Abstract
The authors first examined the concurrent moderating role of lifestyle engagement on the relation between cognitive status (cognitively elite, cognitively normal [CN], and cognitively impaired [CI]) and executive functioning (EF) in older adults. Second, the authors examined whether baseline participation in lifestyle activities predicted differential 4.5-year stabilities and transitions in cognitive status. Participants (initial N = 501; 53–90 years) were from the Victoria Longitudinal Study. EF was represented by a 1-factor structure. Lifestyle activities were measured in multiple domains of engagement (e.g., cognitive, physical, and social). Two-wave status stability groups included sustained normal aging, transitional early impairment, and chronic impairment. Hierarchical regressions showed that baseline participation in social activities moderated cognitive status differences in EF. CI adults with high (but not low) social engagement performed equivalently to CN adults on EF. Longitudinally, logistic regressions showed that engagement in physical activities was a significant predictor of stability of cognitive status. CI adults who were more engaged in physical activities were more likely to improve in their cognitive status over time than their more sedentary peers. Participation in cognitive activities was a significant predictor of maintenance in a higher cognitive status group. Given that lifestyle engagement plays a detectable role in healthy, normal, and impaired neuropsychological aging, further research in activity-related associations and interventions is recommended.
Keywords: Lifestyle activities, Executive functions, Cognitive status, Aging, Victoria Longitudinal Study
Introduction
According to such hypotheses as “use it or lose it” (Bielak, 2010; Small, Hughes, Hultsch, & Dixon, 2007) or activities enrichment (Carlson et al., 2012; Schooler & Mulatu, 2001), leading a lifestyle rich with engaging activities or environmental complexity may have enhancing effects on brain and cognitive health with aging. Numerous neuropsychological studies using experimental and correlational methods have shown various patterns of associations among aspects of an engaged lifestyle and domains of cognitive functioning in normal aging (e.g., Agrigoroaei & Lachman, 2011; Kramer, Bherer, Colcombe, Dong, & Greenough, 2004; Salthouse, 2006; Schooler & Mulatu, 2001; Small, Dixon, McArdle, & Grimm, 2012) and even dementia (e.g., Fratiglioni, Paillard-Borg, & Winblad, 2004). However, other studies have found little to no activity–cognition relationships (e.g., Aartsen, Smits, van Tilberg, Knipscheer, & Deeg, 2002; Brown et al., 2012; Mackinnon, Christensen, Hofer, Korten, & Jorm, 2003). Among other factors, the mixed pattern of findings may be attributed to (a) how lifestyle engagement has been measured, (b) the presence of unmeasured sample heterogeneity, and (c) the match between specific domains of lifestyle activities and cognitive performance.
Three key lifestyle domains (i.e., cognitive, physical, social) have shown some promising benefits in the cognitive and epidemiological aging literature. First, the benefits of concurrent cognitive stimulation on cognitive functioning in healthy older adults have been demonstrated through self-reported active participation in novel or complex cognitive tasks (Bielak, Hughes, Small, & Dixon, 2007). Recently, longitudinal and dynamic coupling between changes in lifestyle engagement and cognitive neuropsychological performance in healthy older adults has been supported, such that poor cognitive performance was related to future reduction in lifestyle engagement (Mitchell et al., 2012; Schooler & Mulatu, 2001; Small et al., 2012). Reviews of pathological aging (Fratiglioni et al., 2004; Hertzog, Kramer, Wilson, & Lindenberger, 2008) conclude that cognitive engagement may help to delay decline attributed to impairment or dementia. Second, some recent reviews and studies suggest that physical activity may promote or improve cognitive function and perhaps delay the onset of cognitive decline (Brown et al., 2012; Kramer et al., 2004; Lindwall et al., 2012; Rockwood & Middleton, 2007). Third, some studies show that participation in social activities may facilitate continued cognitive health in older adults (Bassuk, Glass, & Berkman, 1999; Hughes, Andel, Small, Borenstein, & Mortimer, 2008; Seeman, Lusignolo, Albert, & Berkman, 2001), but the evidence is mixed in normal aging (e.g., Brown et al., 2012). The exact mechanism underlying these potential protective factors on cognitive health is unclear. However, likely candidates include greater neural reserve capacity, increased cerebral blood flow, decreased neuroinflammation and brain atrophy, and neural or functional compensation (Colcombe et al., 2003; Cotman, Berchtold, & Christie, 2007; Dixon, Garrett, & Bäckman, 2008; Stern, 2009). Regarding stability and change in cognitive health, current issues include the identity and variety of measured activities, the match between activity and the assessed domain of neuropsychological functioning, the longitudinal implications of lifestyle activities, and how they may operate across different levels of cognitive status.
The selection of cognitive domains in prior studies of lifestyle engagement has mainly focused on perceptual speed, verbal speed, visuo-spatial ability, and declarative memory (e.g., Fratiglioni et al., 2004; Ghisletta, Bickel, & Lövdén, 2006; Small et al., 2012). Cognitive processes and products that represent a single domain may not closely match the skill that is used in any given environmental context. The cognitive control processes that are required to effectively monitor and regulate thought and action are referred to as executive functions (EFs; Friedman et al., 2008). These related but separable EF abilities include a system of control processes that reflect response inhibition, updating working memory representations, and set shifting (Luszcz, 2011). Complicating the study of lifestyle activity and EF performance is the fact that EF has been previously represented as either a unitary (i.e., common) or diverse (i.e., multidimensional) set of abilities. A multidimensional structure (i.e., inhibition, shifting, and updating) was supported in samples of healthy young adults (Miyake et al., 2000) and cognitively healthy or elite (CE) older adults, with cognitively normal (CN) older adults producing intermediate evidence (i.e., both single and multiple factor evidence, with the latter accompanied by high inter-factor correlations; de Frias, Dixon, & Strauss, 2009). In contrast, only a unitary structure was representative of the cognitively impaired (CI) older adults, suggesting dedifferentiation of EFs (de Frias, Dixon, & Strauss, 2006; de Frias et al., 2009). Notably, longitudinal data have shown that EF structures display two-wave temporal invariance (i.e., the relations between the observed measure and the underlying construct are equivalent across time; de Frias et al., 2009; McFall et al., 2013). A unitary structure also fit the CN and CE groups, suggesting that quantitative comparisons along a continuum of cognitive status may require treating EF as a single ability. In addition, the two-wave stability of cognitive status was related to EF performance in these three neuropsychological groups (i.e., CE, CN, and CI) such that maintaining a higher cognitive status or improving one's status was related to better concurrent EF (de Frias et al., 2009). These findings indicate that the pattern of individual differences in EF is affected by sample composition (e.g., cognitive status) and a function of individual differences in neural integrity and life experience. Conceivably, the mixed pattern of findings for activity–cognition relationships may be partly attributed not only to baseline cognitive status but also to the extent to which these statuses are stable or changing over a subsequent period. The present study (a) expands the range of cognitive domains to include EF and (b) assesses whether the cognitive benefit of active living is affected by concurrent cognitive status (e.g., cognitively healthy vs. CI at baseline) and cognitive status stability and change.
The goals of the present study are two-fold. The first goal was to test whether initial lifestyle engagement moderated the concurrent effect of cognitive status on EF performance. We hypothesized that greater lifestyle engagement would be beneficial to EF, especially for more cognitively vulnerable adults. The second goal was to test whether lifestyle engagement at baseline predicts the stability of cognitive status (i.e., maintenance vs. non-stability [decline/improvement] in status) and maintenance across cognitive status groups in CI, CN, and CE adults over a 4.5-year period. We hypothesized that greater lifestyle engagement at baseline would aid in the maintenance of classification status (for CN and CE adults) over a two-wave period. We explored whether improvement in classification status over a 4.5-year period was predicted by lifestyle engagement levels.
Method
Participants
Characteristics
The participants were community-dwelling adults from the Victoria Longitudinal Study (VLS), an ongoing multisample sequential study of cognitive, neuropsychological, health, and biological aging. They were originally recruited through advertisements in the public media and from community groups. They were paid nominal fees for their participation. The VLS and all present data collection procedures are in full and certified compliance with prevailing human research ethics guidelines and boards. Written informed consent was obtained from all participants. The data used for this study were from the available first two waves of testing for VLS Sample 3. From the VLS neuropsychological battery, six tasks measuring EF were included. We used this source sample to develop the classification procedures. Briefly, at Wave 1 (W1), the source sample consisted of n = 570 participants (385 women and 185 men) who were aged 53–90 (M age = 68.2 years, SD = 8.6 years). The average level of education was 15.2 years (SD = 3.0 years). At W2, the source sample consisted of n = 399 participants (270 women and 129 men; M age = 72.2 years, SD = 8.5 years). The overall average education was 15.6 years (SD = 2.9). The interval between W1 and W2 was 4.5 years. All participants attained scores above 23 on the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975). Self-reported health was referenced by two indicators: Absolute health (i.e., health relative to a perfect state) and relative health (i.e., health relative to same-age peers). For both indicators, the participants rated their health as being good to very good on a 5-point scale, in which 1 indicated very good health. Published overviews present further background information on the VLS methods, procedures, and samples (Dixon & de Frias, 2004; Hultsch, Hertzog, Dixon, & Small, 1998).
Cognitive status classification: W1
Participants at W1 were classified into three groups representing a continuum of provisional cognitive status: CE, CN, and CI. Cognitive status classification was determined by an objective and replicable procedure adapted from previous VLS and other research, as focused on the separation of older adults experiencing potential mild cognitive impairment from those functioning in the normal range (e.g., Dixon et al., 2007; Dolcos, MacDonald, Braslavsky, Camicioli, & Dixon, 2012; de Frias et al., 2009; Ritchie, Artero, & Touchon, 2001; see also Albert et al., 2011). First, the participants were stratified by age (53–70 or 71–90 years) and level of education (0–12 or 13+ years) and placed into one of four groups: (a) Young–old (YO; low education) n = 58; (b) YO (high education) n = 292; (c) Old–old (OO; low education) n = 49; and (d) OO (high education) n = 171. Second, for each of the four groups, mean performance was calculated for five cognitive reference tests, representing the cognitive domains of perceptual speed, inductive reasoning, episodic memory, verbal fluency, and semantic memory. The resulting distributions served as within-sample norms for cognitive status classification. Third, we classified participants into one of three mutually exclusive cognitive status groups as follows. All members of the CE group (n = 80) scored above the mean on all five reference tests at W1. All members of the CN group (n = 284) scored between −1.5 SD and +1.5 SD on all five reference tests. All members of the CI group (n = 137) scored at least 1.5 SD below the group mean on one or more tests. This procedure resulted in n = 501 successful classifications; n = 69 participants did not fit any of the classifications (e.g., having a score within the −1.5 SD to +1.5 SD band for four tests, but above +1.5 SD on a fifth test) and were excluded from further analyses.
Cognitive status classification: W2 and stability
Next, the above three-step procedure was repeated independently at W2. The norms at W2 were used to classify performance at W2. The resulting W2 classifications permitted us to establish the six respective status stability groups. These included three stable status groups: (a) CE–CE (continued cognitively healthy): n = 31, (b) CN–CN (continued CN): n = 137, and (c) CI–CI (chronic impaired): n = 57. The three non-stable status groups were: (d) CE–CN (declining to normal): n = 20, (e) CN–CI (transitioning to impairment): n = 32, and (f) CI–CN (apparent improvement or variability in status): n = 21. As established, the CI group—and especially the chronic CI–CI subgroup—is comparable with parallel groups classified with mild cognitive impairment (e.g., Albert et al., 2011; Dolcos et al., 2012).
Executive function measures
We used six cognitive neuropsychological tests to measure EFs that represent a common set of abilities that cover three key aspects, namely inhibition (Hayling, Stroop), shifting (Brixton, Color Trails 2), and updating (Computational Span, Reading Span). Psychometric and structural properties of these tests have been reported and all have been used in multiple studies, including healthy aging and clinical populations (Bielak et al., 2007; de Frias et al., 2006, 2009; McFall et al., 2013; Strauss, Sherman, & Spreen, 2006).
Hayling sentence completion test
This test (Burgess & Shallice, 1997) consists of two sets of 15 sentences, each having the last word missing. In Section 1, the examiner reads each sentence aloud and the participant completes the sentence as quickly as possible. This section yields a measure of response speed. In Section 2, the participant completes the sentence with a word that is unconnected to the sentence. The participant has to inhibit a strongly activated (automatic) response before generating a new response. Completing the sentence with a word that is connected to the sentence in meaning would be coded as an error. Scaled scores (range = 1–10) were derived from cutoffs of the raw score latencies and errors. The score used for this analysis was based on a scale ranging from 1 (“impaired”) to 10 (“very superior”).
Stroop test
This task measures inhibition by requiring the respondent to ignore the automatic response of reading a printed word and to instead name the color of ink in which the word is printed (Regard, 1981). In Part A, the participant names as fast as possible the color of 24 dots printed in blue, green, red, or yellow. In Part C, the colored stimuli are the color names (i.e., blue, green, red, and yellow) printed in lower case with the color being incongruent to the color name. The performance score was the interference index ([Part C−Part A]/Part A), with higher scores indicating more interference.
Brixton test
The Brixton Spatial Anticipation Test (Burgess & Shallice, 1997) is a rule-attainment (or shifting) task based on the Wisconsin Card Sorting Task (Psychological Assessment Resources, 1990). This test consists of a 56-page stimulus booklet, with each page showing the same basic array of 10 circles set in two rows of five, with each circle numbered from 1 to 10. On each page, one of the circles is filled with a blue color and its position changes from one page to another. The changes in position are governed by a series of simple rules that vary without warning. The participant is shown one page at a time and is asked to decide (by pointing) the position of the next filled circle. The total errors were recorded and these errors (maximum 54) were converted to scaled scores (e.g., 0–7 raw errors were converted to a scaled score of 10, which is classified as “very superior performance”). An overall standardized scaled score based on a scale ranging from 1 (impaired) to 10 (very superior) was used for analysis.
Color Trails Test
The Color Trails Test (CTT; D'Elia, Satz, Uchiyama, & White, 1996) is a shifting task similar to the Trail Making Test (Reitan & Wolfson, 1985) but it was designed to minimize the influence of language. The second part (CTT-2) shows encircled numbers from 1 to 25 twice (one sequence with a yellow background, the other in pink). The participant is required to connect the numbers from 1 to 25 alternating between pink and yellow circles and disregarding the numbers in circles of the alternate color (D'Elia et al., 1996). The latency score (in seconds) for CTT-2 was used for analysis with higher scores indicating slower performance.
Computational span
The computational span task, an indicator of working memory (updating), requires the storage and simultaneous processing of information (Salthouse & Babcock, 1991). Participants are asked to solve a series of arithmetic problems while holding the final digit from each problem in memory for later recall. The number of problems in a series increases from 1 to 7, with three trials at each series length. The highest span correctly recalled for two of three trials was the measure used.
Reading span
The reading span task, an indicator of working memory (updating), requires participants to answer questions about simple sentences that were orally presented while simultaneously trying to remember the final word of each sentence for later recall. The number of sentences in a passage increases from 1 to 7 with three trials at each series length. The target measure was the highest span correctly recalled for two of three trials.
Each of the six EF tests were converted into T-score units, then a summary EF variable score was computed.
Cognitive Reference Measures
For the cognitive status classification, we used indicators of five cognitive domains (i.e., perceptual speed, inductive reasoning, episodic memory, verbal fluency, and vocabulary). The reliability and validity of these standard and widely used measures are presented elsewhere (e.g., Hultsch et al., 1998). The classification procedure is described above.
Perceptual speed
Perceptual processing speed was assessed with the Wechsler Adult Intelligence Scale-Revised Digit Symbol Substitution task (Wechsler, 1981). Participants are presented with a coding key pairing nine numbers (1–9) with nine different symbols. Printed under the coding key are rows of randomly ordered numbers with empty boxes below. Participants were given 90 s to transcribe as many symbols as possible into the empty boxes on the basis of the digit—symbol associations specified in the coding key. The number of correctly completed items represented the outcome measure.
Inductive reasoning
Inductive reasoning was assessed with the Letter Series test (Thurstone, 1962). Participants are presented with a string of letters forming a distinct pattern. The task requires inductively deciphering the pattern in the target string and providing the next letter in the string congruent with the pattern presented. The outcome measure used was the total number correct out of 20 patterns.
Episodic memory
The word recall task consists of immediate free recall of two lists of 30 English words (Dixon et al., 2004). Each list consists of six words from each of five taxonomic categories and participants had 2 min to study each list and 5 min to write their recall. The number of correctly recalled words averaged across the two lists was used as the measure.
Verbal fluency
Participants' verbal fluency was assessed with the Controlled Associations test from the Educational Testing Service (ETS) kit of factor-referenced cognitive tests (Ekstrom, French, Harman, & Dermen, 1976). The test requires the generation of as many synonyms as possible in response to a set of target words. Participants were given 6 min to complete the test, with the total number of correct synonyms representing the fluency score.
Vocabulary
A recognition vocabulary test represented semantic memory and consisted of a 54-item multiple-choice test from the ETS kit (Ekstrom et al., 1976). Participants were given 15 min, with the total number of correct items representing the vocabulary score.
Lifestyle Engagement Measure
We used the VLS Activity Lifestyle Questionnaire (VLS-ALQ) to measure the frequency of engagement in seven types of everyday activities over the past 2 years (for measurement properties, see Bielak et al., 2007; Hultsch, Hertzog, Small, & Dixon, 1999; Small et al., 2012). Frequency of participation was rated on a 9-point scale (from “never” to “daily”). The seven standard categories were created based on 67 items: (a) physical, such as jogging (n = 4); (b) self-maintenance, such as preparing a meal (n = 6); (c) social, such as visiting friends (n = 7); (d) travel, such as nearby holiday travel (n = 3); (e) passive information processing, such as reading the newspaper (n = 8); (f) integrative information processing, such as playing a musical instrument (n = 12); and (g) novel information processing, such as completing income tax forms (n = 27). Composite variables were created by summing the average responses on items within each subscale. All seven subscales were examined separately.
Analyses
Alpha levels of p < .05 were specified as the threshold to indicate statistical significance. The CTT-2 latency and Stroop interference scores were reverse coded to be commensurate with all other cognitive tasks (i.e., higher score = better performance).
Research Goal 1: Everyday Lifestyle Activities as a Moderator of Cognitive Status Differences
For research goal 1, we applied one hierarchical regression model to examine the possible moderating influences of everyday lifestyle activities. The order of correlates was as follows: (a) One cognitive status (at W1) variable (Block 1), (b) seven everyday lifestyle activities (Block 2), and (c) seven two-way interactions between cognitive status and activity (Block 3). Prior research using the present six EF tasks (de Frias et al., 2009) has shown that the three-factor structure of EF does not fit for the CI group, but the single-factor model fit for all the cognitive status groups. Therefore, EF will be assessed as a unidimensional construct. The purpose was to examine the independent and relative contribution of cognitive status and activity levels, and their two-way interactions on EF performance.
Research Goal 2: Does Lifestyle Engagement Affect the Stability of Cognitive Status Over Time?
Six logistic regression models were conducted as follows: (a) CE–CE versus CE–CN; (b) CN–CN versus CN–CI; (c) CI–CI versus CI–CN; (d) CE–CE versus CN–CN; (e) CE–CE versus CI–CI; and (f) CN–CN versus CI–CI to examine whether baseline lifestyle engagement predicted group membership (i.e., maintenance within a cognitive status group, and also the odds of being in a maintenance group with higher cognitive status). All seven activity subscales were included together in each of the six logistic regression models.
Results
Research Goal 1: Everyday Lifestyle Activities as a Moderator of Cognitive Status Differences
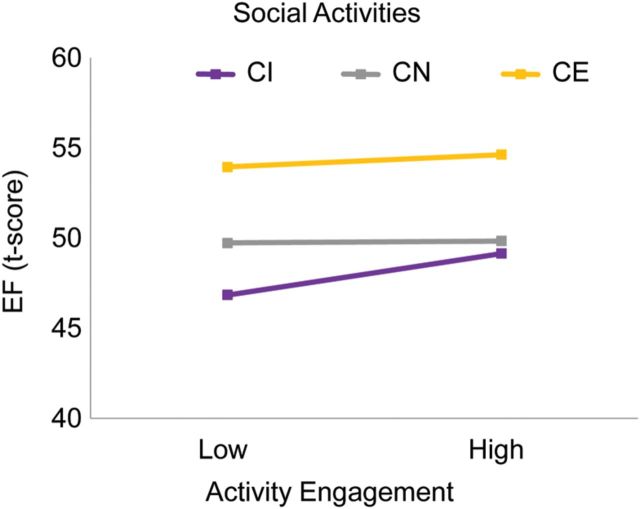
We applied a hierarchical regression model as described above. Table 1 presents a summary of the regression results. Cognitive status accounted for 12% of the variance in executive functioning (EF). Higher cognitive status was correlated with better EF. Overall, the everyday lifestyle activities accounted for an additional 7% of the variance after controlling for cognitive status. One activity subscale was significant: Greater engagement in novel information processing activities was related to better EF. Interactions between cognitive status and each activity did not significantly account for additional variance. However, self-maintenance and socialactivities independently moderated cognitive status differences in EF. To further explore these two effects, we used median split to create low and high activity levels (i.e., for self-maintenance and social activities). Follow-up general linear models showed no differences within groups for self-maintenance. For social activities, the 95% CI around the lower and upper bounds showed differences in level of social activities for the CI group only. As shown in Fig. 1, CI adults who are more engaged in social activities have better EF than those who are less involved. CE adults performed highest irrespective of the activity level. CN adults performed lower than CE.
Table 1.
Interaction effects among cognitive status (W1) and everyday lifestyle activities using hierarchical multiple regressions
| Correlates | EF factor |
|---|---|
| Cognitive status (CS) | 0.34*** |
| R2 | 0.12*** |
| F-Change | 37.60*** |
| Activities | |
| Physical | 0.07 |
| Self-maintenance | 0.05 |
| Travel | 0.07 |
| Passive Information Processing | −0.06 |
| Social | −0.08 |
| Integrative Information Processing | 0.02 |
| Novel Information Processing | 0.22** |
| R2 | 0.18 |
| ΔR2 | 0.07** |
| F-Change | 3.17** |
| Two-way interactions | |
| CS × Physical | −0.13 |
| CS × Self-maintenance | 0.37* |
| CS × Travel | 0.29 |
| CS × Passive Information Processing | 0.06 |
| CS × Social | −0.42* |
| CS × Integrative Information Processing | 0.04 |
| CS × Novel Information Processing | 0.17 |
| R2 | 0.21 |
| ΔR2 | 0.03 |
| F-Change | 1.40 |
Note: EF = executive functioning. N = 469.
*p < .05.
**p < .01.
***p < .001.
Fig. 1.
CI older adults who are engaged in social activities have better EF than those who are less socially engaged. CE adults performed highest irrespective of the activity level. CN adults performed lower than CE.
Research Goal 2: Lifestyle Engagement and Maintenance of Cognitive Status Over Time
Six logistic regression models were conducted: CE–CE versus CN–CN; CE–CE versus CI–CI; CN–CN versus CI–CI; CE–CE versus CE–CN; CN–CN versus CN–CI; and CI–CI versus CI–CN. For the CE–CE versus CN–CN comparison, engagement in novel information processing activities predicted group membership, Exp(β) = 3.52, 95% CI = 1.49–8.28, p < .01. The probability of being in the CE stability group (rather than the CN stability group) over the two-wave period increased 3.52 times per unit increase in activity. For the CE–CE versus CI–CI comparison, engagement in novel information processing activities also predicted group membership, Exp(β) = 5.23, 95% CI = 1.49–8.28, p < .001. The probability of being in the CE stability group (rather than the CI stability group) over the two-wave period increased 5.23 times per unit increase in activity. For the CN–CN versus CI–CI comparison, engagement in novel information processing activities also predicted group membership, Exp(β) = 1.97, 95% CI = 1.05–3.67, p < .05. The probability of being in the CN stability group (rather than the CI stability group) over the two-wave period increased nearly two times per unit increase in activity. For the CI–CI versus CI–CN comparison, engagement in physical activities predicted group membership, Exp(β) = 1.63, 95% CI = 0.99–2.69, p < .05; and engagement in travel predicted group membership, Exp(β) = 0.46, 95% CI = 0.22–0.94, p < .05. The probability of improving cognitive status over the two-wave period increased 1.63 times per unit increase in physical activity in the CI group. The probability of improving cognitive status over the two-wave period in the same group increased 0.46 times per unit decrease in travel activities. Physical activities and travel did not predict group membership between CE–CE versus CE–CN and also CN–CN versus CN–CI comparisons. None of the other lifestyle activity variables were significant in predicting change in cognitive status. Table 2 presents a summary of the regression results.
Table 2.
Using logistic regression analyses to examine everyday lifestyle activities as a predictor of stability transition of cognitive status over a two-wave period
| Cognitive status | Β | Odds ratio Exp(β) | CI | p |
|---|---|---|---|---|
| CE–CE versus CE–CN | ||||
| Physical Activities | 0.04 | 1.04 | 0.51–2.12 | .91 |
| Travel | 0.28 | 1.32 | 0.47–3.70 | .60 |
| CN–CN versus CN–CI | ||||
| Physical Activities | 0.16 | 1.17 | 0.87–1.58 | .30 |
| Travel | 0.16 | 1.17 | 0.68–2.02 | .57 |
| CI–CI versus CI–CN | ||||
| Physical Activities | 0.49 | 1.63 | 0.99–2.69 | .05 |
| Travel | −0.78 | 0.46 | 0.22–0.94 | .03 |
Note: Comparisons between stability groups are reported in the text.
Discussion
We investigated two research goals, both pertaining to the potential effects of lifestyle activities on performance or stability related to cognitive status: (a) the potential moderating effects of lifestyle engagement on cognitive status differences in EF performance, and (b) whether lifestyle engagement predicts two-wave maintenance (vs. transitions) of cognitive status. Our three cognitive status groups were defined using our standard objective classification procedure (e.g., Dixon et al., 2007; Dolcos et al., 2012; de Frias et al., 2009). Namely, we empirically defined a CN older group (i.e., CN), differentiating it from a relatively CI group (i.e., CI) and cognitively higher performing group (i.e., CE) of older adults. The EF domain was handled as a single factor as per our prior findings showing that a unidimensional structure fit all three groups (de Frias et al., 2009) and other samples from the VLS (McFall et al., 2013).
The first research goal was to examine the moderating role of lifestyle engagement. We found support for social engagement as a moderator of cognitive status differences in general EF performance. Specifically, CI adults who have more engaged social lives also have better EF when compared with their CI counterparts who are less socially active. In contrast, the CE group performed the highest on EF and steadily across two levels (above/below median) of the social engagement domain. The CN group performed lower on EF yet also at a steady level across the same activity domain. These findings extend other studies by supporting the social engagement hypothesis such that higher social activity among CE, CN, and CI older adults is associated with better EF (Fratiglioni et al., 2004; Hughes et al., 2008; Krueger et al., 2009; Seeman et al., 2001; Small et al., 2012), whereas low levels of social activity in CI adults is related to an EF deficit. Our results suggest that the ability to maintain a healthy level of social activity (e.g., managing relationships or skill in the cognitive component of social interactions or variety in activity) may be challenging in some CI adults which may further compromise their EF performance. Future research should assess the exact social mechanism (e.g., changes in access to activities or social support, motivational shifts) that may become vulnerable in CI adults. In addition, however, it is possible that related cognitive mechanisms (and perhaps especially executive functions) may support social engagement, at least in the sense that high levels of social activities offer ample opportunities for practice in essential cognitive processes. Decline in these skills (e.g., memory, planning) may affect level, variety, and challenge of social activities (Small et al., 2012). Conceivably, since the CI adults may develop more severe deficits further compromising their independence, it becomes vital to ensure that they remain socially connected. Since the benefits of cognitive collaboration have been demonstrated in older adults (Dixon, 2011), future research should focus on cognitive interventions geared toward facilitating socially charged or collaborative tasks for CI adults. Similar to other studies, we did not find that physical activity was a significant moderator after accounting for variance in all other lifestyle activity categories (see Kramer et al., 2004, for review). The kind and intensity of physical activity may need to be assessed in future research on this topic.
The second research goal was to examine whether maintenance of cognitive status over a two-wave (4.5 years) period was predicted by the frequency of participating in activities at baseline. Using logistic regression models, we found that among the CI group, those individuals who reported greater participation in physical activities were more likely to improve their cognitive status than their more sedentary peers. The cognitive trajectory of CI adults is variable, with improvement, maintenance, and decline as viable pathways (de Frias et al., 2009; Dixon et al., 2007). Our findings show that one mechanism promoting long-term cognitive status improvement is physical activity, especially among already cognitively less healthy older adults (see Fratiglioni et al., 2004; Kramer et al., 2004, for reviews). We also found that among individuals who maintained their classification status over a two-wave period, membership into a higher cognitive status group (i.e., CE–CE relative to CN–CN, CE–CE relative to CI–CI, and CN–CN relative to CI–CI) was consistently predicted by cognitive activities that involve novel information processing. The largest effect was the five-fold increase (odds ratio = 5.23) in being in the CE–CE (sustained cognitively healthy) group than the CI–CI (chronic CI) group with greater involvement in this form of cognitive activity. The odds of being in the CN–CN (stable normal aging) group, relative to the CI–CI group, were nearly two-fold with every increase in the frequency of participating in cognitive activities that demand novel information processing. The protective cognitive effects of cognitively stimulating activities that demand novel information processing has been consistently supported (e.g., Mitchell et al., 2012). Formal cognitive interventions targeting novel information processing skills compounded with a boost in cognitively stimulating activities in everyday life may be most effective in preserving cognition.
Several limitations of this study are noteworthy. First, our measure of lifestyle engagement specifically addressed frequency of participation in a given activity. Recent research by Carlson and colleagues (2012) has shown that participation in a variety of lifestyle activities is also a powerful predictor of cognitive health, at least supplementing the standard frequency of participation in any single activity. To some extent, we observed a result consistent with this perspective initially in the first research goal. The full (and diverse) complement of lifestyle activities were a significant predictor of EF performance even after controlling for cognitive status. Notably, more domain-specific relationships were subsequently also observed. Second, although the overall sample size is adequate, the subgroups that are formed through classification are relatively small for some subgroups. Third, although EF was adequately sampled, the five cognitive (non-EF) domains used for classification had fewer indicators for each domain listed.
Fourth, while the analysis for the second research goal focused on change in cognitive classification, another approach could be to examine change in the cognitive level. Fifth, while environmental/lifestyle factors provide important influences on healthy cognitive aging, biological/mood/genetic factors or their interactions are also viable contributors. For example, it is plausible that physical activity may moderate the cognitive status-EF relation for specific genotypes.
In sum, we found that different but potentially complementary domains of lifestyle activities (social, physical, cognitive) operated to moderate cognitive status effects both at baseline (in terms of the specific cognitive outcome of EF) and over time (in terms of the stability or transitions in status). Regarding the former, cognitive status differences in EF were moderated by social engagement for CI adults. Regarding the latter, two results are especially noteworthy. First, among CI adults, improvement in cognitive status over a two-wave period was provided by greater participation in physical activities. Second, among the stable groups, being in a higher cognitive status group was associated with by greater participation in novel cognitive activities. We examined cognitive status along a continuum which allowed us to pinpoint the critical stage that is most conducive to the benefits of active living (e.g., CI adults). Notably, our results confirm the general trend in the literature to focus on the lifestyle engagement domains of cognitive, social, and physical activities, as the other domains covered by the VLS-ALQ did not play a role in these results. Future clinical research on cognitive interventions in normal and impaired aging should target lower performing and perhaps clinically notable groups of CI older adults, perhaps with a tripartite engagement regimen, examining either separately or interactively elements of physical, cognitive, and social activities or training, namely, a holistic approach.
Funding
The VLS is supported by grants from the U.S. National Institute on Aging (R37 AG008235) and the Canada Research Chairs program to R.A.D.
Conflict of Interest
None declared.
Acknowledgements
We thank VLS staff and participants for their many contributions to the project. For further information about the VLS, see http://www.ualberta.ca/~vlslab/. The information in this manuscript and the manuscript itself has never been published either electronically or in print.
References
- Aartsen M. J., Smits C. H. M., Van Tilburg T. G., Knipscheer C. P. M., Deeg D. J. H. Activity in older adults: Cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. Journal of Gerontology. 2002;2:153–162. doi: 10.1093/geronb/57.2.p153. doi:10.1093/geronb/57.2.P153. [DOI] [PubMed] [Google Scholar]
- Agrigoroaei S., Lachman M. E. Cognitive functioning in midlife and old age: Combined effects of psychosocial and behavioral factors. Journal of Gerontology: Psychological Science. 2011;66B:130–140. doi: 10.1093/geronb/gbr017. doi:10.1093/geronb/gbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. S., DeKoskey S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease. Alzheimer's & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk S. S., Glass T. A., Berkman L. F. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine. 1999;131:165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- Bielak A. A. How can we ‘lose it’ if we still don't understand how to ‘use it’? Unanswered questions about the influences of activity participation on cognitive performance in older age—A mini-review. Gerontology. 2010;56:507–519. doi: 10.1159/000264918. doi:10.1159/000264918. [DOI] [PubMed] [Google Scholar]
- Bielak A. A. M., Hughes T. F., Small B. J., Dixon R. A. It's never too late to engage in lifestyle activities: Significant concurrent but not change relationships between lifestyle activities and cognitive speed. Journal of Gerontology: Psychological Sciences. 2007;62B:P331–P339. doi: 10.1093/geronb/62.6.p331. doi:10.1093/geronb/62.6.P331. [DOI] [PubMed] [Google Scholar]
- Brown C. L., Gibbons L. E., Kennison R. F., Robitaille A., Lindwall M., Mitchell M. B., et al. Social activity and cognitive functioning over time: A coordinated analysis of four longitudinal studies. Journal of Aging Research. 2012;2012:12. doi: 10.1155/2012/287438. Article ID 287438 doi:10.1155/2012/287438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. W., Shallice T. The Hayling and Brixton Tests. Thurston, England: Thames Valley Test Company; 1997. [Google Scholar]
- Carlson M. C., Parisi J. M., Xia J., Xue Q-L., Rebok G. W., Bandeen-Roche K., et al. Lifestyle activities and memory: Variety may be the spice of life. The Women's Health and Aging Study II. Journal of the International Neuropsychological Society. 2012;18:286–294. doi: 10.1017/S135561771100169X. doi:10.1017/S135561771100169X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S. J., Erickson K. I., Scalf P. E., Kim J. S., Prakash R., McAuley E., et al. Aerobic exercise training increases brain volume in aging humans. Journals of Gerontology: Medical Sciences. 2003;58A:176–180. doi: 10.1093/gerona/61.11.1166. doi:10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Berchtold N. C., Christie L. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. doi:10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- D'Elia L. F., Satz P., Uchiyama C. L., White T. Color trails test. Odessa, Fl: PAR; 1996. [Google Scholar]
- Dixon R. A. Evaluating everyday competence in older adult couples: Epidemiological considerations. Gerontology. 2011;57:173–179. doi: 10.1159/000320325. doi:10.1159/000320325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., de Frias C. M. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating patterns and predictors of changes in memory compensation. Aging, Neuropsychology, and Cognition. 2004;11:346–376. doi:10.1080/13825580490511161. [Google Scholar]
- Dixon R. A., Garrett D. D., Bäckman L. Principles of compensation in cognitive neuroscience and neurorehabilitation. In: Stuss D. T., Winocur G., Robertson I. H., editors. Cognitive neurorehabilitation. 2nd ed. New York: Cambridge University Press; 2008. pp. 22–38. [Google Scholar]
- Dixon R. A., Garrett D. D., Lentz T. L., MacDonald S. W. S., Strauss E., Hultsch D. F. Neurocognitive markers of cognitive impairment: Exploring the roles of speed and inconsistency. Neuropsychology. 2007;21:381–399. doi: 10.1037/0894-4105.21.3.381. doi:10.1037/0894-4105.21.3.381. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Wahlin Å., Maitland S. B., Hultsch D. F., Hertzog C., Bäckman L. Episodic memory change in late adulthood: Generalizability across samples and performance indices. Memory and Cognition. 2004;32:768–778. doi: 10.3758/bf03195867. doi:10.3758/BF03195867. [DOI] [PubMed] [Google Scholar]
- Dolcos S., MacDonald S. W., Braslavsky A., Camicioli R., Dixon R. A. Mild cognitive impairment is associated with selected functional markers: Integrating concurrent, longitudinal, and stability effects. Neuropsychology. 2012;26:209–223. doi: 10.1037/a0026760. doi:10.1037/a0026760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom R. B., French J. W., Harman H. H., Dermen D. Manual for kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. doi:10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L., Paillard-Borg S., Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. doi:10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Friedman N. P., Miyake A., Young S. E., DeFries J. C., Corley R. P., Hewitt J. K. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias C. M., Dixon R. A., Strauss E. Structure of executive functioning tests in healthy older adults. Neuropsychology. 2006;20:206–214. doi: 10.1037/0894-4105.20.2.206. doi:10.1037/0894-4105.20.2.206. [DOI] [PubMed] [Google Scholar]
- de Frias C. M., Dixon R. A., Strauss E. Characterizing executive functioning in older special populations: From cognitively elite to cognitively impaired. Neuropsychology. 2009;23:778–791. doi: 10.1037/a0016743. doi:10.1037/a0016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P., Bickel J., Lövdén M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2006;61B:P253–P261. doi: 10.1093/geronb/61.5.p253. doi:10.1093/geronb/61.5.P253. [DOI] [PubMed] [Google Scholar]
- Hertzog C., Kramer A. F., Wilson R. S., Lindenberger U. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest. 2008;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. doi:10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hughes T. F., Andel R., Small B. J., Borenstein A. R., Mortimer J. A. The association between social resources and cognitive change in older adults: Evidence from the Charlotte County Healthy Aging Study. Journal of Gerontology Series: Psychological Sciences. 2008;63B:P241–P244. doi: 10.1093/geronb/63.4.p241. doi:10.1093/geronb/63.4.P241. [DOI] [PubMed] [Google Scholar]
- Hultsch D. F., Hertzog C., Dixon R. A., Small B. J. Memory change in the aged. New York: Cambridge University Press; 1998. [Google Scholar]
- Hultsch D. F., Hertzog C., Small B. J., Dixon R. A. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. doi:10.1037/0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Kramer A. F., Bherer L., Colcombe S. J., Dong W., Greenough W. T. Environmental influences on cognitive and brain plasticity during aging. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2004;59A:940–957. doi: 10.1093/gerona/59.9.m940. doi:10.1093/gerona/59.9.M940. [DOI] [PubMed] [Google Scholar]
- Krueger K. R., Wilson R. S., Kamenetsky J. M., Barnes L. L., Bienias J. L., Bennett D. A. Social engagement and cognitive function in old age. Experimental Aging Research. 2009;35:45–60. doi: 10.1080/03610730802545028. doi:10.1080/03610730802545028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall M., Cimino C. R., Gibbons L. E., Mitchell M. B., Benitez A., Brown C. L. Dynamic associations of change in physical activity and change in cognitive function: Coordinated analyses of four longitudinal studies. Journal of Aging Research. 2012;2012:12. doi: 10.1155/2012/493598. Article ID 493598 doi:10.1155/2012/493598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszcz M. Executive function and cognitive aging. In: Schaie K. W., Willis S. L., editors. The handbook of the psychology of aging. 7th ed. San Diego, CA: Academic Press; 2011. pp. 59–72. doi:10.1016/B978-0-12-380882-0.00004-8. [Google Scholar]
- Mackinnon A., Christensen H., Hofer S. M., Korten A., Jorm A. F. Use it and still lose it? The association between activity and cognitive performance established using latent growth techniques in a community sample. Aging, Neuropsychology, and Cognition. 2003;10:215–229. doi:10.1076/anec.10.3.215.16451. [Google Scholar]
- McFall G. P., Wiebe S. A., Vergote D., Westaway D., Jhamandas J., Dixon R. A. IDE (rs6583817) polymorphism and type 2 diabetes differentially modify executive function in older adults. Neurobiology of Aging. 2013;34:2208–2216. doi: 10.1016/j.neurobiolaging.2013.03.010. doi:10.1016/jneurobiolaging.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. B., Cimino C. R., Benitez A., Brown C. L., Gibbons L. E., Kennison R. F., et al. Cognitively stimulating activities: Effects on cognition across four studies with up to 21 years of longitudinal data. Journal of Aging Research. 2012;2012:12. doi: 10.1155/2012/461592. Article ID 461592 doi:10.1155/2012/461592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howeter A., Wager T. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. doi:10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Psychological Assessment Resources. Wisconsin card sorting test: Scoring program (version 3.0) Odessa, FL: 1990. [Google Scholar]
- Regard M. Cognitive rigidity and flexibility: A neuropsychological study. 1981 Unpublished doctoral dissertation, University of Victoria, Victoria, British Columbia, Canada. [Google Scholar]
- Reitan R. M., Wolfson D. The Halstead–Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Ritchie K., Artero S., Touchon J. Classification criteria for mild cognitive impairment: A population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. doi:10.1212/WNL.56.1.37. [DOI] [PubMed] [Google Scholar]
- Rockwood K., Middleton L. Physical activity and the maintenance of cognitive function. Alzheimer‘s and Dementia. 2007;3:S38–S44. doi: 10.1016/j.jalz.2007.01.003. doi:10.1016/j.jalz.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. Mental exercise and mental aging. Perspectives on Psychological Science. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. doi:10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Salthouse T. A., Babcock R. L. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. doi:10.1037/0012-1649.27.5.763. [Google Scholar]
- Schooler C., Mulatu M. S. The reciprocal effects of leisure time activities and intellectual functioning in older people: A longitudinal analysis. Psychology and Aging. 2001;16:466–482. doi: 10.1037//0882-7974.16.3.466. doi:10.1037/0882-7974.16.3.466. [DOI] [PubMed] [Google Scholar]
- Seeman T. E., Lusignolo T. M., Albert M., Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychology. 2001;20:243–255. doi: 10.1037//0278-6133.20.4.243. doi:10.1037/0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- Small B. J., Dixon R. A., McArdle J. J., Grimm K. J. Do changes in lifestyle engagement moderate cognitive decline in normal aging: Evidence from the Victoria Longitudinal Study. Neuropsychology. 2012;26:144–155. doi: 10.1037/a0026579. doi:10.1037/a0026579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small B. J., Hughes T. F., Hultsch D. F., Dixon R. A. Lifestyle activities and late-life changes in cognitive performance. In: Stern Y., editor. Cognitive reserve. New York: Taylor and Francis Group; 2007. pp. 173–186. [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. doi:10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E., Sherman E. M., Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- Thurstone T. G. Primary mental abilities: Grades 9–12, 1962 revision. Chicago: Science Research Associates; 1962. [Google Scholar]
- Wechsler D. WAIS–R manual. New York: Psychological Corporation; 1981. [Google Scholar]