Abstract
OBJECTIVES
Mitral valve repair techniques for degenerative disease typically entail leaflet resection or neochordal construction, which may require extensive resection, leaflet detachment/reattachment, reliance on diseased native chords or precise neochordal measuring. Occasionally, impaired leaflet mobility, reduced coaptation surface and systolic anterior motion (SAM) may result. We describe a novel technique for addressing posterior leaflet prolapse/flail, which both simplifies repair and addresses these issues.
METHODS
Fifty-four patients (age 62 ± 11 years) with degenerative MR underwent this new repair, 36 of whom minimally-invasively. A CV5 Gore-Tex suture was placed into the posterior left ventricular myocardium underneath the prolapsing segment as an anchor. This suture was then used to imbricate a portion of the prolapsed segment into the ventricle, creating a smooth, broad, non-prolapsed coapting surface on a leaflet with preserved mobility, additional neochordal support and posteriorly positioned enough to preclude SAM.
RESULTS
Repair was successful in all patients. The mean MR grade was reduced from +3.8 to +0.1 with 50 of 54 patients having zero MR and 4 of the 54 having trace or mild MR. All patients had proper antero-posterior location of the coaptation line of a mean length of 10.2 mm, and preserved posterior leaflet mobility. No patients had SAM or mitral stenosis. All patients were discharged and are currently doing well.
CONCLUSION
This new technique facilitated efficient single-suture repair of the prolapsed posterior leaflet mitral regurgitation without the need for resection or sliding annuloplasty. It precluded the need for precise neochordal measurement and preserved the leaflet coaptation surface.
Keywords: Mitral valve repair, Mitral regurgitation, Minimally invasive
INTRODUCTION
Mitral valve repair techniques continue to evolve and a detailed understanding and skillful manipulation of the leaflet, subvalvular apparatus and annular geometric relationships can yield highly reproducible repair of even complex multisegmental bileaflet disease [1, 2]. The classic Carpentier leaflet resection and newer neochordal replacement techniques have proven highly efficacious [3–6] yet possess some limitations. Resection is irreversible, sliding reconstruction is time-consuming and excessive resection yields monoleaflet function, while insufficient resection can yield systolic anterior motion (SAM). Neochordal construction limits these issues, but requires precise measurements and risks SAM if neochords are too long [7]. Addressing some of these issues, we previously reported a non-resectional leaflet remodelling technique whereby excessive prolapsed leaflet tissue is imbricated into the left ventricle, generating a smooth leaflet coaptation surface [8]. This technique carries a theoretical concern of basing the repair on potentially diseased native chords and the possibility that the preserved mobile posterior leaflet can advance too anteriorly, risking SAM. We developed and report here a novel mitral leaflet repair technique for posterior leaflet prolapse that efficiently utilizes a single Gore-Tex suture to posteriorly anchor and support, while remodelling the posterior leaflet, thereby addressing these two concerns. The efficiency of this single-suture remodelling and anchoring repair also facilitates minimally invasive approaches that are gaining widespread application [9–14].
MATERIALS AND METHODS
This study was approved by the UPenn IRB (Protocol 810968). Between June 2011 and November 2012, 54 patients underwent posterior ventricular anchoring neochordal repair of degenerative mitral regurgitation. Preoperative characteristics are summarized in Table 1.
Table 1:
Patient characteristics and outcomes
| No. of patients | 54 |
| Gender (male) | 32 (59%) |
| Age | 61.5 years ± 11.2 |
| Ejection fraction, mean | 59.6% |
| NYHA functional class, median | 2 |
| Hypertension | 26 (48.1%) |
| Smoking history | 24 (44.4%) |
| Diabetes | 6 (11.1%) |
| Previous cardiac operation | 1 |
| Minimally invasive approach | 36 (66.7%) |
| Concomitant procedures | 26 procedures in 21 patients (38.9%) |
| Cardiopulmonary bypass time, mean | 88.7 min ± 23.9 (41–155 min) |
| Cross-clamp time, mean | 59.7 min ± 17.2 (16–99 min) |
| Posterior leaflet prolapsed/flail | 45 |
| Barlow's valve | 9 |
| Grade of MR, preop, mean | +3.8/4 |
| Grade of MR, postop, mean | +0.1/4 |
| Number of patients with zero MR, postop | 50 of 54 |
| Ring size, median | 32 (28–40 mm) |
| Ejection fraction, postop, mean | 54.4% |
| Perioperative complications | |
| Death | 0 |
| Stroke | 1 (neurologically fully recovered) |
| Myocardial infarction | 0 |
| Re-exploration | 2 (POD3 sternal instability, POD6 pericardial effusion) |
| Length of stay, median | 7 days |
Operative technique
Cardiopulmonary bypass, cardioplegic arrest and atriotomy are conducted in a standard fashion. Drawings representing the steps of this operation with en face and lateral views are presented (Fig. 1). A patient with ruptured posterior leaflet chords and a prolapsed/flail middle scallop is shown (Fig. 1A). The prolapsed segment is retracted into the atrium to reveal the left ventricular endocardium directly beneath the leaflet, and a CV5 Gore-Tex suture is passed into the myocardium to an approximate depth of 3–4 mm (Fig. 1B). This suture is then loosely tied, and the needles are passed through the leading edge of the diseased segment at a width of approximately one half of the prolapsed region size (Fig. 1C). The leading edge is grasped and inverted into the left ventricle, imbricating a portion of the leaflet and generating two near-apposing tissue folds emanating radially (Fig. 1D). These folds are sutured together with the Gore-Tex suture in a double-running fashion, thereby fixating the prolapsed leaflet tissue into the ventricular side and generating a smooth coaptation surface in the correct plane. If necessary, the second half of the suture can be used to fold additional tissue into the left ventricle to further imbricate prolapsed tissue (Fig. 1E). The valve is then sized for ring annuloplasty.
Figure 1:
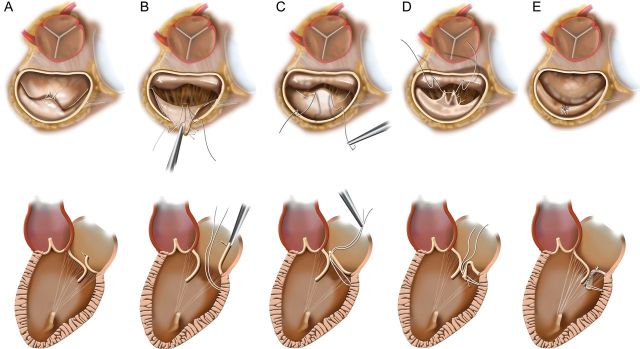
(A) Short- and long-axis drawings depicting degenerative mitral disease with posterior leaflet ruptured chords and prolapse/flail. (B) The prolapsed segment is retracted into the atrium to expose the posterior ventricle just beneath the leaflet, and an anchoring CV5 Gore-Tex suture has been placed. (C) The suture has been loosely tied and the ends brought through the leading edge of the prolapsed segment. (D) The prolapsed segment edge is inverted into the left ventricle, generating two near-apposing radial folds that are then sutured together. (E) This eliminates the prolapse, yielding a smooth broad coaptation surface that is mobile yet positioned posteriorly enough to preclude anterior displacement and SAM.
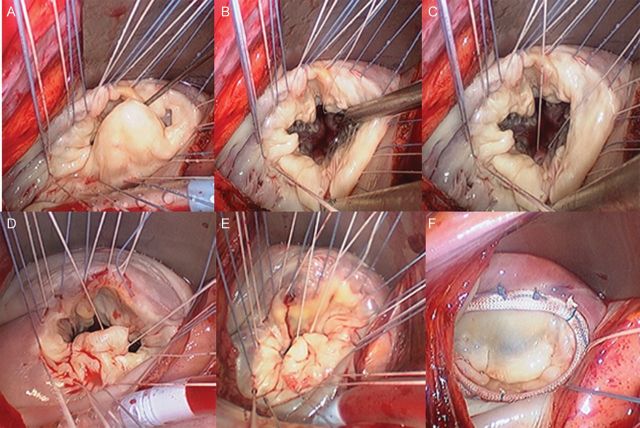
Intraoperative images of a patient undergoing this repair are shown in Fig. 2. To fully expose and facilitate inspection of the mitral valve, annuloplasty sutures are first placed circumferentially and retracted under tension (Fig. 2A). The prolapsed segment has been retracted to facilitate the placement of the anchor suture(Fig. 2B and C). The suture has been brought through the prolapsed segment leading edge (Fig. 2D). The prolapsed tissue has been inverted into the left ventricle generating two near-apposing folds to be sutured together (Fig. 2E). Typically, a semi-rigid complete ring is utilized, and final confirmation of the competence of mitral repair and a proper anterior/posterior height relationship are demonstrated (Fig. 2F). Atriotomy closure, de-airing and weaning from cardiopulmonary bypass are all conducted in standard fashion.
Figure 2:
(A) Intraoperative endoscopic image of minimally invasive mitral valve repair in progress. Annuloplasty sutures have been placed first to aid in exposing valve leaflets. Inspection reveals a large, wide prolapsed P2 segment. (B) The P2 segment is retracted against the posterior annulus to expose the posterior ventricular endocardium directly beneath the P2 region. (C) A CV5 Gore-Tex suture is placed into the posterior ventricular myocardium and then tied loosely. (D) The needles are then passed through the leading edge of P2 at a width of approximately half the width of the amount of prolapsed segment. (E) The central portion of this leading edge is pushed into the left ventricle, and the suture is double run towards the posterior annulus for approximately one-half the distance to the annulus, thereby inverting the prolapsed segment into the LV and creating a smooth and broad coaptation surface well-positioned posteriorly. (F) The resulting repair after ring annuloplasty and pressure testing reveals a completely competent valve with the proper anterior and posterior leaflet size relationship and positioning.
RESULTS
Fifty-four of 54 patients were successfully repaired and none required a second cross-clamp period. There were no mitral valve replacements. Furthermore, in this time period, there were no patients who went to the operating room for planned mitral valve repair and then underwent mitral valve replacement (overall 100% repair rate). Most of the patients had isolated P2 posterior leaflet disease. Eighteen patients had P1 or P3 disease. Nine patients had Barlow's pathology and concomitant anterior leaflet disease, which were treated with papillary muscle-based anterior neochords or a non-resectional leaflet remodelling technique previously reported for anterior leaflet disease [8]. Outcomes are presented in Table 1. Twenty-six concomitant procedures were performed in 21 patients: 9 atrial fibrillation (AF) ablations, 8 atrial septal defects/patent foramen ovale closures, 4 coronary artery bypass grafts, 2 tricuspid valve repairs, 1 aortic valve replacement and 1 transmitral ventricular septal myomectomy. MR grade was reduced from 3.8 preoperatively to 0.1 postoperatively with 50 of 54 patients having zero MR and the other 4 having trace or mild MR. TEE examination of the repair geometry typically reveals a moderately mobile, posteriorly positioned leaflet and a long coaptation length of mean 10.2 ± 2.9 mm. No patient had SAM. There were no perioperative myocardial infarctions or deaths, and 1 patient had a mild stroke from which he recovered. Two patients returned to the operating room. One manifested sternal instability on POD3 and underwent rewiring. Another developed a moderate pericardial effusion on POD6 after therapeutic re-anticoagulation for AF and underwent resternotomy and drainage of serosanguineous fluid. Median length of stay was 7 days, and all patients were discharged home. All patients underwent subsequent clinical follow-up. Outpatient surveillance echocardiography occurred at the discretion of the referring cardiologists, and 25 patients had such echos. There were no deaths, no reoperations for any cause and no patients had recurrent mitral regurgitation. Long-term clinical follow-up is still absent, as this is a very new procedure. However, the patients who underwent this procedure early in this timeframe have greater than 1 year of follow-up, and all have intact mitral valve repairs with no regurgitation.
DISCUSSION
With the drive to treat degenerative MR ever earlier in the disease timecourse and to even routinely operate on asymptomatic patients, the need for extremely successful repair rates is of paramount importance [15–17]. Further compounding this is the rapid development of catheter-based repair devices [2, 18]. The classic quadrangular resection is highly reproducible and associated with great long-term durability with freedom from reoperation as high as 98% at 14 years [19]. This technique has some minor disadvantages that may be overcome with a posterior ventricular anchoring neochordal remodelling technique. Resection is irreversible, whereas, if the appearance or quality of the remodelling repair is suboptimal, the Gore-Tex suture can easily be removed and repositioned without leaflet damage. Resection requires reapproximation, often with an involved sliding annuloplasty. The single-suture remodelling repair is highly efficient. Patients in our cohort undergoing isolated mitral repair had a mean cross-clamp time of 47.0 and bypass time of 65.6 min, which compare favourably to those reported for isolated mitral repair in the Society of Thoracic Surgeons national database (cross-clamp 86.9 and bypass 113.0 min). Our times also favourably compare with reported times for isolated posterior leaflet quadrangular resection of 80 min for cross-clamp and 110 min for bypass [20]. Our overall cross-clamp time of 59.7 and bypass time of 88.7 min, which include concomitant procedures, compare very favourably with studies of degenerative MVRepair with reported cross-clamp times of 86.5 and bypass times of 114 min and a large institutional study of over 1000 degenerative MR patients with a mean cross-clamp time of 78 and bypass time of 112 min [21, 22].
After quadrangular resection, the reapproximated posterior leaflet is supported by potentially diseased native chords. Our posterior ventricular anchoring neochord provides some additional chordal support. Moreover, after quadrangular resection, the residual posterior leaflet is often diminutive, with minimal mobility. This monoleaflet valve results in smaller annuloplasty rings and the potential for dynamic mitral stenosis [23]. Leaflet remodelling preserves tissue and function and yields a larger mitral orifice area. Our median ring size of 32 is larger than the reported median ring size of 30 after quadrangular resection by a group that also observed that the majority of their patients received size 28 or 30 rings [22]. Finally, the preservation of posterior leaflet tissue has been shown experimentally to be biomechanically advantageous [24].
Papillary muscle-based neochordal replacement techniques are also highly successful, but may have minor disadvantages, such as the need for precise length measurement and the potential for leaving excess residual leaflet tissue that may promote SAM. Premeasured chords may obviate intraoperative measurement; however, placement even a few millimeters further up or down the papillary muscle or leaflet edge from where the echo measurement was taken can significantly alter the effective chord length. A long chord could yield residual prolapse/regurgitation and an anteriorly positioned posterior leaflet, risking SAM [7]. The posterior ventricular anchoring neochordal remodelling technique may have its greatest advantage in addressing this issue by ensuring a posteriorly positioned, yet functional leaflet devoid of the risk of SAM. Our postoperative echocardiograms demonstrate proper posterior positioning and leaflet mobility. The abundant mean coaptation length of 10.2 mm compares very favourably to the minimum 5 mm recommended for repair durability [25].
A potential limitation of our repair is the strength of the ventricular anchoring point. This could be strengthened with pledgets. However, this may not be necessary. Leading-edge chords serve primarily to position the coaptation surfaces and upon coaptation, the opposing forces on the ventricular side of the coapted leaflet edges essentially cancel each other out, and the ventricular systolic force is distributed on the remaining leaflet surfaces and their underlying secondary chordae [26–29]. This biomechanical concept is evidenced by the progressive thickness of chords as one moves from the free margin towards the leaflet mid-body. Our posterior ventricle-based neochord aids in positioning the coaptation surface, but should only be needed for a short period of time in early systole. Upon coaptation, the force on this neochord and its anchor point should markedly diminish as forces are transferred to secondary chords. On routine post-bypass TEEs, we did not observe any evidence of suture disruption in any patients. In the patients who have had subsequent outpatient surveillance echos, we likewise did not observe suture disruption.
This represents an initial series of 54 patients with severe, degenerative mitral regurgitation successfully repaired with an efficient single-suture non-resectional leaflet-inversion remodelling and posterior ventricular anchoring neochord to preserve function while ensuring proper positioning and support of the posterior leaflet. This repair facilitated the right chest minimally invasive approach. As this is a newly developed procedure, long-term clinical follow-up is absent. However, follow-up to date has been very encouraging. The patients are clinically well and without recurrent MR.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr H. Najm (Riyadh, Saudi Arabia): This technique you presented certainly adds to the armamentarium of cardiac surgeons who are interested in mitral valve repair and definitely adopts the concept of respect rather than resect leaflet tissue for reconstruction of the mitral valve.
The technique you described still involves significant subjectivity in conducting the repair, such as how far the ventricular suture should be placed along the posterior ventricular wall in order to anchor the prolapsed leaflet, and how wide the imbrications of the posterior leaflet should be performed in order to achieve competency. I have a technical concern on leaving a loosely-tied suture to the posterior myocardium anchoring the posterior leaflet, which as you have mentioned, continues to be mobile, therefore allowing for repetitive movement and exerting pressure with the possibility of cutting through the muscle as time progresses, since it is not fixed with a pledget to the posterior myocardium.
I have two questions for you. First, you have not mentioned in the manuscript the total number of valves which have been repaired during the same period of time, which is the denominator because repairability of 100%, as you mentioned in the manuscript, is only correct if it has been applied to all comers of the degenerative mitral valve repairs you accepted during that time period.
Dr Woo: As you know, all papers dealing with mitral valve repair series may only be looking at successful mitral valve repairs. Thus it is necessary examine all mitral valve operations during that timeframe. We have done that on a larger scale and also for this series. In this series, there were no patients that went to the operating room with an intended mitral valve repair who ended up with a mitral valve replacement. So, this was a 100% repair rate. For our larger series that was just accepted for publication in JTCVS, we had a 99% repair rate, and that was across a larger spectrum of patients. So basically it is essentially a near 100% repair rate.
Dr Najm: On that selected patient group?
Dr Woo: Yes. This obviously does not account for patients where you decide up front in the office that you are going to do a mitral valve replacement. That is a different sort of population. But to address your other technical points, the general answer that I have right now is that the suture is placed in line directly with the middle of the prolapse approximately to the length down from the annulus equal to the leaflet height. I have found that we do not need a pledget, in part because of some of that biophysics modeling that Nazari published. This is mostly a positioning issue and very quickly in early systole, the forces on the leading chords are transferred onto the secondary and tertiary chords. So we will have to see over time whether or not any of these sutures will pull out.
Dr Najm: Is this technique applicable in the calcified or heavily fibrotic, bulky, prolapsed posterior leaflet which is not infrequently seen in such pathology? You have mentioned that your follow-up is recent; how recent is that? Could you quantity in terms of weeks or months or years?
Dr Woo: You are right, the calcium would make this a little more difficult, especially with a fully calcified leaflet where you cannot fold the leaflet at all. In a patient with a heavily calcified leaflet that we are trying to repair, we would typically resect a portion of that calcium. In terms of the follow-up, as I mentioned, the first patient was done in June 2011, so it is a little over a year. We are in close contact with all the referring cardiologists, and thus we have all of the outpatient clinic notes on every single one of these patients, and every time they get an echo, that goes into our database. That is the full extent of our follow-up to date. It is obviously a short mean follow-up because we have only been doing this for about a year, but so far there have been no problems that we can detect.
Dr Najm: We are looking forward to a longer follow-up.
Dr A. Gaafar (Cairo, Egypt): What is the difference between anchoring the leaflet to the posterior ventricular wall compared to anchoring it to the tip of the papillary muscle?
Dr Woo: The issue with any papillary muscle based chord is the necessity for very accurate measurement. Now, some people may say that is not important, but if you are a little too short, you will have a retraction issue, and if you are too long, you will have a prolapse issue. This is very simple. There is really not much measurement that is necessary because part of the distance that you are travelling is the fold, so you really just need to anchor this suture directly in the myocardium, and the rest of it, most of it is taken up by the folded leaflet. So there is really not the necessity for super-accurate measurement here.
REFERENCES
- 1.Borger MA, Mohr FW. Repair of bileaflet prolapse in Barlow syndrome. Semin Thorac Cardiovasc Surg. 2010;22:174–8. doi: 10.1053/j.semtcvs.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Rosengart TK, Feldman T, Borger MA, Vassiliades TA, Jr, Gillinov AM, Hoercher KJ, et al. American Heart Association Council on Cardiovascular S, Anesthesia, American Heart Association Council on Clinical C, Functional G, Translational Biology Interdisciplinary Working G, Quality of C, and Outcomes Research Interdisciplinary Working G. Percutaneous and minimally invasive valve procedures: a scientific statement from the American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology, Functional Genomics and Translational Biology Interdisciplinary Working Group, and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2008;117:1750–67. doi: 10.1161/CIRCULATIONAHA.107.188525. [DOI] [PubMed] [Google Scholar]
- 3.Carpentier A. Cardiac valve surgery—the ‘French correction. J Thorac Cardiovasc Surg. 1983;86:323–37. [PubMed] [Google Scholar]
- 4.Risteski PS, Aybek T, Dzemali O, Doss M, Scherer M, Dogan S, et al. Artificial chordae for mitral valve repair: mid-term clinical and echocardiographic results. Thorac Cardiovasc Surg. 2007;55:239–44. doi: 10.1055/s-2006-955947. [DOI] [PubMed] [Google Scholar]
- 5.Falk V, Seeburger J, Czesla M, Borger MA, Willige J, Kuntze T, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg. 2008;136:1205. doi: 10.1016/j.jtcvs.2008.07.028. discussion 1205–6. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Yozu R, Kokaji K, Iwanaga S. Feasibility of mitral valve repair using the loop technique. Ann Thorac Cardiovasc Surg. 2007;13:21–6. [PubMed] [Google Scholar]
- 7.Perier P, Hohenberger W, Lakew F, Batz G, Urbanski P, Zacher M, et al. Toward a new paradigm for the reconstruction of posterior leaflet prolapse: midterm results of the ‘respect rather than resect’ approach. Ann Thorac Surg. 2008;86:718–25. doi: 10.1016/j.athoracsur.2008.05.015. discussion 718–25. [DOI] [PubMed] [Google Scholar]
- 8.Woo YJ, MacArthur JW., Jr Simplified nonresectional leaflet remodeling mitral valve repair for degenerative mitral regurgitation. J Thorac Cardiovasc Surg. 2012;143:749–53. doi: 10.1016/j.jtcvs.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Grossi EA, Galloway AC, LaPietra A, Ribakove GH, Ursomanno P, Delianides J, et al. Minimally invasive mitral valve surgery: a 6-year experience with 714 patients. Ann Thorac Surg. 2002;74:660–3. doi: 10.1016/s0003-4975(02)03754-2. discussion 663–4. [DOI] [PubMed] [Google Scholar]
- 10.Woo YJ. Minimally invasive valve surgery. Surg Clin North Am. 2009;89:923–49. doi: 10.1016/j.suc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Daneshmand MA, Milano CA, Rankin JS, Honeycutt EF, Swaminathan M, Shaw LK, et al. Mitral valve repair for degenerative disease: a 20-year experience. Ann Thorac Surg. 2009;88:1828–37. doi: 10.1016/j.athoracsur.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 12.McClure RS, Cohn LH, Wiegerinck E, Couper GS, Aranki SF, Bolman RM, III, et al. Early and late outcomes in minimally invasive mitral valve repair: an eleven-year experience in 707 patients. J Thorac Cardiovasc Surg. 2009;137:70–5. doi: 10.1016/j.jtcvs.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 13.Casselman FP, Van Slycke S, Dom H, Lambrechts DL, Vermeulen Y, Vanermen H. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg. 2003;125:273–82. doi: 10.1067/mtc.2003.19. [DOI] [PubMed] [Google Scholar]
- 14.Modi P, Hassan A, Chitwood WR., Jr Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2008;34:943–52. doi: 10.1016/j.ejcts.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 15.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–83. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 16.Kang DH, Kim JH, Rim JH, Kim MJ, Yun SC, Song JM, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009;119:797–804. doi: 10.1161/CIRCULATIONAHA.108.802314. [DOI] [PubMed] [Google Scholar]
- 17.Samad Z, Kaul P, Shaw LK, Glower DD, Velazquez EJ, Douglas PS, et al. Impact of early surgery on survival of patients with severe mitral regurgitation. Heart. 2011;97:221–4. doi: 10.1136/hrt.2010.202432. [DOI] [PubMed] [Google Scholar]
- 18.Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 19.Kasegawa H, Shimokawa T, Horai T, Takeuchi S, Nishimura K, Ozawa N, et al. Long-term echocardiography results of mitral valve repair for mitral valve prolapse. J Heart Valve Dis. 2008;17:162–7. [PubMed] [Google Scholar]
- 20.Lange R, Guenther T, Noebauer C, Kiefer B, Eichinger W, Voss B, et al. Chordal replacement versus quadrangular resection for repair of isolated posterior mitral leaflet prolapse. Ann Thorac Surg. 2010;89:1163–70. doi: 10.1016/j.athoracsur.2009.12.057. discussion 1170. [DOI] [PubMed] [Google Scholar]
- 21.Vohra HA, Whistance RN, Bezuska L, Livesey SA. Initial experience of mitral valve repair using the Carpentier-Edwards Physio II annuloplasty ring. Eur J Cardiothorac Surg. 2011;39:881–5. doi: 10.1016/j.ejcts.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 22.DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg. 2010;139:76–83. doi: 10.1016/j.jtcvs.2009.08.058. discussion 83–4. [DOI] [PubMed] [Google Scholar]
- 23.Magne J, Senechal M, Mathieu P, Dumesnil JG, Dagenais F, Pibarot P. Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis. J Am Coll Cardiol. 2008;51:1692–701. doi: 10.1016/j.jacc.2007.11.082. [DOI] [PubMed] [Google Scholar]
- 24.Padala M, Powell SN, Croft LR, Thourani VH, Yoganathan AP, Adams DH. Mitral valve hemodynamics after repair of acute posterior leaflet prolapse: quadrangular resection versus triangular resection versus neochordoplasty. J Thorac Cardiovasc Surg. 2009;138:309–15. doi: 10.1016/j.jtcvs.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams DH, Anyanwu AC, Sugeng L, Lang RM. Degenerative mitral valve regurgitation: surgical echocardiography. Curr Cardiol Rep. 2008;10:226–32. doi: 10.1007/s11886-008-0038-9. [DOI] [PubMed] [Google Scholar]
- 26.Lawrie GM. Mitral valve: toward complete repairability. Surg Technol Int. 2006;15:189–97. [PubMed] [Google Scholar]
- 27.Goetz WA, Lim HS, Lansac E, Saber HA, Pekar F, Weber PA, et al. Anterior mitral basal ‘stay’ chords are essential for left ventricular geometry and function. J Heart Valve Dis. 2005;14:195–202. discussion 202–3. [PubMed] [Google Scholar]
- 28.Lomholt M, Nielsen SL, Hansen SB, Andersen NT, Hasenkam JM. Differential tension between secondary and primary mitral chordae in an acute in-vivo porcine model. J Heart Valve Dis. 2002;11:337–45. [PubMed] [Google Scholar]
- 29.Nazari S, Carli F, Salvi S, Banfi C, Aluffi A, Mourad Z, et al. Patterns of systolic stress distribution on mitral valve anterior leaflet chordal apparatus. A structural mechanical theoretical analysis. J Cardiovasc Surg (Torino) 2000;41:193–202. [PubMed] [Google Scholar]