Abstract
Purpose.
To develop and evaluate automated computerized algorithms for differentiation of normal and keratoconus corneas based solely on epithelial and stromal thickness data.
Methods.
Maps of the corneal epithelial and stromal thickness were generated from Artemis-1 very high-frequency ultrasound arc-scans of 130 normal and 74 keratoconic subjects diagnosed by combined topography and tomography examination. Keratoconus severity was graded based on anterior curvature, minimum corneal thickness, and refractive error. Computer analysis of maps produced 161 features for one randomly selected eye per subject. Stepwise linear discriminant analysis (LDA) and neural network (NN) analysis were then performed to develop multivariate models based on combinations of selected features to correctly classify cases. The sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) were determined for each classifier.
Results.
Stepwise LDA resulted in a six-variable model that provided an AUC of 100%, indicative of complete separation of keratoconic from normal corneas. Leave-one-out analysis resulted in 99.2% specificity and 94.6% sensitivity. Neural network analysis using the same six variables resulted in an AUC of 100% for the training set. Test set performance averaged over 10 trials gave a specificity of 99.5 ± 1.5% and sensitivity of 98.9 ± 1.9%. The LDA function values correlated with keratoconus severity grade.
Conclusions.
The results demonstrate that epithelial remodeling in keratoconus represents an independent means for differentiation of normal from advanced keratoconus corneas.
Keywords: keratoconus, pachymetry, high-frequency ultrasound, corneal epithelium
Features automatically extracted from layered epithelial and stromal pachymetric maps generated from three-dimensional high-resolution digital ultrasound scans of 130 normal and 74 keratoconic corneas were analyzed by multivariate classifiers. A six-variable model provided 100% classification accuracy of keratoconus from normal.
Introduction
Keratoconus is a progressive, noninflammatory corneal dystrophy that manifests as corneal thinning and formation of a cone-shaped protrusion. Because laser refractive surgery may lead to accelerated postoperative ectasia,1,2 the accurate detection of early keratoconus is a major safety concern. The prevalence of keratoconus in the Caucasian population is approximately 1/2000.3 The incidence of undiagnosed keratoconus presenting to refractive surgery clinics tends to be much higher than this, as keratoconics develop astigmatism that is more difficult to correct by contact lenses or glasses, leading them to consider refractive surgery.4 The challenge for keratoconus screening is to have high sensitivity but for this to be combined with high specificity to minimize the number of atypical normal patients who are denied surgery.
Significant efforts have been made to develop methods for screening of early keratoconus over the last 30 years. In 1984, Klyce5 described generation of corneal surface topographic maps from digitized keratoscope photos and demonstrated severe distortion from sphericity in keratoconus compared to emmetropic eyes. Placido-based instruments producing maps of anterior surface topography and curvature became available by the early 1990s, and their use in keratoconus screening has been demonstrated.6–9 Characterization of corneal thickness and topography of both corneal surfaces using scanning slit tomography was introduced commercially in the mid-1990s by the Orbscan (Bausch & Lomb, Rochester, NY) scanning slit system10–12 and later by the Pentacam (Oculus Optikgeräte, Wetzlar, Germany) Scheimpflug-based system.13,14 Wavefront assessment15 and the Ocular Response Analyzer16 have been employed as a means for detecting early keratoconus.
The current standard for keratoconus screening for refractive surgery varies across practices, but in general includes a thorough clinical history, pachymetry, topography, and tomography.17 Topographic and tomographic evaluation has evolved from qualitative observation8 to quantitative measurements, and many parameters have been described to aid the differentiation of normal from keratoconus eyes.18,19 Several statistical and machine-based or computerized learning models have been employed for keratoconus detection, and automated systems for screening based on front and back surface topography and whole cornea tomography have been developed.7,20–26
Although these approaches have improved the effectiveness of keratoconus screening, there still remain equivocal cases in which a confident diagnosis cannot be made. The addition of quantitative parameters that are independent of those now obtained by topographic and tomographic analysis could potentially improve screening.
The corneal epithelial and stromal thickness profiles may represent such an independent parameter. Reinstein et al.27 described how the epithelial thickness profile, derived from high-frequency ultrasound scans, remodels in response to underlying stromal irregularities, for example, thickening over a LASIK ablation zone. Similar findings have been reported using confocal microscopy,28 optical coherence tomography (OCT),29 ultrasound,30 and slit optical pachometry.31 After describing normal epithelial32 and stromal thickness33 profiles, Reinstein et al.34 reported keratoconic corneas to have a donut epithelial pattern characterized by compensatory thinning over the localized stromal surface cone with a surrounding annulus of thicker epithelium. This was later supported in a study by Vinciguerra et al.,35 who reported higher anterior curvature in keratoconus corneas with epithelium removed (prior to cross-linking) than with epithelium intact. It is also confirmed by a recent study by Li et al.,36 who described differences in the epithelial thickness pattern in normal versus keratoconus corneas based on spectral-domain OCT data, albeit for data only within the central 6-mm diameter. Reinstein et al.37 suggested that epithelial remodeling may be great enough in early keratoconus to mask stromal surface changes from anterior surface topography, but might also serve as an early disease indicator.
In this study, we developed a series of quantitative parameters aimed at capturing the patterns in epithelial and stromal thickness maps of keratoconic eyes described above. We evaluated the effectiveness of multivariate classifiers to distinguish normal and advanced keratoconic corneas based solely on epithelial and stromal thickness maps. This study represents a first step of deriving quantitative parameters from epithelial thickness data as a proof of concept in known keratoconus eyes; the second step will be application of the algorithm to equivocal cases, which will be carried out in a future study.
Methods
We randomly selected and analyzed one eye from 130 normal subjects and one eye from 74 consecutive keratoconic subjects from a population of patients seeking refractive surgery at the London Vision Clinic. All subjects were scanned with the Artemis-1 (ArcScan, Inc., Morrison, CO) very high-frequency (VHF) digital ultrasound system.
Preoperative assessment of all patients included manifest refraction, logMAR corrected distance visual acuity (CDVA) (CSV-1000; Vector Vision, Inc., Greenville, OH), and cycloplegic refraction using one drop of tropicamide 1% (Alcon Laboratories UK Ltd., Hemel Hempstead, UK. Tomography was assessed using the Orbscan II (Bausch & Lomb, Claremont, CA), and topography and simulated keratometry (K) were assessed using the Atlas corneal topography system (Carl Zeiss Meditec AG, Dublin, CA). Dynamic pupillometry was carried out using the Procyon P2000 pupillometer (Haag-Streit, Bern, Switzerland). Wavefront assessment was performed using the WASCA aberrometer (Carl Zeiss Meditec AG). Single-point pachymetry was performed with the Corneo-Gage Plus (50 MHz) handheld ultrasound pachymeter (Sonogage, Cleveland, OH) by determining the minimum of 10 consecutive central total corneal measurements. Three-dimensional epithelial, stromal, and total corneal thickness for the central 8- to 10-mm diameter was measured using the Artemis-1 technology.
Keratoconus diagnostic criteria included (a) patients previously diagnosed with keratoconus and (b) patients whose diagnosis was confirmed by clinical signs of keratoconus such as microscopic signs at the slit lamp, corneal topographic changes, high refractive astigmatism, reduced CDVA and contrast sensitivity, and significant level of higher-order aberrations, in particular coma. The population included eyes at different stages of the disease, ranging from cases with indisputable keratoconus by topography, but without microscopic signs at the slit lamp, to extreme cases approaching the need for corneal transplant, demonstrating clear microscopic signs such as Vogt striae and substantial corneal thinning. In patients in whom the second eye demonstrated suspected keratoconus (i.e., a diagnosis of keratoconus could not be confirmed), the second eye was excluded. Patients with pellucid marginal degeneration and eyes with pathology other than ectatic degeneration (e.g., corneal scarring) or hydrops were also excluded.
Contact lens wearers (65% of normal and 45% of keratoconus eyes) were not excluded; however, the majority of these had not worn their contact lenses over a suitable washout period (i.e., 1 week for soft contact lenses and 1 month per decade of wear for rigid contact lenses). In total, only 8% of normal eyes and 27% of keratoconus eyes had worn contact lenses within the washout period.
Keratoconus eyes were graded by the Krumeich criteria,38 which depend on mean anterior curvature K-readings, minimum corneal thickness, and refractive error.
The research followed the tenets of the Declaration of Helsinki. Informed consent for research, analysis, and publication of data was obtained from patients. The research was conducted under protocols approved by Western Institutional Review Board and the Institutional Review Board of the Columbia University Medical Center.
Artemis-1 Scanning and Postprocessing
Artemis-1 scanning is performed with the patient in a sitting position. The eye is acoustically coupled to the focused 50-MHz ultrasound transducer via a normal saline-filled chamber. With the patient gazing at a fixation light, scans are centered on the vertex based on simultaneous camera views of the pupil and adjustment for maximum echo amplitude, which occurs in the focal plane at normal incidence. Scans are performed by moving the transducer in an arc approximately matching corneal curvature so that the cornea is maintained in the focal zone. Phase-resolved echo data were acquired in four meridional B-scans (128 vectors over a 60° arc) at 45° increments, comprising eight semimeridians. Scan data were then analyzed with ArtPro software, which was developed in our laboratories. ArtPro processes digitized phase-resolved ultrasound data that are stored during the examination in the context of scan geometry parameters attached to each record. The software automatically identifies each surface of the cornea and processes each scan line using Fourier methods (deconvolution and analytic signal envelope detection) to optimally determine the positions of the anterior and posterior surfaces as well as Bowman's membrane.23 From these data, epithelial and stromal thickness are determined along each scan line using speed-of-sound constant of 1640 m/s to convert time-delay measurements to thickness values. A polar/radial interpolation function is applied to produce a 10- × 10-mm Cartesian matrix (ordered horizontally from temporal to nasal) depicting the epithelial and stromal thicknesses in 0.1-mm steps.
Feature Extraction
The epithelial and stromal thickness maps were processed in MATLAB version 7.11 (The MathWorks, Inc., Natick, MA). Maps were smoothed by averaging over 400-μm-diameter spots. The zone within a 3.5-mm radius of the center of the scan (i.e., corneal vertex of the coaxially fixating eye) was then processed for feature extraction. Features included values and locations of minimum and maximum corneal, epithelial, and stromal thickness and automatic detection of the epithelial pattern described by Reinstein et al.34 as characteristic of keratoconus, that is, with central thinning and surrounded by thickened epithelium. To characterize this pattern, six 0.5-mm-thick (outer–inner radius) annular rings centered on the thinnest points in the epithelium were delineated. The mean epithelial thickness of each ring, its standard deviation (SD), and the difference between the ring's mean thickness and the central, minimum epithelial thickness in absolute and normalized SD units were calculated. The gradients of mean epithelial and stromal thicknesses in the six annuli were also calculated. We also determined the thicknesses of ring segments in the superior and inferior quadrants. A total of 161 variables were extracted. A summary of the variables is provided in Table 1.
Table 1.
Parameters Extracted From Layered Corneal Pachymetric Maps and Entered Into Stepwise Multivariate Analysis for Distinguishing Normal From Keratoconus Corneas
|
Variable Description |
N
Variables |
| Minimum and maximum thickness of cornea, stroma, and epithelium, and x, y positions | 18 |
| Mean, median, SD, and difference from minimum in μm and SD units of thicknesses of cornea, stroma, and epithelium | 24 |
| Number of points on corneal, stromal, and epithelial thickness > 2 SD from value of mean normal map at equivalent position | 3 |
| Mean epithelial thickness superiorly and inferiorly and their difference | 3 |
| Distance between minimum thickness locations of epithelium and stroma | 1 |
| Mean, SD, and difference from minimum in μm and SD units of each of six epithelial thickness annuli centered on point of minimum epithelial thickness | 24 |
| Mean, SD, and difference from minimum in μm and SD units of superior half of each of six epithelial thickness annuli centered on point of minimum epithelial thickness | 24 |
| Mean, SD, and difference from minimum in μm and SD units of inferior half of epithelial thickness annuli centered on point of minimum epithelial thickness | 24 |
| Mean, SD, and difference from minimum in μm and SD units of each of six stromal thickness annuli centered on point of minimum stromal thickness | 24 |
| Rate of change of corneal annuli thickness around thinnest point of cornea | 1 |
| Rate of change of stromal annuli thickness around thinnest point of stroma | 1 |
| Rates of change of epithelial annuli thickness around thinnest point of cornea, stroma, and epithelium | 3 |
| Number of measurement points in total and each quadrant of epithelial thickness map | 5 |
| Difference between mean epithelial thickness in 0.5-mm zone about point of minimum epithelial thickness and mean thickness of full, superior, and inferior epithelium | 3 |
| Difference between mean corneal thickness in 0.5-mm zone about points of minimum corneal thickness and mean thickness of full, superior, and inferior epithelium | 3 |
Statistical Analysis
Statistical procedures were performed using IBM SPSS Statistics, version 21 (IBM Corp., Armonk, NY). A stepwise linear discriminant analysis was performed utilizing known classification (normal versus keratoconus) and the values of all variables as input. The discriminant analysis stepwise variable selection process is designed to establish a set of parameters that in combination make statistically significant independent contributions to the classifier. We used a probability-for-entry criterion of P = 0.01 rather than the default P = 0.05 to make the process particularly stringent given the large number of variables. The pooled covariance matrix was used, and the a priori classification probability was set equal for both groups. Receiver operating characteristic (ROC) analysis was then performed based on the discriminant function output and the area under the ROC curve (AUC) determined. The analysis included a leave-one-out validation procedure to estimate prospective performance of the classifier.
Neural network analysis was performed next to assess the relative effectiveness of a nonlinear classifier. A multilayer perceptron with radial basis kernel was used with the same variables selected by stepwise linear discriminant analysis as input and diagnosis as output. Variable values were standardized before input to the neural network by subtraction of the mean and division by the SD. The analysis divided the database into training and test sets consisting of randomly selected 70% and 30% of cases, respectively. Sensitivity and specificity were determined for both sets; while training set classification accuracy may be influenced by overfitting, test set performance reflects how the classifier would be expected to perform with unknown cases. The analysis, including ROC analysis, was repeated 10 times, each with different randomized test and training sets and differently randomized initial link weights. Lastly, we performed the analysis with all cases included in the training set. This procedure will produce a classifier based on and constrained by all available data, but does not allow assessment of prospective performance since there is no independent test set.
We examined the dependence of the linear discriminant classifier on severity of keratoconus by determining the average linear discriminant function value among normal subjects and among keratoconus subjects categorized by Krumeich grade.
Results
Figure 1 shows representative examples of epithelial and stromal thickness maps for one normal and one keratoconus eye. Figure 2 shows pachymetric maps produced by averaging epithelial and stromal thickness maps for all subjects in each population.
Figure 1.
Examples of epithelial and stromal thickness maps for a representative normal and a keratoconus cornea. The horizontal (x) scale is plotted from temporal (T) to nasal (N). In the normal eye, the epithelium is somewhat thickened inferiorly to a maximum of approximately 60 μm, with central thickness of approximately 52 μm. The stroma is thinnest centrally. In the keratoconus eye, a prominent epithelial defect is located 1 mm inferior to center, with the epithelium measuring only 35 μm. The epithelium thickens concentrically about the defect, reaching a maximum of 70 μm superiorly. The stroma shows a defect coincident with the position of the epithelial defect, where it is approximately 350 μm in thickness.
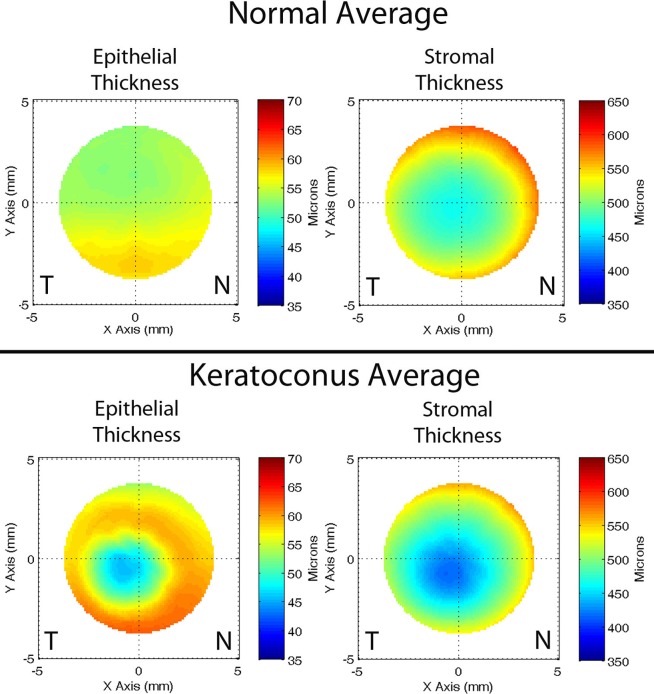
Figure 2.
Epithelial and stromal thickness maps averaged over all normal (n = 130) and keratoconus (n = 74) eyes. The horizontal (x) scale is plotted from temporal (T) to nasal (N). The average maps show patterns much like those shown in Figure 1 in representative individual eyes. The average normal map shows a smooth epithelium, with slight thickening inferiorly. The normal stroma is smooth and symmetric. In contrast, the average keratoconus cornea shows a defect inferotemporally characterized by epithelial thinning, with a surrounding annulus of thickened epithelium. The stroma shows a thinning defect at approximately the same position as the epithelial defect.
Stepwise linear discriminant analysis resulted in a model consisting of six variables (Table 2) with a χ2 of 317.0 (P < 0.001). The canonical discriminant function values for normal and keratoconic corneas showed no overlap, ranging from −5.482 to −0.091 for keratoconic corneas and from 0.082 to 3.390 in normal corneas, and this is reflected in an AUC of 100%. Linear discriminant classifications demonstrated a sensitivity of 95.9% and specificity of 100%. Leave-one-out validation analysis, in which each case is serially treated as an unknown, produced a sensitivity of 94.6% and specificity of 99.2%.
Table 2.
Variables Selected Into Multivariate Model by Stepwise Linear Discriminant Analysis and Their Univariate Statistical Significance in Discrimination of Normal From Clinical Keratoconic Corneas
|
Variable |
Univariate
F |
P
Value |
| Vertical position of minimum corneal thickness, mm | 77.6 | <0.001 |
| Horizontal position of point of minimum stromal thickness, mm | 9.9 | 0.002 |
| Distance between locations of minimum epithelial and stromal thickness, mm | 109.6 | <0.001 |
| Mean thickness of first superior epithelial annulus minus minimum epithelial thickness, SD units | 82.5 | <0.001 |
| Standard deviation of second epithelial annulus, μm | 7.6 | 0.006 |
| Mean thickness of fourth epithelial annulus minus minimum epithelial thickness, μm | 287.2 | <0.001 |
Annuli refer to concentric 0.5-mm-wide rings centered on the point of minimum epithelial thickness.
After 10 trials, neural network AUC averaged 100% ± 0.0% with a classification sensitivity and specificity of 99.4 ± 0.9% and 99.9 ± 0.4%, respectively. The validation set sensitivity and specificity averaged 98.9 ± 1.9% and 99.5 ± 1.5%, respectively. When all cases were included in the training set, 100% classification accuracy was achieved.
Sensitivity and specificity for linear discriminant analysis and neural networks are summarized in Table 3. Mean epithelial thickness maps for normal corneas and each keratoconus grade are presented in Figure 3, demonstrating increasing qualitative deviation from the norm with keratoconus severity. Mean discriminant function values for each of these groups are presented in Table 4 and plotted in Figure 4. Analysis of variance among the four grades of keratoconus eyes demonstrated significant departure from a random distribution (F = 8.53, P < 0.001). The discriminant score, which is positive for normal eyes, becomes increasingly negative with keratoconus severity grade (Spearman's rho = −0.500, P < 0.001).
Table 3.
Performance of Stepwise Linear Discriminant and Neural Network Models
|
Model |
Classification, Training Set |
Validation |
|||
|
Sensitivity, % |
Specificity, % |
AUC, % |
Sensitivity, % |
Specificity, % |
|
| LDA | 95.9 | 100.0 | 100.0 | 94.6 | 99.2 |
| NN | 99.4 ± 0.9 | 99.9 ± 0.4 | 100.0 ± 0.0 | 98.9 ± 1.9 | 99.5 ± 1.5 |
| NN′ | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | - | - |
Validation results refer to leave-one-out analysis for discriminant analysis and test set results for the neural network. Neural network results are the average of 10 repeated analyses ± 1 SD. Each analysis had randomized initial conditions, including training sets (70% of cases) and test sets (30% of cases) with the exception of the last row (NN′), in which all cases were included in the training set.
Figure 3.

Epithelial thickness maps averaged over all normal corneas and each keratoconus (KC) grade. The departure from the normal epithelial distribution is evident even in grade 1 KC, but becomes more obvious with severity.
Table 4.
Mean Discriminant Function Scores and Standard Deviations for Normal Cases (Grade 0) and Keratoconus Eyes Graded From 1 to 4 Using the Krumeich Criteria
|
Grade |
Km, D |
Minimum Thickness, μm |
Spherical Equivalent, D |
Scarring |
N |
Mean Discriminant Score |
SD |
| 0 | 130 | 1.49 | 0.75 | ||||
| 1 | ≤48 | >500 | ≤−5 | None | 11 | −1.46 | 1.18 |
| 2 | 48–53 | 400–500 | −5 to −8 | None | 44 | −2.46 | 1.15 |
| 3 | 54–55 | 200–400 | −8 to −10 | None | 15 | −3.60 | 1.11 |
| 4 | >55 | ≤200 | Not measurable | Central | 4 | −3.68 | 1.59 |
| All keratoconus | 74 | −2.61 | 1.34 |
Km, mean corneal curvature; D, diopters.
Figure 4.
Box and whisker plot of discriminant function value versus keratoconus severity grade. Grade 0 represents normal subjects. Grades 1 to 4 are based on Krumeich classification as defined in Table 4. Boxes represent ±1 quartile about median value (horizontal line), and whiskers represent full range of values for each group. Circles indicate outliers.
Discussion
The aim of this study was to develop and evaluate automated computerized algorithms for differentiation of normal and keratoconus corneas based solely on epithelial and stromal thickness data. We previously demonstrated that qualitative assessment of epithelial and stromal thickness maps derived from high-resolution ultrasound digital scans can help distinguish keratoconus from atypical normal.34 In the current study, we demonstrated that combined quantitative analysis of the epithelial and stromal layers can independently provide information that allows automated differentiation of keratoconic from normal corneas.
We compared linear discriminant analysis with neural networks for classification. Stepwise linear discriminant analysis is advantageous in producing a parsimonious and deterministic classifier, but is restricted to the condition of linear separability. Despite the nonoverlap of discriminant scores between keratoconic and normal corneas, classification was imperfect due to model assumptions regarding Gaussian distributions, equality of covariance matrices, and a priori probabilities for group membership that enter into the classification phase of this parametric procedure. Neural network models are advantageous in their capacity to distinguish nonlinearly separable classes, but do not directly support a stepwise variable selection methodology and are not deterministic in outcome, since the final model depends on gradient descent from randomized initial conditions (link weights and training subset). Linear discriminant function values separated the normal from keratoconus without overlap, although the classifier produced less than perfect results due to Gaussian and Bayesian model assumptions. The neural network, less constrained by such assumptions, gave better classification performance than linear discriminant analysis and perfect classification accuracy when all cases were used for training.
Early automated keratoconus detection systems utilizing Placido-derived video keratometric maps were developed by several investigators. Rabinowitz39 described an index for automated detection of keratoconus based on a combination of central K value, the I–S value (an expression of inferior–superior asymmetry), and SRAX (skew of steepest radial axes). In 1999, Rabinowitz and Rasheed18 demonstrated 100% sensitivity and 98% specificity in identification of clinical keratoconus using the KISA% index, which is derived from the product of the K value, I–S, SRAX, and AST (quantifying regular corneal astigmatism). Maeda et al.7 developed an expert system combining eight indices that provided 89% sensitivity (missing a transplanted cornea) and 99% specificity on a validation set. Mahmoud et al.26 achieved 100% sensitivity and specificity on a validation set of normal and clinically diagnosed keratoconus eyes using a cone location and magnitude index. Smolek and Klyce20 also reported 100% accuracy in automated detection of clinical keratoconus by training a neural network with 10 topographic indices. A potential confounding factor in early Placido-based studies, however, is that classification was based partly on anterior surface curvature. In the present study, cases were classified by clinical, topographic, and tomographic methods that were completely independent of the epithelial and stromal mapping techniques to be evaluated.
Anterior curvature maps may miss early changes that can be detected by elevation-based systems such as the scanning slit-based Orbscan and the rotating Scheimpflug-based Pentacam.40 Differences from the best-fit sphere to either the anterior or posterior surface are particularly useful in depicting ectatic change. Such systems also provide pachymetric maps that are unobtainable by videokeratography. Displacement of the thinnest point from its normally central position, for instance, may be an early indicator of keratoconus. Several studies have taken advantage of the topographic and pachymetric capabilities of scanning slit11,41 and Scheimpflug systems13,14,42–44 to develop improved means for detecting early keratoconus. In particular, the Belin-Ambrosio display of the Pentacam Scheimpflug system provides a detailed quantitative set of measures that characterize the corneal front and back surfaces as well as corneal thickness progression with respect to detecting ectatic changes.13,24,45
While this study was performed with high-frequency ultrasound, spectral-domain OCT has recently been shown capable of imaging and measuring the epithelium and stromal layers in the central cornea.46,47 Epithelial maps produced by this technique were recently shown by Li et al.36 to allow differentiation of normal from advanced keratoconic corneas, which is consistent with our prior observations and current findings. Although the Artemis technology is not currently commercially available, the methods described in the current study could be used or replicated with OCT or any other device that can measure and map epithelium.
In this study, a large percentage of normal and keratoconus subjects were contact lens wearers. In the great majority of cases, a washout period between contact lens use and scanning was present, particularly in normal eyes (92%), in which the potential for misleading epithelial changes is highest. While it is likely that conventional contact lens wear, and warpage in particular, may cause some epithelial change, our findings show that such changes are not sufficient to prevent differentiation of advanced keratoconus from normal corneas. This was also the case in the OCT study by Li et al.,36 in which contact lens wearers were not excluded. In future studies of forme fruste keratoconus, where more subtle alterations in epithelial thickness will need to be assessed, the effect of contact lens wear may need to be taken into account.
Although the present study was restricted to developing algorithms to differentiate normal from clinically confirmed keratoconus (two groups), we nevertheless found that the resulting discriminant function correlated with keratoconus subgrouped by severity grade. While even grade 1 keratoconus was separated from normal, it is plausible that incomplete separation from normals might occur in forme fruste (as is the case for current topographic and tomographic methods). As the primary clinical need is for early diagnosis of such cases, our method might be advantageously combined with topographic and tomographic methods to achieve a more robust means of early diagnosis. Given that early front stromal surface cones may be fully masked by epithelial compensation, it is clear that the earliest keratoconic changes could in some cases be undetectable by corneal surface topography; while concomitant keratoconic posterior corneal surface changes may be present, the specificity of small back surface abnormalities may not be as sensitive a marker for diagnosing keratoconus. However, the combination of epithelial and stromal thickness parameters, together with corneal back surface shape elements, could prove to be an avenue toward improved automatic recognition of early keratoconus; and this will be the focus of our future research in this area.
Acknowledgments
Supported in part by National Institutes of Health Grants EY019055 and P30 EY019007 and an unrestricted grant to the Department of Ophthalmology of Columbia University from Research to Prevent Blindness.
Disclosure: R.H. Silverman, ArcScan, Inc. (I), P; R. Urs, None; A. RoyChoudhury, None; T.J. Archer, None; M. Gobbe, None; D.Z. Reinstein, ArcScan, Inc. (I), P
References
- 1. Ambrosio R, Wilson SE. Complications of laser in situ keratomileusis: etiology, prevention, and treatment. J Refract Surg. 2001; 17: 350–379 [DOI] [PubMed] [Google Scholar]
- 2. Binder PS. Analysis of ectasia after laser in situ keratomileusis: risk factors. J Cataract Refract Surg. 2007; 33: 1530–1538 [DOI] [PubMed] [Google Scholar]
- 3. Krachmer JH, Feder RF, Belin MW. Keratoconus and related non-inflammatory corneal thinning disorders. Surv Ophthalmol. 1984; 28: 293–322 [DOI] [PubMed] [Google Scholar]
- 4. Wilson SE, Klyce SD. Screening for corneal topographic abnormalities before refractive surgery. Ophthalmology. 1994; 101: 145–152 [DOI] [PubMed] [Google Scholar]
- 5. Klyce SD. Computer-assisted corneal topography. High-resolution graphic presentation and analysis of keratoscopy. Invest Ophthalmol Vis Sci. 1984; 25: 1426–1435 [PubMed] [Google Scholar]
- 6. Wilson SE, Lin DT, Klyce SD. Corneal topography of keratoconus. Cornea. 1991; 101: 2–8 [PubMed] [Google Scholar]
- 7. Maeda N, Klyce SD, Smolek MK, Thompson HW. Automated keratoconus screening with corneal topography analysis. Invest Ophthalmol Vis Sci. 1994; 35: 2749–2757 [PubMed] [Google Scholar]
- 8. Rabinowitz YS, McDonnell PJ. Computer-assisted corneal topography in keratoconus. Refract Corneal Surg. 1989; 5: 400–408 [PubMed] [Google Scholar]
- 9. Holladay JT. Keratoconus detection using corneal topography. J Refract Surg. 2009; 25: S958–S962 [DOI] [PubMed] [Google Scholar]
- 10. Auffarth GU, Wang L, Volcker HE. Keratoconus evaluation using the Orbscan Topography System. J Cataract Refract Surg. 2000; 26: 222–228 [DOI] [PubMed] [Google Scholar]
- 11. Rao SN, Raviv T, Majmudar PA, Epstein RJ. Role of Orbscan II in screening keratoconus suspects before refractive corneal surgery. Ophthalmology. 2002; 109: 1642–1646 [DOI] [PubMed] [Google Scholar]
- 12. Tomidokoro A, Oshika T, Amano S, et al. Changes in anterior and posterior corneal curvatures in keratoconus. Ophthalmology. 2000; 107: 1328–1332 [DOI] [PubMed] [Google Scholar]
- 13. Ambrosio R, Alonso RS, Luz A, Velarde LGC. Corneal-thickness spatial profile and corneal-volume distribution: tomographic indices to detect keratoconus. J Cataract Refract Surg. 2006; 32: 1851–1859 [DOI] [PubMed] [Google Scholar]
- 14. de Sanctis U, Loiacono C, Richiardi L, et al. Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratoconus/subclinical keratoconus. Ophthalmology. 2008; 115: 1534–1539 [DOI] [PubMed] [Google Scholar]
- 15. Saad A, Gatinel D. Evaluation of total and corneal wavefront high order aberrations for the detection of forme fruste keratoconus. Invest Ophthalmol Vis Sci. 2012; 53: 2978–2992 [DOI] [PubMed] [Google Scholar]
- 16. Zarei-Ghanavati S, Ramirez-Miranda A, Yu F, Hamilton DR. Corneal deformation signal waveform analysis in keratoconic versus post-femtosecond laser in situ keratomileusis eyes after statistical correction for potentially confounding factors. J Cataract Refract Surg. 2012; 38: 607–614 [DOI] [PubMed] [Google Scholar]
- 17. Gatinel D, Saad A. The challenges of the detection of subclinical keratoconus at its earliest stage. Int J Keratoco Ectatic Corneal Dis. 2012; 1: 36–43 [Google Scholar]
- 18. Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg. 1999; 25: 1327–1335 [DOI] [PubMed] [Google Scholar]
- 19. Klyce SD, Smolek MK, Maeda N. Keratoconus detection with the KISA% method-another view. J Cataract Refract Surg. 2000; 26: 472–474 [DOI] [PubMed] [Google Scholar]
- 20. Smolek MK, Klyce SD. Current keratoconus detection methods compared with a neural network approach. Invest Ophthalmol Vis Sci. 1997; 38: 2290–2299 [PubMed] [Google Scholar]
- 21. Accardo PA, Pensiero S. Neural network-based system for early keratoconus detection from corneal topography. J Biomed Inform. 2002; 35: 151–159 [DOI] [PubMed] [Google Scholar]
- 22. Klyce SD, Karon MD, Smolek MK. Screening patients with the corneal navigator. J Refract Surg. 2005; 21: 617–622 [DOI] [PubMed] [Google Scholar]
- 23. Souza MB, Medeiros FW, Souza DB, et al. Evaluation of machine learning classifiers in keratoconus detection from Orbscan II examinations. Clinics (Sao Paulo). 2010; 65: 1223–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ambrosio R, Caiado AL, Guerra FP, et al. Novel pachymetric parameters based on corneal topography for diagnosing keratoconus. J Refract Surg. 2011; 27: 753–758 [DOI] [PubMed] [Google Scholar]
- 25. Saad A, Gatinel D. Topographic and tomographic properties of forme fruste keratoconus corneas. Invest Ophthalmol Vis Sci. 2010; 51: 5546–5555 [DOI] [PubMed] [Google Scholar]
- 26. Mahmoud AM, Roberts CJ, Lembach RG, et al. CLMI: the cone location and magnitude index. Cornea. 2008; 27: 480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reinstein DZ, Silverman RH, Coleman DJ. Very high-frequency ultrasound corneal analysis identifies anatomic correlates of optical complications of lamellar refractive surgery. Ophthalmology. 1999; 105: 474–482 [DOI] [PubMed] [Google Scholar]
- 28. Erie JC, Patel SV, McLaren JW, et al. Effect of myopic laser in situ keratomileusis on epithelial and stromal thickness. A confocal microscopy study. Ophthalmology. 2002; 109: 1447–1452 [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Thomas J, Cox I, Rollins A. Noncontact measurements of central corneal epithelial and flap thickness after laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2004; 45: 1812–1816 [DOI] [PubMed] [Google Scholar]
- 30. Spadea L, Fasciani R, Necozione S, Balestrazzi E. Role of the corneal epithelium in refractive changes following laser in situ keratomileusis for high myopia. J Refract Surg. 2000; 16: 133–139 [DOI] [PubMed] [Google Scholar]
- 31. Gauthier CA, Holden BA, Epstein D, et al. Role of epithelial hyperplasia in regression following photorefractive keratectomy. Br J Ophthalmol. 1996; 80: 545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reinstein DZ, Archer TJ, Gobbe M, et al. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2008; 24: 571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reinstein DZ, Archer TJ, Gobbe M, et al. Stromal thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2009; 25: 776–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009; 25: 604–610 [DOI] [PubMed] [Google Scholar]
- 35. Vinciguerra P, Albe E, Trazza S, et al. Intraoperative and postoperative effects of corneal collagen cross-linking on progressive keratoconus. Arch Ophthalmol. 2009; 127: 1258–1265 [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Tan O, Brass R, et al. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012; 119: 2425–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reinstein DZ, Gobbe M, Archer TJ, et al. Epithelial, stromal, and total corneal thickness in keratoconus: three-dimensional display with Artemis very-high frequency digital ultrasound. J Refract Surg. 2010; 26: 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krumeich JH, Daniel J, Knulle A. Live-epikeratophakia for keratoconus. J Cataract Refract Surg. 1998; 24: 456–463 [DOI] [PubMed] [Google Scholar]
- 39. Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. J Refract Surg. 1995; 11: 371–379 [DOI] [PubMed] [Google Scholar]
- 40. Belin MW, Khachikian SS. An introduction to understanding elevation-based topography: how elevation data are displayed – a review. Clin Experiment Ophthalmol. 2009; 37: 14–29 [DOI] [PubMed] [Google Scholar]
- 41. Bessho K, Maeda N, Kuroda T, et al. Automated keratoconus detection using height data of anterior and posterior corneal surfaces. Jpn J Ophthalmol. 2006; 50: 409–416 [DOI] [PubMed] [Google Scholar]
- 42. Mihaltz K, Kovacs I, Takacs A, Nagy ZZ. Evaluation of keratometric, pachymetric, and elevation parameters of keratoconic corneas with Pentacam. Cornea. 2009; 28: 976–980 [DOI] [PubMed] [Google Scholar]
- 43. Ucakhan OO, Cetinkor V, Ozkan M, Kanpolat A. Evaluation of Scheimpflug imaging parameters in subclinical keratoconus, keratoconus, and normal eyes. J Cataract Refract Surg. 2011; 37: 1116–1124 [DOI] [PubMed] [Google Scholar]
- 44. Pinero DP, Alio JL, Aleson A, et al. Corneal volume, pachymetry, and correlation of anterior and posterior corneal shape in subclinical and different stages of clinical keratoconus. J Cataract Refract Surg. 2010; 36: 814–825 [DOI] [PubMed] [Google Scholar]
- 45. Belin MW, Ambrósio R. Scheimpflug imaging for keratoconus and ectatic disease. Indian J Ophthalmol. 2013; 61: 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prakash G, Agarwal A, Mazhari AI, et al. Reliability and reproducibility of assessment of corneal epithelial thickness by Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 2580–2585 [DOI] [PubMed] [Google Scholar]
- 47. Ge L, Shen M, Tao A, et al. Automatic segmentation of the central epithelium imaged with three optical coherence tomography devices. Eye Contact Lens. 2012; 38: 150–157 [DOI] [PubMed] [Google Scholar]