Abstract
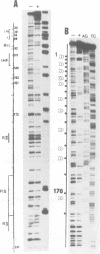
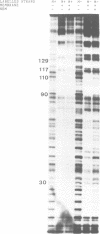
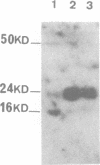
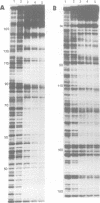
The outer membrane of Escherichia coli binds the origin of DNA replication (oriC) only when it is hemimethylated. We report here the results of a footprinting analysis with the outer membrane which demonstrate that its interaction with oriC occurs mainly at the left moiety of the minimal oriC, where 10 out of 11 Dam methylation sites are concentrated. Two regions, flanking the Integration Host Factor (IHF) sites, are preferentially recognized at the minimum membrane concentration at which oriC plasmid replication is inhibited in vitro. We have identified the putative proteins involved in hemimethylated oriC binding and cloned one of the corresponding genes (hobH). The purified LacZ-HobH fusion protein specifically binds oriC DNA at the same preferential sites as the membrane. A mutant of the hobH gene reveals partial asynchronous initiation of DNA replication.
Full text
PDF
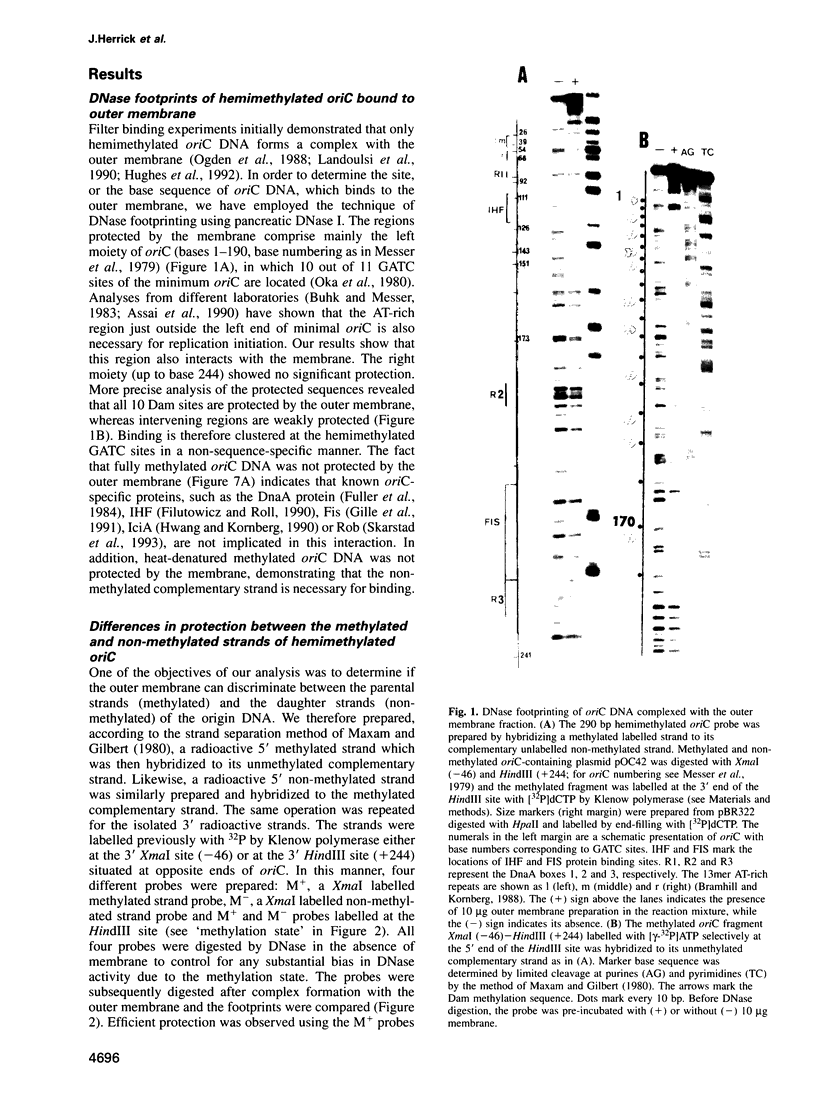





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
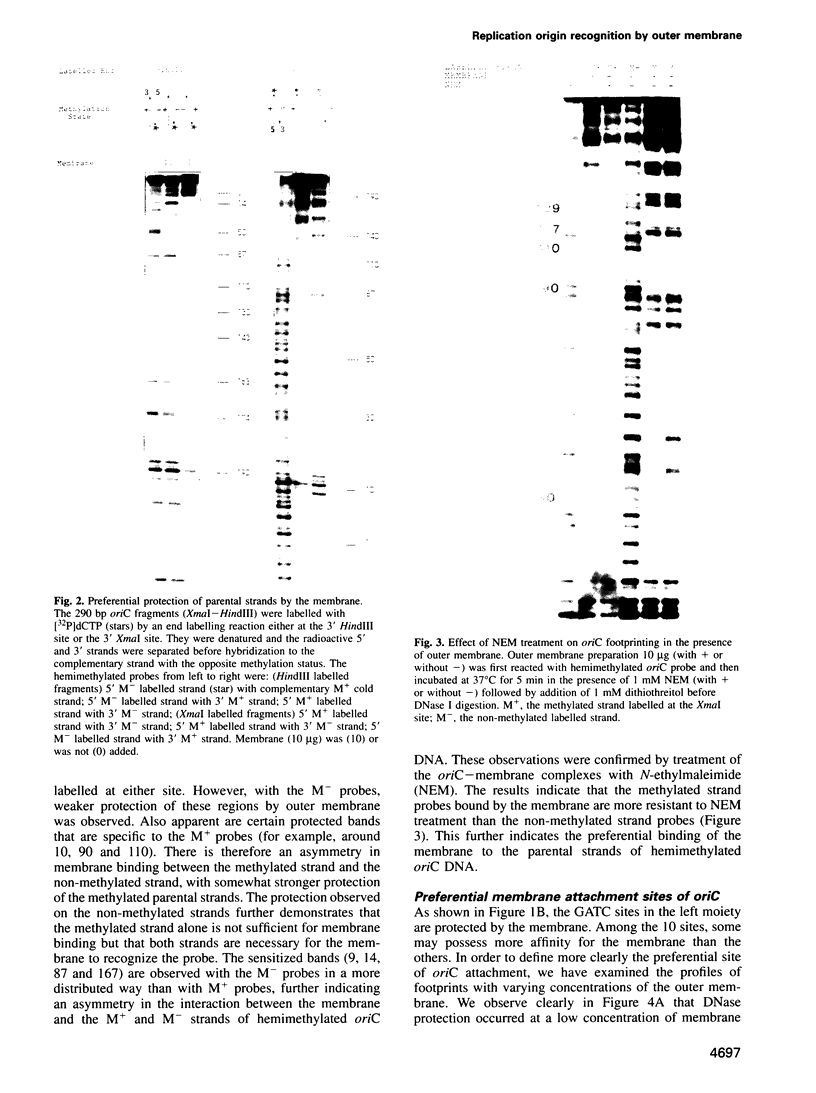
- Asai T., Takanami M., Imai M. The AT richness and gid transcription determine the left border of the replication origin of the E. coli chromosome. EMBO J. 1990 Dec;9(12):4065–4072. doi: 10.1002/j.1460-2075.1990.tb07628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A., Smith D. W. Methylation of GATC sites is required for precise timing between rounds of DNA replication in Escherichia coli. J Bacteriol. 1989 Oct;171(10):5738–5742. doi: 10.1128/jb.171.10.5738-5742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A. The role of dam methyltransferase in the control of DNA replication in E. coli. Cell. 1990 Sep 7;62(5):981–989. doi: 10.1016/0092-8674(90)90272-g. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Braun R. E., Wright A. DNA methylation differentially enhances the expression of one of the two E. coli dnaA promoters in vivo and in vitro. Mol Gen Genet. 1986 Feb;202(2):246–250. doi: 10.1007/BF00331644. [DOI] [PubMed] [Google Scholar]
- Buhk H. J., Messer W. The replication origin region of Escherichia coli: nucleotide sequence and functional units. Gene. 1983 Oct;24(2-3):265–279. doi: 10.1016/0378-1119(83)90087-2. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990 Sep 7;62(5):967–979. doi: 10.1016/0092-8674(90)90271-f. [DOI] [PubMed] [Google Scholar]
- Chakraborti A., Gunji S., Shakibai N., Cubeddu J., Rothfield L. Characterization of the Escherichia coli membrane domain responsible for binding oriC DNA. J Bacteriol. 1992 Nov;174(22):7202–7206. doi: 10.1128/jb.174.22.7202-7206.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firshein W. Role of the DNA/membrane complex in prokaryotic DNA replication. Annu Rev Microbiol. 1989;43:89–120. doi: 10.1146/annurev.mi.43.100189.000513. [DOI] [PubMed] [Google Scholar]
- Fotheringham I. G., Dacey S. A., Taylor P. P., Smith T. J., Hunter M. G., Finlay M. E., Primrose S. B., Parker D. M., Edwards R. M. The cloning and sequence analysis of the aspC and tyrB genes from Escherichia coli K12. Comparison of the primary structures of the aspartate aminotransferase and aromatic aminotransferase of E. coli with those of the pig aspartate aminotransferase isoenzymes. Biochem J. 1986 Mar 15;234(3):593–604. doi: 10.1042/bj2340593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Gille H., Egan J. B., Roth A., Messer W. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli. Nucleic Acids Res. 1991 Aug 11;19(15):4167–4172. doi: 10.1093/nar/19.15.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha S. DNA methyltransferase of Bacillus subtilis Marburg: purification, properties and further evidence of specificity. Gene. 1988 Dec 25;74(1):77–81. doi: 10.1016/0378-1119(88)90256-9. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. G., Kusano T., Yamaki H., Balakrishnan R., King M., Murchie J., Schaechter M. Binding of the origin of replication of Escherichia coli to the outer membrane. Cell. 1982 Oct;30(3):915–923. doi: 10.1016/0092-8674(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Yasuda S., Yamada M., Nishimura A., Sugimoto K., Sugisaki H., Oka A., Takanami M. Structural and functional properties of the Escherichia coli origin of DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):129–138. doi: 10.1101/sqb.1979.043.01.019. [DOI] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Sancar A. Sequences of Escherichia coli uvrA gene and protein reveal two potential ATP binding sites. J Biol Chem. 1986 Apr 15;261(11):4895–4901. [PubMed] [Google Scholar]
- Hwang D. S., Kornberg A. A novel protein binds a key origin sequence to block replication of an E. coli minichromosome. Cell. 1990 Oct 19;63(2):325–331. doi: 10.1016/0092-8674(90)90165-b. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kusano T., Steinmetz D., Hendrickson W. G., Murchie J., King M., Benson A., Schaechter M. Direct evidence for specific binding of the replicative origin of the Escherichia coli chromosome to the membrane. J Bacteriol. 1984 Apr;158(1):313–316. doi: 10.1128/jb.158.1.313-316.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landoulsi A., Hughes P., Kern R., Kohiyama M. dam methylation and the initiation of DNA replication on oriC plasmids. Mol Gen Genet. 1989 Apr;216(2-3):217–223. doi: 10.1007/BF00334359. [DOI] [PubMed] [Google Scholar]
- Landoulsi A., Malki A., Kern R., Kohiyama M., Hughes P. The E. coli cell surface specifically prevents the initiation of DNA replication at oriC on hemimethylated DNA templates. Cell. 1990 Nov 30;63(5):1053–1060. doi: 10.1016/0092-8674(90)90508-c. [DOI] [PubMed] [Google Scholar]
- Leonhardt H., Page A. W., Weier H. U., Bestor T. H. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell. 1992 Nov 27;71(5):865–873. doi: 10.1016/0092-8674(92)90561-p. [DOI] [PubMed] [Google Scholar]
- Lu M., Campbell J. L., Boye E., Kleckner N. SeqA: a negative modulator of replication initiation in E. coli. Cell. 1994 May 6;77(3):413–426. doi: 10.1016/0092-8674(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Carraway M., Frey A. Z., Brown L., Arraj J. A. Insertion mutations in the dam gene of Escherichia coli K-12. Mol Gen Genet. 1983;192(1-2):288–289. doi: 10.1007/BF00327681. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messer W., Meijer M., Bergmans H. E., Hansen F. G., von Meyenburg K., Beck E., Schaller H. Origin of replication, oriC, of the Escherichia coli K12 chromosome: nucleotide sequence. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):139–145. doi: 10.1101/sqb.1979.043.01.020. [DOI] [PubMed] [Google Scholar]
- Nicolaidis A. A., Holland I. B. Evidence for the specific association of the chromosomal origin with outer membrane fractions isolated from Escherichia coli. J Bacteriol. 1978 Jul;135(1):178–189. doi: 10.1128/jb.135.1.178-189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden G. B., Pratt M. J., Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988 Jul 1;54(1):127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Skarstad K., Baker T. A., Kornberg A. Strand separation required for initiation of replication at the chromosomal origin of E.coli is facilitated by a distant RNA--DNA hybrid. EMBO J. 1990 Jul;9(7):2341–2348. doi: 10.1002/j.1460-2075.1990.tb07406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Thöny B., Hwang D. S., Kornberg A. A novel binding protein of the origin of the Escherichia coli chromosome. J Biol Chem. 1993 Mar 15;268(8):5365–5370. [PubMed] [Google Scholar]
- Skarstad K., von Meyenburg K., Hansen F. G., Boye E. Coordination of chromosome replication initiation in Escherichia coli: effects of different dnaA alleles. J Bacteriol. 1988 Feb;170(2):852–858. doi: 10.1128/jb.170.2.852-858.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelker B., Messer W. The structure of the initiation complex at the replication origin, oriC, of Escherichia coli. Nucleic Acids Res. 1993 Nov 11;21(22):5025–5033. doi: 10.1093/nar/21.22.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Masters M. Deoxyribonucleic acid and outer membrane: strains diploid for the oriC region show elevated levels of deoxyribonucleic acid-binding protein and evidence for specific binding of the oriC region to outer membrane. J Bacteriol. 1979 Oct;140(1):50–58. doi: 10.1128/jb.140.1.50-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Cleary J. M., Brusilow W. S., Harding N. E., Smith D. W. Chromosomal replication origin from the marine bacterium Vibrio harveyi functions in Escherichia coli: oriC consensus sequence. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1164–1168. doi: 10.1073/pnas.80.5.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]