Abstract
ARHI is an imprinted tumor suppressor gene that is downregulated in > 60% of ovarian cancers, associated with decreased progression-free survival. ARHI encodes a 26 kDa GTPase with homology to Ras. Re-expression of ARHI inhibits ovarian cancer growth, initiates autophagy and induces tumor dormancy. Recent studies have demonstrated that ARHI also plays a particularly important role in ovarian cancer cell migration. Re-expression of ARHI decreases motility of IL-6- and EGF-stimulated SKOv3 and Hey ovarian cancer cells, inhibiting both chemotaxis and haptotaxis. ARHI inhibits cell migration by binding and sequestering STAT3 in the cytoplasm, and preventing STAT3 translocation to the nucleus and localization in focal adhesion complexes. Re-expression of ARHI inhibits FAKY397 phosphorylation, disrupts focal adhesions and blocks FAK-mediated RhoA signaling, resulting in decreased levels of GTP-RhoA. Re-expression of ARHI disrupts formation of actin stress fibers in a FAK- and RhoA-dependent manner. Recent studies indicate that re-expression of ARHI inhibits expression of β-1 integrin which may also contribute to inhibition of migration, adhesion and invasion.
Keywords: tumor suppressor gene ARHI, migration suppression, STAT3, RhoA GTPase, cytoskeleton, integrin
Introduction
ARHI is an imprinted tumor suppressor gene that is downregulated in several types of cancer. Decreased ARHI expression in > 60% of ovarian cancers is associated with shortened progression-free survival.1ARHI encodes a 26 kDa GTPase with homology to Ras, but exhibits a distinctive 34 amino acid N-terminal extension that sets it apart from other Ras family members.1 Re-expression of ARHI inhibits growth, adhesion, and migration in ovarian cancer cells and induces autophagy and tumor dormancy.2-6 Loss of ARHI-mediated inhibition of cell migration may be particularly important for pathogenesis of the disease. Ovarian cancer cells can metastasize through lymphatics and blood vessels, but most often metastasize over the surface of the peritoneum. We previously reported that re-expression of ARHI inhibits ovarian cancer cell motility by interfering with JAK-STAT3 signal transduction, inhibiting the FAK/RhoA signaling pathway and decreasing formation of focal adhesion complexes and stress fibers.2 In this article, we review these data and explore the possibility that re-expression of ARHI also inhibits ovarian cancer cell movement by downregulating integrins required for migration and adhesion. We provide data to support this hypothesis and suggest potential mechanisms by which ARHI could decrease cancer cell motility by regulating integrin/STAT3 and integrin/FAK signaling.
ARHI in Cell Migration and Adhesion
ARHI decreases β1 integrin expression
Integrins are a family of heterodimeric transmembrane receptors composed of α and β subunits.7 As transmembrane receptors, integrins have extracellular, transmembrane and intracellular domains that link the extracellular matrix (ECM) to the cytoskeleton.8,9 Integrins expressed in tumor cells can contribute to tumor progression and metastasis by increasing tumor cell migration and invasion.10-12 Integrin adhesion to the ECM provides the traction required for tumor cell invasion. Additionally, integrins also contribute to tumor cell invasion by regulating the localization and activity of matrix-degrading proteases, such as matrix metalloprotease 2 (MMP2) and urokinase-type plasminogen activator (uPA).10,13 Integrin-mediated migration is generally required for focal adhesion kinase (FAK) and Src family kinase signaling.14,15 Integrin-specific mechanisms also regulate cell motility.16-18 Cancer cell invasion during metastasis is a multi-step process that involves cell adhesion to proteolysis of, and migration through the basement membrane. Integrin β1 has a well-established role in cell adhesion and motility in various types of cancers, including ovarian cancer.19-24 In ovarian cancer, overexpression of integrin β1 has been found to be associated with higher clinical stage and poor survival.23,25 Upregulation of integrin β1 expression promotes ovarian cancer cell migration and matrix cell invasion.26-28 Moreover, the integrin β1 subunit has been shown to mediate ovarian cancer adhesion to peritoneal mesothelial cells and to increase peritoneal metastasis.25 This notion is supported by evidence that overexpression of integrin β1 enhances the invasive properties of ovarian cancer cells. We have recently found that re-expression of ARHI downregulates the expression of integrin β1 in a time-dependent manner (Fig. 1), suggesting that ARHI may play an important role in regulating the several β1 integrin-mediated contributions to cancer cells growth, migration and invasion.
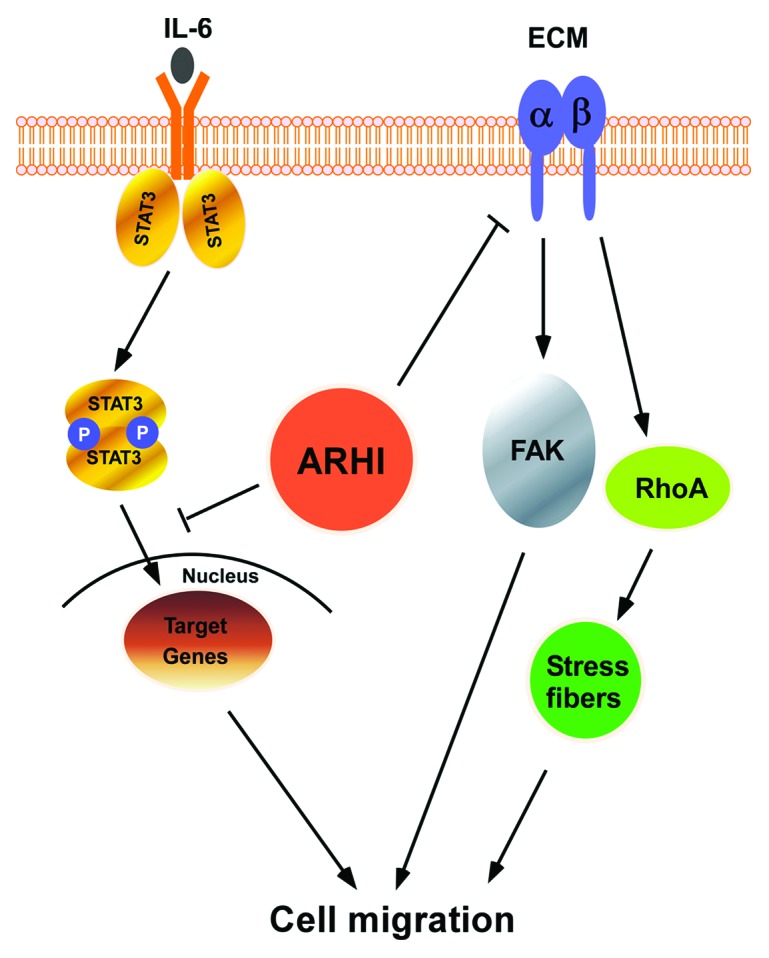
Figure 1. ARHI expression inhibits integrin β1 expression. The re-expression of ARHI decreased the expression of integrin β1 indicated by arrows. SKOv3-ARHI cells were treated with doxycycline (DOX) for the indicated time periods to induce ARHI expression. The expression of integrin β1 was examined by western blotting.
ARHI decreases STAT3 translocation to the nucleus and STAT3 participation in focal adhesion complexes
Constitutive activation of the JAK-STAT pathway has been frequently implicated in cancer and persistent activation of STAT3 is described in many solid tumors, including those of ovarian origin.16,29-32 Recent evidence indicates a role for STAT3 in cell motility. In a significant fraction of ovarian cancers, autocrine stimulation of the secreted IL-6 increases cell proliferation and motility.17,33,34 Non-phosphorylated STAT3 is found primarily in the cytosol, whereas phosphorylated, activated STAT3 translocates to the nucleus to induce gene transcription.35 Nuclear STAT3 has been found in 70% of ovarian cancers, and is associated with a poor prognosis.1 Our group has demonstrated that re-expression of ARHI at physiological levels in two different ovarian cancer cell lines inhibited nuclear translocation of STAT3 and suppressed motility.2 This inhibition relates to physical interaction between ARHI and STAT3, resulting in sequestration of STAT3 in the cytoplasm. In addition, re-expression of ARHI eliminates localization of STAT3 at focal adhesions. In focal adhesions, STAT3 may serve as an adaptor protein in integrin-mediated cell adhesion or could function as a sensor of adhesion, becoming activated in focal adhesion and translocating to the nucleus to alter gene expression in response to cell adhesion.2 Nuclear translocation of activated STAT3 from focal adhesions may induce critical proteins needed for motility. Integrin β1 has a well-established role in cell growth, adhesion and motility in ovarian cancer. Integrin β1 has also been shown to play an important role in IL6 mediated STAT3 survival signaling in several different types of cancer.36-38 Activation of metalloproteinases (MMP-2 and MMP-10) by IL-6 mediated JAK-STAT3-signaling can enhance growth and invasion of pancreatic, brain and lung cancers.39-41 Conversely, one recent report indicated that MMP-2 can complex with integrin α5/β1 to upregulate IL-6 mediated STAT3 phosphorylation and recruitment to cyclin D1 and c-Myc promoters.42 Thus, integrin α5/β1 could contribute to IL-6-mediated constitutive STAT3 activation by directly interacting with MMP-2. Metalloproteinases require activation and there are also multiple integrins with the β1 subunit uniquely implicated in ovarian cancer metastases.43,44 Given the convergence of integrin β1/STAT3 signaling on common downstream signaling targets and their central effect on metastasis-associated entities and the fact of ARHI that inhibits the expression of integrin β1and STAT3, we hypothesize that ARHI may inhibit cooperation between integrins and STAT3 in both signal transduction and modulation of cell motility during ovarian cancer progression and metastasis.
ARHI inhibits FAK/Rho signaling
FAK is activated by a variety of growth factors receptors and integrins and transmits signals downstream to a variety of target molecules to regulate the cycle of focal contact formation and disassembly required for efficient cell movement. Focal adhesion kinase-1 (FAK) is constitutively associated with β-integrin subunits of integrin receptors. The binding to integrins of components of the ECM leads to activation of FAK.45-47 The activation of FAK may be further enhanced by the co-stimulation of growth factor receptors by ECM associated growth factors, such as bFGF, EGF or PDGF.48-50 FAK is a non-receptor protein tyrosine kinase (NRPTK) that is typically, but not always, associated with supramolecular focal adhesion (FA) complexes (FAC). Focal adhesion complex assembly and disassembly are critical for cell attachment and movement. FAK does not appear to phosphorylate other proteins, however, when FAK is activated it autophosphorylates and binds Src kinase which in turn phosphorylates other sites on FAK and the FAK-binding proteins, Cas and paxillin.51,52 Phosphorylated FAK becomes a docking site within focal adhesion complexes for mediators of multiple signaling events that regulate cell motility. FAK mediates cell motility and adhesion turnover through regulation of the RhoGTPases, especially RhoA, Rac-1 and Cdc42.53-55 FAK affects the downregulation of stress fiber formation by activating RhoA activity40 and upregulates the formation of lamellipodia by activating Rac-1 via a Cas-Crk-DOCK-ELMO complex.55 Thus, FAK acts as an integrator of cell motility-associated signaling events. Growth factors stimulate cell motility by inducing the phosphorylation of FAK.56 Previously we reported that the expression of ARHI inhibited FAKY397 phosphorylation and decreased EGF-induced chemotaxis of SKOv3-ARHI cells indicating that ARHI regulates cell migration by reducing FAK-mediated RhoA activation.2 Despite these data strongly implicating ARHI inhibition of FAK as a major signaling pathway leading to decreased cell migration, the inhibition of the cooperative effect between integrins and FAK during cell migration is unexplored. Nevertheless, it is attractive to speculate that ARHI inhibition of integrin expression may reduce phosphorylation of FAK and the phosphor-FAK mediated downstream signaling pathway.
ARHI can inhibit cell migration through several mechanisms
Cell migration can be inhibited by a decrease in cell adhesion, a reduction in focal contacts and alteration in stress fibers—all of which are induced by the re-expression of ARHI2,55,57 (Fig. 2). As re-expression of ARHI downregulates the expression of integrin β1 and FAK, it is possible that ARHI may also regulate cell adhesion. STAT3 has been shown to play a critical role in cell migration through both transcriptional and non-transcriptional mechanisms. The critical target genes transcriptionally regulated by STAT3 include integrin, FN and MMP-3.36,58 Thus, the loss of ARHI-induced blockade of STAT3 signaling may be one critical mechanism regulating motility and invasion, not only in ovarian cancer, but in breast, lung, prostate and pancreatic cancer where ARHI is also downregulated. The engagement of integrin with extracellular matrix proteins results in the regulation of the small GTPase.59-67 In addition, the activity of the small GTPases including Rho A regulate the dynamic organization of the actin cytoskeleton, which determines cell morphology and regulates cell migration. Re-expression of ARHI and subsequent downregulation of integrin β1 may significantly decrease the activity of Rho A. In conclusion, ARHI regulation of integrin β1 expression and STAT3 translocation are likely to provide key mechanisms for inhibiting ovarian cancer cell migration.
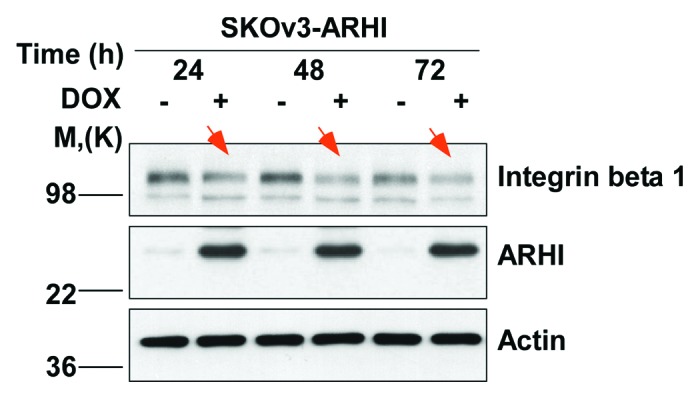
Figure 2. ARHI can inhibit cell migration through several mechanisms. A proposed model of ARHI action in a scheme.
Acknowledgments
This study was supported in part by a grant from the National Cancer Institute (R01 CA135354), by the MD Anderson SPORE in Ovarian Cancer (NCI P50 CA83639) and by philanthropic support from the Zarrow Foundation and Stuart and Gaye Lynn Zarrow.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/23648
References
- 1.Rosen DG, Wang L, Jain AN, Lu KH, Luo RZ, Yu Y, et al. Expression of the tumor suppressor gene ARHI in epithelial ovarian cancer is associated with increased expression of p21WAF1/CIP1 and prolonged progression-free survival. Clin Cancer Res. 2004;10:6559–66. doi: 10.1158/1078-0432.CCR-04-0698. [DOI] [PubMed] [Google Scholar]
- 2.Badgwell DB, Lu Z, Le K, Gao F, Yang M, Suh GK, et al. The tumor-suppressor gene ARHI (DIRAS3) suppresses ovarian cancer cell migration through inhibition of the Stat3 and FAK/Rho signaling pathways. Oncogene. 2012;31:68–79. doi: 10.1038/onc.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Zaman MS, Deng G, Majid S, Saini S, Liu J, et al. MicroRNAs 221/222 and genistein-mediated regulation of ARHI tumor suppressor gene in prostate cancer. Cancer Prev Res (Phila) 2011;4:76–86. doi: 10.1158/1940-6207.CAPR-10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118:3917–29. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Xia W, Luo RZ, Peng H, Zhao S, Dai J, et al. The human ARHI tumor suppressor gene inhibits lactation and growth in transgenic mice. Cancer Res. 2000;60:4913–20. [PubMed] [Google Scholar]
- 6.Yu Y, Luo R, Lu Z, Wei Feng W, Badgwell D, Issa JP, et al. Biochemistry and biology of ARHI (DIRAS3), an imprinted tumor suppressor gene whose expression is lost in ovarian and breast cancers. Methods Enzymol. 2006;407:455–68. doi: 10.1016/S0076-6879(05)07037-0. [DOI] [PubMed] [Google Scholar]
- 7.Bouaouina M, Goult BT, Huet-Calderwood C, Bate N, Brahme NN, Barsukov IL, et al. A conserved lipid-binding loop in the kindlin FERM F1 domain is required for kindlin-mediated αIIbβ3 integrin coactivation. J Biol Chem. 2012;287:6979–90. doi: 10.1074/jbc.M111.330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin J, Wu C. ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol. 2012;24:607–13. doi: 10.1016/j.ceb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stachurska A, Elbanowski J, Kowalczyńska HM. Role of α5β1 and αvβ3 integrins in relation to adhesion and spreading dynamics of prostate cancer cells interacting with fibronectin under in vitro conditions. Cell Biol Int. 2012;36:883–92. doi: 10.1042/CBI20110522. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberger G, Meien S, Kutsche K. Oncogenic HRAS mutations cause prolonged PI3K signaling in response to epidermal growth factor in fibroblasts of patients with Costello syndrome. Hum Mutat. 2009;30:352–62. doi: 10.1002/humu.20855. [DOI] [PubMed] [Google Scholar]
- 11.Pfarrer C, Hirsch P, Guillomot M, Leiser R. Interaction of integrin receptors with extracellular matrix is involved in trophoblast giant cell migration in bovine placentomes. Placenta. 2003;24:588–97. doi: 10.1016/S0143-4004(03)00059-6. [DOI] [PubMed] [Google Scholar]
- 12.Müller-Klingspor V, Hefler L, Obermair A, Kaider A, Breitenecker G, Leodolte S, et al. Prognostic value of beta1-integrin (=CD29) in serous adenocarcinomas of the ovary. Anticancer Res. 2001;21(3C):2185–8. [PubMed] [Google Scholar]
- 13.Wang L, Pedroja BS, Meyers EE, Garcia AL, Twining SS, Bernstein AM. Degradation of internalized αvβ5 integrin is controlled by uPAR bound uPA: effect on β1 integrin activity and α-SMA stress fiber assembly. PLoS One. 2012;7:e33915. doi: 10.1371/journal.pone.0033915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi-Smiraglia A, Paesante S, Bakin AV. Integrin β5 contributes to the tumorigenic potential of breast cancer cells through the Src-FAK and MEK-ERK signaling pathways. Oncogene. 2012 doi: 10.1038/onc.2012.320. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, et al. VEGF-induced vascular permeability is mediated by FAK. Dev Cell. 2012;22:146–57. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson C, Schlaepfer DD. Integrin adhesions: who’s on first? What’s on second? Connections between FAK and talin. Cell Adh Migr. 2012;6:302–6. doi: 10.4161/cam.20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuning U. Integrin αvβ3 promotes vitronectin gene expression in human ovarian cancer cells by implicating rel transcription factors. J Cell Biochem. 2011;112:1909–19. doi: 10.1002/jcb.23111. [DOI] [PubMed] [Google Scholar]
- 18.Lössner D, Abou-Ajram C, Benge A, Reuning U. Integrin alphavbeta3 mediates upregulation of epidermal growth-factor receptor expression and activity in human ovarian cancer cells. Int J Biochem Cell Biol. 2008;40:2746–61. doi: 10.1016/j.biocel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Strell C, Niggemann B, Voss MJ, Powe DG, Zanker KS, Entschladen F. Norepinephrine promotes the β1-integrin-mediated adhesion of MDA-MB-231 cells to vascular endothelium by the induction of a GROα release. Mol Cancer Res. 2012;10:197–207. doi: 10.1158/1541-7786.MCR-11-0130. [DOI] [PubMed] [Google Scholar]
- 20.Schmid MC, Avraamides CJ, Foubert P, Shaked Y, Kang SW, Kerbel RS, et al. Combined blockade of integrin-α4β1 plus cytokines SDF-1α or IL-1β potently inhibits tumor inflammation and growth. Cancer Res. 2011;71:6965–75. doi: 10.1158/0008-5472.CAN-11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny HA, Leonhardt P, Ladanyi A, Yamada SD, Montag A, Im HK, et al. Targeting the urokinase plasminogen activator receptor inhibits ovarian cancer metastasis. Clin Cancer Res. 2011;17:459–71. doi: 10.1158/1078-0432.CCR-10-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spivey KA, Banyard J. A prognostic gene signature in advanced ovarian cancer reveals a microfibril-associated protein (MAGP2) as a promoter of tumor cell survival and angiogenesis. Cell Adh Migr. 2010;4:169–71. doi: 10.4161/cam.4.2.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong Y, Tan OL, Loessner D, Stephens C, Walpole C, Boyle GM, et al. Kallikrein-related peptidase 7 promotes multicellular aggregation via the alpha(5)beta(1) integrin pathway and paclitaxel chemoresistance in serous epithelial ovarian carcinoma. Cancer Res. 2010;70:2624–33. doi: 10.1158/0008-5472.CAN-09-3415. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd TG, Mujoomdar ML, Nachtigal MW. Constitutive activation of BMP signalling abrogates experimental metastasis of OVCA429 cells via reduced cell adhesion. J Ovarian Res. 2010;3:5. doi: 10.1186/1757-2215-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slack-Davis JK, Atkins KA, Harrer C, Hershey ED, Conaway M. Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 2009;69:1469–76. doi: 10.1158/0008-5472.CAN-08-2678. [DOI] [PubMed] [Google Scholar]
- 26.Chen YJ, Wei YY, Chen HT, Fong YC, Hsu CJ, Tsai CH, et al. Osteopontin increases migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Physiol. 2009;221:98–108. doi: 10.1002/jcp.21835. [DOI] [PubMed] [Google Scholar]
- 27.Tan TW, Lai CH, Huang CY, Yang WH, Chen HT, Hsu HC, et al. CTGF enhances migration and MMP-13 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Biochem. 2009;107:345–56. doi: 10.1002/jcb.22132. [DOI] [PubMed] [Google Scholar]
- 28.Baumann F, Leukel P, Doerfelt A, Beier CP, Dettmer K, Oefner PJ, et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. 2009;11:368–80. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee S, Ray D, Lekli I, Bak I, Tosaki A, Das DK. Effects of Longevinex (modified resveratrol) on cardioprotection and its mechanisms of action. Can J Physiol Pharmacol. 2010;88:1017–25. doi: 10.1139/Y10-082. [DOI] [PubMed] [Google Scholar]
- 30.Engel MA, Neurath MF. Anticancer properties of the IL-12 family--focus on colorectal cancer. Curr Med Chem. 2010;17:3303–8. doi: 10.2174/092986710793176366. [DOI] [PubMed] [Google Scholar]
- 31.Kwon EM, Holt SK, Fu R, Kolb S, Williams G, Stanford JL, et al. Androgen metabolism and JAK/STAT pathway genes and prostate cancer risk. Cancer Epidemiol. 2012;36:347–53. doi: 10.1016/j.canep.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, Dimitrov P, Sapinoso LM, Orlowska-Volk M, et al. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2005;24:1053–65. doi: 10.1038/sj.onc.1208298. [DOI] [PubMed] [Google Scholar]
- 33.Dijkgraaf EM, Welters MJ, Nortier JW, van der Burg SH, Kroep JR. Interleukin-6/interleukin-6 receptor pathway as a new therapy target in epithelial ovarian cancer. Curr Pharm Des. 2012;18:3816–27. doi: 10.2174/138161212802002797. [DOI] [PubMed] [Google Scholar]
- 34.Colomiere M, Ward AC, Riley C, Trenerry MK, Cameron-Smith D, Findlay J, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009;100:134–44. doi: 10.1038/sj.bjc.6604794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan Z, Bradner JE, Greenberg E, Levine R, Foster R, Mahoney J, et al. SD-1029 inhibits signal transducer and activator of transcription 3 nuclear translocation. Clin Cancer Res. 2006;12:6844–52. doi: 10.1158/1078-0432.CCR-06-1330. [DOI] [PubMed] [Google Scholar]
- 36.Azare J, Leslie K, Al-Ahmadie H, Gerald W, Weinreb PH, Violette SM, et al. Constitutively activated Stat3 induces tumorigenesis and enhances cell motility of prostate epithelial cells through integrin beta 6. Mol Cell Biol. 2007;27:4444–53. doi: 10.1128/MCB.02404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C, Yang G, Jiang T, Huang K, Cao J, Qiu Z. Effects of IL-6 and AG490 on regulation of Stat3 signaling pathway and invasion of human pancreatic cancer cells in vitro. J Exp Clin Cancer Res. 2010;29:51. doi: 10.1186/1756-9966-29-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shain KH, Yarde DN, Meads MB, Huang M, Jove R, Hazlehurst LA, et al. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009;69:1009–15. doi: 10.1158/0008-5472.CAN-08-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li R, Li G, Deng L, Liu Q, Dai J, Shen J, et al. IL-6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up-regulation of MMP-2 and fascin-1. Oncol Rep. 2010;23:1553–9. doi: 10.3892/or_00000795. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Yin P, Di D, Luo G, Zheng L, Wei J, et al. IL-6 regulates MMP-10 expression via JAK2/STAT3 signaling pathway in a human lung adenocarcinoma cell line. Anticancer Res. 2009;29:4497–501. [PubMed] [Google Scholar]
- 41.Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36(13 Spec No):1621–30. doi: 10.1016/S0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 42.Kesanakurti D, Chetty C, Dinh DH, Gujrati M, Rao JS. Role of MMP-2 in the regulation of IL-6/Stat3 survival signaling via interaction with α5β1 integrin in glioma. Oncogene. 2013;32:327–40. doi: 10.1038/onc.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shield K, Riley C, Quinn MA, Rice GE, Ackland ML, Ahmed N. Alpha2beta1 integrin affects metastatic potential of ovarian carcinoma spheroids by supporting disaggregation and proteolysis. J Carcinog. 2007;6:11. doi: 10.1186/1477-3163-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leroy-Dudal J, Demeilliers C, Gallet O, Pauthe E, Dutoit S, Agniel R, et al. Transmigration of human ovarian adenocarcinoma cells through endothelial extracellular matrix involves alphav integrins and the participation of MMP2. Int J Cancer. 2005;114:531–43. doi: 10.1002/ijc.20778. [DOI] [PubMed] [Google Scholar]
- 45.Hamadi A, Deramaudt TB, Takeda K, Rondé P. Hyperphosphorylated FAK Delocalizes from Focal Adhesions to Membrane Ruffles. J Oncol. 2010;2010 doi: 10.1155/2010/932803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamamura K, Jiang C, Yokota H. ECM-dependent mRNA expression profiles and phosphorylation patterns of p130Cas, FAK, ERK and p38 MAPK of osteoblast-like cells. Cell Biol Int. 2010;34:1005–12. doi: 10.1042/CBI20100069. [DOI] [PubMed] [Google Scholar]
- 47.Zhu H, Liu XW, Cai TY, Cao J, Tu CX, Lu W, et al. Celastrol acts as a potent antimetastatic agent targeting beta1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway. J Pharmacol Exp Ther. 2010;334:489–99. doi: 10.1124/jpet.110.165654. [DOI] [PubMed] [Google Scholar]
- 48.Shi C, Lu J, Wu W, Ma F, Georges J, Huang H, et al. Endothelial cell-specific molecule 2 (ECSM2) localizes to cell-cell junctions and modulates bFGF-directed cell migration via the ERK-FAK pathway. PLoS One. 2011;6:e21482. doi: 10.1371/journal.pone.0021482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabe Y, Jin L, Tsutsumi-Ishii Y, Xu Y, McQueen T, Priebe W, et al. Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Res. 2007;67:684–94. doi: 10.1158/0008-5472.CAN-06-3166. [DOI] [PubMed] [Google Scholar]
- 50.Routray C, Liu C, Yaqoob U, Billadeau DD, Bloch KD, Kaibuchi K, et al. Protein kinase G signaling disrupts Rac1-dependent focal adhesion assembly in liver specific pericytes. Am J Physiol Cell Physiol. 2011;301:C66–74. doi: 10.1152/ajpcell.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miertzschke M, Stanley P, Bunney TD, Rodrigues-Lima F, Hogg N, Katan M. Characterization of interactions of adapter protein RAPL/Nore1B with RAP GTPases and their role in T cell migration. J Biol Chem. 2007;282:30629–42. doi: 10.1074/jbc.M704361200. [DOI] [PubMed] [Google Scholar]
- 52.Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, et al. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–15. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JC, Crary B, Chang YC, Kwon-Chung KJ, Kim KJ. Cryptococcus neoformans activates RhoGTPase proteins followed by protein kinase C, focal adhesion kinase, and ezrin to promote traversal across the blood-brain barrier. J Biol Chem. 2012;287:36147–57. doi: 10.1074/jbc.M112.389676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boccafoschi F, Mosca C, Bosetti M, Cannas M. The role of mechanical stretching in the activation and localization of adhesion proteins and related intracellular molecules. J Cell Biochem. 2011;112:1403–9. doi: 10.1002/jcb.23056. [DOI] [PubMed] [Google Scholar]
- 55.Payne SL, Hendrix MJ, Kirschmann DA. Lysyl oxidase regulates actin filament formation through the p130(Cas)/Crk/DOCK180 signaling complex. J Cell Biochem. 2006;98:827–37. doi: 10.1002/jcb.20792. [DOI] [PubMed] [Google Scholar]
- 56.Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E. Ligand-independent activation of c-Met by fibronectin and α(5)β(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2011;30:1566–76. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 58.Tsareva SA, Moriggl R, Corvinus FM, Wiederanders B, Schütz A, Kovacic B, et al. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia. 2007;9:279–91. doi: 10.1593/neo.06820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camand E, Peglion F, Osmani N, Sanson M, Etienne-Manneville S. N-cadherin expression level modulates integrin-mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J Cell Sci. 2012;125:844–57. doi: 10.1242/jcs.087668. [DOI] [PubMed] [Google Scholar]
- 60.Chang MH, Lee K, Lee KY, Kim YS, Kim YK, Kang JH. Prognostic role of integrin β1, E-cadherin, and rac1 expression in small cell lung cancer. APMIS. 2012;120:28–38. doi: 10.1111/j.1600-0463.2011.02788.x. [DOI] [PubMed] [Google Scholar]
- 61.O’Connor KL, Chen M, Towers LN. Integrin α6β4 cooperates with LPA signaling to stimulate Rac through AKAP-Lbc-mediated RhoA activation. Am J Physiol Cell Physiol. 2012;302:C605–14. doi: 10.1152/ajpcell.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lydolph MC, Morgan-Fisher M, Høye AM, Couchman JR, Wewer UM, Yoneda A. Alpha9beta1 integrin in melanoma cells can signal different adhesion states for migration and anchorage. Exp Cell Res. 2009;315:3312–24. doi: 10.1016/j.yexcr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 63.Rousseau M, Gaugler MH, Rodallec A, Bonnaud S, Paris F, Corre I. RhoA GTPase regulates radiation-induced alterations in endothelial cell adhesion and migration. Biochem Biophys Res Commun. 2011;414:750–5. doi: 10.1016/j.bbrc.2011.09.150. [DOI] [PubMed] [Google Scholar]
- 64.Di Ciano-Oliveira C, Thirone AC, Szászi K, Kapus A. Osmotic stress and the cytoskeleton: the R(h)ole of Rho GTPases. Acta Physiol (Oxf) 2006;187:257–72. doi: 10.1111/j.1748-1716.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- 65.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–86. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sepp KJ, Auld VJ. RhoA and Rac1 GTPases mediate the dynamic rearrangement of actin in peripheral glia. Development. 2003;130:1825–35. doi: 10.1242/dev.00413. [DOI] [PubMed] [Google Scholar]
- 67.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–20. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]


