Abstract
Background
We previously reported isolation of l-isocorypalmine (l-ICP), a mono-demethylated analog of l-tetrahydropalmatine (l-THP), from the plant Corydalis yanhusuo. Here we characterized its in vitro pharmacological properties and examined its effects on cocaine-induced behaviors in mice.
Methods
Receptor binding, cAMP and [35S]GTPγS assays were used to examine pharmacological actions of l-ICP in vitro. Effects of I-ICP on cocaine-induced locomotor hyperactivity and sensitization and conditioned place preference (CPP) in mice were investigated. HPLC was employed to analyze metabolites of I-ICP in mouse serum.
Results
Among more than 40 targets screened, l-ICP and l-THP bound only to dopamine (DA) receptors. l-ICP was a high-affinity partial agonist of D1 and D5 receptors and a moderate-affinity antagonist of D2, D3 and D4 receptors, whereas l-THP bound to only D1 and D5 receptors, with lower affinities than l-ICP. At 10 mg/kg (i.p.), l-ICP inhibited spontaneous locomotor activity for a shorter time than l-THP. Pretreatment with l-ICP reduced cocaine-induced locomotor hyperactivities. Administration of l-ICP before cocaine once a day for 5 days reduced cocaine-induced locomotor sensitization on days 5 and 13 after 7 days of withdrawal. Pretreatment with l-ICP before cocaine daily for 6 days blocked cocaine-induced CPP, while l-ICP itself did not cause preference or aversion. HPLC analysis showed that l-ICP was the main compound in mouse serum following i.p. injection of l-ICP.
Conclusions
l-ICP likely acts as a D1 partial agonist and a D2 antagonist to produce its in vivo effects and may be a promising agent for treatment of cocaine addiction.
Keywords: tetrahydropalmatine, isocorypalmine, cocaine, conditioned place preference, sensitization, dopamine receptor
1. INTRODUCTION
Addiction to drugs such as morphine, heroin, cocaine, amphetamine, and alcohol is serious medical, societal and economic problems around the world. A great deal of effort has been devoted to developing effective pharmacotherapy to combat drug addiction and dependence. To date, only a few medications have been approved by the U.S. Food and Drug Administration for the treatment of addiction, notably for addiction to opioids, nicotine and alcohol. However, there is no effective medication for the treatment of addiction to psychostimulants such as cocaine, methamphetamine and amphetamine (for a review, see Xi and Gardner, 2008).
Herbal medicine may provide new sources for discovery and development of novel agents. Chinese medicinal herbs have been used historically to treat alcohol and drug abuse (Lukas et al., 2005; Tang et al., 2006). Corydalis yanhusuo, a perennial herb in the Papaveraceae family, is among the 10 most frequently used herbs to treat drug addiction in China (Min et al., 2007). Hsu and Kin isolated l-tetrahydropalmatine (l-THP) from C. yanhusuo and did the first pharmacological characterization of the compound (Hsu and Kin, 1962, 1964). l-THP has been used clinically in China for more than 40 years as an analgesic with sedative/hypnotic properties (Jin, 1987). Recently, l-THP has been shown in animal models to reduce the rewarding properties of cocaine, cocaine-induced reinstatement of drug seeking behavior and cocaine-enhanced brain-stimulation reward (Xi et al., 2007; Mantsch et al., 2007, 2010; Figueroa-Guzman et al., 2011; reviewed in Wang and Mantsch, 2012). In a clinical trial in China, treatment of patients addicted only to heroin with l-THP for one month significantly reduced craving and withdrawal symptoms during treatment, and enhanced abstinence rate by three-fold three months after discharge (Yang et al., 2008). l-THP is currently in a clinical trial (phase I) for treatment of cocaine addiction in the US. The trial is directed by Dr. Jia-Bei Wang of the University of Maryland and supported by National Institute on Drug Abuse.
We previously reported isolation of eight known isoquinoline alkaloids from C. yanhusuo, including l-THP and l-isocorypalmine (l-ICP) and examined their binding to the D1 receptor (Ma et al., 2008). l-ICP is a de-methylated analog of l-THP and part of its structure resembles dopamine (Fig. 1). l-ICP has relatively high affinity for the dopamine D1 receptor among the eight isolated compounds (Ma et al., 2008). One of the prominent pharmacological effects of l-THP is sedation, which may be a serious side effect for treatment of drug abuse. Here we found that l-ICP inhibited spontaneous locomotor activity in the mouse for a shorter time (<30 min) than l-THP (> 90 min), suggesting that l-ICP produces less sedation. In addition, we found that l-THP had much lower affinity for the dopamine receptors than l-ICP. We thus further characterized pharmacological properties of l-ICP at cloned human dopamine (DA) D1, D2, D3, D4 and D5 receptors and screened for other pharmacological targets in vitro. In addition, we investigated the effect of l-ICP on cocaine-induced locomotor hyperactivity and development of sensitization, and acquisition of rewarding effects of cocaine in a CPP model. Moreover, we examined its metabolites in mouse serum.
Figure 1. Chemical structures of dopamine and l-isocorypalmine (l-ICP) and l-tetrahydropalmatine (l-THP).
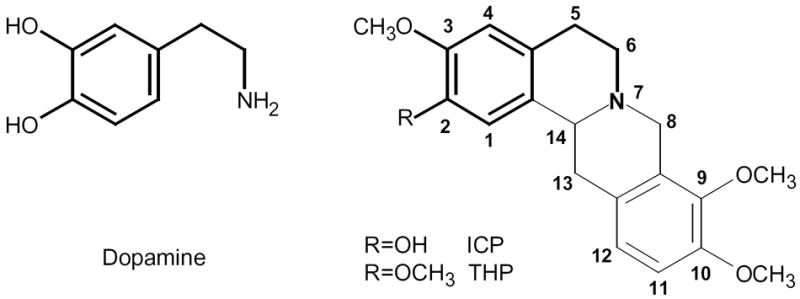
Note that part of the l-ICP structure resembles dopamine (DA) structure.
2. MATERIALS AND METHODS
2.1. Materials
[3H]SCH23390 (73.1 Ci/mmol), [3H]methylspiperone (85.5 Ci/mmol), [3H]cAMP (33.0 Ci/mmol) and [35S]guanosine 5′-3-O-(thio)triphosphate (GTPγS) (1250 Ci/mmol) were purchased from PerkinElmer (Boston, MA). Tetracycline, 1-methyl-3-isobutylxanthine (IBMX), cAMP, guanosine diphosphate (GDP), hygromycin and blasticidin were obtained from Sigma-Aldrich (St. Louis, MO). Cell culture reagents were obtained from Invitrogen (Carlsbad, CA). Cocaine hydrochloride was provided by the National Institute on Drug Abuse.
l-ICP was synthesized and l-THP was purified as we described previously (Ma et al., 2008). Ten mg l-ICP or l-THP was dissolved in 150 μl of 0.1 M H2SO4 and diluted with sterile normal saline or sterile water to 10 mg/ml, and adjusted to pH 4-5 with 0.1 N NaOH. For mice, l- ICP or l-THP was given in a volume of 3 ml/kg body weight. Na2SO4 solution (4.3 mM, pH 4-5) was used as the vehicle control in an equal volume given by the same route as l-ICP. Cocaine was dissolved in a sterile 0.9% saline solution.
2.2. Animals
Eight-week-old male CD-1 mice initially weighing 25-30 g (Charles River Laboratories, Raleigh, NC) were group-housed in a temperature- and humidity-controlled environment on a 12-h light–dark cycle (lights on at 7:00 AM). Animals were given food and water ad libitum. All experiments were conducted in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and with approval from Temple University School of Medicine Institutional Animal Care and Use Committee.
2.3. Cell Culture
Human embryonic kidney 293 cells (HEK293 cells) stably transfected with the human 3HA-D1, FLAG-D2 or FLAG-D3 receptor into the FLP recognition target recombination site were supplied by Dr. Jonathan Javitch of Columbia University Medical Center. HEK293 cells expressing the human DA D4 receptor were supplied by Dr. Bryan Roth of University of North Carolina School of Medicine (who obtained the cell line originally from Dr. Javitch). HEK293 cells transfected with the human DA D5 receptor were supplied by Dr. John Edward Jones of Georgetown University School of Medicine. Cells were grown in 100-mm culture dishes in Minimum Essential Medium supplemented with 10% fetal calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere consisting of 5% CO2 and 95% air at 37°C. In order to maintain the expression of target genes, cells expressing the D1, D2, D3 or D4 receptor were grown in the presence of 100 μg/ml hygromycin and 15 μg/ml blasticidin, and cells expressing the D5 receptor were grown with 15 μg/ml blasticidin. For the D1 receptor, the CMV promoter is controlled by a Tet-on mechanism. After we observed that l-ICP was a high-efficacy partial agonist at the D1 receptor when receptor expression was induced with 1 μg/ml tetracycline, it was necessary to perform the same experiments with lower levels of the D1 receptor for assessment of l-ICP efficacy. HEK cells expressing the D1 receptor were thus induced to have high, moderate and low expression with 1 μg/ml, 6 ng/ml and 1 pg/ml tetracycline, respectively.
2.4. Receptor binding
More than 40 receptors, ion channels, and transporters (see Supplemental Materials1) known to be involved in pain regulation, sedation/hypnosis, and anxiolytic actions were screened for binding to l-ICP and l-THP. Because parts of the l-ICP and l-THP structures resemble dopamine (see Fig. 1), we hypothesized that these two compounds may interact with monoamine receptors and/or transporters. Opioid and dopamine receptor binding assays were done in our laboratory (see below); others were performed by the Psychoactive Drug Screening Program (PDSP) which was supported by National Institute on Mental Health and directed by Dr. Bryan Roth of University of North Carolina School of Medicine. In PDSP, inhibition of radioligand binding to the receptor of interest by 10 μM l-ICP or l-THP was performed and affinities of l-ICP were determined for the receptors which 10 μM l-ICP exhibited > 50% inhibition of ligand binding.
Dopamine receptor binding was performed according to published procedures (Barton et al., 1991; Hummel and Unterwald, 2003). For D1 and D5 receptors, [3H]SCH23390 was used as the radiolabeled ligand and fluphenazine (10 μM) was used to define nonspecific binding, whereas for D2, D3 and D4 receptors, [3H]methylspiperone and (+)-butaclamol (4 μM) were used, respectively. Membranes were prepared from transfected HEK293 cells as described previously (Li et al., 1993). Saturation binding of [3H]SCH23390 or [3H]methylspiperone to the D1 and D5 receptors or D2, D3 and D4 receptors, respectively, was performed with at least 8 concentrations of [3H]SCH23390 (ranging from 20 pM to 20 nM) or [3H]methylspiperone (ranging from 20 pM to 10 nM). Binding was carried out in 50 mM Tris-HCl buffer containing 120 mM NaCl, 5 mM KCl, 2 mM CaCl2 and 1 mM MgCl2 (pH 7.4) at room temperature for 1 h in duplicate in a volume of 250 μl with 10~200 μg membrane protein depending on receptor expression level. Incubations were terminated by filtration through Whatman GF/B filters under vacuum and radioactivity on filters was measured. Competitive inhibition of [3H]SCH23390 (2 nM) binding to the D1 and D5 receptors or [3H]methylspiperone (1 nM) binding to the D2, D3 and D4 receptors by l-ICP was performed with various concentrations (10-11 M to 10-5 M) of l-ICP. Binding data were analyzed with the Prism program (GraphPad, San Diego, CA) and Kd, Bmax and Ki values were determined.
Opioid receptor binding was conducted according to our published procedures (Huang et al., 2001) using [3H]diprenorphine as the radioligand and naloxone to define nonspecific binding (1 μM for the mu opioid receptor and 10 μM for the delta and kappa opioid receptors).
2.5. Activation of D1 and D5 receptors and cAMP assay
D1 and D5 receptors are coupled to Gs proteins and stimulation of adenylyl cyclase (AC) is used as the functional end point. The cAMP assay was performed as described previously (Huang et al., 2001). Briefly, HEK 293 cells stably expressing the D1 or D5 receptors were harvested, re-suspended and counted. Cells (0.6-1×106) were added to assay tubes containing isobutylmethylxanthine (final concentration 2.5 mM) in the Opti-MEM reduced serum medium and 10-11 M to 10-5 M of l-ICP or DA, and incubated at 37°C for 15 min. The reaction was terminated by boiling in a water bath for 5 min. [3H]cAMP was added to all sample tubes and tubes containing known amounts of cAMP to generate a standard curve. cAMP binding protein (regulatory subunits of protein kinase A) was added to each tube. The mixture was incubated for 2 h to overnight at 4°C. Bound and free [3H]cAMP were separated by adsorption of free [3H]cAMP by charcoal suspension. The amounts of cAMP in samples were calculated based on the standard curve using the Prism program and converted to pmol/106 cells/15 min.
2.6. [35S]GTPγS binding assay
Determination of [35S]GTPγS binding to G proteins was carried out using a modification of our published procedure (Zhu et al., 1997). Briefly, membranes of HEK 293 cells stably expressing the D2, D3 or D4 dopamine receptors were prepared. Membranes (10 μg protein) were incubated in reaction buffer containing [35S]GTPγS (80–100 pM) and 10 μM GDP with or without DA (10-10–10-4 M) in a total volume of 0.5 ml for 60 min at 30°C. For antagonist effects of l-ICP on DA-induced increase of [35S]GTPγS binding, membranes were pretreated with the indicated concentration of l-ICP followed by 10-9 M to 10-4 M of DA 10 min later. Nonspecific binding was determined in the presence of 10 μM GTPγS. Bound and free [35S]GTPγS was separated by filtration with vacuum. Data were analyzed and EC50 and Emax values were determined with the Prism program.
2.7. Spontaneous locomotor activity and cocaine-induced locomotor hyperactivity and sensitization
Apparatus
Locomotor activities were measured as we described previously (Huang et al., 2009) using a Digiscan D Micro System (Accuscan, Columbus, OH), an older model similar to the current model of Home Cage - Locomotor Activity Monitor. Each locomotor activity chamber consists of a transparent plastic box (45 cm × 20 cm × 20 cm) set inside a metal frame which has a pair of sensor panels parallel to each other. One panel has a set of 16 infrared light emitters and the other has the same number of detectors. The beam height is 4.5 cm and the distance between two beams is 2.5 cm. Disruption of the light beam by the animals is recorded by computer and the associated software can determine the location of the rodent along the axis perpendicular to the beams. Breaks of two consecutive light beams resulting from horizontal movement of the animal are recorded as ambulatory activity, whereas repeated breaks of the same light beam indicate non-ambulatory repetitive movement with little horizontal movement.
Spontaneous locomotor activity
CD-1 mice were allowed to acclimate for 3 days in home cages after arrival in the animal facility and then adapted to injections by daily intraperitoneal (i.p.) saline injection for 5 days. On the following day, mice were administered with l-ICP or l-THP (10 mg/kg, i.p.) or vehicle and immediately put into locomotor chambers and locomotor activities were measured as described above.
Acute cocaine-induced locomotor hyperactivity
Mice underwent the same acclimation and adaptation to injection as described above. On the following day, after habituation to activity chambers for 50 min, mice were pretreated with l-ICP (1, 3 or 10 mg/kg) or vehicle followed by a cocaine (20 mg/kg, i.p.) or saline injection 10 minutes later. Activity counts (total, ambulatory and non-ambulatory repetitive) were recorded during habituation, during drug or vehicle pretreatment (10 min) and after saline or cocaine treatment (120 min). Data were collected in 5-min intervals.
Repeated cocaine-induced locomotor sensitization
Cocaine-induced sensitization was conducted as described by Miller et al. (2009). The timeline is shown in Figure 6A. Animals were subdivided into 4 groups: vehicle + saline; vehicle + cocaine, l-ICP + saline and l-ICP + cocaine. From day 1 to day 5, mice were placed in locomotor activity chambers for 30 min and injected once daily with vehicle or l-ICP (10 mg/kg, i.p.), followed by saline or cocaine (20 mg/kg, i.p.) 10 min later. Mice were allowed to stay in activity chambers for an additional 30 min. From day 6 to day 12, mice were kept in their home cages without any treatment. On day 13, mice were placed in the same activity chambers for 50 min and then challenged with cocaine (20 mg/kg, i.p.). Locomotor activity was monitored on days 1, 5 and 13 and their total activities were recorded for 60 min or 120 min after cocaine injection.
Figure 6. Effects of l-ICP on development of locomotor sensitization induced by repeated cocaine treatment in mice.
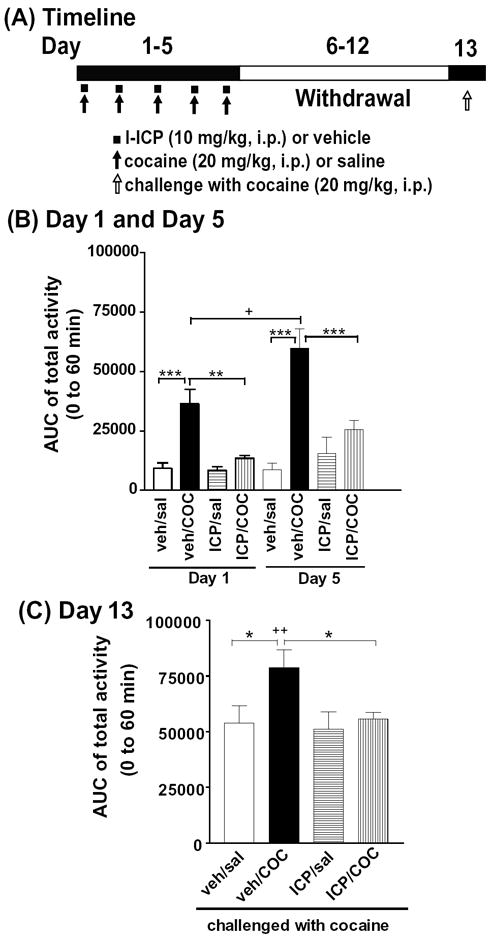
(A) Timeline of the experimental procedure and drug treatment. (B) Locomotor activities on Day 1 and Day 5. (C) Locomotor activities on Day 13. From day 1 to day 5, mice were treated once/day with vehicle or l-ICP (10 mg/kg, i.p.) followed by saline or cocaine (20 mg/kg, i.p.)10 min later as described in Methods. Following withdrawal from day 6 to day 12, on day 13, mice were challenged with cocaine. Activities were monitored on days 1, 5 and 13 for 120 min. The areas under curves of total activities (0-60 minutes) are shown in the B and C. Each value represents mean ± s.e.m (N=8 for each group).
*** P<0.001 and *P<0.05 compared with veh/COC; ++P<0.01 and +P<0.05, compared with day 1 veh/cocaine by two-way ANOVA followed by Bonferroni post hoc test.
2.8. Conditioned place preference
The CPP procedure was used as a model to test the rewarding properties of cocaine and was performed according to published procedures (Huang et al., 2003; Prus et al. 2009) with some modifications.
Apparatus
The Plexiglas chamber (12.7 × 34.7 × 12.7 cm3) consisted of two identically sized compartments with a guillotine door (5 × 5.9 cm2) in between. One compartment was covered with mosaic type paper (checkered) on the three walls and floor with a blue light bulb (5-watt) hung at the top, as two visual cues; the other one was covered with white paper and a red light bulb (5-watt) hung at the top, as the other two distinctive visual cues.
Procedure
Mice were handled and weighed for three days before experiments. The conditioning and testing consisted of three phases (see Fig. 7A for timeline) as follows:
Figure 7. Effects of l-ICP on acquisition of cocaine-induced conditioned place preference (CPP) in mice. (A) Timeline of the CPP paradigm and testing procedure. (B) Pretreatment with l-ICP significantly blocked acquisition of cocaine CPP, but by itself did not induce preference or aversion.
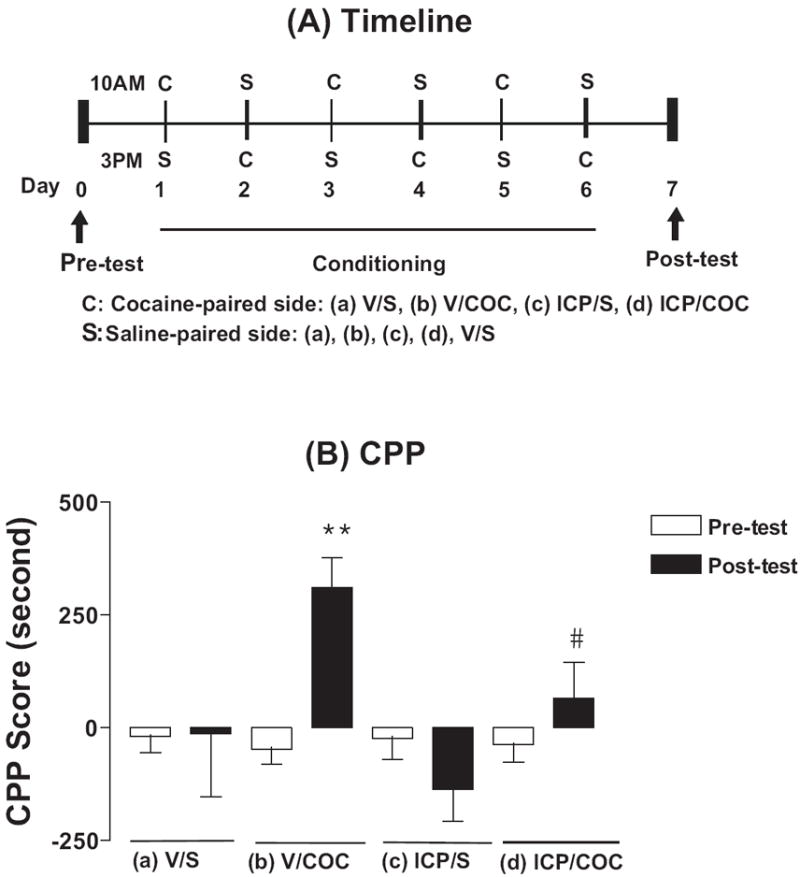
An unbiased and counterbalanced procedure was used for the CPP experiments. At C (cocaine) in the timeline, mice were treated with vehicle or l-ICP (10 mg/kg, i.p.) followed by cocaine (20 mg/kg, i.p.) or saline injection 10 min later and placed in one side of CPP chambers. At S (saline) in the timeline, mice were treated with vehicle followed by saline injection 10 min later and placed in the other side of CPP chambers. Test of preference was conducted on day 7. Results are expressed as mean ± s.e.m. time spent (in seconds) on the cocaine-paired compartment minus the saline-paired compartment. Eight mice in each group were used.
(a)V/S: vehicle/saline, (b)V/C: vehicle/cocaine, (c)I/S: l-ICP/saline, (d)I/C: l-ICP/cocaine.
**P<0.01 compared with the Pre-test of the same group, #P <0.05 compared with the Post-test of the V/C group, both by one-way ANOVA followed by Newman-Keuls multiple comparison test.
Pre-test (Day 0, one session)
Mice were placed in a dark and quiet room with only blue and red light bulbs on for 1 h, and then allowed free access to both conditioning compartments for 15 min. The time spent in each compartment was recorded and initial preference for the compartments was determined. Mice spending over 540 s (60% of total time) in either compartment were excluded.
Conditioning days (Days 1-6, two sessions per day)
Conditioning was conducted using an unbiased and counterbalanced procedure (Prus et al. 2009). For the unbiased method, the compartment in which the animal receives vehicle or drug is randomly assigned regardless of its preference in the pretest. Mice were randomly divided into four groups: vehicle/saline, vehicle/cocaine, l-ICP/saline and l-ICP/cocaine (n = 8 for each group). Half of the mice in each group received an injection and then were confined in the checkered compartment for 40 min, and the other half were confined in the white compartment (counterbalanced design). On days 1, 3 and 5, at 10 AM, mice received an injection of vehicle or l-ICP (10 mg/kg, i.p.) followed by cocaine (20 mg/kg, i.p.) or saline 10 min later and were then confined in the cocaine-paired compartment for 40 min. At 3 PM, mice received an injection of vehicle followed by injection of saline 10 min later and were then confined in the saline-paired compartment for 40 min. On days 2, 4 and 6, the 10 AM and 3 PM procedures were reversed.
Post-test (Day 7, one session)
Each mouse was placed at the door between the two compartments and allowed to freely move between the two. The time that the animal spent in each compartment was recorded for 15 min and their post-drug preference was determined.
2.9. High performance liquid chromatography (HPLC) analysis of l-ICP and its possible metabolites in the mouse serum
Sample preparation
Mice were i.p. injected with 20 mg/kg l-ICP or vehicle (10 mice/group) and killed 30 min later. Blood was collected immediately in tubes, which were placed on ice for at least 30 min to allow blood clot formation, centrifuged at 2,000 × g for 20 min to remove clots and the serum was isolated (~3.5 ml pooled from 10 mice). Serum from untreated mice or sample serum (l-ICP) (1.0 ml) was passed through a C-18 cartridge (100 mg/1ml, Sigma-Aldrich/Supelco, St. Louis, MO), which had previously been conditioned by washing with 1 ml of methanol and 1 ml of deionized distilled water. The cartridge was washed with 0.5 ml of water, and then eluted with 2 ml of methanol. The methanol eluate was evaporated at 40°C in vacuo. The residue was suspended in 1 ml of methanol, and the solution was filtered through a 0.45 μm NYL syringe filter (GE Healthcare Life Sciences / Whatman, Piscataway, NJ). The filtrate was transferred to a 2 ml vial and dried under nitrogen. The residue was dissolved in 50 μl of methanol, and 10.0 μl of the methanol solution was injected into HPLC for analysis.
HPLC apparatus and conditions
HPLC analyses were performed on a Waters Breeze liquid chromatograph system equipped with a Waters 1525 binary pump and a Waters 2487 dual λ absorbance detector. The HPLC system consisted of a YMC ODS-A column (4.6×150 mm I.D., 5 μm particle size) and a security guard cartridge (4.0×20 mm, I.D.) packed with the same material. A mobile phase, consisting of (1) methanol (25-35%) / 0.05% triethylamine water solution (75-65%), 0-20 min and (2) methanol (35%) / 0.05% triethylamine water solution (65%), 20-40 min, was employed. The flow-rate was set at 1.0 ml/min and the UV detection wavelength was at 280 nm.
2.10. Determination of the Protein Content
Protein contents of membranes were determined with the bicinchoninic acid (BCA) method (Smith et al., 1985) with bovine serum albumin as the standard and using the BCA reagents (Pierce Protein Biology Products, Thermo Fisher Scientific, Rockford, IL).
2.11. Statistical Analysis
Data are presented as the mean ± s.e.m. For comparison of multiple groups, data were analyzed by one-way analysis of variance (ANOVA) followed by a Newman-Keuls multiple comparison test or two-way ANOVA followed by Bonferroni post hoc comparisons. For comparison of two groups, Student’s t test was performed. P<0.05 was defined as a statistically significant difference for all analyses.
3. RESULTS
3.1. Characterization of cell lines expressing each of the cloned DA receptors
Saturation binding of [3H]SCH23390 to the DA D1 and D5 receptors and [3H]methylspiperone to the DA D2, D3 and D4 receptors expressed in HEK293 cells was performed and their affinities (Kd) and expression levels (Bmax) were determined2. For the D1 receptor, 1 μg/ml, 6 ng/ml and 1 pg/ml tetracycline were used to induce expression to give high (H) moderate (M) and low (L) levels, respectively. DA D1 and D5 receptors displayed high affinity for [3H]SCH23390 and D2, D3 and D4 receptors had high affinity for [3H]methylspiperone and their Kd values ranged from 0.42 to 1.7 nM, which are similar to those previously reported (Todd et al., 1989; Monsma, Jr. et al., 1990; Grandy et al., 1991; McAllister et al., 1995; Kim et al., 2002; Fiorentini et al., 2008). The Bmax values varied from 0.093 to 25.4 pmol/mg protein.
3.2. Screening for pharmacological targets of l-ICP by binding assays
More than 40 receptors, ion channels, and transporters known to be involved in pain regulation, sedation/hypnosis, and anxiolytic actions were screened for binding to l-ICP. l-ICP had significant affinities only for DA receptors. l-ICP at 10 μM did not bind significantly to alpha1A, 1B, 2A, 2B, and 2C, and beta1 and 2 adrenergic; 5-HT1A, 1B, 1E, 2A, 2C, 3, 4, 5A, 6, and 7 serotonin receptors. In addition, l-ICP at 10 μM did not bind to the dopamine, norepinephrine and serotonin transporters. Moreover, at 10 μM, l-ICP did not bind significantly to any of the following receptors: mu, delta, and kappa opioid; orphanin FQ/nociception; GABAA; histamine H1, 2, 3, and 4; muscarinic M1, 2, 3, 4, and 5; NMDA/MK801. These results suggest that DA receptors may be the key pharmacological targets of l-ICP.
Screening was also done on l-THP and none of over 50 pharmacological targets bound l-THP with high affinity3.
3.3. Affinities and functional activities at dopamine receptors of l-ICP
Further receptor binding and functional assays were conducted on HEK293 cells stably expressing each of the five cloned human DA receptors. Competitive inhibition by l-ICP and l-THP of binding of radiolabeled ligands to dopamine receptors in membranes was conducted and the Ki values were determined. As shown in the Table 1, the affinities of l-ICP for the five dopamine receptors are in the order (high to low) of D1 > D5 > D3 ~ D2 > D4 with the Ki values of 5.1-6.2, 9.5, 37.3, 41.8 and 77.4 nM, respectively. In contrast, l-THP had Ki values of ~150 and ~300 nM for D1 and D5 receptors, respectively, and >1 μM for D2, D3 and D4 receptors.
Table 1. Ki values (nM) of l-ICP or l-THP binding to the human DA D1, D2, D3, D4 and D5 receptors stably expressed in HEK293 cells.
Competitive inhibition by l-ICP or l-THP of [3H]SCH23,390 binding to D1 and D5 receptors and [3H]N-Methyl-spiperone binding to D2, D3 and D4 receptors was conducted and its Ki values were determined. Each value represents mean ± s.e.m. of three experiments performed in duplicate. H, M and L represent D1R high, moderate and low expression, respectively.
| D1 |
D2 | D3 | D4 | D5 | |||
|---|---|---|---|---|---|---|---|
| H | M | L | |||||
|
| |||||||
| l-ICP | 6.2±0.1 | 5.1±1.3 | 5.3±0.6 | 41.8±1.8 | 37.3±3.4 | 77.4±17.5 | 9.5±1.4 |
|
|
|||||||
| l-THP | 153 ± 8.8 | - | - | 1125 ± 97 | 1371 ± 212 | >1,000 | 305 ± 65 |
DA D1-like receptors (D1 and D5) are coupled to Gs proteins and cAMP formation was used as their functional end point. For the D1 receptor, the potency (EC50) and efficacy (Emax) of l-ICP varied with receptor expression level. DA was used as the reference full agonist (Fig. 2 and Table 2). At high D1 expression level (5.0±0.2 pmol/mg protein), l-ICP is a high-efficacy partial agonist with an EC50 of 39 nM and an Emax of 85% of that of DA (Fig. 2A1). At a moderate level of the D1 receptor (1.4±0.2 pmol/mg protein), l-ICP has an EC50 of 263 nM and an Emax of 34% of that of DA (Fig. 2A2). At a low level of the D1 receptor (0.093±0.005 pmol/mg protein), doses of l-ICP up to 10 μM were not active (Table 2). For the D5 receptor, l-ICP is a partial agonist with an EC50 of 20 nM and an Emax of 65% of that of DA (Fig. 2B and Table 2). These results indicate that l-ICP is a partial agonist at the D1 and D5 receptors.
Figure 2. l-ICP acts as a partial agonist at (A1, A2) the dopamine D1 and (B) D5 receptors.
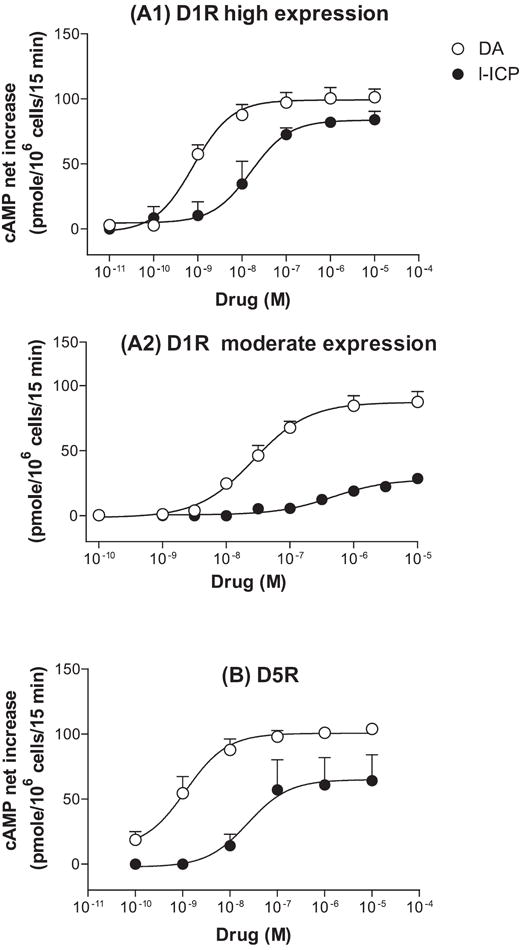
(A1, A2) dopamine- and l-ICP-stimulated cAMP formation in HEK cells expressing a (A1) high or (A2) moderate level of the D1 receptor were performed with different doses and dose-response curves of dopamine and l-ICP were generated. Potencies (EC50) and efficacies (Emax) of l-ICP were determined and shown in Table 2. (B). Dose-response curves of dopamine and l-ICP in stimulating cAMP formation in HEK cells expressing the D5R were generated. EC50 and Emax of l-ICP were shown in Table 2. Each value represents mean ± s.e.m. of three experiments performed in duplicate.
Table 2. Potencies (EC50) and efficacies (Emax) of l-ICP and DA in stimulating D1 and D5 receptors to enhance cAMP formation in HEK cells expressing the human D1 or D5 DA receptor.
H, M and L represent D1R high and moderate expression, respectively. Each value represents mean ± s.e.m. of three experiments performed in duplicate.
| D1R | D5R | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| EC50 (nM) | Emax (% of DA) | EC50 (nM) | Emax (% of DA) | ||||||
|
| |||||||||
| H | M | L | H | M | L | ||||
|
|
|||||||||
| l-ICP | 39±9 | 263±68 | NA+ | 85±3* | 34±5** | - | 20±4 | 65±10* | |
| DA | 0.8±0.1 | 28±7 | 201±55 | 100 | 100 | 100 | 2±1 | 100 | |
P<0.05,
P<0.01, compared with the DA by Student’s t test.
NA: No activity up to 10 μM
DA D2-like receptors (D2, D3 and D4) are coupled to Gi proteins and [35S]GTPγS binding was used as their functional end point. l-ICP at 10 μM did not enhance [35S]GTPγS binding mediated by the D2, D3 or D4 receptor (Fig. 3A, 3B and 3C), indicating that it is not an agonist at these receptors. To examine its antagonist activities, we generated DA dose-response curves in the presence or absence of 1 μM l-ICP. l-ICP significantly shifted the DA-induced D2, D3 and D4 dose-response curves to the right without affecting maximal response (Fig. 3A, 3B and 3C), indicating that l-ICP is an antagonist at D2, D3 and D4 receptors.
Figure 3. l-ICP is an antagonist at dopamine D2, D3 and D4 receptors.
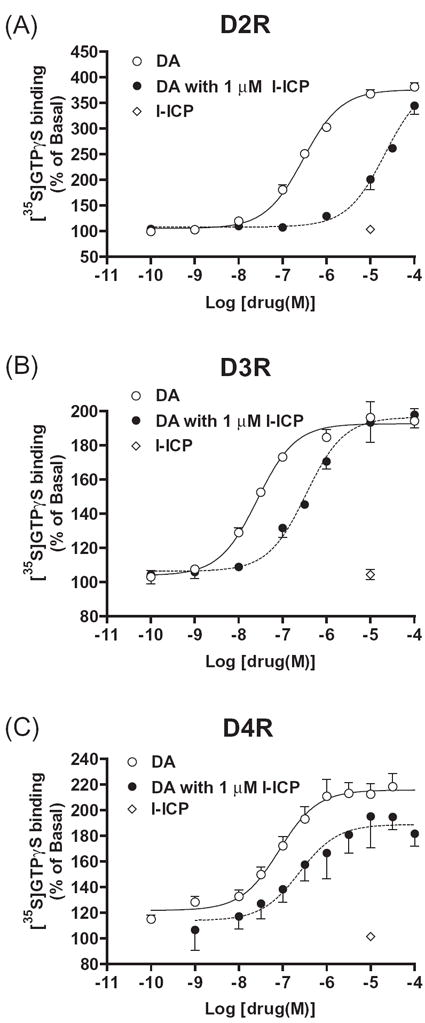
Membranes of HEK293 cells stably transfected with the human dopamine D2, D3 or D4 receptor were prepared. Dose-response curves of dopamine-induced increase in [35S]GTPγS binding were generated in the presence or absence of 1 μM l-ICP. Each value represents the mean ± s.e.m. of three independent determinations performed in duplicate.
3.4. Effect of l-ICP and l-THP on spontaneous locomotor activity
When placed in an activity chamber, all mice displayed gradual reductions in spontaneous locomotor activities during the 50-min habituation period. After habituation, their activities were so low that it was not possible to detect inhibition of locomotor activity. Therefore, to examine the inhibitory effects of l-ICP and l-THP on locomotion, animals did not undergo habituation. After administration of vehicle or 10 mg/kg l-ICP or l-THP (i.p.), they were immediately placed in activity chambers and locomotor activity was monitored for 2 h (Fig. 4). Areas under curves (AUCs) were calculated for 0-30 min and 30-100 min. In the first 30 minutes, 10 mg/kg of both l-ICP and l-THP significantly inhibited locomotor activity by about 55% compared with the vehicle-treated group (Fig. 4). In contrast, between 30 and 100 min, l-ICP had no effect while l-THP still inhibited the locomotor activity by about 60% compared with the vehicle-treated group (P<0.001, compared with that of l-ICP). These results indicate that l-ICP induces a shorter duration of locomotor inhibition than l-THP. It should be noted that as the mice did not undergo habituation to the activity chambers, l-ICP and l-THP inhibited novelty-induced locomotor activity rather than basal activity.
Figure 4. Comparison of the effects of l-ICP and l-THP on spontaneous locomotor activity in mice.
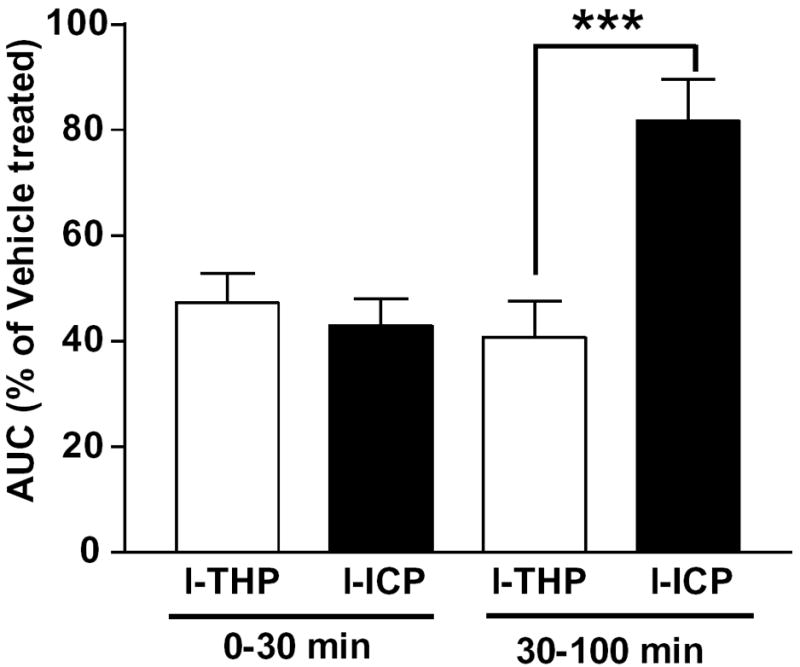
CD-1 mice were injected with vehicle, l-ICP or l-THP (10 mg/kg, i.p.), placed in activity chambers and activity counts were recorded for 120 min as described in Methods. Areas under curves (AUCs) of the total activity of l-ICP or l-THP from 0-30 and 30-100 min were shown as percentage (%) of the vehicle-treated group. Each value represents mean ± s.e.m (n=8 per group).
***P<0.001 by Student’s t test.
3.5. Effects of l-ICP on cocaine-induced increase in locomotor activities
Mice were placed in activity chambers for habituation for 50 minutes and divided randomly into eight groups. Mice were injected with vehicle or l-ICP (1, 3, 10 mg/kg, i.p.) followed by saline or cocaine (20 mg/kg, i.p) 10 min later. Locomotor activities were recorded (Fig. 5).
Figure 5. Effects of l-ICP on cocaine-induced hyperactivity in mice.
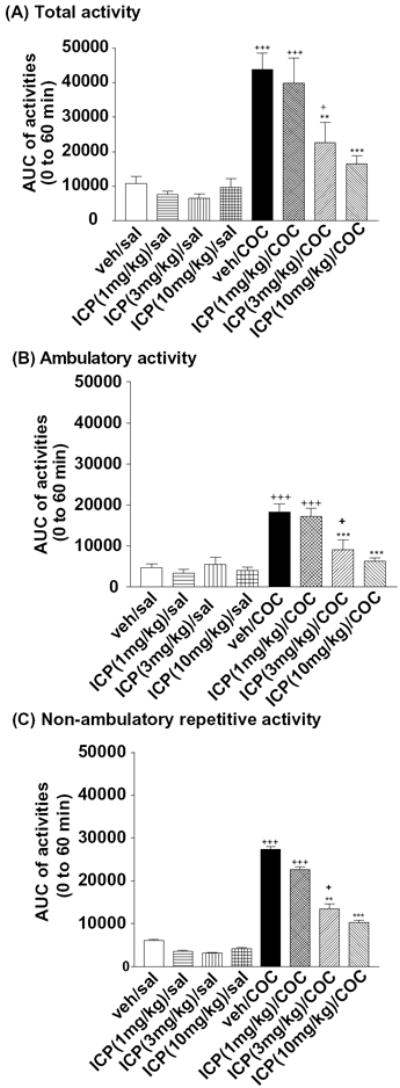
CD-1 mice were habituated to activity chambers for 50 min, pretreated with vehicle or l-ICP (1, 3 and 10 mg/kg, i.p.) for 10 min followed by saline or cocaine (20 mg/kg, i.p.) as described in Methods. Areas under curves (AUCs) were calculated for (A) total activity, (B) ambulatory activity and (C) repetitive non-ambulatory activity. Each value represents mean ± s.e.m (n=16 for veh/saline, n= 10 for all other groups).
**P<0.01, ***P<0.001, compared with the veh/cocaine group; +++P<0.001, +P<0.05 compared with veh/saline, both by two-way ANOVA followed by Bonferroni post hoc test.
3.5.1 Total activity
Two-way ANOVA of the total locomotor activity revealed a main effect of either treatment (cocaine vs saline) [F(1,81)=55.1, P<0.001] or l-ICP doses [F(3,81)=6.1, P<0.001], and a significant interaction between the treatment and l-ICP doses [F(3,81)=4.9, P=0.004]. Bonferroni post hoc comparisons indicated that (1) l-ICP pretreatment had no effect in mice treated with saline (P>0.05); (2) however, at 10 mg/kg and 3 mg/kg, l-ICP dose-dependently decreased cocaine-induced hyperactivities (vehicle vs 10 mg/kg, p<0.001; vehicle vs 3 mg/kg, p=0.002) (1 mg/kg vs 10 mg/kg, p=0.002; 1 mg/kg vs 3 mg/kg, p=0.044); (3) In comparison with saline, cocaine failed to induce hyperactivities in mice pretreated with l-ICP at 10 mg/kg (p=0.265), while it led to significant hyperactivities in mice pretreated with l-ICP at doses of 0 (vehicle) (p<0.001), 1 mg/kg (p<0.001) and 3 mg/kg (p=0.014) (Fig. 5A).
3.5.2 Ambulatory activity
Two-way ANOVA of the ambulatory activity revealed a main effect of either treatment (cocaine vs saline)[F(1,81)=52.2, P<0.001] or l-ICP doses [F(3,81)=6.9, P<0.001], and a significant interaction between the treatment and l-ICP doses [F(3,81)=7.7, P<0.001]. Bonferroni post hoc comparisons indicated that (1) l-ICP pretreatment (1, 3 or 10 mg/kg) had no effect in mice treated with saline (P>0.05); (2) l-ICP dose-dependently decreased cocaine-induced hyperactivities (vehicle vs 10 mg/kg, P<0.001; vehicle vs 3 mg/kg, P<0.001) (1 mg/kg vs 10 mg/kg, P<0.001; 1 mg/kg vs 3 mg/kg, P=0.008); (3) Compared to saline, cocaine failed to induce hyperactivities in mice pretreated with l-ICP at 10 mg/kg (P=0.754), while it led to significant hyperactivities in mice pretreated with l-ICP at doses of 0 (vehicle) (P<0.001), 1 mg/kg (p<0.001) and 3 mg/kg (p=0.024) (Fig. 5B).
3.5.3 Non-ambulatory repetitive activity
Two-way ANOVA of the repetitive activity revealed a main effect of either treatment (cocaine vs saline)[F(1,81)=54.0, P<0.001] or l-ICP doses [F(3,81)=6.1, P<0.001], and a significant interaction between the treatment and l-ICP doses [F(3,81)=3.8, P=0.014]. Bonferroni post hoc comparisons indicated that (1) l-ICP pretreatment (1, 3 or 10 mg/kg) had no effect in mice treated with saline (P>0.05); (2) l-ICP dose-dependently decreased cocaine-induced hyperactivities (vehicle vs 10 mg/kg, P<0.001; vehicle vs 3 mg/kg, P=0.002) (1 mg/kg vs 10 mg/kg, P=0.015); (3) In comparison with saline, cocaine failed to induce hyperactivities in mice pretreated with l-ICP at 10 mg/kg (P=0.129), while it led to significant hyperactivities in mice pretreated with l-ICP at doses of 0 (vehicle) (P<0.001), 1 mg/kg (p<0.001) and 3 mg/kg (p=0.016) (Fig. 5C).
Taken together, these results indicate that l-ICP at 3 and 10 mg/kg significantly reduced acute cocaine-induced hyperlocomotion.
We further evaluated if the locomotor inhibition induced by l-ICP was involved in reducing cocaine-induced hyperlocomotion. Since the effect of l-ICP on spontaneous locomotor activity lasted for only 30 min (see Fig. 4), animals were pretreated with l-ICP (10 mg/kg, i.p.) or vehicle for 30 min followed by cocaine (20 mg/kg, i.p.) and locomotor activities were measured for 2 h. With a 30-min pretreatment, l-ICP still significantly reduced cocaine-induced hyperlocomotion (data not shown), indicating that l-ICP-induced short-term locomotor inhibition does not contribute to its inhibitory effects on cocaine-induced hyperlocomotion.
3.6. Effects of l-ICP on development of cocaine-induced locomotor sensitization
The treatment timeline and test paradigm are shown in Fig. 6A. Mice were injected with l-ICP or vehicle followed by cocaine or saline 10 min later once daily for 5 days. Mice were tested on days 1 and 5. After withdrawal for 7 days, on day 13 mice were injected with cocaine and tested.
Two-way ANOVA of the total activity revealed a main effect of either treatment (cocaine vs saline) [F(5, 84=37.8, P<0.001] or pretreatment (ICP vs vehicle) [F(1, 84=25.3, P<0.001], and a significant interaction between the treatment and pretreatment [F(5, 84)=3.9, P=0.003]. Bonferroni post hoc test showed that on day 1, pretreatment with l-ICP (10 mg/kg, i.p.) for 10 min significantly reduced cocaine (20 mg/kg, i.p.)-induced hyperlocomotion (P=0.003, compared with veh/cocaine) (Fig. 6B), similar to the results shown in Fig. 5. On day 5, cocaine caused significantly greater hyperlocomotion than on day 1 (P=0.027, compared with day 1 veh/cocaine) (Fig. 6B), indicating locomotor sensitization. Pretreatment with l-ICP (10 mg/kg, i.p.) once a day significantly reduced cocaine-induced hyperlocomotion on day 5 (P<0.001, compared with veh/cocaine) (Fig. 6B). After 7 days of withdrawal, cocaine challenge on day 13 caused a greater increase in locomotor activity in mice treated repeatedly with cocaine than those treated with saline (P<0.01, compared with day 1 veh/cocaine), again indicating locomotor sensitization (Fig. 6C). l-ICP pretreatment during days 1-5 blocked cocaine-induced sensitization on day 13 (P<0.05 compared with veh/cocaine)(Fig. 6B and Fig. 6C). These results indicate that repeated l-ICP injection significantly reduced the development of sensitization after repeated cocaine injections and following withdrawal of cocaine.
Repeated l-ICP pretreatment (10 mg/kg, i.p.) in the absence of cocaine during days 1-5 did not affect locomotor activity (Fig. 6B) (ICP/sal group compared with veh/sal group, P=0.724). In addition, l-ICP administration did not affect hyperactivity induced by cocaine challenge on day 13 compared with the veh/saline group (P=0.713) (Fig. 6C), indicating that repeated l-ICP by itself did not affect locomotor activity.
3.7. Effect of l-ICP on the acquisition of cocaine-induced CPP
The CPP procedure was used to investigate if l-ICP affected the rewarding properties of cocaine and if l-ICP by itself induced rewarding or aversive effects. The timeline of the experiments is depicted in Fig. 7A. As shown in Fig. 7B, cocaine (20 mg/kg, i.p., once/day for 6 days) induced a significant CPP with a score of 312 ± 66 s (time spent on the cocaine-paired compartment minus time in the saline-paired compartment) in the post-test compared with the score of -47 ± 33 s in the pre-test (P<0.01). l-ICP pretreatment (10 mg/kg, i.p.,10 min prior to cocaine) once/day for 6 days significantly attenuated cocaine-induced CPP with the score of 65 ± 81 s in the post-test compared with that of veh/cocaine 312 ± 66 s (P<0.05). l-ICP by itself did not induce preference or aversion with the score of -137 ± 70 s in the post-test compared with the score of -24 ± 46 s in the pre-test (P>0.05), These results indicate that l-ICP blocks the acquisition of cocaine-induced CPP and therefore reduces rewarding properties of cocaine.
3.8. HPLC analysis of l-ICP and its possible metabolites in the mouse serum
It is not known whether the in vivo effects of l-ICP are due to l-ICP itself or its metabolites; therefore, we examined the identity of compounds in serum after l-ICP administration. Mice were injected with l-ICP (20 mg/kg, i.p.) and sacrificed 30 min later, when its pharmacological effects are fully manifested. Serum was collected and processed for HPLC analysis. The retention time for l-ICP (10 μg) was 18 min under the conditions used (Fig. 8C). Compared with chromatograms of the standard l-ICP (Fig. 8C) and blank serum (Fig. 8A), only l-ICP was identified in the sample serum (Fig. 8B), indicating that l-ICP is not metabolized to a significant extent in 30 min.
Figure 8. High performance liquid chromatography (HPLC) analysis of l-ICP and its possible metabolites in mouse serum.
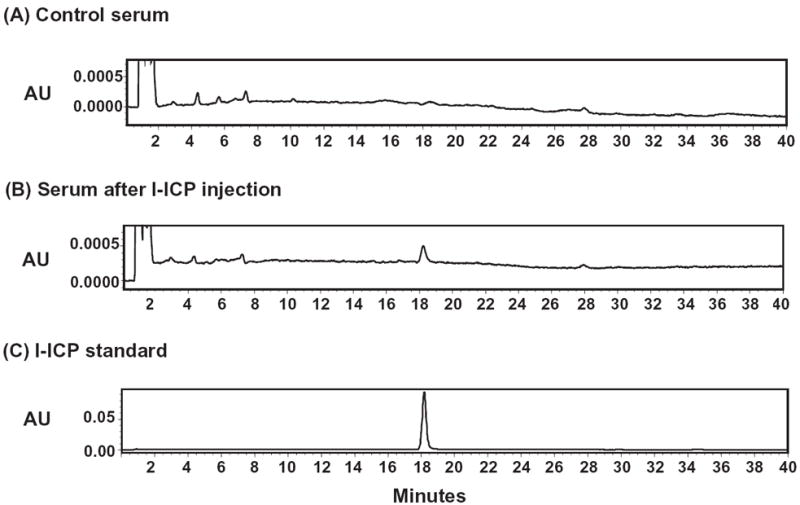
(A) Chromatogram of serum from mice injected with vehicle; (B) Chromatogram of serum from mice injected with l-ICP (20 mg/kg, i.p); (C) Chromatogram of l-ICP standard (10 μg). Male CD-1 mice were injected with vehicle or l-ICP (20 mg/kg, i.p), sacrificed 30 min later and blood was collected. Serum was processed and HPLC was performed using a C18 reverse-phase column and methanol (25-35%) / 0.05% triethylamine water as the mobile phase as described in Methods.
4. DISCUSSION
We have demonstrated that l-ICP is a high-affinity partial agonist of D1/D5 receptors and a moderate-affinity antagonist of D2/D3/D4 receptors in vitro. In vivo, it reduces acute cocaine-induced hyperlocomotion, attenuates development of repeated cocaine-promoted locomotor sensitization, and blocks acquisition of cocaine CPP in mice. Following i.p. injection of l-ICP, the main peak detected in the serum was l-ICP, indicating that it is not metabolized significantly in 30 min when its pharmacological effects are fully manifested. To the best of our knowledge, this is the first report on affinity and efficacy of l-ICP on the cloned DA receptors as well as its lack of affinity to many other pharmacological targets. In addition, this is the first report on its effects on cocaine-induced behaviors, which are likely due to actions on DA receptors. These results indicate that l-ICP may be promising as a novel therapeutic agent for cocaine addiction.
4.1. l-ICP is a D1/D5 partial agonist and D2/D3/D4 antagonist in vitro
l-ICP displays Ki values of 5-10 nM for D1 and D5 receptors and 37-77 nM for D2, D3 and D4 receptors. These Ki values of l-ICP are much lower than those reported by Xu et al. (1989). These researchers found that l-ICP bound to D1-like or D2-like dopamine receptors in calf striatal membranes with the Ki values of 73 nM and 1 μM, respectively. The discrepancy may be due to differences in binding conditions. We used HEK cells transfected with a cloned DA receptor, while they used calf striatal membranes. We used an antagonist, [3H]SCH23390, for D1/D5 binding, but they used an agonist, [3H]dopamine, for binding to D1-like receptors.
The efficacy of l-ICP at the D1 receptor varied with receptor level. At 5.0 and 1.4 pmol/mg of D1 receptor, its Emax values were 85% and 34% of that of DA, respectively, whereas at 0.093 pmol/mg, l-ICP was inactive. The potency of l-ICP is similarly affected by receptor level. At 5.0 and 1.4 pmol/mg of D1 receptor, its EC50 values are 39 nM and 263 nM, respectively. These results indicate that l-ICP is a partial agonist (Kenakin, 1997).
l-Stepholidine, a di-demethylated analog at C2 and C10 of l-THP, has dual D1-like receptor agonist and D2-like receptor antagonist actions [for a review, see (Mo et al., 2007)]. Our finding is the first to show that a mono-demethylated analog of l-THP has similar dual effects on D1-like and D2-like receptors.
4.2. l-THP
Although at least some of the in vivo pharmacological effects of l-THP appear to be mediated by DA receptors (Jin, 1987), we have found that its affinity for DA receptors in vitro is not high. Its Ki values are ~150 and ~300 nM for D1 and D5 receptors, respectively, and >1 μM for D2, D3 and D4 receptors. Our results are the first characterization of its binding affinity for the five cloned dopamine receptors. Xu et al. (1989) reported that the Ki values for D1- and D2-like receptors in bovine striatal membranes were 1.1 μM and 0.85 μM, respectively. Mantsch et al. (2007) reported that l-THP at 10 μM inhibited binding to D1, D2, and D3 by 97, 87, and 83%, respectively; however, Ki values were not determined. Since l-THP did not have high affinity for any of the five (D1-D5) DA receptors, its in vivo effects may be due to its metabolites. This possibility is currently being investigated.
4.3. l-ICP produces its effect on cocaine-induced behaviors likely via its actions at the dopamine receptors
Our HPLC analysis showed that l-ICP was the major peak in serum 30 minutes following injection of l-ICP (20 mg/kg, i.p.), indicating that l-ICP is not metabolized significantly and is likely to be the active compound in the central nervous system. l-Stepholidine penetrates the blood-brain barrier well (Sun et al., 2009). It is likely that l-ICP, which has C10-OCH3 instead of the C10-OH found in l-stepholidine, also has good blood-brain barrier penetration.
We found that l-ICP reduced acute cocaine-induced hyperlocomotion and repeated cocaine-promoted development of sensitization and acquisition of the CPP in mice. Based on the finding that it has significant affinity only for dopamine receptors, we believe that l-ICP produces its effects on cocaine-induced behaviors via dopamine receptors, likely as a partial agonist at D1-like (D1, D5) receptors and an antagonist at D2-like (D2, D3 and D4) receptors.
Our results are consistent with the finding that the D1 receptor is critical for locomotor sensitization and positive rewarding effects of cocaine (Xu et al., 1994; Drago et al., 1996; Tzschentke, 1998; Xu et al., 2000; Berglind et al., 2006; Caine et al., 2007; Chen and Xu, 2010). Cocaine-induced CPP was blocked by D1 receptor antagonists (Tzschentke, 1998) and completely eliminated in D1R knockout mice (Xu et al., 2000; Karlsson et al., 2008; Chen and Xu, 2010). In addition, in D1R knockout mice, acute cocaine failed to induce hyperlocomotion and repeated cocaine did not promote locomotor sensitization (Xu et al., 1994; Drago et al., 1996; Karasinska et al., 2005). The D5 receptor may have only a limited role in cocaine-induced acute or sensitized locomotor hyperactivity as well as CPP since these behaviors were not eliminated in D5R knockout mice (Elliot et al., 2003; Karlsson et al., 2008).
l-ICP is a D1 partial agonist in stimulation of AC activity in vitro, but in vivo it did not cause CPP or hyperlocomotion and it inhibited cocaine-induced hyperlocomotion, behavioral sensitization and CPP. These results are in accordance with the previous findings that D1 partial agonists, as determined by AC stimulation in vitro, do not consistently cause hyperlocomotion and CPP in vivo (Abrahams et al., 1998; Katz et al., 1999; Desai et al., 2005). The D1 partial agonist SKF 77434 did not increase locomotion, nor did it cause CPP (Abrahams et al., 1998; Katz et al., 1999). Desai et al. (2005) studied three D1 partial agonists and found that SKF 38393 produced limited changes in locomotor activity, whereas SKF 75670 and SKF 77434 produced locomotor stimulation similar to full agonists, indicating that there is no close correlation between locomotor activation and AC stimulation. In addition, the D1 partial agonists SKF77434 and SKF38393 with ~50% efficacy of a full agonist produced dose-related decreases in cocaine-induced hyperlocomotion (Katz et al., 1999). Thus in vivo efficacy of the D1 partial agonists (determined in vitro), including l-ICP, can not be predicted from the results of in vitro assays because there may be other factors involved, such as their distribution, metabolism and penetration into the brain and D1 receptor levels at their action sites.
Partial agonists have been shown to be beneficial in treating drug abuse (Johnson and McCagh, 2000; Hays and Ebbert, 2008). Buprenorphine, a high affinity/low efficacy partial agonist at the mu opioid receptor, is used clinically for treatment of heroin addiction (Johnson and McCagh, 2000). Varenicline (Chantix), a partial agonist for α4β2 nicotinic receptor, is used to facilitate smoking cessation (Hays and Ebbert, 2008). D1 receptor partial agonists attenuate cocaine self-administration in animal models at the doses that do not disrupt food-maintained performance (Katz and Witkin, 1992; Caine et al., 2000). Since we show here that l-ICP is also a D1 partial agonist, possibly with D2 antagonist activity, it may be useful for treatment of cocaine abuse.
4.4. Antagonism at D2-like receptor by l-ICP may be involved
Previous studies have shown that D2-like receptors (D2, D3 and D4) are involved in the induction of hyperlocomotion and sensitization (Xu et al., 1997; Chausmer and Katz, 2001, 2002; Karasinska et al., 2005; Chen and Xu, 2010). We have found that l-ICP is a D2/D3/D4 antagonist with moderate affinity in vitro (Ki values ~37 to 78 nM), which is ~4 to 15-fold lower than those to D1-like receptors (Table 1). At the present time, occupancy of D1-like and D2-like receptors in the brain by l-ICP after injection is not known. It is likely that antagonism at D2-like receptors may also contribute to the effects of l-ICP observed in vivo.
4.5. l-ICP produced sedation-like effects with a shorter duration than l-THP
In mice that did not undergo habituation to locomotor chambers, l-ICP at 10 mg/kg inhibited spontaneous locomotor activity in the first 30 min by 57%, but there was no significant inhibition after 30 min (Fig. 4). In contrast, in mice that underwent habituation, l-ICP at 1, 3 or 10 mg/kg did not reduce spontaneous locomotor activity (Fig. 5). These results indicate that l-ICP inhibits novel environment-induced locomotor activation, which is likely dopamine-mediated.
In non-habituated mice, l-ICP at 10 mg/kg inhibited locomotor activity with a shorter duration of action than l-THP at 10 mg/kg. The shorter duration of action is not an intrinsic pharmacokinetic property of l-ICP because l-ICP attenuated cocaine-induced hyperlocomotion for at least 60 min. Therefore, it is more likely due to the pharmacodynamic properties of l-ICP. In addition, this short-term locomotor inhibition or sedation-like effect of l-ICP does not appear to contribute significantly to its inhibition of cocaine-induced behaviors. It is noteworthy that the dose used (10 mg/kg) is pharmacologically relevant for both compounds. For l-THP, 10 mg/kg is in the dose range for attenuation of cocaine self-administration and discrimination as well as cocaine-induced brain stimulation reward and reinstatement of drug seeking (Xi et al., 2007; Mantsch et al., 2007, 2010; Figueroa-Guzman et al., 2011). Similarly, in this study l-ICP at 10 mg/kg reduced cocaine-induced behavioral sensitization and CPP.
Our finding that 10 mg/kg l-THP inhibited locomotor activity is similar to those of Mantsch et al. (2007) and Xi et al. (2007). Pretreatment with l-THP (3.75 or 7.5 mg/kg, i.p.) for 60 min significantly reduced locomotor activity by ~40% or ~90% over 2 h in rats (Mantsch et al., 2007). Xi et al. (2007) found that l-THP, at 10 mg/kg, but not 3 mg/kg, reduced locomotion by ~80% over 2 h.
4.6. Conclusion
Among more than 40 pharmacological targets screened, l-ICP displayed high affinity for only dopamine receptors. We showed that since l-ICP inhibited cocaine-induced behaviors, which is most likely due to its partial agonist activity at the D1 receptors and antagonist activity at the D2 receptors, it may have the potential for treatment to cocaine addiction. Its effects on cocaine self-administration and reinstatement of cocaine seeking following extinction will be investigated.
Supplementary Material
Acknowledgments
We thank Drs. Jonathan Javitch, Bryan Roth and John Edward Jones for providing HEK293 cells transfected with the human DA D1, D2, D3, D4 and D5 receptors. In addition, we thank Dr. Bryan Roth, the director of the PDSP supported by National Institute on Mental Health, for screening l-THP and l-ICP against more than 40 receptors, ion channels, and transporters. We also wish to thank Kelly DiMattio and Dr. Barrie Ashby for editing the manuscript.
Role of Funding Sources
Funding for this study was provided by the NIH grants R01 DA17302 and P30 DA13429 (to LLC) and P01-AT-002038 (to DYL) and R01 AT006899 (to LLC and DYL). The NIH had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the manuscript for publication.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
Drs. Xu, Huang, Wang, Ma, Chiu, Rasakham, Unterwald, Lee and Liu-Chen participated in research design and experimental protocols. Drs Xu, Huang, Wang, Rasakham and Ma conducted the experiments; Drs. Ma and Lee contributed new reagents; Drs Xu, Wang, Rasakham and Ma performed data analysis; Drs Xu and Liu-Chen contributed to the writing of the manuscript. All authors have approved the submission of the manuscript.
Conflict of Interest
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Rutherford JD, Mallet PE, Beninger RJ. Place conditioning with the dopamine D1-like receptor agonist SKF 82958 but not SKF 81297 or SKF 77434. Eur J Pharmacol. 1998;343:111–118. doi: 10.1016/s0014-2999(97)01531-8. [DOI] [PubMed] [Google Scholar]
- Barton AC, Black LE, Sibley DR. Agonist-induced desensitization of D2 dopamine receptors in human Y-79 retinoblastoma cells. Mol Pharmacol. 1991;39:650–658. [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Effects of dopamine D(1-like) and D(2-like) agonists on cocaine self-administration in rhesus monkeys: rapid assessment of cocaine dose-effect functions. Psychopharmacology (Berl) 2000;148:41–51. doi: 10.1007/s002130050023. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausmer AL, Katz JL. The role of D2-like dopamine receptors in the locomotor stimulant effects of cocaine in mice. Psychopharmacology (Berl) 2001;155:69–77. doi: 10.1007/s002130000668. [DOI] [PubMed] [Google Scholar]
- Chausmer AL, Katz JL. Comparison of interactions of D1-like agonists, SKF 81297, SKF 82958 and A-77636, with cocaine: locomotor activity and drug discrimination studies in rodents. Psychopharmacology (Berl) 2002;159:145–153. doi: 10.1007/s002130100896. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu M. Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking. J Neurochem. 2010;114:530–541. doi: 10.1111/j.1471-4159.2010.06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Terry P, Katz JL. A comparison of the locomotor stimulant effects of D1-like receptor agonists in mice. Pharmacol Biochem Behav. 2005;81:843–848. doi: 10.1016/j.pbb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Westphal H, Steiner H. D1 dopamine receptor-deficient mouse: cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience. 1996;74:813–823. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- Elliot EE, Sibley DR, Katz JL. Locomotor and discriminative-stimulus effects of cocaine in dopamine D5 receptor knockout mice. Psychopharmacology (Berl) 2003;169:161–168. doi: 10.1007/s00213-003-1494-y. [DOI] [PubMed] [Google Scholar]
- Figueroa-Guzman Y, Mueller C, Vranjkovic O, Wisniewski S, Yang Z, Li SJ, Bohr C, Graf EN, Baker DA, Mantsch JR. Oral administration of levo-tetrahydropalmatine attenuates reinstatement of extinguished cocaine seeking by cocaine, stress or drug-associated cues in rats. Drug Alcohol Depend. 2011;116:72–79. doi: 10.1016/j.drugalcdep.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Zhang YA, Bouvier C, Zhou QY, Johnson RA, Allen L, Buck K, Bunzow JR, Salon J, Civelli O. Multiple human D5 dopamine receptor genes: a functional receptor and two pseudogenes. Proc Natl Acad Sci U S A. 1991;88:9175–9179. doi: 10.1073/pnas.88.20.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JT, Ebbert JO. Varenicline for tobacco dependence. N Engl J Med. 2008;359:2018–2024. doi: 10.1056/NEJMct0800146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu B, Kin KC. Pharmacological study of tetrahydropalmatine and its analogs. A new type of central depressants. Arch Int Pharmacodyn Ther. 1962;139:318–327. [PubMed] [Google Scholar]
- Hsu B, Kin KC. Some pharmacological properties of corydalis B (tetrahydropalmatine) and its related compounds. Sci Sin. 1964;13:601–609. [PubMed] [Google Scholar]
- Huang EY, Liu TC, Tao PL. Co-administration of dextromethorphan with morphine attenuates morphine rewarding effect and related dopamine releases at the nucleus accumbens. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:386–392. doi: 10.1007/s00210-003-0803-7. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen L-Y. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- Huang P, Liu-Chen LY, Unterwald EM, Cowan A. Hyperlocomotion and paw tremors are two highly quantifiable signs of SR141716-precipitated withdrawal from delta9-tetrahydrocannabinol in C57BL/6 mice. Neurosci Lett. 2009;465:66–70. doi: 10.1016/j.neulet.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M, Unterwald EM. Intra-accumbens pertussis toxin sensitizes rats to the locomotor activating effects of a single cocaine challenge. Brain Res. 2003;965:100–107. doi: 10.1016/s0006-8993(02)04142-2. [DOI] [PubMed] [Google Scholar]
- Jin GZ. l-Tetrahydropalmatine and its analogues as new dopamine receptor antagonists. Trends Pharmacol Sci. 1987;8:81–82. [Google Scholar]
- Johnson RE, McCagh JC. Buprenorphine and naloxone for heroin dependence. Curr Psychiatry Rep. 2000;2:519–526. doi: 10.1007/s11920-000-0012-8. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, Cheng R, O’Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci. 2005;22:1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 2008;200:117–127. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Myers KA, Mitkus RJ, Chider M. Behavioral effects of cocaine: interactions with D1 dopaminergic antagonists and agonists in mice and squirrel monkeys. J Pharmacol Exp Ther. 1999;291:265–279. [PubMed] [Google Scholar]
- Katz JL, Witkin JM. Selective effects of the D1 dopamine receptor agonist, SKF 38393, on behavior maintained by cocaine injection in squirrel monkeys. Psychopharmacology (Berl) 1992;109:241–244. doi: 10.1007/BF02245508. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic Analysis of Drug-Receptor Interaction. Lippincott Williams & Wilkins; New York: 1997. [Google Scholar]
- Kim OJ, Ariano MA, Lazzarini RA, Levine MS, Sibley DR. Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J Neurosci. 2002;22:5920–5930. doi: 10.1523/JNEUROSCI.22-14-05920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhu J, Chen C, Chen YW, DeRiel JK, Ashby B, Liu-Chen L-Y. Molecular cloning and expression of a rat kappa opioid receptor. Biochem J. 1993;295(Pt. 3):629–633. doi: 10.1042/bj2950629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Penetar D, Berko J, Vicens L, Palmer C, Mallya G, Macklin EA, Lee DY. An extract of the Chinese herbal root kudzu reduces alcohol drinking by heavy drinkers in a naturalistic setting. Alcohol Clin Exp Res. 2005;29:756–762. doi: 10.1097/01.alc.0000163499.64347.92. [DOI] [PubMed] [Google Scholar]
- Ma ZZ, Xu W, Jensen NH, Roth BL, Liu-Chen LY, Lee DY. Isoquinoline alkaloids isolated from Corydalis yanhusuo and their binding affinities at the dopamine D1 receptor. Molecules. 2008;13:2303–2312. doi: 10.3390/molecules13092303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Li SJ, Risinger R, Awad S, Katz E, Baker DA, Yang Z. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology (Berl) 2007;192:581–591. doi: 10.1007/s00213-007-0754-7. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Wisniewski S, Vranjkovic O, Peters C, Becker A, Valentine A, Li SJ, Baker DA, Yang Z. Levo-tetrahydropalmatine attenuates cocaine self-administration under a progressive-ratio schedule and cocaine discrimination in rats. Pharmacol Biochem Behav. 2010;97:310–316. doi: 10.1016/j.pbb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister G, Knowles MR, Ward-Booth SM, Sinclair HA, Patel S, Marwood R, Emms F, Patel S, Smith A, Seabrook GR. Functional coupling of human D2, D3, and D4 dopamine receptors in HEK293 cells. J Recept Signal Transduct Res. 1995;15:267–281. doi: 10.3109/10799899509045220. [DOI] [PubMed] [Google Scholar]
- Miller JS, Tallarida RJ, Unterwald EM. Cocaine-induced hyperactivity and sensitization are dependent on GSK3. Neuropharmacol. 2009;56:1116–1123. doi: 10.1016/j.neuropharm.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min X, Lee DT, Jinhua X, Wenjun D, Li C, Bin D, Pingxiang D, Wingho L, Xiaoyin T, Xiaohui Z. A database on treating drug addiction with traditional Chinese medicine. Addiction. 2007;102:282–288. doi: 10.1111/j.1360-0443.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- Mo J, Guo Y, Yang YS, Shen JS, Jin GZ, Zhen X. Recent developments in studies of l-stepholidine and its analogs: chemistry, pharmacology and clinical implications. Curr Med Chem. 2007;14:2996–3002. doi: 10.2174/092986707782794050. [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Jr, Mahan LC, McVittie LD, Gerfen CR, Sibley DR. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci U S A. 1990;87:6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prus AJ, James JR, Rosecrans JA. Conditioned place preference. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. Taylor and Francis Group; Boca Raton, FL: 2009. pp. 59–76. [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sun Y, Dai J, Hu Z, Du F, Niu W, Wang F, Liu F, Jin G, Li C. Oral bioavailability and brain penetration of (-)-stepholidine, a tetrahydroprotoberberine agonist at dopamine D(1) and antagonist at D(2) receptors, in rats. Br J Pharmacol. 2009;158:1302–1312. doi: 10.1111/j.1476-5381.2009.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Zhao D, Zhao C, Cubells JF. Opiate addiction in China: current situation and treatments. Addiction. 2006;101:657–665. doi: 10.1111/j.1360-0443.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- Todd RD, Khurana TS, Sajovic P, Stone KR, O’Malley KL. Cloning of ligand-specific cell lines via gene transfer: identification of a D2 dopamine receptor subtype. Proc Natl Acad Sci U S A. 1989;86:10134–10138. doi: 10.1073/pnas.86.24.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Wang JB, Mantsch JR. l-tetrahydropalamatine: a potential new medication for the treatment of cocaine addiction. Future Med Chem. 2012;4:177–186. doi: 10.4155/fmc.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev. 2008;1:303–327. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Yang Z, Li SJ, Li X, Dillon C, Peng XQ, Spiller K, Gardner EL. Levo-tetrahydropalmatine inhibits cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropharmacol. 2007;53:771–782. doi: 10.1016/j.neuropharm.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- Xu SX, Yu LP, Han YR, Chen Y, Jin GZ. Effects of tetrahydroprotoberberines on dopamine receptor subtypes in brain. Zhongguo Yao Li Xue Bao. 1989;10:104–110. [PubMed] [Google Scholar]
- Yang Z, Shao YC, Li SJ, Qi JL, Zhang MJ, Hao W, Jin GZ. Medication of l-tetrahydropalmatine significantly ameliorates opiate craving and increases the abstinence rate in heroin users: a pilot study. Acta Pharmacol Sin. 2008;29:781–788. doi: 10.1111/j.1745-7254.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo L-Y, Chen C, Liu-Chen L-Y. Activation of the cloned human κ opioid receptor by agonists enhances [35S]GTPγS binding to membranes: determination of potencies and efficacies of ligands. J Pharmacol Exp Ther. 1997;282:676–684. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


