Abstract
Objective
While the standard has been to define motor threshold (MT) using EMG to measure motor cortex response to transcranial magnetic stimulation (TMS), another method of determining MT using visual observation of muscle twitch (OM-MT) has emerged in clinical and research use. We compared these two methods for determining MT.
Methods
Left motor cortex MTs were found in 20 healthy subjects. Employing the commonly-used relative frequency procedure and beginning from a clearly suprathreshold intensity, two raters used motor evoked potentials and finger movements respectively to determine EMG-MT and OM-MT.
Results
OM-MT was 11.3% higher than EMG-MT (p<0.001), ranging from 0-27.8%. In eight subjects, OM-MT was more than 10% higher than EMG-MT, with two greater than 25%.
Conclusions
These findings suggest using OM yields significantly higher MTs than EMG, and may lead to unsafe TMS in some individuals. In more than half of the subjects in the present study, use of their OM-MT for typical rTMS treatment of depression would have resulted in stimulation beyond safety limits.
Significance
For applications that involve stimulation near established safety limits and in the presence of factors that could elevate risk such as concomitant medications, EMG-MT is advisable, given that safety guidelines for TMS parameters were based on EMG-MT.
Keywords: transcranial magnetic stimulation, TMS, motor threshold, electromyography, EMG, safety
INTRODUCTION
Transcranial magnetic stimulation (TMS) is gaining popularity as a therapeutic tool for alleviating depression, with great potential for use in other illnesses as well, and as an experimental method for establishing causal brain-behavior relationships. TMS dosage is generally set relative to the minimum intensity of the magnetic field necessary to elicit a reliable response in a target muscle when stimulating the motor cortex of an individual, the motor threshold (MT), with the assumption made that excitability in non-motor cortex is similar to that of motor cortex, or at least correlated. MT has become the standard for determining TMS dose due to its relationship with safety in regard to the possibility of inadvertent seizure, and to its efficacy and reproducibility in stimulating cortex.
The International Federation of Clinical Neurophysiology (IFCN) defined MT in a resting muscle (Resting MT; RMT) through the use of electromyography (EMG) in two seminal publications, first using an ascending relative frequency method to find the “level which induces reliable (usually around 100 μV) motor evoked potentials (MEPs) in 50% of 10-20 consecutive stimuli” (Rossini et al., 1994) and later using a descending relative frequency method to find a level at which an MEP of at least 50 μV occurs in at least 50% of 10 to 20 consecutive trials (Rothwell et al., 1999). The use of EMG to determine MT (EMG-MT) has the inherent advantage of providing a quantitative measure of muscle response. More important, MT based on EMG has been the basis for establishing IFCN guidelines for the safe use of TMS (Wassermann, 1997; Rossi et al., 2009). Rossi et al. (2009) reviewed the sixteen cases known at the time of inadvertent seizures, the most severe acute adverse effect caused by TMS, and reaffirmed the use of the limits on TMS parameters, established in relation to EMG-MT (Wassermann, 1997), in preventing inadvertent seizures.
However, a second method for determining MT has seen increasing use, in which EMG is not used, and instead the threshold estimation is performed by counting visually-detected movements of the target muscle (observed movement: OM-MT; Pridmore et al., 1998). This method has the advantage of being more convenient to perform, and simpler in a clinical setting since no expertise in EMG is necessary. There is currently no clear agreement among TMS users regarding the two methods (Anderson and George, 2009). A recent international consensus conference on TMS safety did lead to a general agreement that EMG-MT is more precise and that OM-MT may overestimate MT, but only 80% of participants endorsed these ideas and full consensus was not reached (Rossi et al., 2009). One recent study presented evidence that endorsed OM-MT as a reliable method of determining MT (Varnava et al., 2011).
Only four studies have been published in which a direct comparison of the two methods of determining MT has been made, with varying results (Balslev et al., 2007; Conforto et al., 2004; Hanajima et al., 2007; Pridmore et al., 1998). In Pridmore et al. (1998), six subjects were tested, and in five of those six, OM-MT was lower than EMG-MT. In two others, EMG-MT was slightly lower than OM-MT, on average by less than 2% of total stimulator output (Balslev et al., 2007 (4 subjects); Conforto et al., 2004 (14 subjects)). In the fourth study testing ten subjects, EMG-MT was much lower than OM-MT, on average by 6% of total stimulator output (Hanajima et al., 2007). One difficulty in comparison is that in two of these studies EMG was measured from a particular muscle, yet OM was performed counting any motion from the entire hand and wrist (Conforto et al., 2004; Pridmore et al., 1998).
In examining the four studies comparing the two methods for estimating MT, it is concerning that in two of them the reported data indicated that use of OM-MT to establish subsequent TMS dosage could lead to adverse outcomes in some individuals. In Conforto et al. (2004), one subject had an OM-MT much higher than his or her EMG-MT. The difference was 14% of stimulator output, which corresponded (using the group mean EMG-MT, as no individual MTs were provided) to an OM-MT 132% of EMG-MT. In Hanajima et al. (2007), while individual MTs were not reported, on average the OM-MTs were 113% higher than EMG-MTs, presumably with some individuals having even higher percentage differences. Because the parameters for safe use of TMS were based on EMG-MTs (Wassermann, 1998; Rossi et al., 2009), the use of such OM-MTs to establish dosage in subsequent repetitive TMS sessions could result in overstimulation. Overestimation of MT leads to stimulation at a higher intensities above true MT. In single pulse TMS studies, this results in decreased focality. In rTMS studies or clinical settings, this results in stimulation trains that exceed established safety limits and could lead to accidental seizures. For example, if the OM-MT of the subject in Conforto et al. (2004) whose threshold was 32% higher than his/her EMG-MT was used to establish the TMS dose for a typical depression treatment, the treatment parameters used would exceed safe limits. Typically, the device might be set at 100% MT, applying 4 s trains at 10 Hz. As consensus safe limits are based on EMG-MT, the patient would be receiving an intensity of over 130% EMG-MT, where safe train duration is actually 2.9 s (Rossi et al., 2009; Table 5), and would thus be receiving an unsafe, potentially seizure-inducing dose.
With these considerations in mind, we found OM-MT and EMG-MT for each of a larger group of twenty subjects and focused our attention on individual variability in OM-MT and EMG-MT differences, to determine how well OM-MT estimates EMG-MT, and whether OM-MT is adequate to prevent stimulation at potentially unsafe levels. It should be noted that while the most recent IFCN consensus guidelines included other methods for finding MT such as adaptive staircases and the two-threshold method, and recommended using adaptive staircasing where possible (Groppa et al., 2012), we used the traditional relative frequency method (Rothwell et al., 1999) in the present study, as it is still the most commonly used method of estimating MT in both clinical and research situations.
METHODS
Subjects
Twenty healthy adult volunteers (8 female, mean age 40 ±13 years, range 19-62) were recruited, gave written informed consent, and were paid for participation in one of several healthy control TMS studies, approved by the New York State Psychiatric Institute Investigational Review Board. Subjects were excluded if they were over the age of 65, had a history of any Axis I psychiatric disorder including substance abuse or dependence as determined by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Non-Patient Edition (SCID-I/NP; First et. al, 1998) or history of any neurological disease or other illness that would present a risk with TMS. All subjects were screened with physical and neurological examinations, blood and urine testing, urine drug screens, and pregnancy tests for women of childbearing capacity.
Transcranial Magnetic Stimulation (TMS)
This study used a Magstim 200 TMS device (Magstim Co., Whitland, Wales, UK) and a 70 mm figure-8 coil. Consecutive stimuli were separated by 7-10 s to avoid carry-over effects. Stimulation intensity was initially set at 48% of the maximum device intensity, a suprathreshold level for which most individuals. Maintenance of optimal coil orientation was assisted by Brainsight computerized frameless stereotaxy system (Rogue Research, Montreal, Canada). This system uses an infrared camera to monitor the positions of tracking devices attached to the TMS coil and to the subject's head. The relative positions of the coil and the target site(s) on the subject's head were tracked in real time, and allowed the coil to be placed and maintained to within 1 mm of the site chosen during MT determination.
EMG
MEPs were recorded from the right first dorsal interosseous (FDI) muscle with Ag-AgCl KittyCat electrodes (Covidien Corp, Mansfield, MA, USA). Electrode recordings were amplified by James Long (Caroga Lake, NY, USA) Bioamp-4, with gain set to 1000x, bandpass filtering 30-1000 Hz. The signal was then sampled at 5 kHz on a Tektronix TDS-1002 oscilloscope (Beaverton, OR).
Motor Threshold Determination
Motor thresholds were determined visually and using electromyography (EMG) of the right FDI using the relative frequency method (Rothwell et al., 1999), using a 50% frequency criterion to determine MT and beginning at a clearly suprathreshold intensity. The motor threshold using EMG was defined as the lowest setting of TMS machine power at which ≥5 out of 10 MEPs were ≥50μV peak to peak, and for the OM method, it was defined as the lowest setting at which ≥5 out of 10 stimuli resulted in any observable movement of the index finger. The optimal position for stimulating the FDI was defined as the coil position eliciting the largest MEP, with the coil rotated 45° from the sagittal plane so that the induced current would be in the optimal direction for stimulating the motor strip.
An optimal spot was found by moving the coil in 0.5-1 cm increments on the scalp, starting from approximately 4cm lateral and one cm anterior from the vertex of the head, until a movement in the index finger was observed. Using this location as an origin, pulses were applied 0.5 cm away in four cardinal directions using frameless stereotaxy (Brainsight: Rogue Research Inc., Montreal, Canada). On average, three pulses were used at each test location. If no larger EMG resulted at any of these test sites, the origin was considered the optimal site. If one was larger in EMG, it was considered the new origin (as long as the size of index finger movement was not diminished there), and the tests were repeated. This procedure was repeated until an optimal site was found and recorded using frameless stereotaxy and Brainsight software.
At the optimal site, a descending relative frequency method was then utilized to find the MTs. Starting at the intensity at which the optimal site selection had been determined, up to 10 stimuli were applied. One rater, who also held the TMS coil and applied the stimuli, counted the number of times the EMG response exceeded 50uVp-p as recorded on an oscilloscope. A second rater sat near the subject's right hand, watching the index finger carefully for any sign of a twitch occurring with the TMS click, counting each occurrence. Once both raters had counted five positive occurrences, the device intensity was reduced by 2%. If one rater did not count five positive occurrences after ten stimuli, he recorded the MT as 2% above the level just used, while the other rater continued with descending intensities until reaching completion. Trials which had an observable hand or arm movement immediately prior to the stimulus or with peak-to-peak EMG data greater than 50 microvolts in the previous 500 ms before the TMS pulse disqualified that stimulus, as stimuli preceding the test stimuli can facilitate the MEP (Rothwell et al., 1999). OM and EMG raters were randomly assigned amongst a pool of four investigators.
RESULTS
The TMS stimulation was well tolerated and no subjects reported side effects during or after the motor threshold determination procedures.
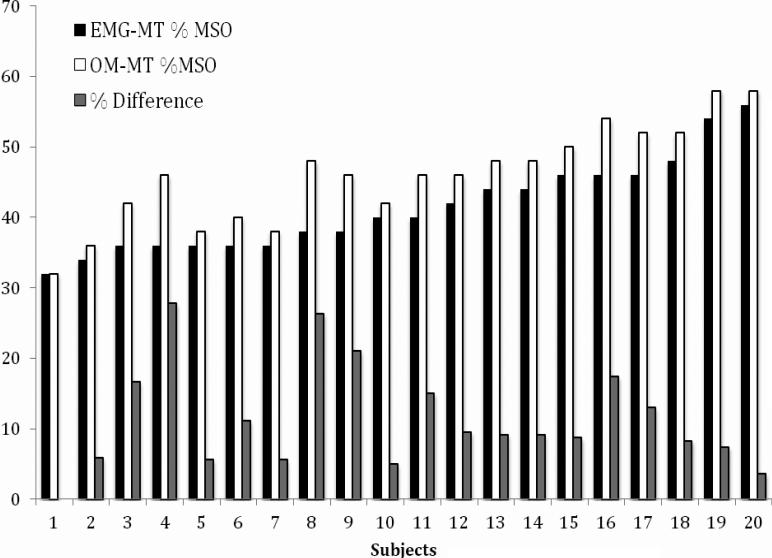
Figure 1 and Table 1 show the results for each subject. Figure 1 shows the EMG-MT, OM-MT, and the % difference of OM from EMG MT for each subject, and is ordered from left to right by increasing EMG-MT. As is evident from the figure, the EMG-MT and the amount that OM-MT differs from it was not correlated (r= 0.17, p=0.46), and thus there was no simple scaling difference between EMG and OM MT. In Table 1, the second and third columns list the raw EMG-MT and OM-MT (in % of maximum device output). In terms of raw device intensity level, the mean motor thresholds as a percentage of total stimulator output were 46.0 (SD = 7.0) for OM-MT and 41.4 (SD = 6.5) for EMG-MT. A paired t-test revealed there was a significant difference between the two means (t(19) = 7.45, p < 0.001).
Figure 1.
EMG-MT and OM-MT (as %MSO: % maximum stimulator output) and the % difference of the OM from the EMG MT for each of the 20 subjects.
Table 1.
For each subject, the MTs for the two methods and their difference (in percentage of maximum device intensity), the percentage of EMG-MT by which the OM value exceeded the EMG, the percentage of EMG-MT if the device is set at 110% OM-MT, and the safe duration of 10 Hz rTMS trains if intensity is set using OM-MT.
| Subject | OM-MT (% device max) | EMG-MT (% device max) | Difference (% device max) | Difference (% EMG-MT) | %EMG-MT at 110% OM-MT | Safe duration at 10 Hz (sec)* |
|---|---|---|---|---|---|---|
| 1 | 50 | 46 | 4 | 8.7 | 120 | 3.2 |
| 2 | 48 | 38 | 10 | 26.3 | 139 | 2.2 |
| 3 | 46 | 36 | 10 | 27.8 | 141 | 2.2 |
| 4 | 48 | 44 | 4 | 9.1 | 120 | 4.2 |
| 5 | 46 | 38 | 8 | 21.1 | 133 | 2.2 |
| 6 | 32 | 32 | 0 | 0.0 | 110 | 5.0 |
| 7 | 52 | 48 | 4 | 8.3 | 119 | 5.0 |
| 8 | 38 | 36 | 2 | 5.6 | 116 | 5.0 |
| 9 | 36 | 34 | 2 | 5.9 | 116 | 5.0 |
| 10 | 42 | 36 | 6 | 16.7 | 128 | 3.2 |
| 11 | 46 | 40 | 6 | 15.0 | 127 | 3.2 |
| 12 | 54 | 46 | 8 | 17.4 | 129 | 3.2 |
| 13 | 46 | 42 | 4 | 9.5 | 120 | 3.2 |
| 14 | 58 | 56 | 2 | 3.6 | 114 | 5.0 |
| 15 | 40 | 36 | 4 | 11.1 | 122 | 3.2 |
| 16 | 38 | 36 | 2 | 5.6 | 116 | 5.0 |
| 17 | 48 | 44 | 4 | 9.1 | 120 | 3.2 |
| 18 | 58 | 54 | 4 | 7.4 | 118 | 5.0 |
| 19 | 42 | 40 | 2 | 5.0 | 116 | 5.0 |
| 20 | 52 | 46 | 6 | 13.0 | 124 | 3.2 |
see Table 5, Rossi et al. (2009)
As can be seen in the fourth column of Table 1, the raw difference in percentage of maximum TMS device intensity (OM-MT – EMG-MT), in none of the 20 subjects was the MT found by the OM method less than that found with EMG (i.e., the difference was positive), although in one case they were the same. The group mean difference in TMS device % maximum intensity level between the methods was 4.6% (± 2.8% SD) higher for OM-MT than for EMG-MT (95% CI 3.2 - 5.9%).
The percentage of EMG-MT by which the OM-MT exceeded the EMG-MT (calculated as 100 × [OMG-MT - EMG-MT]/EMG-MT) are listed for each subject in the fifth column of Table 1. OM-MT was greater than EMG-MT in all cases but one, over a range of 0% to 27.8%, with a group mean difference of 11.3 ± 7.4%. Importantly, eight of the 20 subjects had OMMTs that exceeded EMG-MTs by more than 10%, with two subjects exceeding 25%.
Columns six and seven of Table 1 illustrate the consequences of the OM- and EMG-MT differences of column five. The sixth column calculates the percentage level of EMG-MT for each subject corresponding to 110% of their OM-MT. Note that the safe train duration for 10 Hz rTMS at 110% MT is five seconds (Table 5 of Rossi et al., 2009). Since the safe duration of rTMS at increasing levels of intensity and frequency are expressed in Rossi et al. (2009) for intensity in relation to EMG-MT, the seventh column shows the actual safe duration of 10 Hz rTMS trains for each subject if 110% of their OM-MT is used. Only eight of the twenty subjects would be within safe levels with a five second train, while nine would only be safe with trains of 3.2 s, and three would require trains no longer than 2.2 s.
DISCUSSION
OM-MT was on average 11.3% higher than EMG-MT, covering a range from 0% to 27.8% among twenty subjects. No subject had a lower MT using the OM method compared to the EMG method. This outcome was most similar to that found in Hanajima et al. (2007), where OM-MT averaged 113% of EMG-MT. Of the other three studies comparing the two MT methods, one compared a very low number of subjects (Balslev et al., 2007; N=4), and the other two used a less strict criterion for counting visual movement positives (i.e., they counted any twitch of wrist or hand), in one case resulting in greater OM-MTs, but of smaller magnitude (Conforto et al., 2004), and in the other, greater EMG-MT (Pridmore et al., 1998). We conclude that in a direct comparison of the two techniques, OM-MT of the FDI is on average significantly greater than EMG-MT. Of concern, the results confirm the possibility raised by examination of the data in other studies (Conforto et al., 2004; Hanajima et al., 2007) that the OM-MT method could lead to possibly unsafe overstimulation in some individuals. The OM-MT of eight of twenty subjects exceeded their EMG-MT by more than 10%, with two at more than 25% greater. Were one to use OM-MT to dose rTMS at 110% MT with 10 Hz, 4 s trains typically used in depression treatment protocols, one would on average be stimulating at 122% of EMG-MT with this group. In this scenario, twelve of the twenty subjects would be stimulated beyond the safe levels established using EMG-MT. In a worst case scenario, a 20 Hz train at 120% MT is deemed safe if lasting for 0.8 s (Rossi et al., 2009; Table 5), but two from the present group would actually be receiving stimulation at over 150% EMG-MT and another two at 140%, well out of safe range. While it is true that no seizures have been reported as yet when OM-MT has been used to set TMS intensity, the results found here and in Hanajima et al. (2007) serve as a caution to its accepted use.
While safety issues are of paramount concern, there are a number of other drawbacks to the use of OM-MT. First, using a higher threshold than necessary means causing more superficial TMS effects in the scalp than would have occurred at lower levels of stimulation. This impacts on patient comfort (and dropout rate) due both to greater (and possibly more painful) scalp muscle contraction and scalp nerve stimulation and to a louder clicking sound. Second, using two methods of MT determination that result in differing levels of stimulation makes it more difficult to assess and standardize safe parameters for the field. Third, overstimulation is less focal in its effects, which lowers targeting accuracy. It also results in inadvertent stimulation of non-target cortical regions, which can cause confounding effects in research and clinical settings. While the first three drawbacks to using OM-MT are based on the sort of overstimulation observed in the present study, the fourth has to do with possible understimulation that can occur in some cases without EMG. Spontaneous muscle activity immediately prior to stimulation is typically undetectable in an OM-MT procedure. Spontaneous activity immediately prior to the TMS pulse amplifies the muscle response, in the same way that intentionally activated index finger presses lead to active MTs which are lower than resting MTs. With restless or anxious subjects, spontaneous muscle activity can occur frequently, possibly leading to a threshold estimate lower than the true resting MT and thus to understimulation in those individuals.
On the other hand, the standardized use of EMG avoids these problems. In addition to the practical advantage of determining a lower, presumably more accurate, threshold, EMG-MT provides significant technical advantages over OM-MT. It allows for operator-independent evaluation of the motor response, which may increase inter-rater reliability, and for monitoring of peristimulus subthreshold muscle activity (Rossi et al., 2009). It allows a quantitative measurement, which may be useful additional information to improve the faster, more sophisticated algorithms for determining MT that have been proposed in recent years (e.g., Awiszus, 2003; Mishory et al., 2004, Qi et al., 2011). In the future, as TMS techniques evolve and become more integrated with electrophysiology and brain imaging, physiological methods of measuring cortical excitability may predominate, and standard use of a quantifiable physiological measure such as EMG today will provide continuity in the literature.
Further, the argument that the OM method is easier because it does not employ EMG is not particularly strong. As used in the EMG-MT method, EMG is simple and can be learned quite rapidly. The electrodes, amplifier and oscilloscope needed for EMG represent a relatively inexpensive investment. It takes only a few minutes to prepare a subject with three electrodes. The amplifier and oscilloscope are usually operated with a single set of easily memorized settings, and the EMG response to TMS is fairly easy to interpret. Some TMS devices already have built-in EMG options, similar to modern ECT devices.
A number of methods for estimating MT presently exist beyond the commonly used relative frequency method (Rossini et al., 1994; Rothwell et al., 1999), including adaptive methods such as maximum-likelihood threshold-tracking algorithms (Awiszus, 2003; Mishori et al., 2004) and Bayesian adaptive methods (Qi et al., 2011), the two-threshold method in which lower and upper thresholds are found and averaged (Mills and Nithi, 1997), and supervised parametric estimation, in which MT is estimated from the input/output curve across TMS intensities (Tranulis et al., 2006). The relative frequency method itself can be improved upon, for instance by beginning at a level below the MT of a subject, and increasing in 5% increments of maximum device output until MEPs greater than 50 uV are consistently evoked, and then decrementing in 1% steps until less than five out of ten positive responses are found, as suggested by Groppa et al. (2012). In reviewing these various methods, the most recent IFCN guidelines suggest using adaptive staircasing rather than relative frequency methods if possible (Groppa et al., 2012). Due to the natural fluctuations in excitability of the pyramidal cells and spinal motor neurons, there is a probabilistic component to EMG responses that is directly handled in adaptive algorithms but not in relative frequency methods, which can make them more reliable in estimating MT. In addition, adaptive methods require less TMS pulses to achieve their estimates, and thus MT procedures can be done more quickly (Goetz et al., 2011). That said, the present study employed the relative frequency method, which has a validated scientific background (Groppa et al., 2012), because it is the most widely used at present and to that extent may be most pertinent to the present message concerning safety. Moreover, given that 19 of 20 subjects showed higher OM-MTs than EMG-MTs, it is unlikely that the outcome of the present study would have been different using other MT estimation methods.
It should also be pointed out that whatever the method of MT estimation, the use of MT as the basis for determining minimum cortical excitability and thus dosing intensity for non-motor cortical regions is based on assumptions that are being called into question. For example, one assumption is that thresholds are similar for all cortical areas. However, it was demonstrated using evoked EEG responses that excitability of prefrontal cortex was less than that of motor cortex, although they were positively correlated (Kahkonen et al., 2004). In addition, using phosphenes evoked by TMS to estimate occipital excitability has resulted in phosphene thresholds that were not correlated with MT in the same subjects (Antal et al., 2003; Boroojerdi et al., 2002; Gerwig et al., 2003; Stewart et al., 2001), although when a more systematic approach to measuring the two kinds of thresholds was used, positive correlations were found (Deblieck et al., 2008). Thus, thresholds found using other methods (phosphenes, EEG) to measure cortical excitability have not always converged onto MTs, and there is some controversy over the generality of using MT as a gauge for dosing non-motor cortex. One solution was recently suggested, at least for research situations, in which a fixed dose of TMS is used, but with MTs also measured and used as a correction factor in subsequent analysis (Kaminski et al., 2011).
In conclusion, there is little evidence to support the argument that OM-MT is equivalent to EMG-MT, and our study adds to the evidence that the OM-MT method significantly overestimates MT. For applications that involve stimulation near established safety limits and for populations or stimulation sites that involve increased risk, EMG-MT is the more prudent choice. This may be particularly relevant for patients concurrently using medications that can alter seizure threshold. The present results encourage the use of EMG as the standard in MT determination, both in the interests of patient/subject safety and comfort, and as a research tool that accurately and reliably quantifies physiological response.
Highlights.
This is the largest study to date to systematically compare TMS motor thresholds (MTs) determined via electromyography (EMG) to those determined via observation of hand movement (OM).
MTs determined via OM were on average 111% of those determined via EMG.
OM-MT should not be assumed to be equivalent to EMG-MT, and may lead to stimulation outside of accepted safety standards.
Acknowledgements
This research was in part supported by a grant from the National Institute of Aging (NIA K01AG031912). We thank a former member of our technical staff, Anouk Allart, for assistance in collection of data. Dr. Lisanby has received research support, for topics not presented here, from Magstim, MagVenture, Neuronetics, Cyberonics, and ANS/St. Jude. Columbia University has applied for a patent for novel TMS technology developed in Dr. Lisanby's Lab, for work unrelated to the topic presented here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Work done at the New York State Psychiatric Institute, NY, USA
REFERENCES
- Anderson BS, George MS. Review of Studies Comparing Methods for Determining Transcranial Magnetic Stimulation Motor Threshold: Observation of Movement or Electromyography Assisted. J Am Psychiatr Nurses Assoc. 2009;15:304–313. doi: 10.1177/1078390309347372. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Kincses TZ, Lampe C, Paulus W. No correlation between moving phosphene and motor thresholds: A transcranial magnetic stimulation study. Neuroreport. 2003;15:297–302. doi: 10.1097/00001756-200402090-00017. [DOI] [PubMed] [Google Scholar]
- Awiszus F. TMS and threshold hunting. Suppl Clin Neurophysiol. 2003;56:13–23. doi: 10.1016/s1567-424x(09)70205-3. [DOI] [PubMed] [Google Scholar]
- Balslev D, Braet W, McAllister C, Miall RC. Interindividual variability in optimal current direction for transcranial magnetic stimulation of the motor cortex. J Neurosci Methods. 2007;162:309–313. doi: 10.1016/j.jneumeth.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Barker A, Jalinous R, Freeston I. Non-invasive magnetic stimulation of the human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Meister IG, Foltys H, Sparing R, Cohen LG, Toepper R. Visual and motor cortex excitability: A transcranial magnetic stimulation study. Clin Neurophysiol. 2002;113:1501–1504. doi: 10.1016/s1388-2457(02)00198-0. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Z'Graggen WJ, Kohl AS, Rosler KM, Kaelin-Lang A. Impact of coil position and electro- physiological monitoring on determination of motor thresholds to transcranial magnetic stimulation. Clin Neurophysiol. 2004;115:812–819. doi: 10.1016/j.clinph.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Deblieck C, Thompson B, Iacoboni M, Wu AD. Correlation between motor and phosphine thresholds: A transcranial magnetic stimulation study. Hum Brain Mapp. 2008;29:662–670. doi: 10.1002/hbm.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis I disorders, non-patient edition, Version 2.0-8/98 revision. Biometrics Research Department, NY State Psychiatric Institute; New York: 1998. [Google Scholar]
- Gerwig M, Kastrup O, Meyer B-U, Niehaus L. Evaluation of cortical excitibilty by motor and phosphene thresholds in transcranial magnetic stimulation. J Neurol Sci. 2003;215:75–78. doi: 10.1016/s0022-510x(03)00228-4. [DOI] [PubMed] [Google Scholar]
- Goetz SM, Whiting P, Peterchev AV. Threshold estimation with transcranial magnetic stimulation: algorithm comparison. Clin Neurophysiol. 2011;122:S197. [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: Reort of an IFCN committee. Clin Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Wang R, Nakatani-Enomoto S, Hamada M, Terao Y, Furubayashi T, et al. Comparison of different methods of estimating motor threshold with transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:2020–2122. doi: 10.1016/j.clinph.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Kahkonen S, Wilenius J, Komssi S, Ilmoniemi RJ. Distinct differences in cortical reactivity of motor and prefrontal cortices to magnetic stimulation. Clin Neurophysiol. 2004;115:583–588. doi: 10.1016/j.clinph.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Kaminski JA, Korb FM, Villringer A, Ott DVM. Transcranial magnetic stimulation intensities in cognitive paradigms. PLoS One. 2011;6:e24836. doi: 10.1371/journal.pone.0024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR, Nithi KA. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve. 1997;20(Suppl.):570–6. doi: 10.1002/(sici)1097-4598(199705)20:5<570::aid-mus5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mishory A, Molnar C, Koola J, Li X, Kozel FA, Myrick H, et al. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J ECT. 2004;20:160–165. doi: 10.1097/00124509-200409000-00007. [DOI] [PubMed] [Google Scholar]
- Pridmore S, Filho JAFF, Nahas Z, Liberatos C, George MS. Motor Threshold in Transcranial Magnetic Stimulation: A Comparison of a Neurophysiological Method and a Visualization of Movement Method. J ECT. 1998;14:25–27. [PubMed] [Google Scholar]
- Qi F, Wu AD, Schweighofer N. Fast estimation of transcranial magnetic stimulation motor threshold. Brain Stimul. 2011;4:50–57. doi: 10.1016/j.brs.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM. Pascual-Leone A Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Noninvasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroenceph Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The international federation of clinical neurophysiology. Electroenceph Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- Schutter DJLG, van Honk J. A standardized motor threshold estimation procedure for transcranial magnetic stimulation research. J ECT. 2006;22:176–178. doi: 10.1097/01.yct.0000235924.60364.27. [DOI] [PubMed] [Google Scholar]
- Stewart LM, Walsh V, Rothwell JC. Motor and phosphine thresholds: A transcranial magnetic stimulation correlation study. Neuropsychologia. 2001;39:415–419. doi: 10.1016/s0028-3932(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Tranulis C, Gueguen B, Pham-Scottez A, Vacheron M, Cabelguen G, Costantini A, et al. Motor threshold in transcranial magnetic stimulation: comparison of three estimation methods. Neurophysiol Clin. 2006;36:1–7. doi: 10.1016/j.neucli.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Varnava A, Stokes MG, Chambers CD. Reliability of the ‘observation of movement’ method for determining motor threshold using transcranial magnetic stimulation. J Neurosci Methods. 2011;201:327–332. doi: 10.1016/j.jneumeth.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation : report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, 5-7 June, 1996. Electroenceph Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]