Abstract
Acute myeloid leukemia (AML) is the most frequently diagnosed adulthood leukemia, yet current therapies offer a cure rate of less than 30%. This may be due in part to the fact that the leukemia-initiating cells in AML reside within the rare and highly primitive CD34+CD38- hematopoietic stem/progenitor cell (HSC) population that are often resistant to chemotherapy. Docosahexanoic acid (DHA), a major component of fish oil, has previously been shown to inhibit the induction and progression of breast, prostate and colon cancer, and increase the therapeutic effects of numerous chemotherapeutics, often by enhancing apoptosis. In the present studies, we investigated DHA's effect on the primitive and undifferentiated AML cell line KG1a, to explore the potential of this fatty acid to serve as adjuvant therapy for AML. Treatment of KG1a cells with DHA for 96 hours did not lead to maturation or cell cycle modification when compared to an untreated KG1a control (n = 4). However, DHA treatment of KG1a cells resulted in a progressive loss of viability, DNA fragmentation, and an increase in Annexin V expression, demonstrating DHA-induced apoptosis (n = 4). Moreover, expression of the pro-apoptotic protein Bax was increased, with resultant skewing in the Bax/bcl-2 ratio, providing a mechanistic explanation for the observed DHA-induced increase in apoptosis. Since we also show that DHA does not have a detrimental effect on normal hematopoiesis our results suggest that DHA could potentially serve as an well-tolerated adjuvant in the treatment of AML patients.
Keywords: docosahexaenoic acid, DHA, KG1a, acute myeloid leukemia, apoptosis, Bax, Bcl2
Introduction
Several epidemiologic and clinical studies have shown that docosahexanoic acid (DHA), an essential fatty acid and one of the main constituents of fish oil and marine algae, is able to provide beneficial effect in a wide variety of maladies ranging from autoimmune and inflammatory diseases to neurological and psychiatric disorders and, notably, to several types of malignancies including breast, ovarian, pancreatic, prostate, renal and colorectal cancer.1-5 Although the entire mechanism by which DHA exerts its beneficial effects is not yet fully understood, it is likely that different mechanisms may contribute either individually or in synergy to its broad range of action. For instance, DHA and other essential fatty acids are known to increase cell membrane fluidity, enhance the activity of protein kinase C and other second messenger systems, as well as increase reactive oxygen species and lipid peroxidation.2,6,7 Furthermore, these fatty acids are also able to induce tumor apoptosis through cell cycle gene modulation, or to activate and induce cell death via both a mitochondrial-dependent and a bax-mediated, mitochondrial-independent pathway.8-10 DHA has also been shown to increase the therapeutic effects of Imatinib11 doxorubicin, mitomycin C and cyclophosphamide,12,13 while enhancing arsenic trioxide-mediated apoptosis in arsenic trioxide-resistant leukemia cells.14
Acute myeloid leukemia (AML) is the most common leukemia diagnosed in adults, with two-thirds of the new cases being diagnosed in patients over the age of sixty.15,16 Unfortunately, less than 30% of adult patients are cured by current existing therapies, with the elderly population being linked with a poorer outcome. The leukemia initiating cells have been shown to reside within the CD34+CD38- fraction of the leukemic progenitors.17 These cancer stem-like cells are frequently impervious to intensive chemotherapy and to immune response,18 making them largely responsible for resistance to treatment or relapse of the disease, and suggesting that novel therapies that can target these very early malignant clones would significantly improve existing treatment strategies.
In the present studies, we investigated the effect of DHA on KG1a, an undifferentiated subtype and differentiation resistant, acute myeloid leukemia cell line, expressing P-glycoprotein, and thus meeting the criteria for AML with poor treatment outcome.15,19 We showed that at the doses tested, DHA induced progressive loss of viability, increased expression of AnnexinV and DNA fragmentation. Furthermore, quantification of Bcl-2 and Bax proteins in DHA-treated KG1a cells demonstrated an increased expression of the pro-apoptotic protein Bax, without caspase-3 activation, and an increase in the Bax/Bcl-2 ratio leading to apoptosis. Since we also show that DHA does not have a detrimental effect on normal hematopoiesis and it seems to be well tolerated by elderly patients,20 DHA could therefore have a promising role as an adjuvant in the treatment of AML patients for which successful therapeutic options are currently limited.
Results
DHA induces cell death in KG1a, an undifferentiated AML cell line
Since leukemic precursors in AML have been shown to reside within the CD34+CD38- subpopulation,17 we investigated the effect of DHA on KG1a, a differentiation-resistant and undifferentiated subtype of acute myeloid leukemia, expressing P-glycoprotein, and thus meeting the criteria for AML with poor treatment outcome.15,19 In these experiments, 1.5–2.0 × 106 KG1a cells were cultured with or without DHA (0, 100, 150 μM) for 96 h, removing aliquots every 24 h to evaluate cell numbers and viability. Figure 1A shows the fold increase in numbers of viable KG1a cells with the time in culture (n = 4). KG1a cells cultured in the absence of DHA proliferated gradually between days one (1.4 ± 0.4 fold increase) and three (2.5 ± 0.8 fold increase), followed by a higher proliferative rate between days three and four, expanding a total of 4.3 ± 1.6-fold over the four days of culture. As shown in Figure 1A, KG1a cells cultured in the presence of 100 μM DHA maintained a relatively constant cell number over the first three days of culture, only expanding significantly between days three and four to reach levels of viable cells that were 2.8 ± 0.5-fold higher than the input cell number. Thus, while the kinetics of growth was similar in the two conditions, the magnitude of the expansion was considerably reduced in the presence of 100 μM DHA. The presence of 150 μM DHA in KG1a cultures resulted in absence of expansion (p < 0.05), with cell numbers remaining constant throughout the four day period (Fig. 1A), suggesting that at this higher concentration, DHA either blocked cell division, or that cell division was balanced by cell death. To distinguish between these two potential mechanisms, we examined viability and performed cell cycle analysis on the cell aliquots removed at each of the days of culture. Figure 1B shows that cell viability remained at nearly 100% throughout the four-day period in the cultures without DHA. Overall, a marked reduction in cell viability was observed in cultures containing DHA, with loss of viability correlating directly with the concentration of DHA present. DHA at a concentration of 100 μM caused 20% cell death by day two of culture, while DHA at a concentration of 150 μM was able to induce major (nearly 60%) cell death over the same two day period, with the most pronounced drop in viability (40%) occurring within the first 24 hours. Cell cycle analysis was then performed by flow cytometry on KG1a cells cultured with (150 μM) or without DHA at 0, 2, 4, 6 and 24 h (doubling time of KG1a cells: 24–27 h) to assess the effect of DHA upon cell cycle and ascertain whether cell cycle status was related to DHA-induced cell death. At time point zero, a sample was collected from the KG1a cell culture and the percentage of cells in G0/G1, G2/M and S phase were determined to be 57 ± 1.4, 11.4 ± 1.6, 31.6 ± 0.2, respectively. The culture was then split into two different aliquots, one containing DHA and the other remaining as a control. At the time point of two hours, the percentage of KG1a cells cultured with DHA that was in each phase of the cell cycle was as follows: G0/G1: 57 ± 0.9; G2/M: 13 ± 1.7; and S: 34 ± 0.8. Similarly, the percentage of control cells in each phase of cell cycle was: G0/G1: 53 ± 0.5; G2/M: 13 ± 2.2; and S: 34 ± 2.8. At four hours, the percentage of DHA-treated KG1a cells in G0/G1 phase was 54.6 ± 1.5%; G2/M phase 12 ± 3.7%; and S phase 33.2 ± 5.2%, with the cells in the control cultures exhibiting a similar cycle phase distribution of G0/G1: 53.5 ± 3.9%; G2/M: 14.9% ± 0.3; and S: 31.1 ± 3.6. At the time points of six and 24 h, both cell populations continued to display similar cycling behavior, with no statistically significant differences between those treated with DHA and those that were not. At the time points of six and 24 h, the percentage of G0/G1 cells in DHA-treated populations was 60 ± 1% and 68.08%, respectively, and the control cells, 56.3 ± 2.7% and 55.8 ± 1. At the same time points of six and 24 h, the percentage of DHA-treated cells in G2/M was 8.4 ± 4 and 4.7 ± 1.4, and in the control, the values were 13.6 ± 0.5 and 9 ± 2.2. The percentage of cells in S phase also exhibited no significant change during the time in culture. At six and 24 h, 31.5 ± 6 and 27.5 ± 0.5% of DHA-treated cells and 30.5 ± 0.8 and 35 ± 0.9% of control cells were in S phase. Cell cycle analysis after the 24 h time point, was not possible due to the low viability of the DHA treated cells.
Figure 1.
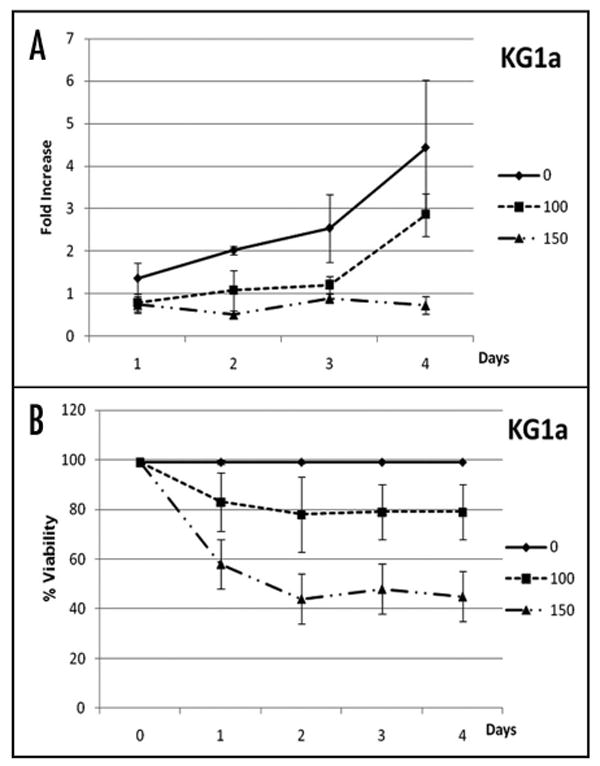
DHA induces cell death in KG1a cells. (A) Fold increase in numbers and (B) viability of KG1a cells with time in culture in the absence of DHA (solid line), in the presence of 100 μM DHA (dotted line with squares) and with 150 μM DHA (dashed line with triangles). While an inverse correlation between cell expansion and DHA concentration was found, cell death correlated directly with the concentration of DHA present. This suggests that at higher concentrations, DHA either blocked cell division, or that cell division was balanced by cell death.
We also investigated whether KG1a, which is resistant to phorbol-diester-induced maturation, was able to undergo differentiation in the presence of DHA. To this end, aliquots were collected daily from cultures with or without DHA (100, 150 μM), and cells were stained with monoclonal antibodies against CD11b, CD13, CD33 and CD34, and analyzed by flow cytometry. During the four days of culture, DHA was unable to induce differentiation of KG1a cells as demonstrated by the lack of expression of CD33, and CD11b, and the maintenance of a phenotypic profile similar to those cultured without DHA, with cells continuing to express CD13 and CD34 (Fig. 2).
Figure 2.
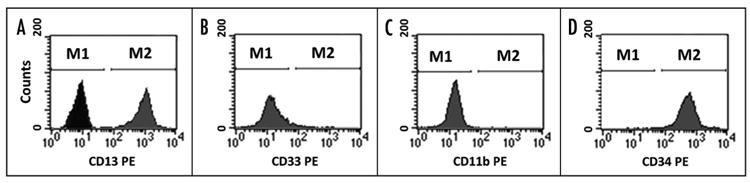
KG1a cells do not undergo differentiation in the presence of DHA. Flow cytometric analysis of KG1a cells using monoclonal antibodies against CD11b, CD13, CD33 and CD34 showed that culture of KG1a cells with 150 μM DHA for four days did not induce differentiation of these cells. In (A) the negative isotype control antibody is shown along with CD13 expression. M1 defines the region with negative cells; M2 defines the region with cells positive for CD 13 (A) and CD34 (D).
DHA induces dose dependent apoptotic cell death on KG1a
In order to investigate the mechanism by which DHA induced cell death in KG1a cells, samples were collected from cultures grown in the absence or presence of DHA (100 and 150 μM) every 24 hours for up to 4 days, and stained with Annexin V and PI and analyzed by flow cytometry (n = 4). The results of a representative experiment are shown in Figure 3. At the first time point of 24 h, 37.4% of KG1a cells cultured with 150 μM DHA had undergone early apoptosis (Annexin V positive/PI negative cell population) and 8.9% cell death (Annexin V positive/PI positive). At this time point, no significant difference was found between cells treated with 100 μM DHA and the untreated controls, with cell death rates of 1.2% and 0.97%, respectively. For the rest of the experimental period, control KG1a cultures continued to have percentages of early apoptotic cells that never exceeded 4%, and cell death rates of less than 1%. By contrast, 2%, 27.4% and 47.4% of KG1a cells cultured with 100 μM DHA were dead at days 2, 3 and 4, respectively. This effect was enhanced at a DHA concentration of 150 μM, which resulted in cell death rates of 30.4%, 99.5 and 98.5%, respectively, during the same time period.
Figure 3.
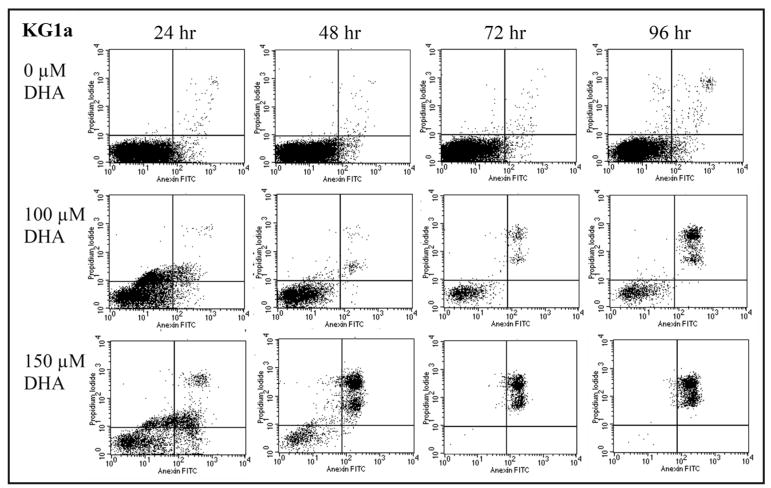
DHA induces dose dependent apoptotic cell death in KG1a. KG1a cells cultured with 0, 100 or 150 μM DHA and stained with Annexin V and PI were analyzed daily by flow cytometry. Shown are the results from a representative experiment. Cells in early apoptosis are Annexin V positive/PI negative and appear in the lower right quadrant. Cell death is characterized by positivity for both Annexin V and PI and these cells appear in the upper right quadrant. While the untreated control had a very low percentage of cell death, KG1a cultures with 150 μM of DHA had cell death rates of 8.9, 30.4, 99.5 and 98.5%, respectively, at the four time points analyzed.
DHA induces internucleosomal DNA fragmentation in KG1a cells
DNA fragmentation into oligonucleosomal fragments by cleavage at internucleosomal sites is a characteristic of apoptosis in mammalian cells. Thus, to further confirm the mechanism of DHA-induced cell death in KG1a cells, DNA fragmentation analysis was performed as described in the materials and methods. As can be seen in Figure 4, a typical nucleosomal DNA ladder pattern was clearly seen at day two of culture in cells treated with 150 μM DHA, while it was also noticeable, although not so clearly, in cultures grown in the presence of 100 μM DHA. However, by day three of culture, DNA fragmentation was clearly evident in cells cultured with both 100 and 150 μM DHA, while in untreated cells this pattern was absent throughout the time in culture.
Figure 4.
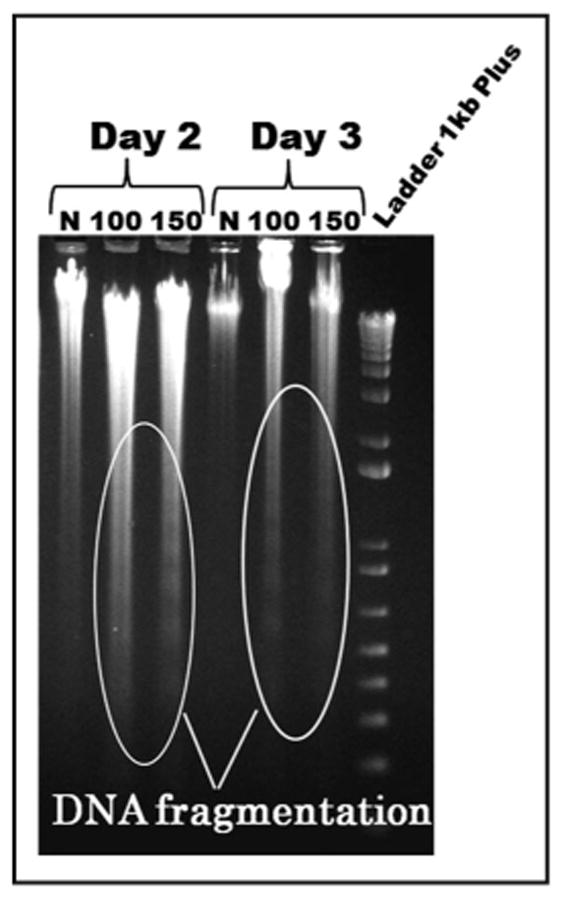
DHA induces DNA fragmentation in KG1a cells. Cells were cultured in the absence (lanes 1 and 4; indicated by “N”) or presence of DHA at concentrations of 100 μM (lanes 2 and 5) or 150 μM (lanes 3 and 6). A typical DNA ladder pattern was clearly evident at day 2 of culture in cells treated with 150 μM DHA, while it was not so noticeable in cultures with 100 mM. At day three of culture, both DHA concentrations induced obvious DNA fragmentation. This pattern was absent in untreated cells throughout the time in culture.
DHA-induced apoptosis involves alteration of the Bcl-2/Bax ratio
In order to delineate the possible mechanism(s) by which DHA induced apoptosis in the DHA-susceptible KG1a leukemic cell line, we performed flow cytometric analyses to examine the cytoplasmic levels of two of the key members in the Bcl-2 family of apoptosis-regulating proteins. We focused our analyses on the anti-apoptotic protein bcl-2 and its biochemical converse, the proapoptotic protein Bax, whose expression is known to be induced in some types of cells by radiation, chemotherapeutic drugs, and other forms of genotoxic stress. Figure 5 shows the fold increase in expression level of each protein as well as the Bax/Bcl-2 ratio. In each case, the fold-increase as a result of DHA treatment was calculated by comparing the levels observed in the DHA-treated cells to those obtained with a negative control culture without DHA, setting the levels obtained with the negative control culture = 1. As can be seen in Figure 5A, DHA treatment at either 100 μM or 150 μM produced no significant change in the levels of cytoplasmic Bcl-2 protein over the course of the 96 hour experiment in the KG1a leukemic cell line. In contrast, as can be seen in Figure 5B, which is data from one of four representative experiments, the presence of 100 μM DHA produced a modest increase (5–8 fold) in the cytoplasmic levels of the pro-apoptotic protein Bax during the 96 hours of study. When the DHA concentration was increased to 150 μM, however, a pronounced increase of approximately 120 fold in the cytoplasmic levels of Bax protein was observed (Fig. 5B). As can be seen in Figure 5C, this dramatic increase in the cytoplasmic levels of Bax resulted in a marked perturbation in the cytoplasmic Bax/ Bcl-2 ratio, an intracellular gauge which plays a key role in maintaining the delicate balance between survival and apoptosis. Indeed, prior studies have shown that the ratio of various pro-apoptotic and anti-apoptotic proteins in the Bcl-2 family determines the ultimate sensitivity or resistance of cells to diverse apoptotic stimuli, including chemotherapeutic drugs and radiation. These results thus provide a possible mechanistic explanation for the high rate of apoptosis we observed when these leukemic cells were exposed to 150 μM DHA. Interestingly, this dramatic increase in the level of cytoplasmic Bax protein and the resultant induction of apoptosis did not appear to involve Caspase 3, since Flow cytometric studies examining Caspase 3 levels in the absence and presence of DHA revealed no difference. This agrees with previous studies showing that Bax-mediated apoptosis can occur in a Caspase 3-independent fashion.
Figure 5.
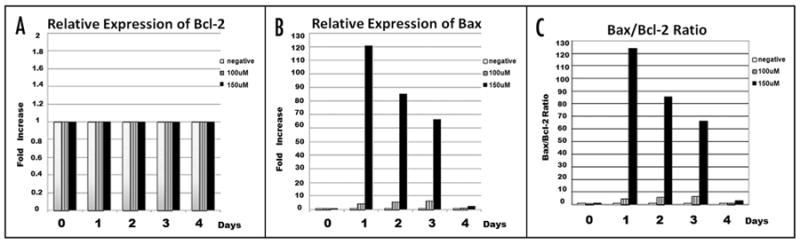
DHA-induced apoptosis involves alteration of the Bcl-2/Bax Ratio. (A) Relative cytoplasmic expression levels of Bcl-2 in cultures in the absence/ presence of DHA. Expression of Bcl-2 was not altered by DHA treatment and remained constant throughout the time in culture. (B) Relative cytoplasmic expression levels of Bax in KG1 cells cultured in the absence/presence of DHA. 150 μM DHA induced a significant increase in cytosolic Bax levels, starting at the first time point of 24 h. (C) Cytoplasmic Bax/Bcl-2 ratio is altered by DHA treatment, with 150 μM DHA producing a much more pronounced effect.
Effects of DHA on human normal hematopoiesis
We next examined whether DHA had a detrimental effect on normal hematopoiesis when used at concentrations similar to those found to induce cell death in KG1a cells. We also wished to investigate whether DHA preferentially targeted more primitive HSC populations such as CD34+ cells, or CD34+CD38- cells. To this end, BM-derived mono-nuclear cells (MNC), CD34+ cells, or CD34+CD38- cells (n = 3) were cultured in methylcellulose with or without DHA at concentrations of 100 and 150 μM and evaluated for colony forming potential as described in the Materials and Methods. We used this assay since it is one of the most universally accepted approaches for quantification of multi-lineage- and single lineage-committed hematopoietic progenitors.21 Since BM- and CB-derived cells have different functional characteristics regarding their cloning efficiency and differentiative capability,22,23 we also investigated the effect of DHA on CB-derived cell populations (n = 3). The results for CB and BM are presented in Figure 6A and B respectively, and are expressed as fold increase/ decrease in clonogenic potential of DHA-treated cells/non-treated cells. To facilitate quantification of the effects of DHA, the colony forming potential of the non-treated cells was set equal to 1, and is indicated by the black line in the figure. When 100 μM of DHA was used in normal HSC cultures, it did not significantly change the clonogenic potential of either CB (Fig. 6A) or BM (Fig. 6B) derived MNC or CD34+ cells. However, DHA at the higher concentration of 150 μM decreased the overall clonogenic potential of both CB- and BM-derived CD34+ and CD34+CD38- cells, reducing the number of colonies in the latter by approximately 60% when compared to the number generated by the untreated population. However, given the limited numbers of more primitive hematopoietic cells that could be obtained, we used only 104 CD34+ and 103 CD34+CD38- cells in the methylcellulose assays, as compared to 105 cells when total MNC. It is thus likely that reduction in colony formation was due, at least in large part, to the higher relative dosage of DHA per cell in the CD34+ and CD34+38- cultures. To test this possibility, we investigated whether lowering the effective DHA dose per cell by reducing the DHA concentration in the media to 50 μM would alter the apparent detrimental effect of DHA in these cultures. As can be seen in Figure 6, when the concentration of DHA was decreased, the adverse effect seen on the CD34+ and CD34+38- populations at higher DHA doses was no longer present. Moreover, low dose DHA actually increased, to some extent, the clonogenic potential of both BM and CB MNC.
Figure 6.
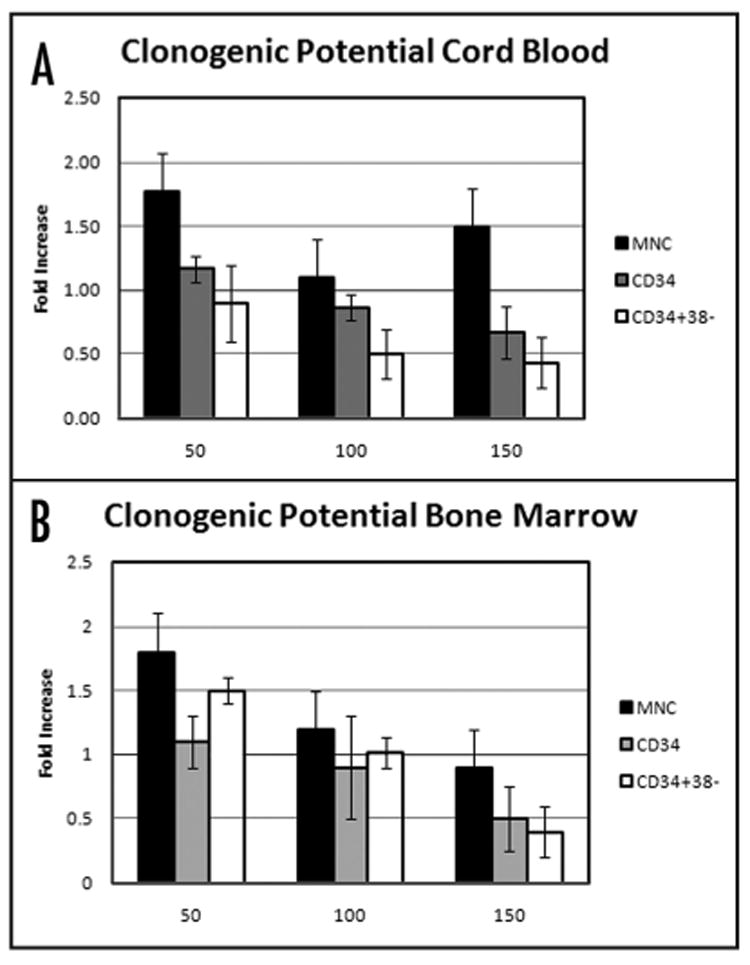
Effect of DHA on clonogenic potential of human HSC. (A) CB or (B) BM-MNC (black bars), CD34+ (gray bars), or CD34+CD38- (white bars) were cultured in the absence or in the presence of DHA at concentrations of 50, 100 or 150 μM and the clonogenic potential evaluated. The results are expressed as fold increase/decrease of the clonogenic potential of DHA treated cells/non-treated cells. To facilitate quantification of the effects of DHA, the colony forming potential of the non-treated cells was set equal to 1, and is indicated by the black line.
Discussion
Despite the prevalence of AML in older adults, very little progress has been made in a treatment of patients older than 60 years using traditional chemotherapy-based approaches. In particular, prognosis is very poor for patients in whom AML presents with myeloblasts with high level expression of CD34, an undifferentiated morphology, a high Bcl2/Bax ratio, or high P glycoprotein expression. In these clinical situations, primitive leukemic cells with self-renewal and high proliferative capabilities frequently evade both intensive chemotherapy and the immune response to cause resistance or relapse.
In the present study, we examined the effects of DHA, one of the primary constituents of fish oil and marine algae, on an undifferentiated subtype and differentiation resistant, acute myeloid leukemia cell line, KG1a.15,19 Although DHA has been shown to exert effects on many types of tumors in vivo as well as on leukemic cell lines in vitro, this is the first report, to our knowledge, to explore the ability of DHA to act on a primitive undifferentiated AML cell line. This cell line is particularly relevant to the patient population most in need of novel therapeutic interventions, since it is undifferentiated and expresses high levels of both CD34 and P glycoprotein.
In this study, we showed that DHA induced dose-dependent apoptosis in a Bax-dependent, Caspase 3-independent fashion, producing roughly 60% cell death within only four days of culture. While DHA, at the concentrations we employed, did not result in elimination of all KG1a cells, it is important to note that this could simply be due to exhaustion of the supply of DHA in the medium, since DHA was only added once at the initiation of the cultures. Thus, it is conceivable that daily dosing of the cells with DHA would have produced higher cell death rates. Importantly, however, the fact that a single treatment with DHA alone resulted in 60% cell death combined with prior studies showing that DHA enhances the tumorspecific toxicity of various chemotherapeutics in other leukemic cell lines supports our premise that DHA could prove to be a valuable adjuvant to existing AML treatments to allow targeting of undifferentiated myeloblasts.
Our finding of dependence on the proapoptotic Bax protein is in agreement with two previous studies conducted in the human myeloid leukemia cell line HL-60 which showed that DHA alone10 or in combination with arsenic trioxide14 resulted in a pronounced upregulation of Bax expression as an integral part of the DHA-induced apoptosis. Interestingly, our present findings and those of these two prior studies differ from another study conducted by Miura et al. in HL-60 cells that used cyclosporine treatment to conclude that DHA-induced apoptosis occurred via a Bax-independent mechanism.8 It is likely that our current results differ from those of this prior study as a result of the differences between the HL-60 cell line employed by these authors and the KG1a cell line we employed in our current studies. It is not clear, however, what differences in experimental approach may have led to the apparent discrepancy amongst investigators using the HL-60 cell line. This study by Miura et al. also provided evidence that Caspase-3 activation was involved in the DHA-induced apoptotic cascade, as did one other study conducted in U937 monocytic leukemia cells.6 Our present findings indicate that this is not the case in the undifferentiated KG1a AML cell line, since Caspase-3 activity levels remained unchanged regardless of the concentration of DHA. Although the increase in Bax does not appear to be a mechanism universally employed by DHA to induce apoptosis in malignant cell lines, the skewing of the Bax/Bcl-2 ratio we observed as a result of DHA-induced upregulation of Bax has also been observed in both MCF-7,24 and KPL-1,25 breast cancer cell lines in response to DHA. However, in these two breast cancer cell lines, the ratio was perturbed as a result of DHA-induced down-regulation of Bcl-2 rather than upregulation of Bax, as we observed in the KG1a AML cell line. Thus, it appears that DHA, depending on the target cell, utilizes two opposing means of achieving the same end of increasing the Bax/Bcl-2 ratio and inducing apoptosis. This issue is clearly of importance from a the standpoint of clinical prognosis, since prior studies have clearly shown that the relative levels of Bcl-2 expression impact AML therapeutic efficacy, not only by regulating apoptotic responses to therapeutic agents, but also by regulating cell cycle responses.26,27 Despite the frequent association between cell cycle disruption and apoptosis, these studies showed that at the concentrations used, DHA has no significant effect on the cell cycle status of KG1a cells. Only one prior study was done investigating DHA's effect on cell cycle in detail,10 and it was performed in HL-60 cells, making a direct comparison between our present study and this prior study difficult. It is important to note, however, that the degree of cell cycle arrest observed in this prior study was modest (12–22%), and the authors relied heavily on gene array analysis to confirm the effects of DHA on cell cycle; a technique we did not employ in the present study. Perhaps future studies using this higher sensitivity technique will allow us to discern some subtle effects of DHA on cell cycle in KG1a cells as well, or possibly, cell cycle arrest does not play a role in DHA's induction of apoptosis in KG1a cells, as suggested by our flow cytometric cell cycle analyses. It also bears mention that our finding of a lack of alteration in the absolute levels of Bcl-2 could in fact preclude an effect on cell cycle progression, since prior studies have suggested that Bcl-2 is pivotal in the arrest of cell cycle.
Importantly, from the standpoint of considering DHA as a potential therapeutic adjuvant, our results also demonstrate that DHA does not have a pronounced effect on normal hematopoiesis. When 100 μM DHA was added to cultures of total MNC or the more primitive fraction of CD34+ cells, the clonogenic potential was not significantly different from the respective untreated control. Of concern was that higher concentrations of DHA appeared to affect, to some extent, the clonogenic potential of both CD34+ and CD34+CD38- cells, with the latter suffering a 60% reduction in colony output. It is likely that this reduction in colony formation is related to a higher relative dosage of DHA per cell in the CD34+ and CD34+38- cultures in which, respectively, only 104 and 103 cells were plated, compared to 105 cells in the cultures of normal MNC or to 106 of the leukemic KG1a cells. Since DHA acts by integrating into the membrane, our efforts to maintain a similar μM concentration in all of the cultures regardless of phenotype or cell number may in fact have resulted in much higher DHA dosage in the cultures plated with fewer cells. Indeed, when the concentration of DHA was decreased to 50 μM, no adverse effect was seen within the CD34+ or CD34+38- population.
In conclusion, our studies support the utility of DHA as a non toxic adjunct to chemotherapy for a wide array of tumors, and leukemia in particular. Our findings also demonstrate for the first time that the effects of DHA could potentially be exploited to improve the clinical outcome in AML, especially in the elderly population whose prognosis is usually poor.
Materials and Methods
Culture of the KG1a leukemic cell line
KG1a (an undifferentiated subtype of acute myeloid leukemia) was obtained from ATCC (Manassas, VA USA), and cultured in: IMDM with 10% fetal bovine serum (FBS) (HyClone Laboratory Inc., Logan, UT USA), Penicillin (100 U/mL), streptomycin (100 g/mL) and Amphotericin B (0.25 g/mL) (Gibco Laboratories, Grand Island, NY USA), and cultured under 5% CO2 in a humidified incubator.
Cell proliferation and differentiation and viability
To examine the effect of DHA on KG1a proliferation, 1.5–2.0 x 106 cells/ml were cultured up to 96 hours with or without DHA, and every 24 hours, samples were removed, cells counted and viability determined by Trypan-blue exclusion. In addition, KG1a cells were also examined for evidence of possible differentiation by staining with monoclonal antibodies against CD11b, CD13, CD14, CD33 and CD34 (BD Biosciences Pharmingen, San Diego, CA USA) and analyzing by flow cytometry.
Annexin V FITC/PI analysis
KG1a cells were incubated with DHA as previously described and cell samples were stained at different time points using the Annexin V-FITC Apoptosis Detection kit I (BD Biosciences, Pharmingen, San Diego, CA) according to the manufacturer's instructions. Briefly, cells were washed twice with cold phosphate-buffered saline (PBS; Gibco BRL, Gaithersburg, MD, USA) and resuspended with 1x Annexin binding buffer at the concentration of 106 cells/ml. Cells were then incubated with Annexin V-FITC and PI for 15 minutes at room temperature. Each sample was then analyzed by flow cytometry immediately after staining.
DNA fragmentation analysis
DNA fragmentation analysis was performed using the Quick Apoptotic DNA Ladder Detection kit (BioSource International, Inc., Camarillo CA) according to the manufacturer's instructions. DNA was isolated from KG1a cells cultured for various lengths of time with DHA. Isolated DNA was analyzed by running on a 1% agarose gel at 5 V/cm for two hrs. DNA was visualized by adding 0.5 mg/mL ethidium bromide to both the gel and running buffer.
Determination of Bcl-2/Bax expression
Expression levels of cytoplasmic Bcl-2 and Bax proteins were analyzed using Immunotech IntraPrep Permeabilization/Fixation Reagent kit (Beckman Coulter Immuno-Tech, Hialeah, FL. Coulter). Cells were removed from culture, washed with ice-cold PBS, and fixed/permeabilized according to the manufacturer's instructions. Permeabilized cells were stained with either an anti-human Bcl-2 FITC-conjugated monoclonal antibody (Dako Cytomation, Carpinteria, CA, USA) or an unconjugated mouse anti-human Bax monoclonal antibody (Beckman Coulter, Fullerton, CA, USA), which was subsequently detected with a conjugated rat anti-mouse secondary antibody (BD Biosciences, Pharmingen, San Diego, CA, USA). The stained cells were fixed and analyzed (at least 10,000 events) by flow cytometry.
Determination of caspase 2, 3, 6, 8 and 9 activation
Caspase activity was measured using the ApoTarget CPP32 Colorimetric Protease Assay (BioSource International, Camarillo, CA) according to manufacturer's guidelines. KG1a cells were cultured in the absence or in the presence of 100 μM or 150 μM DHA for 96 h, and samples were collected every 24 h. The cells were washed with PBS and resuspended in 50 ml of cold lysis buffer. Cell lysates were then centrifuged, and the protein concentration adjusted to 3 g/l, 2X reaction buffer was added, and samples were incubated with 5 ml of DEVD-pNA at 37°C for two hrs. Released p-NA was determined in a spectrophotometer at 400 nm.
Cell isolation and preparation of human bone marrow and cord blood cells
Heparinized human bone marrow was obtained by aspiration from the posterior iliac crest of healthy volunteers after informed consent. Umbilical cord blood samples were obtained from the Ohio State University Comprehensive Cancer Center, Columbus, OH. After obtaining maternal donor consent, fresh blood was collected from the umbilical cord vein using the method previously described in the National Heart, Lung and Blood Institute (NHLBI) for Cord Blood Transplantation. Low density bone marrow (BM) or cord blood (CB) mononuclear cells (MNC) were isolated by Ficoll-Hypaque density gradient centrifugation (1.077 g/ml) (Sigma, St Louis, MO USA) and washed twice in Iscove's Modified Dulbecco's Medium (IMDM) with Penicillin (100 U/mL), streptomycin (100 mg/mL) and Amphotericin B (0.25 mg/mL) (Gibco Laboratories, Grand Island, NY USA). BM MNC and CB MNC from each donor were enriched for CD34+ or CD34+CD38- cells by using magnetic cell sorting (Miltenyi Biotec Inc., Auburn, CA USA) according to the manufacturer's instructions. All protocols were approved from the Human Subjects Committee of the University of Nevada, Reno.
Clonogenic assays for hematopoietic stem/progenitor cells
Clonogenic assays for hematopoietic stem/progenitors were performed in triplicate using MethoCult GF H4434 (Stem Cell Technologies Inc., Vancouver, BC Canada) according to the manufacturer's instructions. A total of 105 mononuclear cells, 104 CD34+ cells, 103 CD34+CD38- cells from CB, or BM were cultured with or without DHA (Sigma, St. Louis, MO) at concentrations of 50, 100 or 150 μM, and incubated in a humidified incubator at 37°C in 5% CO2 in air. After 14 days of incubation, the colonies (BFU-E, CFU-GM, and CFU-Mix) were enumerated and categorized according to standard criteria.
Statistical analysis
All the results are expressed as mean ± standard error of the mean (SEM). Student's t-test and Mann-Whitney test were used to determine statistical significance. Statistical significance was defined as p < 0.05.
Acknowledgments
HL70566, HL73737, National Institutes of Health.
Abbreviations
- DHA
docosahexaenoic acid
- AML
acute myeloid leukemia
- HSC
hematopoietic stem/progenitor cell
- KG1a
human caucasian bone marrow acute myelogenous leukaemia
- Bax
BCL2-associated X protein
- BCL-2
B-cell CLL/lymphoma 2, integral outer mitochondrial membrane protein that blocks the apoptotic death of some cells
- PI
propidium iodide
References
- 1.Baumgartner M, Sturlan S, Roth E, Wessner B, Bachleitner-Hofmann T. Enhancement of arsenic trioxide-mediated apoptosis using docosahexaenoic acid in arsenic trioxide-resistant solid tumor cells. Int J Cancer. 2004;112:707–12. doi: 10.1002/ijc.20462. [DOI] [PubMed] [Google Scholar]
- 2.Das UN. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol J. 2006;1:420–39. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]
- 3.Moyad MA. An introduction to dietary/supplemental omega-3 fatty acids for general health and prevention: Part II. Urol Oncol. 2005;23:36–48. doi: 10.1016/j.urolonc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Kato T, Kolenic N, Pardini RS. Docosahexaenoic acid (DHA), a primary tumor suppressive omega-3 fatty acid, inhibits growth of colorectal cancer independent of p53 mutational status. Nutr Cancer. 2007;58:178–87. doi: 10.1080/01635580701328362. [DOI] [PubMed] [Google Scholar]
- 5.Pardini RS, Wilson D, Schiff S, Bajo SA, Pierce R. Nutritional intervention with omega-3 Fatty acids in a case of malignant fibrous histiocytoma of the lungs. Nutr Cancer. 2005;52:121–9. doi: 10.1207/s15327914nc5202_2. [DOI] [PubMed] [Google Scholar]
- 6.Aires V, Hichami A, Filomenko R, Ple A, Rebe C, Bettaieb A, et al. Docosahexaenoic acid induces increases in [Ca2+]i via inositol 1,4,5-triphosphate production and activates protein kinase C gamma and -delta via phosphatidylserine binding site: Implication in apoptosis in U937 cells. Mol Pharmacol. 2007;72:1545–56. doi: 10.1124/mol.107.039792. [DOI] [PubMed] [Google Scholar]
- 7.Kong X, Ge H, Hou L, Shi L, Liu Z. Induction of apoptosis in K562/ADM cells by gammalinolenic acid involves lipid peroxidation and activation of caspase-3. Chem Biol Interact. 2006;162:140–8. doi: 10.1016/j.cbi.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Miura Y, Takahara K, Murata Y, Utsumi K, Tada M, Takahata K. Docosahexaenoic acid induces apoptosis via the Bax-independent pathway in HL-60 cells. Biosci Biotechnol Biochem. 2004;68:2415–7. doi: 10.1271/bbb.68.2415. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Vosseler CA, Weber PC, Erl W. Docosahexaenoic acid induces apoptosis in proliferating human endothelial cells. J Cell Physiol. 2005;204:881–8. doi: 10.1002/jcp.20351. [DOI] [PubMed] [Google Scholar]
- 10.Chiu LCM, Wong EYL, Ool VEC. Docosahexaenoic acid modulates different genes in cell cycle and apoptosis to control grouwth of human leukemia HL-60 cells. Int J Oncol. 2004;25:737–44. [PubMed] [Google Scholar]
- 11.de Lima TM, Amarante-Mendes GP, Curi R. Docosahexaenoic acid enhances the toxic effect of imatinib on Bcr-Abl expressing HL-60 cells. Toxicol in vitro. 2007;21:1678–85. doi: 10.1016/j.tiv.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Kinsella JE, Black JM. Effects of polyunsaturated fatty acids on the efficacy of antineoplastic agents toward L5178Y lymphoma cells. Biochem Pharmacol. 1993;45:1881–7. doi: 10.1016/0006-2952(93)90447-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Li L, Jiang W, Yang Z, Zhang Z. Synthesis and preliminary antitumor activity evaluation of a DHA and doxorubicin conjugate. Bioorg Med Chem Lett. 2006;16:2974–7. doi: 10.1016/j.bmcl.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 14.Sturlan S, Baumgartner M, Roth E, Bachleitner-Hofmann T. Docosahexaenoic acid enhances arsenic trioxide-mediated apoptosis in arsenic trioxide-resistant HL-60 cells. Blood. 2003;101:4990–7. doi: 10.1182/blood-2002-08-2391. [DOI] [PubMed] [Google Scholar]
- 15.Lu C, Hassan HT. Human stem cell factor-antibody [anti-SCF] enhances chemotherapy cytotoxicity in human CD34+ resistant myeloid leukaemia cells. Leuk Res. 2006;30:296–302. doi: 10.1016/j.leukres.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Krause DS, Van Etten RA. Right on target: Eradicating leukemic stem cells. Trends Mol Med. 2007;13:470–81. doi: 10.1016/j.molmed.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 18.Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, et al. Human acute myeloid leukemia CD34+/CD38- progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403–11. [PubMed] [Google Scholar]
- 19.Koeffler HP. Induction of differentiation of human acute myelogenous leukemia cells: Therapeutic implications. Blood. 1983;62:709–21. [PubMed] [Google Scholar]
- 20.Burns CP, Halabi S, Clamon GH, Hars V, Wagner BA, Hohl RJ, et al. Phase I clinical study of fish oil fatty acid capsules for patients with cancer cachexia: Cancer and leukemia group B study 9473. Clin Cancer Res. 1999;5:3942–7. [PubMed] [Google Scholar]
- 21.Pereira C, Clarke E, Damen J. Hematopoietic colony-forming cell assays. Methods Mol Biol. 2007;407:177–208. doi: 10.1007/978-1-59745-536-7_14. [DOI] [PubMed] [Google Scholar]
- 22.Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks GM. A functional comparison of CD34+ CD38- cells in cord blood and bone marrow. Blood. 1995;86:3745–53. [PubMed] [Google Scholar]
- 23.Cardoso AA, Li ML, Batard P, Hatzfeld A, Brown EL, Levesque JP, et al. Release from quiescence of CD34+ CD38- human umbilical cord blood cells reveals their potentiality to engraft adults. Proc Natl Acad Sci U S A. 1993;90:8707–11. doi: 10.1073/pnas.90.18.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu LC, Wong EY, Ooi VE. Docosahexaenoic acid from a cultured microalga inhibits cell growth and induces apoptosis by upregulating Bax/Bcl-2 ratio in human breast carcinoma MCF-7 cells. Ann N Y Acad Sci. 2004;1030:361–8. doi: 10.1196/annals.1329.045. [DOI] [PubMed] [Google Scholar]
- 25.Tsujita-Kyutoku M, Yuri T, Danbara N, Senzaki H, Kiyozuka Y, Uehara N, et al. Conjugated docosahexaenoic acid suppresses KPL-1 human breast cancer cell growth in vitro and in vivo: Potential mechanisms of action. Breast Cancer Res. 2004;6:291–9. doi: 10.1186/bcr789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banker DE, Groudine M, Willman CL, Norwood T, Appelbaum FR. Cell cycle perturbations in acute myeloid leukemia samples following in vitro exposures to therapeutic agents. Leuk Res. 1998;22:221–39. doi: 10.1016/s0145-2126(97)00174-4. [DOI] [PubMed] [Google Scholar]
- 27.Garrido SM, Willman C, Appelbaum FR, Banker DE. Three-color versus four-color multi-parameter cell cycle analyses of primary acute myeloid leukemia samples. Cytometry. 2000;42:83–94. doi: 10.1002/(sici)1097-0320(20000415)42:2<83::aid-cyto1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]


