Abstract
Purpose.
The purpose of this study was to determine how increasing ocular surface stimulation affected blinking and sensation, while controlling task concentration.
Methods.
Ten healthy subjects concentrated on a task while a custom pneumatic device generated air flow toward the central cornea. Six flow rates (FRs) were randomly presented three times each and subjects used visual analog scales to record their sensory responses. The interblink interval (IBI) and the FR were recorded simultaneously and the IBI, sensory response, and corresponding FR were determined for each trial. The FR associated with a statistically significant decrease in IBI, the blink increase threshold (BIT), was calculated for each subject.
Results.
Both the mean and SD of IBI were decreased with increasing stimulation, from 5.69 ± 3.96 seconds at baseline to 1.02 ± 0.37 seconds at maximum stimulation. The average BIT was 129 ± 20 mL/min flow rate with an IBI of 2.33 ± 1.10 seconds (permutation test, P < 0.001). After log transformation, there was a significant linear function between increasing FR and decreasing IBI within each subject (Pearson's r ≤ −0.859, P < 0.05). The IBI was highly correlated with wateriness, discomfort, and cooling ratings (Pearson's r ≤ −0.606, P < 0.001).
Conclusions.
There was a dose-response–like relationship between increased surface stimulation and blinking in healthy subjects, presumably for protection of the ocular surface. The blink response was highly correlated with ocular surface sensation, which is not surprising given their common origins. The BIT, a novel metric, may provide an additional end point for studies on dry eye or other conditions.
Keywords: blinking, ocular surface stimulation, sensation
Despite the importance of the blink, its control by ocular surface inputremains controversial. In this study, the authors tested how the increasing ocular surface stimulation affected blinking andsensation, while controlling task concentration.
Introduction
Dry eye affects millions in the United States1,2 and worldwide.3,4 It is considered to be a multifactorial condition driven by tear film instability and hyperosmolarity,5 with reduced blink rate as a potential risk factor that may exacerbate the condition through increased tear film evaporation.5,6 The blink acts to spread the tear film and rewet the ocular surface,7–9 so that the quantity and quality of the tear film also may be affected by the blink.10,11 However, despite its importance for ocular surface wetting and the dry eye condition, the ocular surface controls over blinking remain controversial.12–15
Previous studies have shown that the blink rate (BR) or interblink interval (IBI) is affected by central dopamine level,13,16 cognitive state,17,18 and ocular surface input.12,19–21 Reading, working on computer, or other visual tasks requiring concentration are known to decrease blink frequency,19,22–24 whereas irritation or stimulation of the ocular surface increases the BR.12,21,25 The dry eye condition is associated with an increased BR,23,25 presumably due the ocular surface irritation and stress provided by an unstable or hyperosmolar tear film.26,27 Likewise, air blowing to the ocular surface, which induces mechanical force onto and evaporation of the tear film, has been shown to increase BR under a variety of experimental conditions and in subjects with and without dry eye.12,19,21,25 Pneumatic stimulation provides the possibility of a controlled, laboratory-based method to further study the effect of ocular surface stimulation on blinking.
The neural pathways involved in ocular surface controls over reflex blinking arise in ocular surface sensory nerves that project to the motor neuron of the seventh cranial nerve through trigeminal sensory fibers.28,29 Recently, Kaminer et al.14 hypothesized that the spinal trigeminal complex plays an important role in generating the spontaneous blink by modulating direct signals from the ocular surface and indirect signals from the basal ganglia to vary the blink pattern. Furthermore, stimulation of the ocular surface by the dry eye condition, in both humans and animal models, increased both BR and the regularity of blinking.30,31 Thus, ocular surface afferent input appeared to be responsible for increasing blink frequency and regularity.21 However, the effect of varying the level of ocular surface stimulation on BR has not been explored under controlled experimental conditions.
Sensations of eye discomfort also begin with stimulation of ocular surface afferent sensory neurons, although the reflex blink and sensory pathways diverge at the level of the spinal trigeminal complex.32 Although the changes in the BR12,23,25 and symptoms of ocular irritation33–35 are both associated with the dry eye condition, few studies have used human subjects to systematically examine their relationship to each other, despite common origins. Therefore, in this study, we used a human-based laboratory model to investigate the effect of varying levels of pneumatic stimulation of the ocular surface on blinking and ocular sensation. During the study, all subjects were engaged in a visual task to minimize the effect of variations in concentration on blinking to better isolate the effect of ocular surface stimulation. Only young, healthy subjects were included in this study to determine the responses within a normal physiological range (i.e., healthy ocular surface and asymptomatic), thus avoiding the potential variability that putative sensory and other nerve damage associated with dry eye or other conditions could add.36–40
Methods
Subjects
The study was conducted at the Borish Center for Ophthalmic Research at the Indiana University School of Optometry, Bloomington, IN. It adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board at Indiana University. Informed consent was obtained from each subject before beginning the study.
Ten young, healthy subjects were recruited for the study. Subjects reporting ophthalmic disorders, including dry eye, ocular or systemic allergies, any systemic disease, or contact lens wear were excluded.
All subject visits were scheduled for approximately the same time of the day (between 1:00 and 1:30 PM).41 At the beginning of the study, subjects were told that the reason for the study was to examine the tear film while they were engaged a computer task. They were not informed that the purpose included monitoring of blinking until the study was completed to avoid any potential cognitive or affective contaminating effects on blinking.8
Procedures
There was one visit in this study. After filling out the Dry Eye Questionnaire (DEQ)33 to assess habitual symptoms of ocular irritation and dry eye, subjects were seated behind a slit lamp biomicroscope (Zeiss 20SL, ×8 magnification; Carl Zeiss, Oberkochen, Germany) with a custom-attached camera (Basler piA640-210gm, 30 Hz; Basler AG, Ahrensburg, Germany), recording the movement of the upper lid. To quantify eyelid movement, a self-adhesive 2-mm diameter reflective white dot (3M Company, St. Paul, MN) was gently positioned as close as possible to the margin of the right upper lid. Subjects looked straight ahead and played a computer game (Tetris) viewed through a beam splitter. Only the right eye was tested. The other eye was manually held shut by the subject.
An instrument similar to a pneumatic esthesiometer was used to stimulate the cornea with air flow.42,43 It consisted essentially of an air pump (using atmospheric air), a voltage-regulated valve to control the flow rate (FR), an approximately 1-L reservoir to minimize the slight irregularities in flow from the pump, and a sensor measuring the actual flow.44 Air was delivered through a hypodermic syringe with a 0.5-mm diameter mounted on a slit lamp biomicroscope. The air stimulus was aimed toward the center of cornea, but at a slight angle (12 degrees from horizontal and 5 degrees from vertical), so that it did not block the slit lamp view of the cornea or the subject's vision while playing the computer game. The distance between the tip of the syringe and the cornea was 15 mm and its position was constantly monitored by a calibrated side-mounted camera. The FR from the stimulus tip was recorded by a customized LabVIEW 5.1 program (National Instruments, Austin, TX) and time stamps were used to relate stimulus timing with blink data.
To estimate the level of pneumatic stimulus that triggered a higher BR, the stimulus FR was systematically increased from zero every 30 seconds in a step size of 50 mL/min. The experimenter initially (by simple observation) estimated the level that appeared to produce consistently increased BR. After 5 minutes, this procedure was repeated and a final estimate obtained from the average of the two trials. This estimate was then used to set the six levels of pneumatic stimuli to be tested for each individual subject in the study by multiplying this initial estimate with 0, 0.25, 0.50, 0.75, 1.00, and 1.25; thereby estimating a range of sub- to suprathreshold stimuli producing an increased BR for each subject.
A randomly ordered presentation of these six FRs was used to determine the effect of each level of stimulation on the IBI for each subject. Each trial began with no stimulus for 1 minute, continued with a stimulus for 2 minutes, and was followed another minute without the stimulus (Figs. 1, 3). Three sets of randomly presented stimuli (six levels, three repeats, total 18 trials) were applied with at least a 1-minute break between trials. During all testing, overhead lights in the testing room were turned off and infrared light was used to image the lid, in an attempt to avoid reflex blinking and tearing from visible light. The subject was asked to blink twice before each trial began. Immediately after each trial, using visual analog scales (VAS), subjects rated specific ocular sensations (cooling, wateriness, discomfort, burning, and dryness) that they experienced during the stimulus period. Each VAS consisted of a continuous line with zero labeled as no sensation and 10 labeled as the severe sensation. Subjects viewed the VAS on a computer screen and used a mouse to position the cursor on the line to quantify their experience. At the end of the study, a Schirmer's I tear test (without anesthetic) and a fluorescein tear break-up time (TBUT) test were performed.
Figure 1.
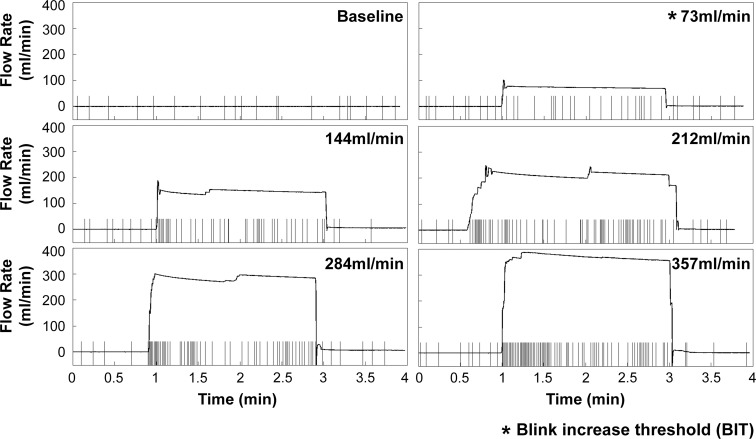
Blink response (Subject 1) to six levels of air stimulation from 0 (baseline) to 305 mL/min. Within each individual graph, the small vertical bars denote the timing of blinks and the horizontal line indicates the flow rate over the trial, with the average flow rate during the central 2 minutes shown at the top right corner. The BIT for this trial was 73 mL/min.
Figure 3.
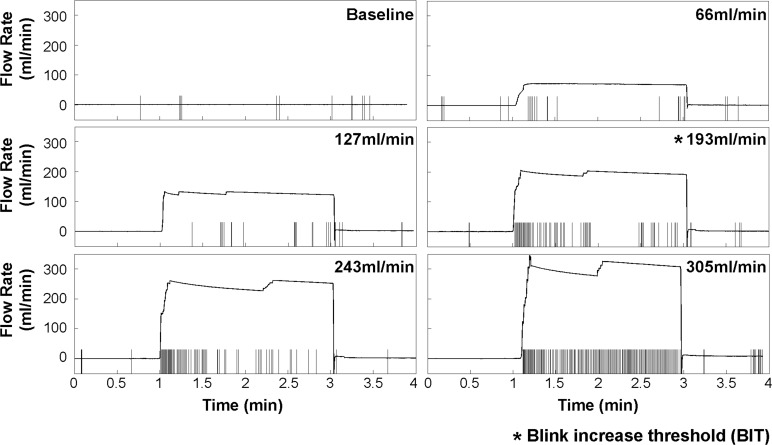
Blink response (Subject 2) to six levels of air stimulation from 0 (baseline) to 357 mL/min. Within each individual graph, the small vertical bars denote the timing of blinks and the horizontal line indicates the flow rate over the trial, with the average flow rate during the central 2 minutes shown at the top right corner. The BIT for this trial is 193 mL/min.
Blink Analysis
A custom MATLAB (The Mathworks, Natick, MA) program was used to track the Purkinje I image,45 located approximately at the center of the cornea. A blink was registered if the Purkinje I image was covered by the eyelid. All the detected blinks, including full and partial blinks that covered the Purkinje image, were treated as identical event markers to measure the temporal pattern of blinks.14 During each trial, the blink activity and FR were overlaid using time stamps and the IBI was calculated.
Statistical Analysis
The IBI data from the three repeated sets were pooled due to the variability inherent in the blink response.14 Because IBIs are often not normally distributed with unbalanced sample size and are highly variable among subjects,17 we used a permutation test46 to determine the FR at which the median of IBI was significantly changed from the baseline for each subject (corrected for multiple comparisons to P ≤ 0.0033). We considered this point, the blink increase threshold (BIT), to mark the level at which there was significant blink change associated with pneumatic stimulation in our model. The Brown-Forsythe test was used to test whether the IBI variability differed among the six stimulus levels within each subject.47
For the sensory VAS data, the three repeated trials were combined for each subject, and the Stevens' power function48 was fitted between FR and sensory rating. We did not test for a “threshold” similar to the BIT for sensory data due to its more subjective nature. The Pearson's correlation coefficient was used to determine associations among the flow rate, IBI, and sensory ratings.
Results
Subjects
The average (±SD) age of study subjects was 23.8 ± 3 years (range, 19–29 years). Five were female and five were male. The median DEQ-5 score49 was 2.5 (range, 0–14), and none of the subjects reported a previous dry eye diagnosis on the DEQ or thought they had dry eye.50 The average (±SD) Schirmer's I tear test and TBUT were 21.2 ± 11.9 mm/5 min (range, 2–41 mm) and 7.22 ± 4.55 seconds (range, 2–58 seconds), respectively. The average (±SD) of the temperature and humidity in exam room were 24.2 ± 0.5°C and 28.4% ± 6.7%, respectively.
Blink Response to Air Stimulation
Figure 1 shows an individual example (Subject 1) of the blink response to six levels of stimulation. During baseline testing, the IBI was irregular with an average (±SD) of 12.5 ± 6.9 seconds. With increasing stimulation, the IBI and its variability markedly decreased from the 73-mL/min to the 357-mL/min air stimulus. Corresponding BRs were 10.5, 19.5, 34.0, 29.0, and 40.5 blinks per minute, respectively. Figure 2A shows a histogram of the IBIs from Subject 1, with pooled the results from the three repeated sets of six stimulus levels. As reported in previous studies, the IBI distribution was asymmetric with a J-shaped positive skew at baseline.51–53 The distribution becomes less skewed with more short IBIs and fewer long IBIs while increasing stimulation. Figure 2B shows that both IBI and its variability decreased with increasing stimulation. Log transformation (Figs. 2C, 2D) of IBI data reduces the skew of the data and it appears to be a normal distribution,52 thus improving visualization and showing a leftward shift in IBI as stimulation increased.
Figure 2.
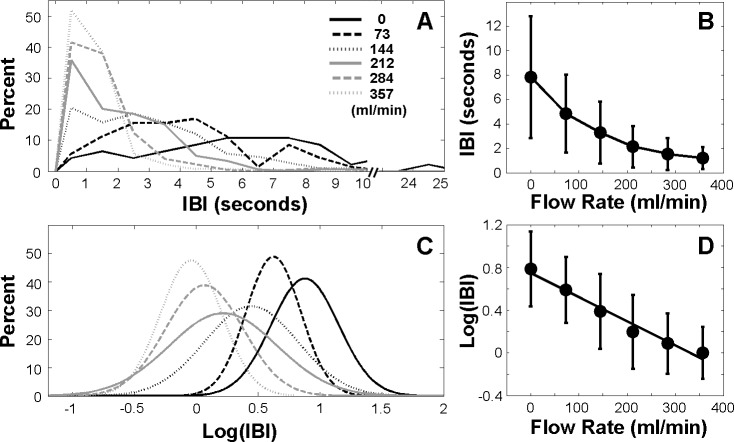
Individual example (Subject 1) using pooled data from three sets of trials. (A) IBI distributions under different flow rates. (B) Mean IBI as a function of flow rate. (C) Log transformation of IBI distributions under different flow rates. (D) Log transformation of mean IBI as a function of flow rate. A linear regression line was fitted to the data.
The decrease in the median of IBIs with FR of 73 mL/min was statistically significant compared with the baseline (permutation test, P < 0.001), and thus represented what we defined as the threshold stimulus intensity to produce a significant change in IBI (BIT) in this case (Fig. 1). In addition, the variability of the IBI decreased significantly with increasing stimulation (Brown-Forsythe test, P < 0.001). Figure 2D shows a significant linear correlation between FR and the mean of log IBI (r = −0.987, P = 0.0002). Log transformation of the data in Figure 2D also demonstrates that, as the IBI decreased with increasing FR, its variability decreased proportionally, making the SDs of IBI similar in the log scale for this subject.
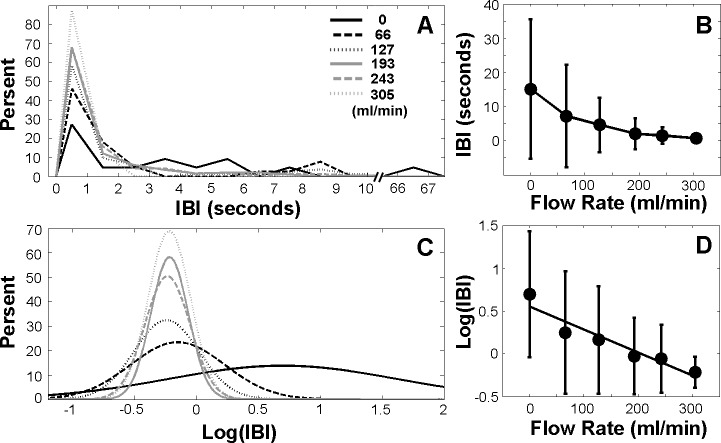
Subject 2 in Figure 3 demonstrates a very different initial blinking pattern, much more infrequent and irregular at baseline. The pooled IBI histogram in Figure 4A shows the positive skew of the data at baseline, with some IBIs as long as 67 seconds. With increasing stimulation, the IBI decreased (Fig. 4B) and the BIT was 193 mL/min (permutation test, P < 0.001). The IBI variability also decreased significantly with increasing stimulation (Brown-Forsythe test, P < 0.001). Log transformation of IBI data (Figs. 4C, 4D) shows a leftward shift and increasingly peaked data with increasing stimulation. In contrast to Subject 1, the variability was less proportional to the decreasing mean, leading to the more variable standard deviations of the IBI in the log scale (Fig. 4D). The IBI and FR were highly correlated (r = −0.953, P = 0.0033).
Figure 4.
Individual example (Subject 2) using pooled data from three sets of trials. (A) IBI distributions under different flow rates. (B) Mean IBI as a function of flow rate. (C) Log transformation of IBI distributions under different flow rates. (D) Log transformation of mean IBI as a function of flow rate. A linear regression line was fitted to the data.
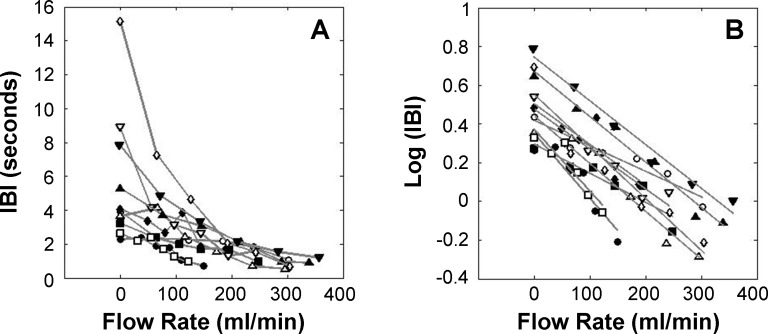
Figure 5 shows the relationship between IBI and FR for all subjects. The average BIT (±SE) was 129 ± 20 mL/min, ranging from 65 to 193 mL/min, with an average IBI of 2.33 ± 1.10 seconds. Although there was high individual variation of IBI during baseline (IBI = 5.69 ± 3.96 seconds), the IBI decreased to 1.02 ± 0.37 seconds at maximum FR, resulting in similar, short IBIs. The variability of the IBI significantly decreased with increasing surface stimulation within all subjects (P < 0.001, Brown-Forsythe test). After log transformation (Fig. 5B), significant linear functions were fitted to each subject's data, with an average (±SD) slope of −0.0023 ± 0.0006 and Pearson's r values ranging from −0.859 to −0.988 (P < 0.05).
Figure 5.
Blink response for all subjects. (A) IBI under different flow rates. (B) Log of IBI under different flow rates with linear regression line fitted for each subject.
Sensory Response to Stimulation
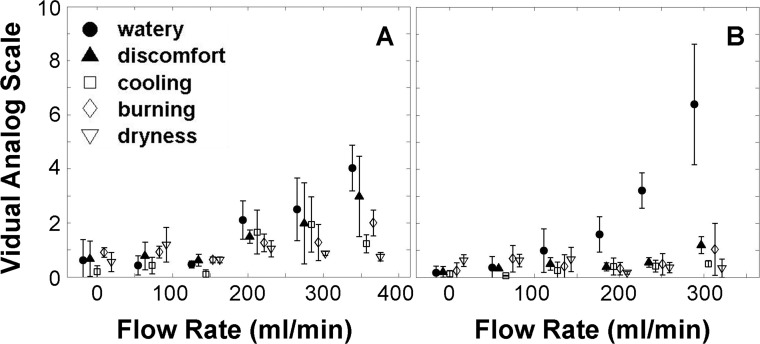
Figure 6 shows the VAS scores for watery, discomfort, cooling, burning, and dryness at different FRs for subjects 1 and 2. Each data point represents an average and SD from the three repeated trials. Subject 1 (Fig. 6A) showed the greatest response for watery and discomfort, whereas Subject 2 (Fig. 6B) reported mostly wateriness. Other subjects (data not shown) showed similarly increasing ratings for each of the sensory attributes with increasing stimulation.
Figure 6.
Sensory response to different flow rates. Markers represent the average sensation from three trials with error bars. (A) Subject 1. (B) Subject 2.
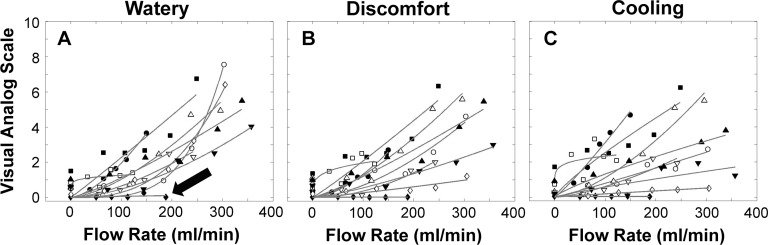
The Stevens' power functions were fit to the average sensory data for each subject from the three repeated trials.48 Individual subject data for watery, discomfort, and cooling are shown in Figure 7. In Figure 7A, the watery response for nine subjects increased with increasing air stimulation, but one subject showed no response (closed arrow). Discomfort (Fig. 7B) was similar, but typically lower for many subjects, and cooling (Fig. 7C) exhibited more variation. The subject indicated with the arrow in Figure 7A followed a similar response for discomfort and cooling (not indicated with an arrow).
Figure 7.
Sensory response of all subjects (fitted with the Stevens' power function) to different flow rates. (A) Watery. (B) Discomfort. (C) Cooling. The arrow indicates a subject with low responses to the stimuli.
The Table shows average exponent and constant of Stevens' power function for nine subjects. One subject (Fig. 7A, closed arrow) was excluded due to an overall lack of sensory response to stimulation. As the Table shows, r2 values were generally high, suggesting a good fit for the other nine subjects with the power function. As expected, there was some individual variation of fitted power functions among subjects. The exponents of these power functions for watery and discomfort ratings were greater than 1, suggesting acceleration of these sensory responses. Cooling, burning, and dryness fitted power functions had average exponents approximately equal to 1, and there was also variation among subjects for these attributes.
Table.
The Sensory Response to Different Flow Rates Fitted With the Stevens' Power Function
|
a, Constant |
b, Exponent |
r2 |
|
| Watery | 0.082 ± 0.238 | 1.722 ± 1.099 | 0.826 |
| Discomfort | 0.075 ± 0.214 | 1.303 ± 0.492 | 0.780 |
| Cooling | 0.153 ± 0.413 | 0.975 ± 0.415 | 0.714 |
| Burning | 0.208 ± 0.328 | 0.929 ± 0.858 | 0.531 |
| Dry | 0.458 ± 0.617 | 0.850 ± 0.835 | 0.587 |
The average parameters for the Stevens' power function (y = a × xb, where a is the constant, and b is the exponent) are shown.
Correlation Between Blink and Sensory Responses
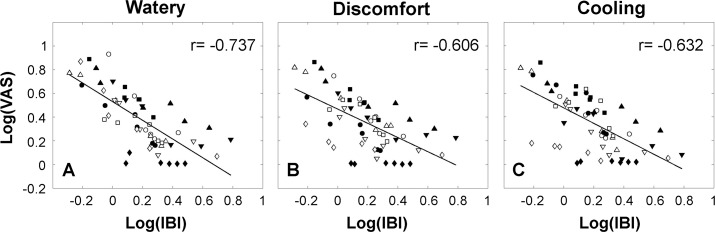
Figure 8 shows the relationship between the log of the IBI and the log of the sensory data for all subjects (data pooled). As Figure 8 illustrates, this was statistically significant for the watery, discomfort, and cooling sensations (Pearson's r = −0.737, −0.606, and −0.632, respectively, all P < 0.001). Burning and dryness were also statistically significant, but the correlations were lower and are not shown (r = −0.470 and −0.466 respectively, both P < 0.001).
Figure 8.
Correlation between IBI and the sensory response. A linear regression line was fitted for each sensation (Pearson's correlation coefficient, P < 0.001). (A) Watery. (B) Discomfort. (C) Cooling.
Discussion
The results of this experiment support the hypothesis that ocular surface stimulation increases the blink rate and its regularity, presumably as a protective mechanism.21,30,31 When task concentration was controlled, there was a linear relationship between stimulus flow rate and IBI, further suggesting a dose-response relationship between ocular surface input and blinking. The ocular sensory response was highly correlated with the blink response, as might be expected, considering that they share the same initial input from the ocular surface.32
Previous studies have found that blinking increased with air stimulation to the ocular surface12,19,21 and other presumed stimuli, such as wearing contact lenses54 or the dry eye condition.12,23,25 Although many of these studies showed an effect on blinking, the level of surface stimulation was often difficult to quantify.23,54 The effect of concentration on a visual task is especially important, as it is known to exert a sizeable inhibitory effect on the blink rate.22,24 In this study, we attempted to control task concentration as much as possible while delivering known stimuli at several levels, thus emphasizing the measurable effect of ocular surface stimulation on blinking.
One of the main purposes of this study was to explore the relationship between ocular stimulation and blink response. As previous studies have shown, there was some variation in IBI among healthy subjects when no stimulus was applied (Fig. 5A).51,53 Subjects in this study were engaged in a visual task and showed the typical “J”-shaped distribution with some longer IBIs, which might be expected when concentrating on a computer game (Figs. 2A, 4A).51 However, with application of the pneumatic stimulus, the blink response among subjects became increasingly similar, as was the linear slope of the decrease in IBI (Fig. 5B). Given that this study involved young healthy subjects, the similar slope of changes in IBI appears to reflect a comparable, and relatively uniform, physiological response, perhaps “designed” to quantify external stimulation and respond with appropriate blinking to protect the ocular surface.9
Although the young healthy subjects in this study showed linear relationships between ocular surface stimulation and blinking, in those experiencing more stimulation due to pathological dry eye conditions might be expected to produce an altered response, depending on the condition of the ocular surface. Previous studies have shown both increased and decreased sensory thresholds in dry eye, presumably due to damage or injury to sensory neurons.36–40 Thus, an altered blink response to increasing ocular surface stimulation might be expected in dry eye, although not tested in this study.
In this study, we quantified the pneumatic FR that associated with a statistically significant change in the blinking and introduced a new term: BIT. Given the linear relationship between IBI and flow rate, this may seem an unnecessary and artificial distinction. However, the purpose is to establish it as an additional end point for later experimental manipulation of testing conditions that may be expected to affect BR. Because the input for triggering an ocular surface–stimulated blink depends on the integrity of surface nerves,32 this may be a useful measure for understanding the sensory response in a number of circumstances or in subjects with a number of conditions, such as dry eye. However, because this threshold might be expected to vary among subjects with differing levels of concentration,22 this extraneous (or confounding) variable would need to be well controlled when using the blink-change threshold as an experimental outcome variable.
In this study, increasing stimulation of the ocular surface affected both the IBI and its variability. As the rate of blinking increased with the stimulation, so did the regularity of blinking, although the increase was proportional to the IBI in some subjects when the results were scaled (compare Figs. 2B, 4B). Similarly, in animal and human models, stimulation of the ocular surface or supraorbital nerve was associated with an increased spontaneous blink rate and enhanced regularity of the blink pattern.14,30,31 Irritation of the ocular surface is suggested to produce trigeminal reflex blink excitability, which is associated with extra blinks at relatively constant intervals after an initial reflex blink, termed blink oscillations.31 In addition, we21,23,54 and others51 have noted cluster blinks in dry eye or healthy subjects, which may be a similar phenomenon. Both blink oscillations and cluster blinks may transiently increase blink rate and regularity in response to surface stimulation, perhaps as an adaptive modulation of the blink response to provide both ocular surface protection and more rapid tear film renewal.30,31
The sensory response also increased with increasing ocular surface stimulation, but the results were more variable than the IBI among subjects for all sensations tested. Although the initial afferent for blinking and sensation is the same up to the level of the trigeminal ganglion complex, blink and sensory pathways then diverge.32 Judgments of sensation involve higher centers in the brain, which are perhaps an additional basis for differing responses among individuals. However, despite these differences in sensory reports among subjects, the correlations between IBI and the sensory ratings were quite high for watery, discomfort, and cooling sensations, underscoring the possibility of a common origin of blinking and the sensory response at the ocular surface.
The sensory input at the level of the ocular surface in this study is likely to be the result of stimulation of multiple types of neurons.55 The air stimulus was presented at room temperature and thus was likely to stimulate both mechanical and thermal receptors, through surface deformation and cooling.56,57 Recent evidence has linked tear secretion with stimulation of cooling receptors,58,59 which may account for the relatively uniform watery response among subjects. Cooling sensations were possibly linked to stimulus air temperature, although tear film evaporation with air flow could also stimulate these “cold” receptors.60 More rapid evaporation of the tears also could lead to tear film hyperosmolarity, which may stimulate chemical polymodal nociceptors55 and result in the burning sensation reported by some subjects.26,27 Discomfort is considered a global sensation and may be due to a mixed input from sensory neurons. The origin of the sensation of dryness is poorly understood, and reports of this sensation were variable in this group of subjects. However, although we used a potentially mixed stimulus to gauge the ocular surface–derived blink response, the results were surprisingly similar in this group of healthy young subjects. This may be due to the robustness of the protective blink and sensory response, both of which are designed to induce the individual to blink and move quickly away from adverse stimuli.9
This study involved several limitations that may have affected our results. We used a custom-built device, similar to Belmonte or Murphy's esthesiometer,42,56 to stimulate the cornea. However, the pneumatic stimulus could also strike the lids during the blink, which could affect the blink rate. In addition, subjects were asked to hold one eye shut to avoid any stimulation from the nontested eye, which may have affected the subject's natural blink response. However, all experiments were performed under this condition, so comparisons between trials within a subject should minimize this effect.
The study addressed whether ocular surface stimulation affects blinking, a controversial question important in dry eye research.12,14,19,20,22 Although previous studies have yielded differing results,12,19 we controlled task concentration and demonstrated a dose-response–like relationship between ocular surface stimulation and the blink response in healthy subjects. In addition, we showed high correlations between the blink response and some ocular surface sensations, which highlights their common origin.32 These methods and the novel metric, BIT, hold promise for understanding ocular surface sensory input in dry eye and other related conditions and may provide a basis for connecting sensory data to more objective, measurable outputs, such as blinking.
Acknowledgments
Supported in part by Grant R01EY021794 (CGB) from the National Eye Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health. The authors alone are responsible for the content and writing of the paper.
Disclosure: Z. Wu, None; C.G. Begley, None; P. Situ, None; T. Simpson, None
References
- 1. Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009; 127: 763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003; 136: 318–326 [DOI] [PubMed] [Google Scholar]
- 3. McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998; 105: 1114–1119 [DOI] [PubMed] [Google Scholar]
- 4. Uchino M, Nishiwaki Y, Michikawa T, et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011; 118: 2361–2367 [DOI] [PubMed] [Google Scholar]
- 5. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop ( 2007). Ocul Surf. 2007; 5: 75–92 [DOI] [PubMed] [Google Scholar]
- 6. Tsubota K, Nakamori K. Effects of ocular surface area and blink rate on tear dynamics. Arch Ophthalmol. 1995; 113: 155–158 [DOI] [PubMed] [Google Scholar]
- 7. Palakuru JR, Wang J, Aquavella JV. Effect of blinking on tear dynamics. Invest Ophthalmol Vis Sci. 2007; 48: 3032–3037 [DOI] [PubMed] [Google Scholar]
- 8. Doane MG. Interactions of eyelids and tears in corneal wetting and the dynamics of the normal human eyeblink. Am J Ophthalmol. 1980; 89: 507–516 [DOI] [PubMed] [Google Scholar]
- 9. Oyster CW. The Human Eye: Structure and Function. Sunderland, MA: Sinauer Associates; 1999: xxviii, 766, 729 [Google Scholar]
- 10. Harrison WW, Begley CG, Liu H, Chen M, Garcia M, Smith JA. Menisci and fullness of the blink in dry eye. Optom Vis Sci. 2008; 85: 706–714 [DOI] [PubMed] [Google Scholar]
- 11. Hirota M, Uozato H, Kawamorita T, Shibata Y, Yamamoto S. Effect of incomplete blinking on tear film stability. Optom Vis Sci. 2013; 90: 650–657 [DOI] [PubMed] [Google Scholar]
- 12. Nakamori K, Odawara M, Nakajima T, Mizutani T, Tsubota K. Blinking is controlled primarily by ocular surface conditions. Am J Ophthalmol. 1997; 124: 24–30 [DOI] [PubMed] [Google Scholar]
- 13. Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983; 106: 643–653 [DOI] [PubMed] [Google Scholar]
- 14. Kaminer J, Powers AS, Horn KG, Hui C, Evinger C. Characterizing the spontaneous blink generator: an animal model. J Neurosci. 2011; 31: 11256–11267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doughty MJ, Naase T. Further analysis of the human spontaneous eye blink rate by a cluster analysis-based approach to categorize individuals with ‘normal' versus ‘frequent' eye blink activity. Eye Contact Lens. 2006; 32: 294–299 [DOI] [PubMed] [Google Scholar]
- 16. Taylor JR, Elsworth JD, Lawrence MS, Sladek JR Jr, Roth RH, Redmond DE Jr. Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Exp Neurol. 1999; 158: 214–220 [DOI] [PubMed] [Google Scholar]
- 17. Doughty MJ. Consideration of three types of spontaneous eyeblink activity in normal humans: during reading and video display terminal use, in primary gaze, and while in conversation. Optom Vis Sci. 2001; 78: 712–725 [DOI] [PubMed] [Google Scholar]
- 18. Patel S, Henderson R, Bradley L, Galloway B, Hunter L. Effect of visual display unit use on blink rate and tear stability. Optom Vis Sci. 1991; 68: 888–892 [DOI] [PubMed] [Google Scholar]
- 19. Acosta MC, Gallar J, Belmonte C. The influence of eye solutions on blinking and ocular comfort at rest and during work at video display terminals. Exp Eye Res. 1999; 68: 663–669 [DOI] [PubMed] [Google Scholar]
- 20. Naase T, Doughty MJ, Button NF. An assessment of the pattern of spontaneous eyeblink activity under the influence of topical ocular anaesthesia. Graefes Arch Clin Exp Ophthalmol. 2005; 243: 306–312 [DOI] [PubMed] [Google Scholar]
- 21. Wu Z, Begley CG, Situ P, Simpson T, Liu H. The effects of mild ocular surface stimulation and concentration on spontaneous blink parameters. Curr Eye Res. 2014; 39: 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cardona G, Garcia C, Seres C, Vilaseca M, Gispets J. Blink rate, blink amplitude, and tear film integrity during dynamic visual display terminal tasks. Curr Eye Res. 2011; 36: 190–197 [DOI] [PubMed] [Google Scholar]
- 23. Himebaugh NL, Begley CG, Bradley A, Wilkinson JA. Blinking and tear break-up during four visual tasks. Optom Vis Sci. 2009; 86: E106–E114 [DOI] [PubMed] [Google Scholar]
- 24. Schlote T, Kadner G, Freudenthaler N. Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefes Arch Clin Exp Ophthalmol. 2004; 242: 306–312 [DOI] [PubMed] [Google Scholar]
- 25. Tsubota K, Hata S, Okusawa Y, Egami F, Ohtsuki T, Nakamori K. Quantitative videographic analysis of blinking in normal subjects and patients with dry eye. Arch Ophthalmol. 1996; 114: 715–720 [DOI] [PubMed] [Google Scholar]
- 26. Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009; 50: 3671–3679 [DOI] [PubMed] [Google Scholar]
- 27. Begley C, Simpson T, Liu H, et al. Quantitative analysis of tear film fluorescence and discomfort during tear film instability and thinning. Invest Ophthalmol Vis Sci. 2013; 54: 2645–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pellegrini JJ, Horn AK, Evinger C. The trigeminally evoked blink reflex. I. Neuronal circuits. Exp Brain Res. 1995; 107: 166–180 [DOI] [PubMed] [Google Scholar]
- 29. Hiraoka M, Shimamura M. Neural mechanisms of the corneal blinking reflex in cats. Brain Res. 1977; 125: 265–275 [DOI] [PubMed] [Google Scholar]
- 30. Evinger C, Bao JB, Powers AS, et al. Dry eye, blinking, and blepharospasm. Mov Disord. 2002; 17: S75–S78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peshori KR, Schicatano EJ, Gopalaswamy R, Sahay E, Evinger C. Aging of the trigeminal blink system. Exp Brain Res. 2001; 136: 351–363 [DOI] [PubMed] [Google Scholar]
- 32. Stapleton F, Marfurt C, Golebiowski B, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the Subcommittee on Neurobiology. Invest Ophthalmol Vis Sci. 2013; 54: TFOS71–TFOS97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Begley CG, Caffery B, Chalmers RL, Mitchell GL. Use of the dry eye questionnaire to measure symptoms of ocular irritation in patients with aqueous tear deficient dry eye. Cornea. 2002; 21: 664–670 [DOI] [PubMed] [Google Scholar]
- 34. Nichols KK, Begley CG, Caffery B, Jones LA. Symptoms of ocular irritation in patients diagnosed with dry eye. Optom Vis Sci. 1999; 76: 838–844 [DOI] [PubMed] [Google Scholar]
- 35. Schein OD, Tielsch JM, Munoz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997; 104: 1395–1401 [DOI] [PubMed] [Google Scholar]
- 36. Situ P, Simpson TL, Jones LW, Fonn D. Conjunctival and corneal hyperesthesia in subjects with dryness symptoms. Optom Vis Sci. 2008; 85: 867–872 [DOI] [PubMed] [Google Scholar]
- 37. Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005; 46: 2341–2345 [DOI] [PubMed] [Google Scholar]
- 38. Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren's syndrome. Exp Eye Res. 2008; 86: 879–885 [DOI] [PubMed] [Google Scholar]
- 39. Toda I, Kato-Asano N, Hori-Komai Y, Tsubota K. Dry eye after LASIK enhancement by flap lifting. J Refract Surg. 2006; 22: 358–362 [DOI] [PubMed] [Google Scholar]
- 40. De Paiva CS, Chen Z, Koch DD, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006; 141: 438–445 [DOI] [PubMed] [Google Scholar]
- 41. Barbato G, Ficca G, Muscettola G, Fichele M, Beatrice M, Rinaldi F. Diurnal variation in spontaneous eye-blink rate. Psychiatry Res. 2000; 93: 145–151 [DOI] [PubMed] [Google Scholar]
- 42. Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999; 40: 513–519 [PubMed] [Google Scholar]
- 43. Situ P, Simpson TL, Fonn D, Jones LW. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness. Invest Ophthalmol Vis Sci. 2008; 49: 2971–2976 [DOI] [PubMed] [Google Scholar]
- 44. Vega JA, Simpson TL, Fonn D. A noncontact pneumatic esthesiometer for measurement of ocular sensitivity: a preliminary report. Cornea. 1999; 18: 675–681 [DOI] [PubMed] [Google Scholar]
- 45. Freudenthaler N, Neuf H, Kadner G, Schlote T. Characteristics of spontaneous eyeblink activity during video display terminal use in healthy volunteers. Graefes Arch Clin Exp Ophthalmol. 2003; 241: 914–920 [DOI] [PubMed] [Google Scholar]
- 46. Berger VW. Pros and cons of permutation tests in clinical trials. Stat Med. 2000; 19: 1319–1328 [DOI] [PubMed] [Google Scholar]
- 47. Brown MB, Forsythe AB. Robust tests for the equality of variances. J Am Stat Assoc. 1974; 69: 364–367 [Google Scholar]
- 48. Stevens SS. Neural events and the psychophysical law. Science. 1970; 170: 1043–1050 [DOI] [PubMed] [Google Scholar]
- 49. Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010; 33: 55–60 [DOI] [PubMed] [Google Scholar]
- 50. Begley CG, Chalmers RL, Abetz L, et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci. 2003; 44: 4753–4761 [DOI] [PubMed] [Google Scholar]
- 51. Doughty MJ. Further assessment of gender- and blink pattern-related differences in the spontaneous eyeblink activity in primary gaze in young adult humans. Optom Vis Sci. 2002; 79: 439–447 [DOI] [PubMed] [Google Scholar]
- 52. Borges FP, Garcia DM, Cruz AA. Distribution of spontaneous inter-blink interval in repeated measurements with and without topical ocular anesthesia. Arq Bras Oftalmol. 2010; 73: 329–332 [DOI] [PubMed] [Google Scholar]
- 53. Carney LG, Hill RM. The nature of normal blinking patterns. Acta Ophthalmol (Copenh). 1982; 60: 427–433 [DOI] [PubMed] [Google Scholar]
- 54. Jansen ME, Begley CG, Himebaugh NH, Port NL. Effect of contact lens wear and a near task on tear film break-up. Optom Vis Sci. 2010; 87: 350–357 [DOI] [PubMed] [Google Scholar]
- 55. Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001; 42: 2063–2067 [PubMed] [Google Scholar]
- 56. Murphy PJ, Patel S, Marshall J. A new non-contact corneal aesthesiometer (NCCA). Ophthalmic Physiol Opt. 1996; 16: 101–107 [PubMed] [Google Scholar]
- 57. Golebiowski B, Lim M, Papas E, Stapleton F. Understanding the stimulus of an air-jet aesthesiometer: computerised modelling and subjective interpretation. Ophthalmic Physiol Opt. 2013; 33: 104–113 [DOI] [PubMed] [Google Scholar]
- 58. Acosta MC, Peral A, Luna C, Pintor J, Belmonte C, Gallar J. Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Invest Ophthalmol Vis Sci. 2004; 45: 2333–2336 [DOI] [PubMed] [Google Scholar]
- 59. Parra A, Madrid R, Echevarria D, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010; 16: 1396–1399 [DOI] [PubMed] [Google Scholar]
- 60. Craig JP, Singh I, Tomlinson A, Morgan PB, Efron N. The role of tear physiology in ocular surface temperature. Eye (Lond). 2000; 14: 635–641 [DOI] [PubMed] [Google Scholar]