Abstract
Ehlers–Danlos syndrome (EDS) is a rare disorder caused by abnormalities in the synthesis and structure of collagen. The resultant tissue fragility and weakness can lead to multiple surgical conditions. In this report we present the very rare and life threatening case of a massive spontaneous diaphragmatic rupture in a 35-year-old man with EDS and reflect on the literature, highlighting points to consider when managing such complex patients.
Keywords: Ehlers–Danlos, Diaphragmatic rupture, Acute, Emergency
Ehlers–Danlos syndrome (EDS) is a rare disorder caused by abnormalities in the synthesis and structure of collagen. The resultant tissue fragility and weakness can lead to multiple surgical conditions. In this report we present the very rare and life threatening case of a massive spontaneous diaphragmatic rupture in a 35-year-old man with EDS and reflect on the literature, highlighting points to consider when managing such complex patients.
Case History
A normally well 35-year-old man presented to his local accident and emergency (A&E) department with a 2-day history of severe right lower quadrant abdominal pain, constant in nature and associated only with anorexia. There were no other abdominal symptoms. There was no history of trauma or injury. The significant medical history was that of EDS of type unknown. In addition, the patient previously underwent an appendicectomy and a bilateral inguinal hernia repair some years earlier. He was a non-smoker and consumed alcohol occasionally.
On initial observation he appeared to be uncomfortable and in pain, and acutely unwell but non-toxic and not in extremis. Physical examination revealed he was tachycardic at 118 beats per minute and pyrexial at 38°C but was otherwise cardiovascularly stable, with a respiratory rate of 14 breaths per minute and an oxygen saturation of 97% on air. Auscultation of the chest revealed only mildly diminished breath sounds on the left side. Abdominal examination revealed a soft abdomen and tenderness in the right lower quadrant, without rebound or guarding but with bowel sounds present.
Blood analysis revealed a leucocytosis (white cell count: 16.7 x 103/µl) and an elevated C-reactive protein (222mg/l). The patient was given intravenous (IV) fluids and analgesia, and empirical IV antibiotics were initiated. He proceeded to deteriorate rapidly, becoming hypotensive with worsening hypoxia, and required intensive care unit (ICU) admission and intubation for ventilation. A series of chest x-rays (CXRs) with contrast showed the stomach to be within the left chest (Fig 1). Computed tomography (CT) confirmed this, demonstrating herniation of the stomach into the left hemithorax through what appeared to be a defect in the left hemidiaphragm (Fig 2).
Figure 1.
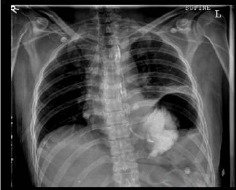
Chest x-ray demonstrating oral contrast administered via nasogastric tube within the stomach located in the left hemithorax
Figure 2.
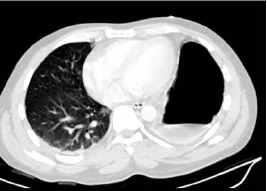
Computed tomography (lung view) showing air/fluid interface in the stomach within the left side of the chest above the level of the diaphragm
The patient was stabilised sufficiently for an ICU transfer to our facility, a tertiary upper gastrointestinal referral centre, for definitive care approximately 24 hours after the initial admission. He was taken to theatre soon after arrival. Operative findings (Fig 3) included rupture of the left triangular ligament of the liver and the left crus of the diaphragm, which extended 5cm laterally. There was gastric translation into the thorax with associated avulsive damage to the short gastric vessels, with an estimated 3l of blood loss into the abdomen. Repair was undertaken with gastric reduction and fixation via gastropexy to the anterior abdominal wall. The left and right crura were opposed, sutured and re-enforced by an Ultrapro® (Ethicon Inc, Somerville, NJ, US) mesh tacked onto the inferior domes of the diaphragm.
Figure 3.
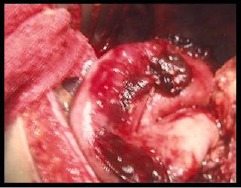
Intraoperative image demonstrating a large hiatal defect after retraction of the stomach from the thorax back into the abdominal cavity
The immediate postoperative period was uncomplicated. Our patient was discharged home five days after surgery on a soft diet and was seen six weeks later as an outpatient. He was improving his oral intake, managing all types of food and was gaining weight. Further follow up involved an outpatient referral to an Ehlers–Danlos unit for further investigation and familial testing.
discussion
EDS is a rare hereditary disorder caused by abnormal collagen synthesis. The key features include poor wound healing, tissue friability, recurrent hernias, vascular fragility, coagulation disorders and joint hyperextensibility.1 There are six major types of EDS based on the underlying aetiology of the syndrome.2 Although the estimated incidence of EDS is 1 in 5,000, it is theorised that the true incidence may be higher with a large number of undiagnosed cases.1 Due to the spectrum of features, the affected patients often present with a variety of surgical conditions and challenges.1
Diaphragmatic herniation is not an uncommon finding in EDS, with a reported incidence of up to 22%,1 and complications of such herniations are well described. However, acute diaphragmatic rupture is a rare event and only a handful of reports can be found in the literature.2,3 Acute rupture of the diaphragm is most commonly the result of thoracic or abdominal trauma. Nevertheless, it has been seen during pregnancy and after excessive exertion, coughing or vomiting.2 It has also been associated with an established diaphragmatic defect or weakness.3 The weakness and laxity of tissues associated with EDS predisposed our patient to his previous development of bilateral inguinal hernias and this recent episode of spontaneous diaphragmatic rupture. We believe this to be the first reported European case of a spontaneous rupture of the diaphragm in an EDS patient without a precipitating event.
Typical presentation of a diaphragmatic rupture can include nausea, vomiting, epigastric pain and dyspnoea but there are no specific pathognomonic signs or symptoms. A standard CXR may mislead diagnosis through misinterpretation as a pneumothorax, pleural effusion or pneumatocoele,4 and with non-specific symptoms the true pathology can be masked. In an acute rupture where diagnosis is delayed and time to definitive treatment extended, morbidity and mortality are increased.
Diaphragmatic rupture without trauma is a life threatening condition that requires prompt diagnosis and surgical intervention to reduce mortality. Owing to the potential risk of rupture of fragile intestinal vessels, EDS associated with a congenital diaphragmatic hernia theoretically carries an even higher surgical risk.1 In such patients, the surgical approach requires careful surgical technique due to the effect abnormal collagen has on tissue handling, healing and haemostasis.5
Conclusions
For any patient with EDS presenting with abdominal pain, thought must be given to diaphragmatic rupture as part of the differential diagnostic process. CXRs and CT with contrast are useful investigations but simple measures such as insertion of a nasogastric tube followed by a CXR may also be diagnostic. Delay to definitive surgical treatment for non-emergent investigations should, however, be minimised. Although this complication of EDS is very rare, it is important for both A&E and surgical staff to be aware of its existence and display a high index of suspicion as management often requires immediate aggressive resuscitative treatment, including ventilation and oxygenation, with timely surgical intervention to reduce morbidity and mortality.
References
- 1.Lin IC, Ko SF, Shieh CS, et al. Recurrent congenital diaphragmatic hernia in Ehlers–Danlos syndrome. Cardiovasc Intervent Radiol. 2006;29:920–923. doi: 10.1007/s00270-005-0154-5. [DOI] [PubMed] [Google Scholar]
- 2.Levine M, Adler J. Acute diaphragmatic rupture in a patient with Ehlers–Danlos syndrome. J Emerg Med. 2008 Apr 23; doi: 10.1016/j.jemermed.2008.03.004. {Epub ahead of print} [DOI] [PubMed] [Google Scholar]
- 3.Iglesias JL, Renard T. Diaphragmatic hernia in an 8-year-old with Ehlers–Danlos syndrome. Pediatr Surg Int. 1998;13:553–555. doi: 10.1007/s003830050401. [DOI] [PubMed] [Google Scholar]
- 4.Akbar A, Parikh DH, Alton H, et al. Spontaneous rupture of the diaphragm. Arch Dis Child. 1999;81:341–342. doi: 10.1136/adc.81.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wesley JR, Mahour H, Woolley MM. Multiple surgical problems in two patients with Ehlers–Danlos syndrome. Surgery. 1980;87:319–324. [PubMed] [Google Scholar]


