Abstract
With the global population predicted to grow by at least 25 per cent by 2050, the need for sustainable production of nutritious foods is critical for human and environmental health. Recent advances show that specialized plant membrane transporters can be used to enhance yields of staple crops, increase nutrient content and increase resistance to key stresses, including salinity, pathogens and aluminium toxicity, which in turn could expand available arable land.
Of The Present Global Population Of Seven Billion People, Almost One billion are undernourished and lack sufficient protein, fats and carbohydrates in their diets1. An additional billion people are malnourished because their diets lack required micronutrients such as iron, zinc and vitamin A (ref. 2). These dietary deficiencies have an enormous negative impact on global health, resulting in increased susceptibility to infection and diseases, as well as increasing the risk of significant mental impairment3. During the next four decades, an expected additional two billion humans will require nutritious food. Along with growing urbanization, increased demand for protein in developing countries, coupled with impending climate change and population growth, will impose further pressures on agricultural production4. Global demand for food is predicted to increase by 40% by 2030 (ref. 4). Innovative solutions are required to increase production on the land currently used for agriculture, because we are already close to the sustainable limit of 15% of the Earth’s surface that can be exploited for crop production5.
Analysis of crop yields globally shows that in those developing regions where humans are most susceptible to malnutrition, the availabilities of inorganic nutrients and water are the principal factors that determine crop productivity6,7. Simply increasing inorganic fertilizer use and water supply or applying organic farming systems to agriculture8 will be unable to satisfy the joint requirements of increased yield and environmental sustainability. Increasing food production on limited land resources will rely on innovative agronomic practices coupled to the genetic improvement of crops9.
Transport proteins embedded within membranes are key targets for improving the efficiency with which plants take up and use water and nutrients. These proteins not only transport mineral nutrients and control drought tolerance but are also essential for moving sucrose, the energy currency of plants, to where it is needed. Furthermore, transporters are also central to mechanisms that allow plants to tolerate adverse environments such as saline or acid soils. Advances driven by physiology, genetics and biophysics over the past 20 years have dramatically improved our understanding of the molecular basis of plant nutrition and how plants respond to stress. Genome sequencing and the development of experimental systems for studying transporter function have allowed many of the major families of membrane transporters to be characterized. Next-generation sequencing is leading to an understanding of how the natural genetic diversity of plant membrane transporters can be exploited for agriculture, whether by marker-assisted breeding or through genetic engineering. Breakthrough approaches involving transcriptional-activator-like effectors and genome editing10 can provide non-genetically modified (non-GM) yet molecularly directed rapid solutions to crop improvement.
Here we report on findings demonstrating that understanding the biology of plant membrane transporters can be a key contributor to the goal of global food security. We discuss examples where fundamental research is already being translated into practical applications such as enhancing the micronutrient content of grain and improving the plant tolerance to saline and acidic soils. We further discuss potential applications linked to breakthroughs in basic research that are yet to be applied to crop plants. This Perspective reviews the extent to which the rapid advances in plant transport research address global aspects of food security, and how we can potentially reduce the time between trait identification in the laboratory and exploitation in the field.
Transporters, stress resistance and yield
Aluminium-tolerant crops for acid soils
Acid soils comprise 30% of Earth’s ice-free land and thus constrain agricultural production, given that only a small proportion of these soils is suitable for crops11. At soil pH values above 5, aluminium exists in the soil in non-toxic complexed forms. However, when soils are acidic, Al3+ ions are freed in the soil, resulting in plant toxicity. Once in the soil solution, Al3+ damages the root tips of susceptible plants and inhibits root growth, which impairs the uptake of water and nutrients. Natural genetic variation in Al3+ tolerance exists within major cereal crops. The efflux of organic anions from roots was discovered to be a naturally occurring tolerance mechanism of several species12. Transport proteins are central to this mechanism, with members of two families of transport proteins responsible for exporting the organic anions from inside root cells to the external medium surrounding roots. The organic anions secreted by roots chelate Al3+ into a non-toxic form, thus protecting the sensitive tips and allowing the roots to grow unimpeded (Fig. 1).
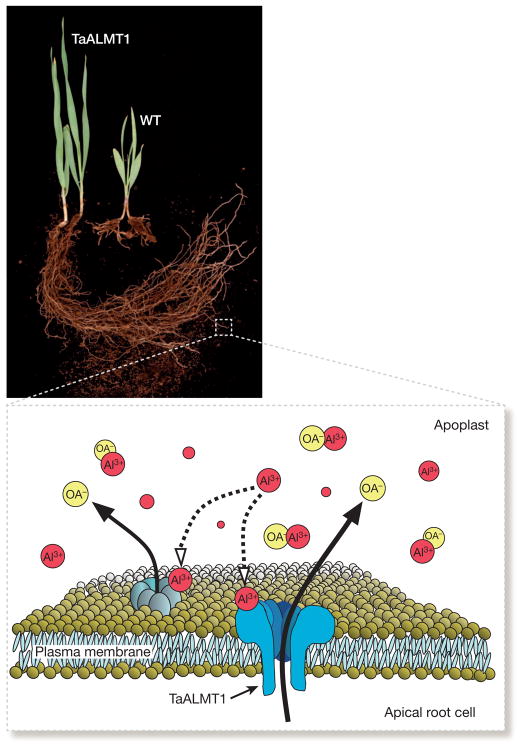
Figure 1. Engineering plants for enhanced aluminium (Al3+) tolerance.
The photograph shows barley seedlings grown on an acid soil that contains high concentrations of toxic Al3+. One seedling has been genetically engineered with an Al3+-tolerance transporter gene from wheat (TaALMT1), whereas the other seedling is the non-transgenic parental line (wild type, WT). The diagram shows the TaALMT1 anion channel (blue structure) embedded within the plasma membrane of apical root cells. In acid soils, Al3+ activates TaALMT1 (dashed line) resulting in malate efflux into the apoplast (cell wall) external to the cytoplasm. Malate molecules (OA−, yellow circles) bind Al3+ in the apoplast to protect cells from aluminium toxicity at the root apex. The diagram is modified from figure 2 in ref. 92.
In wheat, the Triticum aestivum aluminium-activated malate transporter 1 gene TaALMT1 encodes an Al3+-gated anion channel that facilitates malate efflux from roots13 (Fig. 1). Molecular markers based on the TaALMT1 gene can be used in marker-assisted breeding to select for Al3+ tolerance in wheat germplasm. The TaALMT1 gene itself can be used to genetically modify susceptible species for improved Al3+ tolerance. When expressed in barley, one of the most Al3+-sensitive cereal crops, TaALMT1 confers substantially improved grain yields in acid soil14 (Fig. 1). Similarly, a unique subgroup of the large family of plant multidrug and toxic compound extrusion (MATE) transporters mediates citrate efflux from root cells15,16. In sorghum16, barley17, and maize18, MATE transporters located at the root tip confer Al3+-activated citrate efflux and represent the primary Al3+ tolerance proteins. Genetic studies in sorghum recently identified markers associated with Al3+-tolerant alleles of the Sorghum bicolor MATE gene SbMATE. These markers have been used by breeders to introgress rapidly the most favourable SbMATE alleles into sorghum germplasm, which is currently being field-tested in acid soils16.
Although genes encoding organic anion transporters are already established as a means of enhancing the Al3+ tolerance of crops, other recently discovered mechanisms provide additional options. For instance, rice is the most Al3+ tolerant of the cereal crops and uses mechanisms distinct from the efflux of organic anions. One of these mechanisms involves transporters working in concert ultimately to sequester Al3+ into the vacuole, thus removing Al3+ from mainstream metabolism19,20. Genetic analysis has identified susceptible and tolerant variants of one of the transporter genes (natural resistance-associated macrophage protein aluminium transporter 1, Nrat1) that explains a large proportion of the variation in Al3+ tolerance within the rice aus subpopulation. This finding provides a promising tool for marker-assisted breeding21.
The discovery of transporters that mediate Al3+ tolerance has identified two principal strategies that plants use to deal with this toxic cation. In one strategy, Al3+ is excluded from cells by chelating the toxic ion external to plants and in the other Al3+ is sequestered within cells in the vacuole. The genes encoding these transporters can be used to develop Al3+-tolerant crops and represent an important component—along with management practices such as soil liming to increase soil pH—of a strategy for improving yields on acid soils.
HKT transporters improve salt tolerance
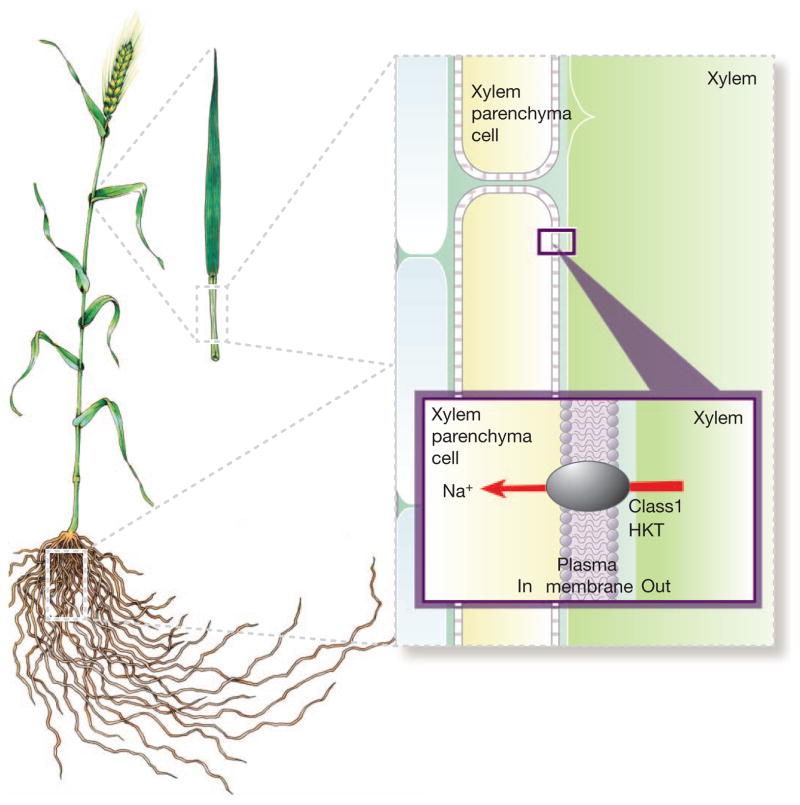
Approximately 7% of the world’s land including agricultural lands is affected by either salinity or sodium toxicity. Production in over 30% of irrigated crops and 7% of dryland agriculture worldwide is limited by salinity stress. Crop irrigation is increasing soil salinity, owing to trace amounts of salt in irrigation waters. Plant plasma membrane transporters in the HKT family transport sodium (Na+) and potassium (K+) (ref. 22) and play an essential part in salt tolerance23. Research in the reference plant Arabidopsis showed that the ‘class 1’ HKT transporters are Na+ selective and protect plant leaves from salinity stress by prohibiting toxic sodium over-accumulation in leaves23. Class 1 HKT transporters are expressed in veins23 that connect nutrient flux between roots and leaves. These transporters are expressed in the living cells surrounding the xylem, which are vessels that carry nutrients and water to the leaves. Class 1 HKT transporters remove excess Na+ from the xylem in Arabidopsis and rice, thereby keeping Na+ below toxic levels in the photosynthetic leaf tissues24–26 (Fig. 2). Analogous mechanisms have been demonstrated in wheat for the HKT1;4 and HKT1;5 genes27,28 (Fig. 2). Remarkably, the recent introgression of an ancestral form of the HKT1;5 gene from the more Na+-tolerant wheat relative Triticum monococcum into susceptible commercial durum wheat (Triticum turgidum ssp durum) increased grain yields on saline soil by 25% in the field, illustrating the immense potential of this mechanism28.
Figure 2. HKT transporter-mediated salt tolerance in plants.
The drawing illustrates the function of class I HKT transporters in protecting plants from salinity stress. These HKT transporters mediate Na+ unloading from the xylem under salinity stress, which prevents Na+ over-accumulation in leaves, thereby protecting photosynthetic organs. An example of this mechanism in wheat plants is shown.
Some crops are salt tolerant through the effective sequestration of Na+ in leaf vacuoles by Na+/H+ antiporters29. Specific ‘class 2’ HKT transporters30 mediate cation influx into roots31. These class 2 HKT transporters, together with transporters that sequester sodium and potassium in the vacuole32,33, have the potential to improve the production of cereals such as barley, a species that copes with high Na+ loads in leaves by compartmentation in the vacuole34. Thus combining (pyramiding) HKT transporter traits with vacuolar Na+ sequestration mechanisms provides a potentially powerful platform for molecular breeding and transgenic approaches to improve the salinity tolerance of crops.
SWEET transporters and pathogen resistance
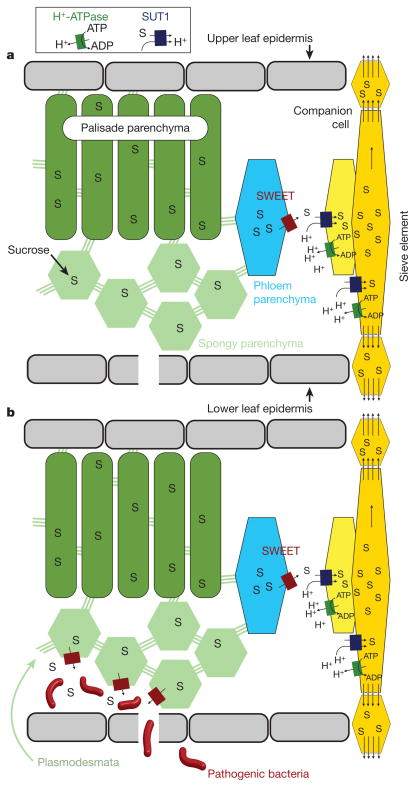
Photosynthesis in leaves produces sugars, which are distributed through the veins to support growth of roots, meristems and seeds. The translocation rates and relative distribution critically determine the yield potential of crops. Sucrose is a key energy-carrying molecule that also acts as the driver for translocation of all other nutrients and signalling molecules in veins, but until recently, the molecular players were unknown. In the early 1990s the sucrose-proton co-transporter SUT1 was identified as the key transporter that loads sugar into veins35, yet the mechanism for sucrose release from the leaf cells that synthesize it remained elusive. Recently, the ‘SWEET’ sugar transporters were identified with the help of sugar sensors based on Förster (fluorescence) resonance energy transfer technology36,37. Known crop genomes possess about 20 SWEET genes. SWEETs are plasma membrane proteins located in the phloem parenchyma, a cell type inside the veins that exports sucrose to the SUT1 sugar loaders (Fig. 3). The import and export of sucrose from vein cells is controlled by hormones, turgor feedback and sugar levels38. Knowledge of this machinery could provide a new starting point to engineer yield by modifying energy and carbon distribution within the plant.
Figure 3. The role of SWEET sugar transporters in efflux of sucrose into the cell-wall space and induction by pathogenic bacteria.
a, SWEETs (red) localized in the phloem parenchyma (a cell type of the plant vasculature), export sucrose produced by photosynthesis into the cell wall, from where it is loaded actively, with the help of the transporter SUT1 and energized by H+-ATPases into the actual conduits, the sieve element companion cell complex for translocation to seeds. Photosynthesis mainly occurs in the palisade parenchyma. b, The role of SWEETs as the ‘Achilles’ heel’ (susceptibility factors) of host plants during pathogen infection. SWEETs are induced directly as a consequence of the injection of transcriptional-activator-like effectors from pathogens via type III secretion systems into the infected plant cell, leading to release of sugars as a critical source of nutrition for the pathogens.
Notably, SWEET sugar efflux transporters have been identified as pathogen resistance loci, leading to a new understanding of disease development in plants39–42. The growth of pathogens in leaves and stems depends on nutrient supply from their plant hosts. Blight bacteria directly induce SWEET gene expression in rice in infected cells through transcriptional-activator-like effectors (bacterial transcription factors that directly target SWEET promoters). Inhibiting the induction of SWEET genes with an innovative technology such as chromosomal editing of the promoters of SWEETs with TALENs (artificial transcriptional-activator-like effector nucleases) or through cell-specific expression of microRNAs in cells outside the phloem has now enabled blight resistance to be engineered in rice43,44. The discovery of these key players in combination with TALENs promises new ways of engineering both crop yield and pathogen resistance, without the introduction of foreign genetic material, to produce plants with significantly improved performance in the field.
Human health and plant nutrition
Pumping iron and zinc
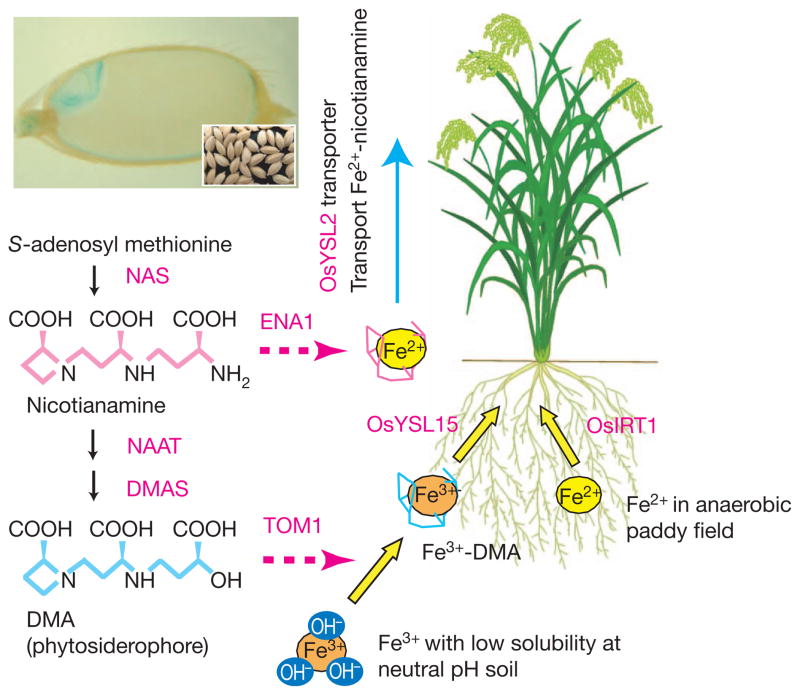
Over two billion people suffer from iron and zinc deficiencies because their plant-based diets are not a sufficiently rich source of these essential elements. Developing crop cultivars with increased micronutrient concentrations, an approach known as biofortification, is challenging because metal ion concentrations in various tissues and compartments are maintained within narrow physiological limits by coordinated uptake, translocation and storage. Furthermore, for crops like rice, removal of the outer layers of the grain during polishing essentially removes all of the micronutrients, leaving only the starchy endosperm. By expressing key genes involved in the mobilization of micronutrients from the soil to the seed, scientists have biofortified rice, a staple food consumed by half the world population every day (Fig. 4). Enhancing iron translocation through overproduction of the metal chelator nicotianamine and phytosiderophores45–47 or enhancing iron influx into the endosperm by means of the iron-nicotianamine transporter Oryza sativa yellow-stripe-like-2 (OsYSL2)48, has resulted in greenhouse-grown rice with three- to fourfold higher levels of iron (Fe) in polished grain. Combining overproduction of nicotianamine with enhanced expression of the iron storage protein ferritin increased the iron content more than sixfold49, and combining all three approaches has resulted in paddy-field-grown polished rice with Fe concentrations 4.4-fold higher than those found in non-transgenic seeds, with no yield penalty50. Although these results are impressive and bring iron levels close to those recommended by nutritionists, only a handful of studies have tested whether these enhanced levels of nutrients are bioavailable. Most encouragingly, enhancing the nicotianamine concentration does increase the levels of bioavailable iron46 and zinc51 in polished rice.
Figure 4. Iron transport in rice.
Rice takes up iron from the soil as Fe3+ deoxymugineic acid (DMA) by the OsYSL15 transporter. Rice also uses the OsIRT1 transporter to take up Fe2+, which is abundant in submerged and anaerobic conditions. DMA, which is the primary phytosiderophore that aids in iron transport, is synthesized from S-adenosyl methionine through three sequential enzymatic reactions mediated by nicotianamine synthase (NAS), nicotianamine aminotransferase (NAAT), and DMA synthase (DMAS), and then secreted by the efflux transporter TOM1 to solubilize iron in the soil. Nicotianamine, which is the biosynthetic precursor of DMA, is a chelator of divalent metals and plays a part in translocation of metals within plants. Nicotianamine is secreted into the cell wall by the nicotianamine efflux transporter ENA1. The iron–nicotianamine transporter OsYSL2 mediates iron influx into rice grains. The photograph shows iron staining (blue coloration) of a rice seed (inset shows rice seeds). Iron is mainly localized to the embryo and the outer layers of the grain.
Vacuolar sequestration is another mechanism to enhance the concentrations of iron and zinc (Zn) in seeds52. Transporters belonging to several different families transport metals between the cytoplasm and the vacuole53–55, including the Arabidopsis vacuolar iron transporter VIT1 protein, which is highly expressed in developing seeds and transports iron and manganese into the vacuole54. Disruption of the rice VIT orthologues (OsVIT1 and OsVIT2) increases Fe/Zn accumulation in rice seeds and decreases Fe/Zn in the source organ flag leaves, probably because VIT genes are highly expressed in rice flag leaves. Without a sink, there is enhanced Fe/Zn translocation to the seed, providing another strategy with which to biofortify Fe/Zn in staple foods56. Metal tolerance protein 1 (MTP1) also transports divalent cations into the vacuole and is another promising candidate for use in biofortification57. Thus several strategies are being used to enhance iron and zinc micronutrients in edible plant tissues, but more improvements are needed. We can use our growing knowledge of the transporters that take up micronutrients from the soil, such as iron-regulated transporter 1 (IRT1)58, the major entry point for Fe in many plant species. Enhanced nutrient content is a crucial goal in the light of the world’s growing population and the central roles of staple crops in human diets.
Enhancing phosphate use efficiency
Phosphorus (P) is a macro-element that is essential for plant growth and of vital importance to crop yield. The availability of inorganic P, or orthophosphate (the only form of P directly accessible to plants), is influenced by the biogeochemical properties of the soil and limits crop production on nearly 70% of the world’s agricultural soils59. Consequently, global crop production depends on orthophosphate fertilizers, which are produced from rock phosphate, a finite, non-renewable mineral resource (Fig. 5a). Only 20–30% of the P fertilizer applied is used by cultivated plants and at the current rate of use, it is estimated that rock phosphate reserves will be consumed within the next 70–200 years60, so ensuring the sustainable use of orthophosphate is of paramount importance for human nutrition. Improving orthophosphate acquisition and use-efficiency in plants is a complex problem and recent solutions have included modifications to root growth and architecture61,62, and novel engineering strategies to use alternative P sources60. An understanding of plant transporter proteins may offer additional approaches. Plants possess several families of orthophosphate transporter proteins, and both high- and low-affinity transporters are important for orthophosphate uptake into roots63,64. Phosphate transporters are also critical for orthophosphate distribution throughout the plant, and for remobilization between source and sink tissues65,66. A phosphate efflux transporter (PHO1), essential for orthophosphate transfer to the shoot, is a major player in the regulation of orthophosphate homeostasis67 and may provide strategies for optimizing orthophosphate distribution within plants.
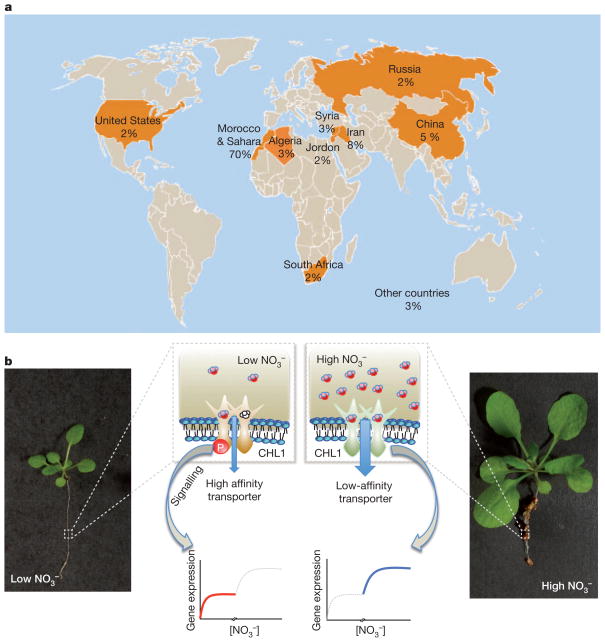
Figure 5. Global phosphate availability and nitrate sensing.
a, Global distribution map of reserves of rock phosphate. The percentage of effective reserves is illustrated (data from ref. 93). b, Dual functions of the nitrate transporter and nitrate sensor, CHL1, in nitrate uptake and sensing. Photographs show Arabidopsis seedlings at low (left) and high (right) nitrate. At low nitrate, CHL1 is phosphorylated at a specific amino acid, T101, converting it to a high-affinity nitrate uptake transporter, enabling nitrate accumulation at limiting soil nitrate concentrations. At high nitrate, the T101 amino acid is not phosphorylated, and CHL1 functions as a low-affinity transporter.
In addition to the direct acquisition of orthophosphate from soil, most crop species have the capacity to form symbiotic associations (called arbuscular mycorrhizae) with soil fungi. These fungi capture orthophosphate through extensive hyphal networks and deliver it to symbiotic compartments in the root, where plant orthophosphate transporters transfer this ion into the root cells. Plant symbiotic orthophosphate transporter function is essential for this process and is also required to maintain the symbiosis68,69. Phosphate transporters are important targets for breeding plants with improved orthophosphate acquisition and use-efficiency, and that benefit maximally from their fungal symbionts.
Nitrate sensing and transport
The application of nitrogen (N) fertilizers has greatly increased crop yields and alleviated hunger over the past five decades. However, N fertilizer production consumes 1% of global energy usage and poses the highest input cost for many crops. Nevertheless, only 30% to 50% of the N fertilizer applied is used by plants. The remainder can lead to production of the greenhouse gas N2O, or to eutrophication of aquatic ecosystems through water run-off. Therefore, enhancing crop nitrogen utilization efficiency is an important goal70. For most crops, nitrate is the primary nitrogen source and so enhancing nitrate uptake is one strategy for improving nitrogen utilization efficiency.
Multiple nitrate uptake transporters of the NRT1 and NRT2 families work together to enable nitrogen uptake in plants71,72. Most NRT1 transporters are low-affinity nitrate transporters, meaning that they function mainly when nitrate is abundant. In contrast, chlorate resistant 1 (CHL1, also known as NRT1.1) is a dual-affinity nitrate transporter involved in nitrate acquisition73,74. A recent study found that CHL1 also functions as a nitrate sensor, thus regulating nitrate-induced gene expression75, which implies that plants use this transporter to monitor changes in external nitrate concentration to trigger proper metabolic acclimation. CHL1 has therefore become a paradigm for how nutrient transporters may also serve as nutrient sensors, and how optimization of transport and signalling can be used simultaneously to improve nutrient efficiency.
Using dual-affinity binding, and with the help of two protein kinases (CIPK23 and CIPK8), CHL1 senses a wide range of nitrate concentration changes in the soil to alter its own transport properties75,76 (Fig. 5b). CHL1 and NRT2.1 are also important for nitrate-regulated root development77,78. Vigorous root development is important for plants to compete for nutrients and sustain crop yield61,79. Therefore, nitrate transporters and other proteins that regulate nitrate uptake and sensing provide potential tools for engineering crops with tailored N uptake activity, N metabolism and improved root growth for enhanced nitrogen-use efficiency and reduced-N-fertilizer requirements.
Future outlook
Our knowledge of the molecular nature and regulation of transporters has expanded vastly over the past twenty years. As shown in the examples here, fundamental research into transport mechanisms in plants is leading to rapid innovations for improving yields, extending the range of arable land that can be used for crops and improving the performance of plants under stress. This research also points to new solutions for more sustainable use of limited soil nutrients and for enhanced human nutrition through biofortification.
Recent advances on other plant transporters add to the list of potentially innovative applications in agriculture. For example, plants in the Brassica family, which includes oilseed rape (canola) and mustard, produce glucosinolates, which are potent defence compounds against herbivores and plant pathogens. A transporter controls the distribution of glucosinolates in Arabidopsis80 and may be engineered to enhance herbivore resistance, as a way of reducing the application of pesticides. In another example, toxic heavy metal and arsenic accumulation in edible plant tissues, including rice in the United States, poses a threat to human health81. Plant transporters have recently been identified that control toxic metal and arsenic accumulation in seeds and other tissues82–84, pointing directly to potential applications for developing plants with reduced levels of toxicant accumulation in grain and other edible tissues. Rice with nearly cadmium-free grain has been produced by identifying cadmium transporters and also using a molecular marker to select for genotypes that accumulate low cadmium concentrations85.
Drought tolerance and desiccation avoidance by plants is critical for conserving water during drought periods. Plants lose over 90% of their water by transpiration from stomatal pores in the epidermis of leaves. Stomatal pores are also the gateways for CO2 intake into plants for photosynthesis and have a key role in determining the water-use efficiency of plants. Research has shown that modification in the expression level of ion channels in guard cells (which control the opening and closing of stomatal pores) can be used to reduce water loss, enhance water-use efficiency and regulate efficient CO2 intake for photosynthesis86–88. Moreover, transporters for the plant abiotic stress resistance hormone, abscisic acid, have been identified89,90, and these may be used to target drought resistance responses. Targeting drought tolerance to particularly drought-sensitive tissues or organs during particularly susceptible stages of reproductive development (for example, grain filling and pollen meiosis) could become an important strategy during prolonged droughts and other predicted consequences of climate change.
Transport processes are key to photosynthesis. Since Peter Mitchell’s groundbreaking ‘chemiosmotic hypothesis’ in 1961, the relevance of transporters in photosynthesis has become abundantly clear. Major advances have been made in identifying metabolite transporters across chloroplast membranes91. Yet many of the key transporters in chloroplast membranes remain unidentified. Discovery of the many predicted transporters in subcellular compartments, specifically in chloroplasts, has potential for improving energy capture.
A major challenge in future agriculture is establishing which genetic traits can be combined, or ‘pyramided’, without adversely affecting yield. Many transport processes reviewed here enhance plant performance via defined functions in specific tissues or cell types, so these may be particularly amenable to pyramiding. For instance, salinity tolerance that operates by removal of toxic sodium ions from the xylem sap23–25,28 could be combined with traits that enhance sequestration of sodium into vacuoles32,33, to confer additional salt tolerance. More work will be needed to determine whether or not traits will be compatible when combined. Moreover, many fundamental mechanisms for essential transport processes remain to be uncovered and many essential transporters undoubtedly remain to be discovered. Therefore, knowledge-targeted pyramiding of traits will require future advances in fundamental research into plant membrane transport processes.
Recent advances have identified plant membrane transporters and underlying mechanisms that increase the stress resistance and yield of staple crops. We expect that research into fundamental mechanisms of plant membrane transport processes will continue to produce surprises and breakthroughs that will provide new avenues towards a more sustainable and productive agriculture in the face of impending challenges.
Acknowledgments
Research in our laboratories was supported by: the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences at the US Department of Energy (DOE) under grant numbers DE-FG02-03ER15449 (to J.I.S.), DE-FG02-04ER15542 (to W.B.F.) and DE-FG-2-06ER15809 (to M.L.G.); by the Grains Research and Development Corporation, Australia (to R.M. and E.D.); by the US National Science Foundation under grant numbers IOS:0842720 (to M.J.H.), MCB0918220 (to J.I.S.) and IOS-091994 and DBI 0701119 (to M.L.G.); by the UK Biotechnology and Biological Sciences Research Council under grant number BB/J004561/1 (to D.S.); by the National Institutes of Health under grant numbers GM060396-P42ES010337 (to J.I.S.) and GM078536 and P42ES007373 (to M.L.G.); by the US Department of Agriculture under grant number 2009-02273 (to L.V.K.); by a Generation Challenge Grant under grant number G7010.03.06 (to L.V.K.); by the Howard Hughes Medical Institute under grant number 55005946 (to L.H.E.); the CREST Japan Science and Technology Agency (to N.K.N.); by the Ministry of Education, Culture, Sports, Science and Technology, Japan under grant number 23119507 (to T.H.); and by the Academia Sinica, Taiwan and the National Science Council, Taiwan under grant number NSC 101-2321-B-001-005 (to Y.F.T.).
Footnotes
Author Contributions The project was conceived and outlined by J.I.S. The manuscript was planned by J.I.S. and D.S. All authors contributed to writing sections of the manuscript and all authors commented on versions of the manuscript.
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
References
- 1.The Food and Agriculture Organization of the United Nations. The State of Food Insecurity in the World 2010. FAO; 2010. pp. 8–11. [Google Scholar]
- 2.The World Bank. Repositioning Nutrition as Central to Development: A Strategy for Large-Scale Action. Vol. 2. The International Bank for Reconstruction and Development/The World Bank; 2006. pp. 42–61. http://siteresources.worldbank.org/NUTRITION/Resources/281846-1131636806329/NutritionStrategy.pdf. [Google Scholar]
- 3.World Health Organization/FAO. Diet, Nutrition and the Prevention of Chronic Diseases. 2003:4–12. http://whqlibdoc.who.int/trs/who_trs_916.pdf. [PubMed]
- 4.Foresight The Future of Food and Farming: Final Project Report. The Government Office for Science; 2011. pp. 49–74. http://www.bis.gov.uk/assets/foresight/docs/food-and-farming/11-546-future-of-food-and-farming-report.pdf. [Google Scholar]
- 5.Rockström J, et al. A safe operating space for humanity. Nature. 2009;461:472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 6.Foley JA, et al. Solutions for a cultivated planet. Nature. 2011;478:337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 7.Mueller ND, et al. Closing yield gaps through nutrient and water management. Nature. 2012;490:254–257. doi: 10.1038/nature11420. [DOI] [PubMed] [Google Scholar]
- 8.Connor DJ. Organic agriculture cannot feed the world. Field Crops Res. 2008;106:187–190. [Google Scholar]
- 9.Conway G. One Billion Hungry: Can We Feed the World? Vol. 7. Cornell Univ. Press; 2012. pp. 125–142. [Google Scholar]
- 10.Sanjana NE, et al. A transcription activator-like effector toolbox for genome engineering. Nature Protocols. 2012;7:171–192. doi: 10.1038/nprot.2011.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Uexküll HR, Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171:1–15. [Google Scholar]
- 12.Ryan PR, Delhaize E, Jones DL. Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:527–560. doi: 10.1146/annurev.arplant.52.1.527. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki T, et al. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. These authors identified and characterized the plant aluminium tolerance protein, TaALMT1, an Al-activated anion channel that mediates the efflux of Al-detoxifying malate anion from the wheat root tip. [DOI] [PubMed] [Google Scholar]
- 14.Delhaize E, et al. Transgenic barley (Hordeum vulgare L) expressing the wheat aluminium resistance gene (TaALMT1) shows enhanced phosphorus nutrition and grain production when grown on an acid soil. Plant Biotechnol J. 2009;7:391–400. doi: 10.1111/j.1467-7652.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- 15.Rogers EE, Guerinot ML. FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell. 2002;14:1787–1799. doi: 10.1105/tpc.001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magalhaes JV, et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nature Genet. 2007;39:1156–1161. doi: 10.1038/ng2074. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa J, et al. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007;48:1081–1091. doi: 10.1093/pcp/pcm091. [DOI] [PubMed] [Google Scholar]
- 18.Maron LG, et al. Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J. 2010;61:728–740. doi: 10.1111/j.1365-313X.2009.04103.x. [DOI] [PubMed] [Google Scholar]
- 19.Xia J, Yamaji N, Kasai T, Ma JF. Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci USA. 2010;107:18381–18385. doi: 10.1073/pnas.1004949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CF, Yamaji N, Chen Z, Ma JF. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012;69:857–867. doi: 10.1111/j.1365-313X.2011.04837.x. [DOI] [PubMed] [Google Scholar]
- 21.Famoso AN, et al. Genetic architecture of aluminum tolerance in rice (Oryza sativa) determined through genome-wide association analysis and QTL mapping. PLoS Genet. 2011;7:e1002221. doi: 10.1371/journal.pgen.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 23.Mäser P, et al. Alteredshoot/root Na+ distribution andbifurcating saltsensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 2002;531:157–161. doi: 10.1016/s0014-5793(02)03488-9. [DOI] [PubMed] [Google Scholar]
- 24.Ren ZH, et al. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nature Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- 25.Sunarpi, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- 26.Moller IS, et al. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell. 2009;21:2163–2178. doi: 10.1105/tpc.108.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, et al. A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol. 2006;142:1718–1727. doi: 10.1104/pp.106.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munns R, et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nature Biotechnol. 2012;30:360–364. doi: 10.1038/nbt.2120. A class 1 HKT transporter gene that prevents sodium accumulation in leaves was transferred from a wheat ancestor into modern durum wheat, with a resulting 25% increase in grain yield on saline soils. [DOI] [PubMed] [Google Scholar]
- 29.Blumwald E, Poole R. Na+/H+-antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol. 1985;78:163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- 31.Horie T, et al. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 2007;26:3003–3014. doi: 10.1038/sj.emboj.7601732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- 33.Barragan V, et al. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell. 2012;24:1127–1142. doi: 10.1105/tpc.111.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mian A, et al. Over-expression of an Na+-and K+-permeable HKT transporter in barley improves salt tolerance. Plant J. 2011;68:468–479. doi: 10.1111/j.1365-313X.2011.04701.x. [DOI] [PubMed] [Google Scholar]
- 35.Riesmeier JW, Willmitzer L, Frommer WB. Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J. 1994;13:1–7. doi: 10.1002/j.1460-2075.1994.tb06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen LQ, et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468:527–532. doi: 10.1038/nature09606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen LQ, et al. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012;335:207–211. doi: 10.1126/science.1213351. A FRET (fluorescence (Förster) resonance energy transfer) sucrose nanosensor was used to identify the missing link in phloem loading, that is, the phloem-parenchyma-expressed SWEET sucrose transporters, which are also key susceptibility factors for bacterial pathogens in rice. [DOI] [PubMed] [Google Scholar]
- 38.Patrick JW. Phloem unloading: sieve element unloading and post-sieve element transport. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:191–222. doi: 10.1146/annurev.arplant.48.1.191. [DOI] [PubMed] [Google Scholar]
- 39.Antony G, et al. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell. 2010;22:3864–3876. doi: 10.1105/tpc.110.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu Z, et al. Targeting xa13, a recessive gene for bacterial blight resistance in rice. Theor Appl Genet. 2006;112:455–461. doi: 10.1007/s00122-005-0145-6. [DOI] [PubMed] [Google Scholar]
- 41.Chu Z, et al. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006;20:1250–1255. doi: 10.1101/gad.1416306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q, et al. A paralog of the MtN3/saliva family recessively confers race-specific resistance to Xanthomonas oryzae in rice. Plant Cell Environ. 2011;34:1958–1969. doi: 10.1111/j.1365-3040.2011.02391.x. [DOI] [PubMed] [Google Scholar]
- 43.Li C, Wei J, Lin Y, Chen H. Gene silencing using the recessive rice bacterial blight resistance gene xa13 as a new paradigm in plant breeding. Plant Cell Rep. 2012;31:851–862. doi: 10.1007/s00299-011-1206-8. [DOI] [PubMed] [Google Scholar]
- 44.Li T, Liu B, Spalding MH, Weeks DP, Yang B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nature Biotechnol. 2012;30:390–392. doi: 10.1038/nbt.2199. Synthetic transcriptional-activator-like effectors were used to develop rice plants that are resistant to the blight pathogen Xanthomonas oryzae, such that the pathogen can no longer induce SWEET transporters, thus starving the pathogen by reducing the rice-derived sugar supply to the pathogen. [DOI] [PubMed] [Google Scholar]
- 45.Masuda H, et al. Increase in iron and zinc concentrations in rice grains via the introduction of barley genes involved in phytosiderophore synthesis. Rice. 2008;1:100–108. [Google Scholar]
- 46.Lee S, et al. Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc Natl Acad Sci USA. 2009;106:22014–22019. doi: 10.1073/pnas.0910950106. These authors showed that increasing nicotianamine levels resulted in increased levels of iron in polished rice and also that the iron is bioavailable using animal feeding studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, et al. Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol Cells. 2012;33:269–275. doi: 10.1007/s10059-012-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishimaru Y, et al. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010;62:379–390. doi: 10.1111/j.1365-313X.2010.04158.x. [DOI] [PubMed] [Google Scholar]
- 49.Wirth J, et al. Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol J. 2009;7:631–644. doi: 10.1111/j.1467-7652.2009.00430.x. [DOI] [PubMed] [Google Scholar]
- 50.Masuda H, et al. Iron biofortification in rice by the introduction of multiple genes involved in iron nutrition. Sci Rep. 2012;2:543–549. doi: 10.1038/srep00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S, et al. Bio-available zinc in rice seeds is increased by activation tagging of nicotianamine synthase. Plant Biotechnol J. 2011;9:865–873. doi: 10.1111/j.1467-7652.2011.00606.x. [DOI] [PubMed] [Google Scholar]
- 52.Palmgren MG, et al. Zinc biofortification of cereals: problems and solutions. Trends Plant Sci. 2008;13:464–473. doi: 10.1016/j.tplants.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Lanquar V, et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005;24:4041–4051. doi: 10.1038/sj.emboj.7600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SA, et al. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science. 2006;314:1295–1298. doi: 10.1126/science.1132563. These authors used X-ray fluorescence microprobe spectroscopy to localize iron in seedsand showed that failure to store iron properly in the vacuole via theVIT1 transporter leads to seedling lethality under iron limitation. [DOI] [PubMed] [Google Scholar]
- 55.Morrissey J, et al. The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell. 2009;21:3326–3338. doi: 10.1105/tpc.109.069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Xu YH, Yi HY, Gong JM. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012;72:400–410. doi: 10.1111/j.1365-313X.2012.05088.x. [DOI] [PubMed] [Google Scholar]
- 57.Podar D, et al. Metal selectivity determinants in a family of transition metal transporters. J Biol Chem. 2012;287:3185–3196. doi: 10.1074/jbc.M111.305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cakmak I. Plant nutrition research: priorities to meet human needs for food in sustainable ways. Plant Soil. 2002;247:3–24. [Google Scholar]
- 60.López-Arredondo DL, Herrera-Estrella L. Engineering phosphorus metabolism in plants to produce a dual fertilization and weed control system. Nature Biotechnol. 2012;30:889–893. doi: 10.1038/nbt.2346. [DOI] [PubMed] [Google Scholar]
- 61.Gamuyao R, et al. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature. 2012;488:535–539. doi: 10.1038/nature11346. [DOI] [PubMed] [Google Scholar]
- 62.Beebe SE, et al. Quantitative trait loci for root architecture traits correlated with phosphorus acquisition in common bean. Crop Sci. 2006;46:413–423. [Google Scholar]
- 63.Shin R, Schachtman DP. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA. 2004;101:8827–8832. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Remy E, et al. The Pht1;9 and Pht1;8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytol. 2012;195:356–371. doi: 10.1111/j.1469-8137.2012.04167.x. [DOI] [PubMed] [Google Scholar]
- 65.Versaw WK, Harrison MJ. A chloroplast phosphate transporter, PHT2;1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell. 2002;14:1751–1766. doi: 10.1105/tpc.002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagarajan VK, et al. Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol. 2011;156:1149–1163. doi: 10.1104/pp.111.174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arpat AB, et al. Functional expression of PHO1 to the Golgi and trans-Golgi network and its role in export of inorganic phosphate. Plant J. 2012;71:479–491. doi: 10.1111/j.1365-313X.2012.05004.x. [DOI] [PubMed] [Google Scholar]
- 68.Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJA. Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA. 2007;104:1720–1725. doi: 10.1073/pnas.0608136104. A phosphate transporter (MtPT4) was shown to be necessary for Medicago truncatula plants to obtain phosphate delivered via the fungal symbiont and furthermore, that MtPT4 transporter function is essential to maintain the symbiosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang SY, et al. Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the PHOSPHATE TRANSPORTER1 gene family. Plant Cell. 2012;24:4236–4251. doi: 10.1105/tpc.112.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McAllister CH, Beatty PH, Good AG. Engineering nitrogen use efficient crop plants: the current status. Plant Biotechnol J. 2012;10:1011–1025. doi: 10.1111/j.1467-7652.2012.00700.x. [DOI] [PubMed] [Google Scholar]
- 71.Wang YY, Hsu PK, Tsay YF. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012;17:458–467. doi: 10.1016/j.tplants.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 72.Kiba T, et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell. 2012;24:245–258. doi: 10.1105/tpc.111.092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu KH, Tsay YF. Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 2003;22:1005–1013. doi: 10.1093/emboj/cdg118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang R, Liu D, Crawford NM. The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc Natl Acad Sci USA. 1998;95:15134–15139. doi: 10.1073/pnas.95.25.15134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ho CH, Lin SH, Hu HC, Tsay YF. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. These authors provided the first report of a nutrient transporter in plants that also acts as a sensor for its own substrate, nitrate, over a wide range of concentrations. [DOI] [PubMed] [Google Scholar]
- 76.Hu HC, Wang YY, Tsay YF. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. 2009;57:264–278. doi: 10.1111/j.1365-313X.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 77.Little DY, et al. The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci USA. 2005;102:13693–13698. doi: 10.1073/pnas.0504219102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krouk G, et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Ruffel S, et al. Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc Natl Acad Sci USA. 2011;108:18524–18529. doi: 10.1073/pnas.1108684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nour-Eldin HH, et al. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature. 2012;488:531–534. doi: 10.1038/nature11285. [DOI] [PubMed] [Google Scholar]
- 81.Gilbert-Diamond D, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci USA. 2011;108:20656–20660. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ueno D, et al. Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA. 2010;107:16500–16505. doi: 10.1073/pnas.1005396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song WY, et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA. 2010;107:21187–21192. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma JF, et al. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA. 2008;105:9931–9935. doi: 10.1073/pnas.0802361105. These authors demonstrated that arsenite, a toxic form of soil arsenic, is transported into and within the rice plant by two novel transporters, providing new possible strategies for minimizing arsenic entry into the food chain by altering these transporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishikawa S, et al. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci USA. 2012;109:19166–19171. doi: 10.1073/pnas.1211132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kusumi K, Hirotsuka S, Kumamaru T, Iba K. Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J Exp Bot. 2012;63:5635–5644. doi: 10.1093/jxb/ers216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu H, et al. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nature Cell Biol. 2010;12:87–93. doi: 10.1038/ncb2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuromori T, et al. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc Natl Acad Sci USA. 2010;107:2361–2366. doi: 10.1073/pnas.0912516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang J, et al. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc Natl Acad Sci USA. 2010;107:2355–2360. doi: 10.1073/pnas.0909222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weber AP, Brautigam A. The role of membrane transport in metabolic engineering of plant primary metabolism. Curr Opin Biotechnol. doi: 10.1016/j.copbio.2012.09.010. http://dx.doi.org/10.1016/j.copbio.2012.09.010 (4 October 2012) [DOI] [PubMed]
- 92.Delhaize E, Gruber BD, Ryan PR. The roles of organic anion permeases in aluminium tolerance and mineral nutrition. FEBS Lett. 2007;581:2255–2262. doi: 10.1016/j.febslet.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 93.US Geological Survey. Mineral Commodity Summaries 2012. US Geological Survey; 2012. pp. 118–119. http://minerals.usgs.gov/minerals/pubs/mcs/2012/mcs2012.pdf. [Google Scholar]