Abstract
Study Objectives:
To investigate the mechanisms by which auditory targeted memory reactivation (TMR) during slow wave sleep (SWS) influences the consolidation of emotionally negative and neutral memories.
Design:
Each of 72 (36 negative, 36 neutral) picture-location associations were encoded with a semantically related sound. During a subsequent nap, half of the sounds were replayed in SWS, before picture-location recall was examined in a final test.
Setting:
Manchester Sleep Laboratory, University of Manchester.
Participants:
15 adults (3 male) mean age = 20.40 (standard deviation ± 3.07).
Interventions:
TMR with auditory cues during SWS.
Measurements and Results:
Performance was assessed by memory accuracy and recall response times (RTs). Data were analyzed with a 2 (sound: replayed/not replayed) × 2 (emotion: negative/neutral) repeated measures analysis of covariance with SWS duration, and then SWS spindles, as the mean-centered covariate. Both analyses revealed a significant three-way interaction for RTs but not memory accuracy. Critically, SWS duration and SWS spindles predicted faster memory judgments for negative, relative to neutral, picture locations that were cued with TMR.
Conclusions:
TMR initiates an enhanced consolidation process during subsequent SWS, wherein sleep spindles mediate the selective enhancement of reactivated emotional memories.
Citation:
Cairney SA; Durrant SJ; Hulleman J; Lewis PA. Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. SLEEP 2014;37(4):701-707.
Keywords: Emotional memory, memory consolidation, reactivation, sleep spindles, slow wave sleep
INTRODUCTION
Research spanning several decades has suggested that sleep plays both an active and critical role in declarative memory consolidation.1–7 Sleep-dependent changes in memory are thought to be driven by a process of neural replay, whereby newly acquired information is reactivated during slow wave sleep (SWS), as indexed by spontaneous neural activity in the brain regions where it was encoded.8 Both animal and human studies have provided evidence to support this proposal,9–12 but recent work has suggested that memory reactivations can be externally induced, or triggered, by reexposing individuals to learning-associated cues while they sleep,13–19 a process known as targeted memory reactivation (TMR).
In a seminal study by Rudoy et al.,20 human participants formed associations between picture-locations and semantically related sounds (i.e., a picture of a dog and a barking sound) immediately before taking a nap, during which they were re-exposed to half of the sounds in SWS. At subsequent testing, picture-location memories were remembered more accurately if the associated sound had been replayed during SWS, suggesting that auditory cues had stimulated the reactivation of individual memory representations. While compelling, this and other memory replay studies13–19,21 have adopted experimental stimuli with an entirely neutral tone, thereby raising the important question of how emotionally salient memories, which have been shown to undergo preferential consolidation during postlearning sleep,21,22 are affected by TMR. Because recent work has suggested that sleep facilitates an offline mnemonic tradeoff, whereby the emotional components of a memory are fortified at the expense of their neutral counterparts,22,23 it is possible that TMR also promotes the selective strengthening of emotional memories, even when both neutral and emotional representations are reactivated.
Critically, despite the reported benefits of TMR for consolidation, precisely how new memories are influenced by TMR in SWS remains to be investigated. One possibility is that cue reexposure provides an immediate boost to consolidation, and another is that cued memory reactivations elicit a targeted consolidation process, which unfolds throughout the ensuing SWS. If the second possibility is correct, then the extent to which new memories are enhanced by TMR should be dependent on the amount of SWS that is obtained after such reactivation. Moreover, because electroencephalographic (EEG) sleep spindles (12-15Hz) occur in SWS,24,25 and recently have been implicated in emotional memory consolidation,26 TMR-related enhancements of emotional memory may depend more specifically on SWS spindle oscillations.
In this study, we examined how auditory TMR in SWS affects the consolidation of both emotionally negative and neutral picture-location memories. Participants were trained on a picture-location task in which each picture was paired with a semantically related sound. After training, participants took a nap and half of the sounds were replayed during SWS. We used polysomnographic recordings of the nap in combination with performance on a subsequent picture-location retrieval test to distinguish between immediate consolidation during TMR and consolidation that follows TMR, related to both SWS and sleep spindles.
METHODS
Participants
Fifteen (three male) healthy participants aged 18-28 y (mean age 20.40 [standard deviation (SD) ± 3.07]) were recruited on a voluntary basis. As evaluated with prestudy screening questionnaires and telephone interviews, participants had no history of sleep, psychiatric, or neurological disorders, and reported regular sleep-wake patterns across the preceding month. Participants were not using any psychologically active medications and agreed to abstain from alcohol and caffeine from 24 h prior to the study start. To control for differences in prestudy sleep, participants followed a standardized sleep schedule for 3 days prior to the study, during which they went to bed at 11:00 and rose the following morning at 07:30. Participants recorded bed and wake times on each day of the scheduled period, together with subjective estimations of hours slept, in laboratory-issued sleep diaries. To assess alertness levels, participants completed the Stanford Sleepiness Scale27 at the beginning of each experimental session. Written informed consent was obtained from all participants in line with the School of Psychological Sciences Research Ethics Committee, University of Manchester, who also approved the study.
Materials and Equipment
Stimuli
Seventy-two pictures (36 negative and 36 neutral) were selected from the International Affective Picture System (IAPS).28 IAPS pictures range from images of everyday scenes to images of rotten food, violence, and death, and each are rated on 9-point scales of emotional valence (1 = negative, 5 = neutral, 9 = positive) and emotional arousal (1 = boring, 9 = exciting). The negative and neutral picture sets were significantly different in terms of both mean IAPS valence rating (negative: 2.74 [SD ± 0.62]; neutral: 5.60 [SD ± 0.77], t(35) = 16.24; P < 0.0001) and mean IAPS arousal rating (negative: 5.81 [SD ± 0.74]; neutral: 4.00 [SD ± 0.85], t(35) = 8.55; P < 0.0001), but were balanced for human content. Each picture was paired with a semantically related 6000-ms sound taken from the International Affective Digitized Sounds (IADS) battery29 to create 36 negative and 36 neutral picture-sound pairs. IADS sounds are rated on the same 9-point scales as IAPS pictures, with the negative and neutral sound sets showing significant differences in terms of both mean IADS valence rating (negative: 3.60 [SD ± 1.33]; neutral: 5.32 [SD ± 1.23], t(35) = 7.19; P < 0.0001) and mean IADS arousal rating (negative: 6.17 [SD ± 1.16]; neutral: 5.29 [SD ± 1.00], t(35) = 3.16; P = 0.003). A comprehensive account of the picture-sound pairs used in this study is available in Table S1. All study pictures were adjusted to a standardized size for the experimental tasks (height, 85mm; width, 70mm).
Experimental Tasks and Sound Replay
Computerized tasks and a sound replay algorithm were written and implemented with Presentation version 14.1 (Neurobehavioral Systems, Inc.). During experimental tasks, sounds were heard through a pair of headphones (Sennheiser, model: HD 207), and participant responses were recorded with a joystick (Logitech, model: Attack 3). During sleep, sounds were replayed via PC speakers (Dell, model: A425) placed 1 m from the bed. Sounds were integrated into unobtrusive background brown noise (approximately 39 dB overall sound-pressure level), which was presented throughout the entire napping period.
Polysomnography and Sleep Spindles
Sleep monitoring was carried out using a Polysomnography (PSG) system (Embla, model: N7000) with RemLogic 1.1 software (Embla). Silver-silver chloride (Ag-AgCl) electrodes were attached using EC2 electrode cream (Grass Technologies) after the scalp was cleaned with NuPrep exfoliating agent (Weaver and Company). EEG scalp electrodes were attached according to the international 10-20 system at six standardized locations: central (C3 and C4), frontal (F3 and F4), and occipital (O1 and O2), and each was referenced to the contralateral mastoid (A1 or A2). Left and right electrooculogram, left, right, and upper electromyogram, and a ground electrode were also attached. All electrodes were verified to have a connection impedance of less than 5 kOhms. All signals were digitally sampled at a rate of 200 Hz.
To detect the emergence of SWS in real time, online sleep scoring was conducted on the referenced central electrodes (C3-A2 and C4-A1) according to the standardized sleep scoring criteria of Rechtschaffen and Kales,30 with sleep stages three and four collectively scored as SWS. To confirm that sound replay had occurred during SWS, sleep data were subsequently partitioned into 30-sec epochs and scored offline by a second researcher who was unaware of when the sounds were presented again. Epochs scored as SWS were extracted from each EEG channel and, following artefact rejection, bandpass filtered at 12-15 Hz using a linear finite impulse response filter in EEGLAB version 10.0. An automated detection algorithm31 counted discrete spindle events as amplitude fluctuations within the filtered time series, which exceeded a predetermined threshold (eight times the mean channel amplitude). This algorithm has been used in prior studies of sleep and memory consolidation.32,33
Procedure
Session One (12:00)
The first experimental session began with a modified version of the object-location task used in Takashima et al.34 On each randomized trial, participants were presented with a gray rectangular mask (height, 85mm; width, 70mm) in the center of the computer screen for 1000 ms before one of the 72 study pictures was presented in its place for 6000 ms, with its associated sound heard through headphones. One of six gray circles surrounding the picture on the bottom left/right, center left/ right, and top left/right of the screen then flashed green for 1000 ms, before the picture moved across the screen to its location. The picture remained at this location for 2000 ms until the next trial began. Participants were instructed to memorize the pictures and their associated screen locations for a future test, and to move a joystick in the direction that each picture moved.
Immediately after the encoding phase, participants' memory was probed with a picture-location retrieval task. For each randomized trial, participants were re-presented with one of the 72 study pictures in the center of the computer screen, along with its associated sound, for 6000 ms. The picture then disappeared and participants were required to indicate its associated screen location by moving a cursor to the appropriate gray circle with the joystick. Participants were instructed to make their responses as quickly as possible, and no feedback was provided. Participants were then required to indicate how certain they were that their response was correct by moving the cursor to one of five new circles, each depicting a different level of certainty (for reference: 1 = very uncertain; 5 = very certain), before moving on to the next trial. To facilitate learning, the procedures of session one were carried out twice.
Targeted Memory Reactivation (14:00)
After PSG setup, participants were given an opportunity to sleep for approximately 90 min in a darkened bedroom. Approximately 2 min after SWS onset, one half of the sound cues (18 negative and 18 neutral, counterbalanced across participants) were replayed to participants in random order. The sounds were replayed once, with each sound separated by a 3000-ms interval. After waking, electrodes were removed and participants were given a 30-min break to recover from the general effects of sleep inertia.
Session Two (16:00)
Participants completed the picture-location retrieval task for a third and final time. At the end of the session, participants were asked if they were aware of any sound replay during sleep, before completing a questionnaire in which they were required to discriminate between the replayed and nonreplayed sounds. Experimental procedures and tasks are illustrated in Figure 1.
Figure 1.
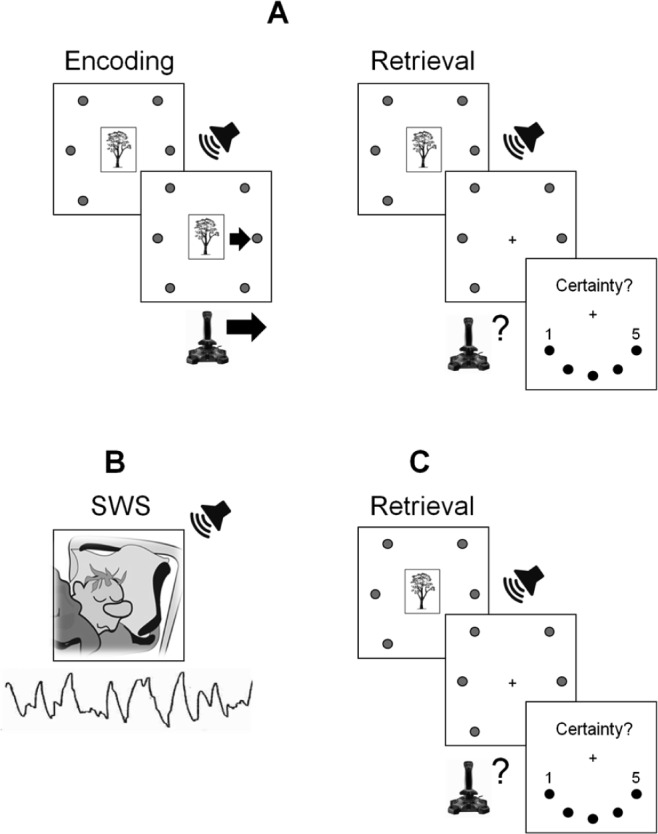
(A) Session one (12:00): encoding and retrieval tasks. (B) Targeted memory reactivation (02:00): half of the sounds were replayed during slow wave sleep (SWS). (C) Session two (04:00): retrieval task.
Data Analysis
We examined memory accuracy and response times (RTs) for correctly recalled picture locations in the final retrieval phase. In keeping with prior research,34,35 we focused on confident correct memory responses, and therefore included only trials with a high certainty rating of 4 or 5. To control for response bias, retrieval scores were calculated as the percentage of high-certainty responses that were correctly answered. Data was analyzed with a 2 (sound: replayed/not replayed) × 2 (emotion: negative/neutral) repeated-measures analysis of variance (ANOVA). To examine how SWS and sleep spindles influenced the effects of TMR, we repeated these analyses with time spent in SWS (min), and then spindle count averaged across all six EEG channels during SWS, as separate mean-centered covariates (analysis of covariance, ANCOVA).36 Analyses were conducted with SPSS statistical software version 16 (SPSS, Inc.), and a two-tailed P < 0.05 was considered significant.
RESULTS
Sleep Data and Alertness
Sleep diaries showed that participants obtained a mean of 7.37 h [SD ± 1.03 h] sleep during the night preceding the study. There was no signifi-cant difference between this value and subjective estimations of hours slept in a typical night: 7.60 (mean) [SD ± 0.91] (t(14) = 1.20; P = 0.25), confirming that participants' prestudy sleep had not deviated from their usual practices. PSG sleep data scored offline confirmed that sound replay had occurred for all participants during a definitive period of SWS (for sleep stage data, see Table 1). The Stanford Sleepiness Scale revealed no difference in mean levels of alertness between session one and session two, which took place before and after sleep, respectively (session one: 2.40 [SD ± 0.99]; session two: 2.27 [SD ± 0.96], t(14) = 0.56; P = 0.58).
Table 1.
Sleep stage data (means)

Targeted Memory Reactivation
Memory Accuracy
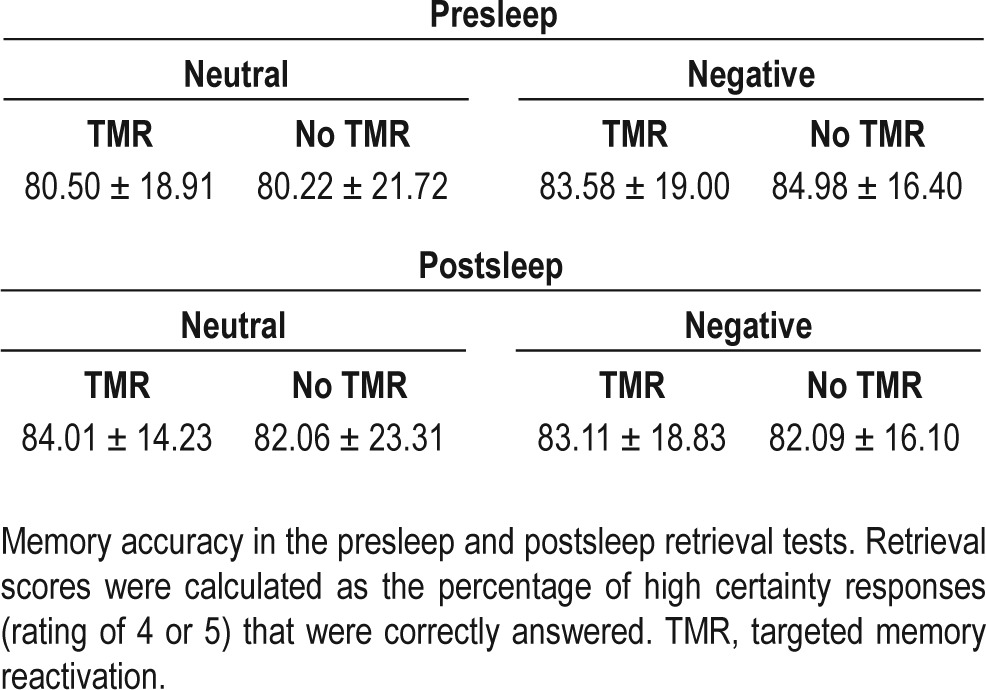
Retrieval scores were calculated as the percentage of high-certainty responses (rating of 4 or 5) that were correctly answered (see Table 2). There was no difference in memory accuracy between negative and neutral items in the presleep retrieval session (t(14) = 1.19; P = 0.26), and no change in accuracy between the presleep and postsleep retrieval sessions, irrespective of emotional valence (negative: t(14) = 0.88; P = 0.39, neutral: t(14) = 1.00; P = 0.33). For postsleep retrieval, a 2 (sound: replayed/not replayed) × 2 (emotion: negative/ neutral) repeated-measures ANOVA revealed no difference in accuracy between memories that were replayed relative to those that were not (F(1,14) = 0.21; P = 0.65), no difference between negative and neutral memories (F(1,14) = 0.01; P = 0.92), and no interaction between factors (F(1,14) = 0.02; P = 0.90). Accuracy data is available in Table 3.
Table 2.
Certainty data (means)
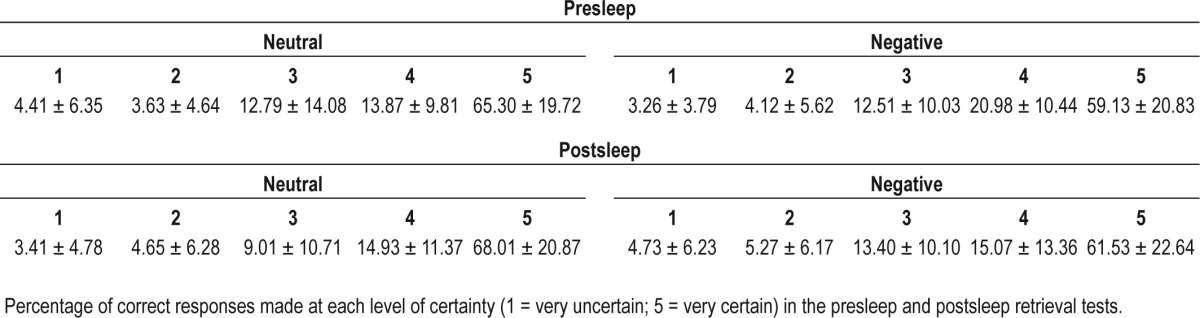
Table 3.
Memory accuracy (means)
Response Times
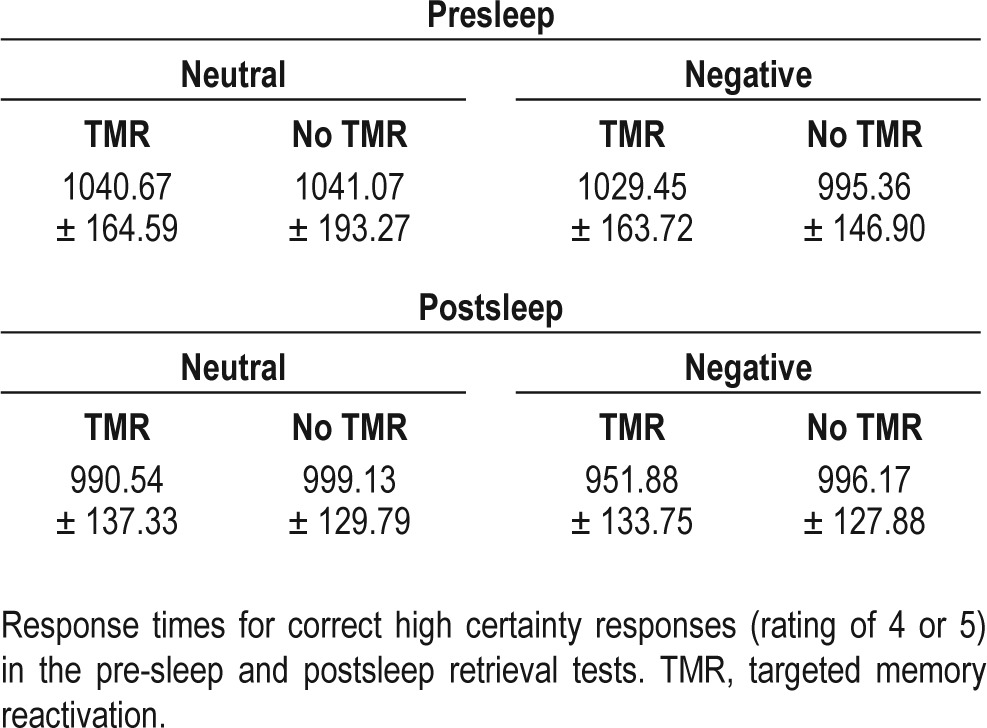
Like our analysis of memory accuracy, our RT analyses included only high-certainty correct responses. RTs in the presleep retrieval session were equivalent for negative and neutral items (t(14) = 0.76; P = 0.46), and no significant difference was observed between RTs in the presleep and postsleep retrieval sessions (negative: t(14) = 1.49; P = 0.16, neutral: t(14) = 1.89; P = 0.08). The same 2 × 2 repeated-measures ANOVA previously mentioned was conducted on postsleep RT data, and revealed no main effect of sound (F(1,14) = 2.00; P = 0.18), no main effect of emotion (F(1,14) = 0.48; P = 0.50) and no interaction between these factors (F(1,14) = 0.65; P = 0.43). RT data is available in Table 4.
Table 4.
Response times (means)
Slow Wave Sleep
To examine how TMR is influenced by SWS, we repeated the aforementioned analyses with SWS duration (min) as a mean-centered covariate, thereby producing a 2 × 2 repeated-measures ANCOVA. For memory accuracy, there was no main effect of SWS (F(1,13) = 0.29; P = 0.60) or interaction between SWS and any of the experimental factors (SWS × sound: F(1,13) = 0.42; P = 0.53, SWS × emotion: F(1,13) = 0.03; P = 0.87, SWS × sound × emotion: F(1,13) = 1.66; P = 0.22).
For RTs, however, this ANCOVA revealed a three-way interaction between SWS, sound, and emotion (F(1,13) = 9.04; P = 0.01). To better understand this interaction, we calculated an RT difference measure for each participant, which paralleled the emotion factor in the ANCOVA (emotion effect = neutral RT – negative RT), and tested for correlations between this measure and time spent in SWS. Thus, a positive emotion effect represented faster memory judgements for emotional (relative to neutral) picture locations, whereas a negative emotion effect corresponded to faster memory judgements for neutral (relative to emotional) picture locations. Among picture-location memories that were reactivated during sleep, SWS duration predicted a strongly positive emotion effect (r = 0.66; P = 0.008) (see Figure 2). Examination of the simple effects of emotion showed that this relationship was driven by negative reactivated picture locations, as SWS predicted faster RTs for these items (r = -0.52; P = 0.05), whereas no significant relationship was found between SWS and RTs for neutral reactivated picture locations (r = 0.34; P = 0.21). Importantly, SWS did not predict any emotion effect for nonreactivated picture locations (r = 0.06; P = 0.83). These findings suggest that emotional memories, but not neutral memories, were more strongly consolidated during SWS when TMR had taken place.
Figure 2.
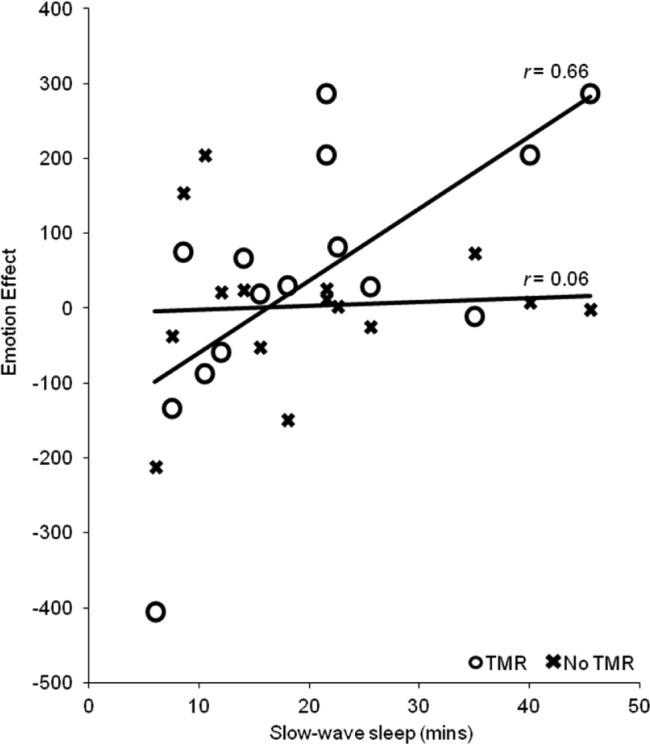
The influence of targeted memory reactivation (TMR) on the relationship between slow wave sleep and the emotion effect (neutral response time – negative response time).
In addition to the three-way interaction, this ANCOVA of RTs revealed an interaction between SWS and emotion (F(1,13) = 4.99; P = 0.044), suggesting that SWS had influenced negative and neutral memories in generally distinct manners. No interaction was found between SWS and sound (F(1,13) = 0.58; P = 0.46) for RTs, and there was no main effect of SWS (F(1,13) = 0.42; P = 0.53).
SWS Spindles
To examine the role that sleep spindles play in SWS-TMR interactions, we repeated the ANCOVA for the aforementioned RTs, but this time included the spindle count averaged across all six EEG channels during SWS as the mean-centered covariate. This revealed a three-way interaction between spindles, sound, and emotion (F(1,13) = 5.12; P = 0.043), with spindles predicting a positive emotion effect for reactivated picture locations (r = 0.51; P = 0.05), but not nonreactivated picture locations (r = 0.02; P = 0.93). No interaction was found between spindles and either sound (F(1,13) = 0.01; P = 0.92) or emotion (F(1,13) = 2.02; P = 0.18), and there was no main effect of spindles (F(1,13) = 0.04; P = 0.85). These findings suggest that SWS-related enhancements of reactivated emotional memories may be mediated by the spindle oscillations that occur in this sleep stage.
Sound Awareness
All participants confirmed that they were unaware of any sound replay during sleep and performed at chance when asked to discriminate between the sounds that were replayed and those that were not (t(14) = 0.26; P = 0.80).
DISCUSSION
In this study, we investigated how TMR influences emotional memory consolidation during SWS and the mechanisms that underpin such effects. Our findings suggest that when emotionally negative and neutral memories are reactivated together, the ensuing SWS supports the consolidation of negative memories alone, with such effects mediated by sleep spindle oscillations.
The Mechanism of TMR
The notion that SWS-dependent memory reactivations can be triggered with sensory cues, leading to superior retrieval performance, has been well supported in recent years,13,15–18,20,21,37 but the mechanisms underpinning this effect have been largely uncharacterized. Our observation that time spent in SWS predicts faster memory judgments for reactivated picture locations suggests that the explicit effects of TMR do not occur instantaneously, but are instead driven by the SWS that follows. Crucially, such RT speeding was also predicted by sleep spindles recorded in SWS, suggesting that spindle oscillations play a critical role in TMR-related memory enhancements. These findings join a wealth of behavioral and physiological evidence indicating an active role for both SWS and sleep spindles in memory consolidation.33,35,38–43 Building on this research, the active systems model of consolidation1,5,8 proposes that neocortical slow oscillations (< 1 Hz) drive the synchronized occur-rence of hippocampal memory replay, together with associated sharp-wave ripple events in the hippocampus, and thalamocortical sleep spindles throughout SWS. As a result, sharp-wave ripples and reactivated memory information are thought to become nested into single oscillatory troughs of the spindle, which is collectively fed back to the neocortex in the form of a spindle-ripple event, and thereby facilitates the transfer of hippocampal-dependent memories to neocortical sites for long-term storage. Using this model, it is tempting to interpret our findings as evidence for an augmented consolidation process, whereby TMR initiates focused hippocampal replays and thus triggers an enhanced series of spindle-ripple events, which occur at a greater frequency than those generated endogenously. Accordingly, as a result of TMR, recently acquired memories may become rapidly independent of the hippocampus during SWS, and more effectively stabilized within long-term neocortical memory networks.
Selective Emotional Memory Processing
Rather than strengthening all newly encoded memories equally, sleep-dependent consolidation processes are thought to operate in a discriminatory manner, such that new information is either lost or retained on the basis of its relevance for future recall.8,44,45 Emotional memory research has provided considerable evidence for this selective gating of relevant and irrelevant memories during sleep, with several studies demonstrating that affective representations are better remembered over sleep than their neutral counterparts,22,23,46–48 although these findings are also discussed by Baran et al.49 and Lewis et al.50 Our current findings add to this literature by showing that consolidation processes occurring in SWS as a result of TMR also operate in a highly selective manner. Although both emotionally negative and neutral memories were reactivated in this study, only negative memories showed evidence of enhanced consolidation during the SWS that proceeded TMR, suggesting that SWS had preferentially affected reactivated memories with the highest degree of emotional salience. Moreover, our data revealed that this selective enhancement of negative reactivated memories was also predicted by sleep spindles recorded during SWS. In addition to being previously implicated in the memory effects of TMR,13,21 recent work has suggested that spindles support discriminatory memory processes over sleep,51 and facilitate emotional memory consolidation.26 Importantly, Wilhelm et al.52 found an increase in SWS spindles after newly encoded object-location memories were made more salient, and the extent of this increase predicted memory performance in a subsequent test. However, because Wilhelm et al. did not examine emotional memories, care should be taken when drawing comparisons between their findings and those reported here. Selective memory processes are thought to be governed by neural salience tags, which are attached to relevant information at encoding and stimulate a targeted consolidation of such information during sleep, while unimportant or irrelevant memories are left to decay.8,45 In the current study, the salience tags attached to emotionally aversive memories at encoding may have become boosted during TMR, resulting in a subsequently enhanced consolidation process that was mediated by SWS-specific spindle oscillations. Future research should therefore focus on whether the sleep spindle can be harnessed to maximize the selective influences of TMR.
Study Limitations
Although the current study provides insight to the neural mechanisms underpinning TMR effects in human memory, we did not observe the TMR-related changes in memory accuracy reported in previous studies of this type.15,16,18,20 This caveat may be reasonably attributed to substantial differences between the experimental task used here and those adopted by others. According to Rudoy et al.,20 for example, the distance between the encoded and recalled screen locations of each study item provided an index of memory accuracy that was particularly sensitive to the effects of TMR. Instead, our data indicate that our six-option forced choice task provides greater insight to TMR-related changes in RTs, which have not been appropriately examined in prior research. It is important to note, however, that our significant findings were limited to the postsleep retrieval phase, rather than a change in performance between presleep and postsleep retrieval sessions, as reported in prior studies of auditory TMR.17,20,37
Several studies have reported a predictive relationship between rapid eye movement (REM) sleep and emotional memory retention, suggesting that this sleep stage may support the consolidation of affective representations,23,47,53,54 but Baran et al.49 also provide insight. Although we found no association between REM and subsequent emotional memory performance, studies that have shown such a relationship did not involve TMR during sleep. Thus, TMR-related changes in emotional memory performance may, in part, result from an atypical pattern of offline consolidation, in which REM-dependent processes are superseded by those occurring in SWS. Moreover, seven of our participants achieved no REM during their afternoon nap, reducing the plausibility of our attempts to correlate time spent in REM with behavioral changes.
DISCLOSURE STATEMENT
Financial support was provided by the Engineering and Physical Sciences Research Council (EPSRC) and Unilever for investigational use. All work was performed at the School of Psychological Sciences, University of Manchester, United Kingdom. Dr. Cairney has received research support from Unilever UK and Ireland. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Björn Rasch, Andrew Mayes, and James Cousins for fruitful discussions of the data, and to two anonymous reviewers who provided invaluable comments on the manuscript.
SUPPLEMENTAL MATERIAL
Picture-sound pairs
REFERENCES
- 1.Born J. Slow-wave sleep and the consolidation of long-term memory. World J Biol Psychiatry. 2010;11:16–21. doi: 10.3109/15622971003637637. [DOI] [PubMed] [Google Scholar]
- 2.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–24. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 3.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 4.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 5.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 7.Walker MP. Sleep-dependent memory processing. Harv Rev Psychiatry. 2008;16:287–98. doi: 10.1080/10673220802432517. [DOI] [PubMed] [Google Scholar]
- 8.Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–7. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 10.Peigneux P, Laureys S, Fuchs S, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10:180–6. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 13.Antony JW, Gobel EW, O'Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012;15:1114–6. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012;15:1439–44. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diekelmann S, Biggel S, Rasch B, Born J. Offline consolidation of memory varies with time in slow wave sleep and can be accelerated by cuing memory reactivations. Neurobiol Learn Mem. 2012;98:103–11. doi: 10.1016/j.nlm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Diekelmann S, Buchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci. 2011;14:381–6. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 17.Oudiette D, Antony JW, Creery JD, Paller KA. The role of memory reactivation during wakefulness and sleep in determining which memories endure. J Neurosci. 2013;33:6672–8. doi: 10.1523/JNEUROSCI.5497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 19.van Dongen EV, Takashima A, Barth M, et al. Memory stabilization with targeted reactivation during human slow-wave sleep. Proc Natl Acad Sci U S A. 2012;109:10575–80. doi: 10.1073/pnas.1201072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuentemilla L, Miró J, Ripollés P, et al. Hippocampus-dependent strengthening of targeted memories via reactivation during sleep in humans. Curr Biol. 2013;23:1769–75. doi: 10.1016/j.cub.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19:781–8. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne JD, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Front Integr Neurosci. 2012;6:108. doi: 10.3389/fnint.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 25.Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–76. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 26.Kaestner EJ, Wixted JT, Mednick SC. Pharmacologically Increasing Sleep Spindles Enhances Recognition for Negative and High-arousal Memories. J Cogn Neurosci. 2013;25:1597–610. doi: 10.1162/jocn_a_00433. [DOI] [PubMed] [Google Scholar]
- 27.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 28.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville, FL: University of Florida; 2005. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- 29.Bradley MM, Lang PJ. Technical report B-3. Gainesville, FL: University of Florida; 2007. The International Affective Digitized Sounds, 2nd Edition (IADS-2): Affective ratings of sounds and instruction manual. [Google Scholar]
- 30.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. US Department of Health, Education and Welfare Public Health Service -NIH/NIND. 1968 [Google Scholar]
- 31.Ferrarelli F, Huber R, Peterson M, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–92. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 32.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010;30:14356–60. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takashima A, Nieuwenhuis IL, Jensen O, Talamini LM, Rijpkema M, Fernández G. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29:10087–93. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takashima A, Petersson KM, Rutters F, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–61. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delaney HD, Maxwell SE. On using analysis of covariance in repeated measures designs. Multivariate Behav Res. 1981;16:105–23. doi: 10.1207/s15327906mbr1601_6. [DOI] [PubMed] [Google Scholar]
- 37.Oudiette D, Paller KA. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn Sci. 2013;17:142–9. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 39.Marshall L, Molle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24:9985–92. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 41.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–35. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Clemens Z, Fabo D, Halasz P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett. 2006;403:52–6. doi: 10.1016/j.neulet.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 43.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 44.Stickgold R. Parsing the role of sleep in memory processing. Curr Opin Neurobiol. 2013;23:847–53. doi: 10.1016/j.conb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16:139–45. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–8. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- 47.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–9. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry. 2006;60:788–90. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 49.Baran B, Pace-Schott EF, Ericson C, Spencer RMC. Processing of emotional reactivity and emotional memory over sleep. J Neurosci. 2012;32:1035–42. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis PA, Cairney S, Manning L, Critchley HD. The impact of overnight consolidation upon memory for emotional and neutral encoding contexts. Neuropsychologia. 2011;49:2619–29. doi: 10.1016/j.neuropsychologia.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saletin JM, Goldstein AN, Walker MP. The role of sleep in directed forgetting and remembering of human memories. Cereb Cortex. 2011;21:2534–41. doi: 10.1093/cercor/bhr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilhelm I, Diekelmann S, Mozlow I, Ayoub A, Mölle M, Born J. Sleep selectively enhances memory expected to be of future relevance. J Neurosci. 2011;31:1563–9. doi: 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–66. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: Evidence from behavior and event-related potentials. Neurobiol Learn Mem. 2013;99:1–9. doi: 10.1016/j.nlm.2012.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Picture-sound pairs


