin interaction may be required for these forces since, without Vt, vinculin does not follow the retrograde flow of actin.42 The need for vinculin to bind F-actin and transduce force is further supported through measurements of force fluctuations, and correlating them to the degree of coupling displayed by the speckle motions of F-actin and vinculin during protrusion and retraction events at the leading edge of cells.43 Further, recent studies have shown that vinculin mutants deficient in actin binding are unable to generate traction on the ECM within the leading edge of cells.44 However, vinculin-mediated F-actin bundling appears to be a key factor in regulating how cells respond to external force since expression of a vinculin variant deficient in actin bundling, ΔC5 vinculin, prevented cells from responding to pulses of force when a magnetic bead coated in fibronectin (FN) was applied.31 These data suggest that it is not only the connections between vinculin and F-actin that are necessary to respond to extracellular stimuli, but also the vinculin-mediated F-actin bundles that are needed to properly transduce external forces.
F-Actin Bundles Mediated by Vinculin During Migration and Spreading Events
During migration and early stages of cell spreading, the leading edges of cells are defined by the balance between dynamics in actin polymerization and formation and turnover of adhesions. Only through the interplay of these two components can effective migration and spreading be achieved. Following integrin engagement and recruitment of key cytoskeletal proteins, including talin and vinculin that establishes the link between active integrins and F-actin, actin flow slows and transmits force on the ECM to enable cells to move45 and allow adhesions to mature.46 In studies examining motility and spreading in the absence of vinculin, spreading is severely impaired but the cells are able to migrate at a higher rate.47 These data suggest that vinculin is needed to stabilize adhesions in order to spread instead of impacting rapid actin polymerization and depolymerization that is necessary for effective migration. Recent studies have suggested that, in addition to bundling F-actin, vinculin can promote actin polymerization and cap filaments.31,48,49 However, it has been hard to observe the result of these different functions in cells due to the use of large and destabilizing vinculin C-terminal deletion variants. Humphries et al. showed that expression of vinculin tail domain alone was sufficient for vinculin to be recruited to areas of high contractility that were under mechanical tension, such as FAs, but not to the lamellipodia.50 An actin deficient deletion mutant lacking helix 2 and 3 within the tail domain of vinculin displayed not only larger and denser FAs, but also prevented an invasive phenotype,51 indicating that the F-actin/vinculin interaction may regulate cellular functions pertaining to organizing the actin network during migration. However, given the size of this deletion and likelihood that Vt is destabilized, this variant likely possesses multiple defects.
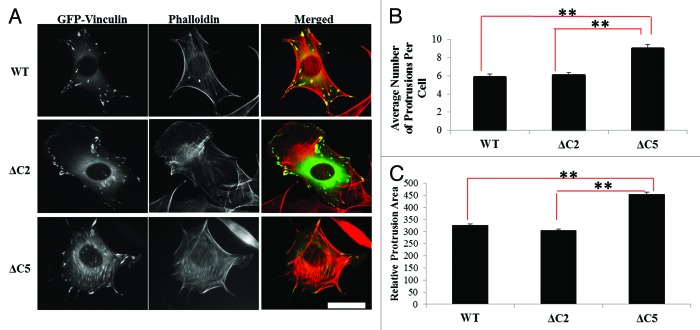
One question that remains unanswered is how does a vinculin mutation that retains actin binding but possesses a specific defect in actin-bundling differ from other variants that have been previously used to probe vinculin/F-actin interactions? It has been shown that a vinculin variant containing a mutation in the proposed actin-induced dimerization site (Y1065) is slightly deficient in its ability to spread on FN.52 Moreover, as mentioned earlier, we have recently shown that vinculin null murine embryo fibroblasts (Vin−/− MEFS) re-expressing ΔC5 vinculin are smaller in cell area and have significantly fewer adhesions when cells are allowed to spread on FN.31 The ability of Vin−/− MEFs expressing either WT-, ΔC2- or ΔC5 vinculin to spread on FN over time was quantified to determine differences in spreading and overall cell morphology (Fig. 3). Although significantly more and larger protrusions were observed for ΔC5 vinculin expressing cells in comparison to cells expressing WT vinculin (Fig. 3B and C), cells expressing ΔC2 vinculin had the same number and size of protrusions as WT vinculin (Fig. 3B and C). Thus, despite their similar defects in actin bundling in vitro,31 ΔC2- and ΔC5 vinculin display significant differences during spreading and in their overall cell morphology, as well as from WT vinculin. These data suggest differential roles for the last two vs. five amino acids in the C-terminal hairpin of vinculin.
Figure 3. Vinculin C-terminal hairpin deletions alter cell morphology. (A) Vin−/− MEFs were transfected with GFP-tagged WT-, ΔC2- or ΔC5 vinculin and plated on FN for two hours as previously described.31 (B) The number of protrusions and (C) the relative protrusion area were measured with NIH ImageJ. Cells expressing ΔC5 vinculin show significantly more and larger protrusions per cell, relative to WT vinculin and ΔC2 vinculin. Results shown represent ~60 cells from three independent experiments. Statistical significance was determined by the Student’s t-test. **p ≤ 0.001 comparing WT vinculin and ΔC2 vinculin to ΔC5 vinculin. Scale bar is 30 µm.
The F-Actin Induced Vinculin Dimer: Could It Be a Scaffold Too?
Activation of vinculin is believed to promote its scaffold function. At sites of adhesion, vinculin activation facilitates recruitment of additional proteins enabling vinculin-mediated regulation of FA dynamics, efficient cell spreading or migration. While a number of binding partners have been identified, some of these proposed interactions have not been verified in cells. Moreover, new interaction partners may yet be identified.53 Since vinculin is an essential scaffolding protein that both senses and regulates mechanical tension, the following question is raised: Could the structures formed by activated vinculin mediate tension (i.e., F-actin bundles) and contribute to its signaling properties? While this question remains to be explored fully, the phenotypes that are observed in the presence of the different vinculin mutants deficient in actin-bundling (Fig. 3) raise interesting questions. Is the primary role of the C-terminal hairpin to promote F-actin bundling via generation of the actin-induced dimer or does the C-terminus also function as a binding site for interaction partners? Furthermore, could the dimer itself serve a scaffolding function? Currently there are no binding partners that are known to bind to residues 1,061–1,066 in vinculin. However, in the full-length protein there are a number of contacts between the residues in the C-terminus with amino acids in the proline-rich region including P878, the docking site for the Arp2/3 complex (Fig. 4).21 It has also been suggested that vinexin-α, an adaptor that influences actin cytoskeletal rearrangements and promotes cell spreading, also interacts with vinculin in the proline-rich linker close to the contacts described in Figure 4.18,54,55 Perhaps residues in the C-terminal hairpin regulate binding of interaction partners to the proline-rich region?
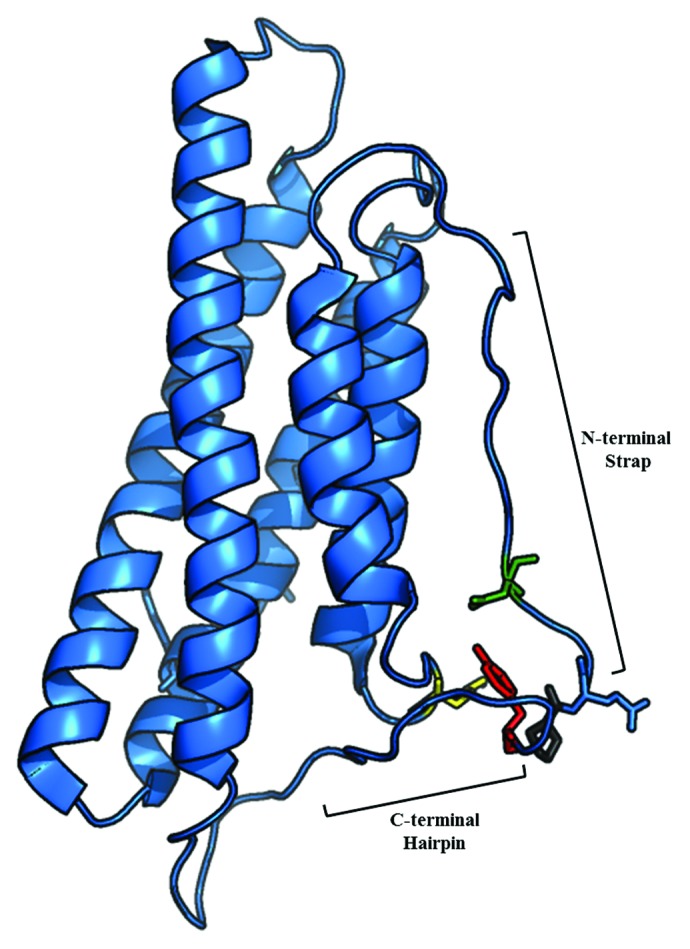
Figure 4. Ribbon diagram of Vt (PDB ID 1ST6) highlighting interactions of the Y1065 side chain. The side chain of Y1065 (red) in the C-terminal hairpin forms hydrogen bonds with D882 (green) in the strap, K915 (yellow) in the helix 1/helix 2 loop and P878 (gray) in the proline-rich linker region.
To assess the scaffolding function of our mutants, we isolated adhesions from cells expressing either WT-, ΔC2-, ΔC5 vinculin or untransfected cells, to monitor their ability to recruit known binding partners to integrin-containing adhesions. Following a short incubation time of 30 min with magnetic beads coated with FN, complexes that formed around the beads were isolated as previously described.56 Adhesions isolated from cells containing WT vinculin were able to efficiently recruit vinexin-α and the Arp2/3 complex, as indicated by western blot detection of the p34-Arc subunit (Fig. 5). Cells expressing either ΔC2-, ΔC5- or lacking vinculin were able to recruit the Arp2/3 complex but not with as high of efficiency as cells expressing WT vinculin (Fig. 5). However, cells expressing the mutants or were untransfected were severely impaired in their ability to not only recruit vinexin-α, but also lacked a significant amount of actin (Fig. 5). These results suggest that cells expressing the actin-bundling deficient mutants are impaired in their ability to act as a scaffold, and also show less actin accumulated at adhesion complexes.31 While it remains to be determined whether the actin-induced dimer itself or residues in the C-terminus regulate these interactions, it will be interesting to further explore this possibility through more selective mutations within the C-terminus. Additionally, future studies will examine phenotypic differences between the ΔC2- and ΔC5 vinculin mutations and how these differences impact the formation of the actin-induced vinculin dimer.
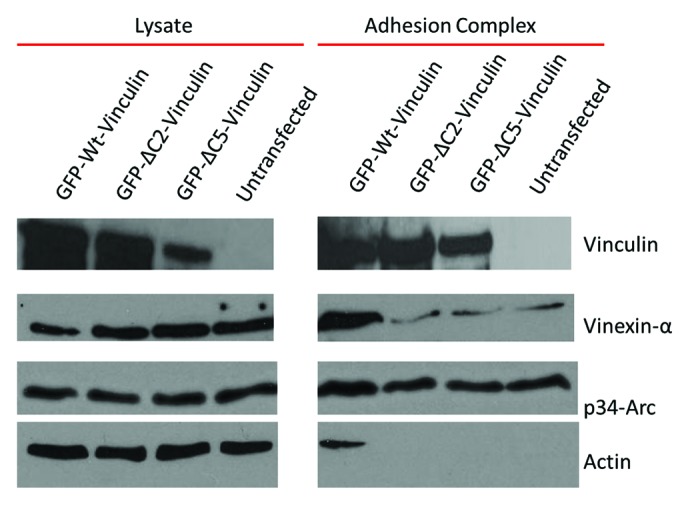
Figure 5. Vinculin mutants deficient in F-actin bundling also alter vinculin’s scaffold function and prevent actin accumulation at adhesion complexes. Adhesion complexes were isolated from Vin−/− MEFs expressing either WT-, ΔC2-, ΔC5 vinculin or untransfected, after incubation with FN coated beads for 30 min.
Conclusions
Results from our studies have demonstrated the importance of the C-terminal hairpin in vinculin tail mediated F-actin bundling both in vitro and in cells. In particular, vinculin residues Y1065 and Q1066 appear to be crucial for the formation of the actin-induced Vt dimer. Moreover, distinct phenotypic differences between ΔC2- and ΔC5 vinculin are observed in cells, even though the C-terminal deletion variants display the same actin-bundling deficiencies in vitro. Furthermore, the C-terminal hairpin may play a role in the recruitment of binding partners to FAs in cells by contributing to vinculin’s scaffolding function as both of the C-terminal deletion mutants appear defective in recruitment of vinexin-α. The C-terminal hairpin deletion mutations also prevent the accumulation of actin at adhesion complexes. Given these results, it will be interesting to further explore the following:
(1) Refine the F-actin/Vt interaction and dimerization model.
(2) Examine if there are other vinculin interacting proteins that require the C-terminal hairpin for binding.
(3) Further investigate how the C-terminal hairpin or actin-induced dimer may contribute to vinculin’s scaffolding function.
Acknowledgments
We thank Peter M. Thompson (University of North Carolina at Chapel Hill) for his helpful comments and suggestions. This work was supported by grants from the National Institute of Health 1RO1GM029860 to K.B. and 1RO1GM081764 and 1RO1GM080568 to S.L.C.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/23184
References
- 1.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–43. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–60. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–37. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- 4.Xu W, Coll JL, Adamson ED. Rescue of the mutant phenotype by reexpression of full-length vinculin in null F9 cells; effects on cell locomotion by domain deleted vinculin. J Cell Sci. 1998;111:1535–44. doi: 10.1242/jcs.111.11.1535. [DOI] [PubMed] [Google Scholar]
- 5.Zemljic-Harpf AE, Ponrartana S, Avalos RT, Jordan MC, Roos KP, Dalton ND, et al. Heterozygous inactivation of the vinculin gene predisposes to stress-induced cardiomyopathy. Am J Pathol. 2004;165:1033–44. doi: 10.1016/S0002-9440(10)63364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne BJ, Kaczorowski YJ, Coutu MD, Craig SW. Chicken vinculin and meta-vinculin are derived from a single gene by alternative splicing of a 207-base pair exon unique to meta-vinculin. J Biol Chem. 1992;267:12845–50. [PubMed] [Google Scholar]
- 7.Maeda M, Holder E, Lowes B, Valent S, Bies RD. Dilated cardiomyopathy associated with deficiency of the cytoskeletal protein metavinculin. Circulation. 1997;95:17–20. doi: 10.1161/01.CIR.95.1.17. [DOI] [PubMed] [Google Scholar]
- 8.Olson TM, Illenberger S, Kishimoto NY, Huttelmaier S, Keating MT, Jockusch BM. Metavinculin mutations alter actin interaction in dilated cardiomyopathy. Circulation. 2002;105:431–7. doi: 10.1161/hc0402.102930. [DOI] [PubMed] [Google Scholar]
- 9.Coll JL, Ben-Ze’ev A, Ezzell RM, Rodríguez Fernández JL, Baribault H, Oshima RG, et al. Targeted disruption of vinculin genes in F9 and embryonic stem cells changes cell morphology, adhesion, and locomotion. Proc Natl Acad Sci U S A. 1995;92:9161–5. doi: 10.1073/pnas.92.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, Hahn KM. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J Cell Biol. 2004;165:371–81. doi: 10.1083/jcb.200308011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, et al. Structural basis for vinculin activation at sites of cell adhesion. Nature. 2004;430:583–6. doi: 10.1038/nature02610. [DOI] [PubMed] [Google Scholar]
- 12.Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL. Vinculin is associated with the E-cadherin adhesion complex. J Biol Chem. 1997;272:32448–53. doi: 10.1074/jbc.272.51.32448. [DOI] [PubMed] [Google Scholar]
- 13.Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, et al. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–57. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wachsstock DH, Wilkins JA, Lin S. Specific interaction of vinculin with alpha-actinin. Biochem Biophys Res Commun. 1987;146:554–60. doi: 10.1016/0006-291X(87)90564-X. [DOI] [PubMed] [Google Scholar]
- 15.Gingras AR, Ziegler WH, Frank R, Barsukov IL, Roberts GC, Critchley DR, et al. Mapping and consensus sequence identification for multiple vinculin binding sites within the talin rod. J Biol Chem. 2005;280:37217–24. doi: 10.1074/jbc.M508060200. [DOI] [PubMed] [Google Scholar]
- 16.Tran Van Nhieu G, Ben-Ze’ev A, Sansonetti PJ. Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J. 1997;16:2717–29. doi: 10.1093/emboj/16.10.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandai K, Nakanishi H, Satoh A, Takahashi K, Satoh K, Nishioka H, et al. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J Cell Biol. 1999;144:1001–17. doi: 10.1083/jcb.144.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kioka N, Sakata S, Kawauchi T, Amachi T, Akiyama SK, Okazaki K, et al. Vinexin: a novel vinculin-binding protein with multiple SH3 domains enhances actin cytoskeletal organization. J Cell Biol. 1999;144:59–69. doi: 10.1083/jcb.144.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawabe H, Hata Y, Takeuchi M, Ide N, Mizoguchi A, Takai Y. nArgBP2, a novel neural member of ponsin/ArgBP2/vinexin family that interacts with synapse-associated protein 90/postsynaptic density-95-associated protein (SAPAP) J Biol Chem. 1999;274:30914–8. doi: 10.1074/jbc.274.43.30914. [DOI] [PubMed] [Google Scholar]
- 20.Brindle NP, Holt MR, Davies JE, Price CJ, Critchley DR. The focal-adhesion vasodilator-stimulated phosphoprotein (VASP) binds to the proline-rich domain in vinculin. Biochem J. 1996;318:753–7. doi: 10.1042/bj3180753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881–91. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood CK, Turner CE, Jackson P, Critchley DR. Characterisation of the paxillin-binding site and the C-terminal focal adhesion targeting sequence in vinculin. J Cell Sci. 1994;107:709–17. [PubMed] [Google Scholar]
- 23.Ziegler WH, Tigges U, Zieseniss A, Jockusch BM. A lipid-regulated docking site on vinculin for protein kinase C. J Biol Chem. 2002;277:7396–404. doi: 10.1074/jbc.M110008200. [DOI] [PubMed] [Google Scholar]
- 24.Hüttelmaier S, Illenberger S, Grosheva I, Rüdiger M, Singer RH, Jockusch BM. Raver1, a dual compartment protein, is a ligand for PTB/hnRNPI and microfilament attachment proteins. J Cell Biol. 2001;155:775–86. doi: 10.1083/jcb.200105044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hüttelmaier S, Bubeck P, Rüdiger M, Jockusch BM. Characterization of two F-actin-binding and oligomerization sites in the cell-contact protein vinculin. Eur J Biochem. 1997;247:1136–42. doi: 10.1111/j.1432-1033.1997.01136.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RP, Niggli V, Durrer P, Craig SW. A conserved motif in the tail domain of vinculin mediates association with and insertion into acidic phospholipid bilayers. Biochemistry. 1998;37:10211–22. doi: 10.1021/bi9727242. [DOI] [PubMed] [Google Scholar]
- 27.Cohen DM, Chen H, Johnson RP, Choudhury B, Craig SW. Two distinct head-tail interfaces cooperate to suppress activation of vinculin by talin. J Biol Chem. 2005;280:17109–17. doi: 10.1074/jbc.M414704200. [DOI] [PubMed] [Google Scholar]
- 28.Cohen DM, Kutscher B, Chen H, Murphy DB, Craig SW. A conformational switch in vinculin drives formation and dynamics of a talin-vinculin complex at focal adhesions. J Biol Chem. 2006;281:16006–15. doi: 10.1074/jbc.M600738200. [DOI] [PubMed] [Google Scholar]
- 29.Bakolitsa C, de Pereda JM, Bagshaw CR, Critchley DR, Liddington RC. Crystal structure of the vinculin tail suggests a pathway for activation. Cell. 1999;99:603–13. doi: 10.1016/S0092-8674(00)81549-4. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RP, Craig SW. Actin activates a cryptic dimerization potential of the vinculin tail domain. J Biol Chem. 2000;275:95–105. doi: 10.1074/jbc.275.1.95. [DOI] [PubMed] [Google Scholar]
- 31.Shen K, Tolbert CE, Guilluy C, Swaminathan VS, Berginski ME, Burridge K, et al. The vinculin C-terminal hairpin mediates F-actin bundle formation, focal adhesion, and cell mechanical properties. J Biol Chem. 2011;286:45103–15. doi: 10.1074/jbc.M111.244293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen ME, Kim E, Liu H, Fujimoto LM, Bobkov A, Volkmann N, et al. Three-dimensional structure of vinculin bound to actin filaments. Mol Cell. 2006;21:271–81. doi: 10.1016/j.molcel.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Palmer SM, Playford MP, Craig SW, Schaller MD, Campbell SL. Lipid binding to the tail domain of vinculin: specificity and the role of the N and C termini. J Biol Chem. 2009;284:7223–31. doi: 10.1074/jbc.M807842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janmey PA, Weitz DA. Dealing with mechanics: mechanisms of force transduction in cells. Trends Biochem Sci. 2004;29:364–70. doi: 10.1016/j.tibs.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng X, Nelson ES, Maiers JL, DeMali KA. New insights into vinculin function and regulation. Int Rev Cell Mol Biol. 2011;287:191–231. doi: 10.1016/B978-0-12-386043-9.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mierke CT, Kollmannsberger P, Zitterbart DP, Smith J, Fabry B, Goldmann WH. Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys J. 2008;94:661–70. doi: 10.1529/biophysj.107.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boccafoschi F, Mosca C, Bosetti M, Cannas M. The role of mechanical stretching in the activation and localization of adhesion proteins and related intracellular molecules. J Cell Biochem. 2011;112:1403–9. doi: 10.1002/jcb.23056. [DOI] [PubMed] [Google Scholar]
- 39.Coyer SR, Singh A, Dumbauld DW, Calderwood DA, Craig SW, Delamarche E, et al. Nanopatterning Reveals an ECM Area Threshold for Focal Adhesion Assembly and Force Transmission that is regulated by Integrin Activation and Cytoskeleton Tension. J Cell Sci. 2012;125:5110–23. doi: 10.1242/jcs.108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 41.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–6. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–57. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji L, Lim J, Danuser G. Fluctuations of intracellular forces during cell protrusion. Nat Cell Biol. 2008;10:1393–400. doi: 10.1038/ncb1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thievessen I, Berlemont S, Plevock KM, Plotnikov SV, Thompson PM, Zemljic-Harpf A, Ross RS, et al. Vinculin-actin interaction mediates engagement of actin retrograde flow to focal adhesions, but is dispensable for actin-dependent focal adhesion maturation. doi: 10.1083/jcb.201303129. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–33. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfenson H, Henis YI, Geiger B, Bershadsky AD. The heel and toe of the cell’s foot: a multifaceted approach for understanding the structure and dynamics of focal adhesions. Cell Motil Cytoskeleton. 2009;66:1017–29. doi: 10.1002/cm.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ezzell RM, Goldmann WH, Wang N, Parashurama N, Ingber DE. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. [DOI] [PubMed] [Google Scholar]
- 48.Wen KK, Rubenstein PA, DeMali KA. Vinculin nucleates actin polymerization and modifies actin filament structure. J Biol Chem. 2009;284:30463–73. doi: 10.1074/jbc.M109.021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Clainche C, Dwivedi SP, Didry D, Carlier MF. Vinculin is a dually regulated actin filament barbed end-capping and side-binding protein. J Biol Chem. 2010;285:23420–32. doi: 10.1074/jbc.M110.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–57. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marg S, Winkler U, Sestu M, Himmel M, Schönherr M, Bär J, et al. The vinculin-DeltaIn20/21 mouse: characteristics of a constitutive, actin-binding deficient splice variant of vinculin. PLoS One. 2010;5:e11530. doi: 10.1371/journal.pone.0011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Izaguirre G, Lin SY, Lee HY, Schaefer E, Haimovich B. The phosphorylation of vinculin on tyrosine residues 100 and 1065, mediated by SRC kinases, affects cell spreading. Mol Biol Cell. 2004;15:4234–47. doi: 10.1091/mbc.E04-03-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carisey A, Ballestrem C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur J Cell Biol. 2011;90:157–63. doi: 10.1016/j.ejcb.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Li X, Yao B, Shen W, Sun H, Xu C, et al. Solution structure of the first SH3 domain of human vinexin and its interaction with vinculin peptides. Biochem Biophys Res Commun. 2007;357:931–7. doi: 10.1016/j.bbrc.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 55.Roignot J, Soubeyran P. ArgBP2 and the SoHo family of adapter proteins in oncogenic diseases. Cell Adh Migr. 2009;3:167–70. doi: 10.4161/cam.3.2.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–7. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]