Abstract
Here we investigated the potential mechanism of capsaicin-mediated apoptosis in pancreatic cancer cells. Capsaicin treatment phosphorylated JNK, FOXO1 and BIM in BxPC-3, AsPC-1 and L3.6PL cells. The expression of BIM increased in response to capsaicin treatment. Capsaicin treatment caused cleavage of caspase-3and PARP indicating apoptosis. Antioxidants tiron and PEG-catalase blocked capsaicin-mediated JNK/FOXO/BIM activation and protected the cells from apoptosis. Furthermore, capsaicin treatment caused a steady increase in the nuclear expression of FOXO-1 leading to increased DNA binding. Capsaicin-mediated expression of BIM was found to be directly dependent on the acetylation of FOXO-1. The expression of CBP was increased whereas SirT-1was reduced by capsaicin treatment. Using acetylation mimic or defective mutants, our result demonstrated that phosphorylation of FOXO-1 was mediated through acetylation by capsaicin treatment. JNK inhibitor attenuated the phosphorylation of FOXO-1, activation of BIM and abrogated capsaicin-induced apoptosis. Moreover, silencing FOXO1 by siRNA blocked capsaicin-mediated activation of BIM and apoptosis whereas overexpression of FOXO-1augmented its effects. Silencing Bim drastically reduced capsaicin-mediated cleavage of caspase-3 and PARP, indicating the role of BIM in apoptosis. Oral administration of 5mg/kg capsaicin substantially suppressed the growth of BxPC-3 tumor xenografts in athymic nude mice. Tumors from capsaicin-treated mice showed an increase in the phosphorylation of JNK, FOXO-1, BIM, and levels of CBP, cleavage of caspase-3, PARP and decreased SirT-1 expression. Taken together, our results suggest that capsaicin activated JNK and FOXO-1, leading to the acetylation of FOXO-1 through CBP and SirT-1. Acetylated FOXO1 induced apoptosis in pancreatic cancer cells through BIM activation.
Keywords: Pancreatic cancer, Capsaicin, Apoptosis, Bim, FOXO-1 acetylation
Introduction
The forkhead box transcription factor, class O (FoxO) proteins play important role in the cellular response to growth signals and nutrients, oxidative stress and genomic damage (1). These proteins regulate the transcription of genes which contribute to DNA repair, cell cycle and initiate apoptosis. Each of these functions are activated in response to signals that induce the appropriate post-translation modification of FOXO proteins by phosphorylation or acetylation. These extracellular signals translate into activities that direct regulation of pro-apoptotic genes Bim and Fas Ligand (1-3). Nuclear and cytoplasmic shuttling of FOXO proteins is a dynamic process where nuclear export depends on the growth signal and nuclear import is due to stress signal. JNK activation phosphorylates cytoplasmic FOXO proteins at the C-terminal, initiate nuclear import of FOXO proteins and activate transcriptional regulatory genes such as proapoptotic protein Bim (1, 4-7). Nuclear translocation of FOXO proteins by oxidative stress results in association of FOXO with p300/CBP and p300/CBP-associated factor (PCAF). This interaction not only assembles the transcriptional activation complex of FOXO but also results in the acetylation of FOXO (1, 4, 8, 9). SIRT-1, a nicotinamide adenine dinucleotide -dependent protein deactylase is known to interact with acetylated FOXO in the nucleus (4, 10-14). In addition, SIRT-1 has been reported to be directly involved in the acetylation of FOXOs and expression of Bim (4, 14).
Bim was reported to play an important role in apoptosis (15). Recently published studies show that cell death induced by FOXO3a is mediated by FOXO targeted gene Bim (16). Phosphorylation of FOXO, which is a post translational modification, is considered to play an important role in activating Bim (2, 17-19). Previous study reported that atorvastatin prevents apoptosis induced by H2O2 by increasing the phosphorylation of FOXO4 and reducing the protein levels of Bim (19). It is important to know whether phosphorylation of FOXO through oxidative stress is involved in Bim-induced apoptosis. Many histone deacetylase inhibitors were also found to induce apoptosis in tumor cells through Bim and Noxa but the exact mechanism underling this effect was not clear (20).
Capsaicin, a homovanillic acid derivative (N-vanillyl-8-methyl-nonenamide) has been used as spices in South Asian and Latin-American countries (21-24). In our previous study, we have shown that capsaicin induced apoptosis in pancreatic cancer cells was associated with the generation of ROS and persistent disruption of mitochondrial membrane potential without affecting the normal cells (25, 26). In the present study, we explored the mechanism of FOXO phosphorylation and acetylation by capsaicin in BxPC-3, AsPC-1 and L3.6PL pancreatic cancer cells.
Materials and Methods
Chemicals and Antibodies
Capsaicin (purity>99%), anti-actin, PEG-catalase, tiron and nicotinamide were obtained from Sigma (St. Louis, MO). The antibodies against Cl-caspase-3, Cl-PARP, JNK, p-JNK (Thr 183/Tyr185), FOXO-1, p-FOXO-1 (Ser256), FOXO-3a, p-FOXO-3a (Ser253), BIM, p-BIM (Ser69), PI3K, p-PI3K (Tyr 458), Akt, p-Akt (Ser473), 14-3-3 binding motif, CBP/p300, SirT-1, SirT-2, SirT-3, acetylated lysine and acetylated CBP/p300 were purchased from Cell Signaling (Danvers, MA). Lamin B was purchased from Santa Cruz Biotechnology, Inc (CA, USA). Apoptosis detection kit annexin V-FITC was procured from BD Bio-Sciences, Inc. (La Jolla, CA). Alexa fluor 488 (green) and alexa fluor 594 (red) conjugated goat-anti-rabbit secondary antibody and DAPI were obtained from Invitrogen. SP60025, a specific JNK inhibitor was procured from Calbiochem (San Diego, CA) and LY294002 from Cayman Chemicals (Ann Arbor, MI).
Cell Culture
Human pancreatic cancer cell lines AsPC-1and BxPC-3 were obtained from ATCC (Rockville, MD) in 2004 and L3.6PL was a generous gift from Dr. Ming-Hai Wang (Texas Tech. University of Healthy Science Center, TX) in 2011. AsPC-1 and BxPC-3 cells were authenticated using STR analysis by the core facility at the Cancer Center of Texas Tech University Health Sciences Center (Lubbock, TX). However, L3.6PL cells were not authenticated during the course of studies but now are being authenticated. Monolayer cultures of AsPC-1 and BxPC-3 cells were maintained in RPMI medium and L3.6PL was maintained in MEM medium supplemented with 10% fetal bovine serum, PSN antibiotic mixture (10 ml/l), 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate and 20% glucose. All the cultures were maintained at 37°C in a humidified chamber with 95% air and 5% CO2.
Transient Transfection
The L3.6PL and AsPC-1 cells were transiently transfected with WT-FOXO-1 or FOXO-1 acetylation mutant (3KQ and 3KR) plasmid (a generous gift from Dr. Akiyoshi Fukamizu, University of Tsukuba, Japan) using FuGENE-6 transfection reagent (Roche Diagnostics, Indianapolis, USA). Briefly 0.3 × 106 L3.6PL and AsPC-1 cells were transfected with 2μg WT FOXO-1,2μg of 3KQ (acetylation mimic) and 2μg of 3KR (acetylation defective mutant) respectively using Opti-MEM serum free medium. Cells were incubated with plasmid-FuGENE-6 mixture for 6h and after that media were replaced with fresh RPMI media. Transfected cells were treated with DMSO or 150μM capsaicin for indicated time period.
Determination of BIM and CBP mRNA transcripts
To determine the effect of capsaicin on BIM and CBP gene transcription, BxPC-3 cells were treated with 150μM capsaicin for 24h. RNA was extracted from control and capsaicin treated cells using Trizol RNA extraction reagent (Life Technologies, Inc., Carlsbad, CA) RNA samples were prepared for RT-PCR as described by us previously (27). The following primer sets were used: Bim-F, 5′-GAGAAGGTAGACAATTGCAG-3′, Bim-R, 5′-GACAATGTAACGTAACAGTCG-3′; CBP-F, 5′-GGGCCTGTCATCAACACCC-3′, CBP-R, 5′-CGTAGTCCTCGCACACAGTG-3′. RT-PCR was performed in 50 μL reactions using 100 ng RNA, 0.5 μM of each primer and an annealing temperature of 53°C for 40 cycles for BIM primer and 58°C for 40 cycles for CBP primer. The PCR products were separated on a 1.5% agarose gel, stained with 0.5mg/ml ethidium bromide, and visualized under UV light.
Tumor therapy model
Tumor therapy experiment was performed as described by us previously (28, 29) with minor modifications. The use of athymic nude mice and their treatment was approved by the Institutional Animal Care and Use Committee (IACUC), Texas Tech University of Health Science Center. All the experiments were carried out in strict compliance with their regulations. Exponentially growing BxPC-3 (1×106) cells were injected subcutaneously into the right flanks of 20 athymic nude mice. When the tumors reached a size of approximately 70mm3, mice were randomly segregated into 2 groups with 10 mice in each group. Test group of mice received 5mg/kg body weight capsaicin in PBS by oral gavage every day for 35 days, whereas control mice received vehicle alone. Tumor volume and animal weights were taken as we have described previously (28, 29).
Small Interfering RNA Transfection
The AsPC-1 or BxPC-3 cells were transfected with FOXO-1 siRNA or BIM siRNA for 48h using siPORT™ NeoFX™ transfection reagent (Ambion INC, TX). Briefly 0.3 × 106 AsPC-1 or BxPC-3 cells were transfected with 60nM of FOXO-1 siRNA or BIM siRNA using Opti-MEM serum free medium. Cells were incubated with plasmid-siPORT mixture for 6h and after that media were replaced with fresh RPMI media. Transfected cells were treated with 150μM capsaicin for indicated time period.
Immunoprecipitation assay
Immunoprecipitaion assay was performed to examine the effect of capsaicin on the interaction of SirT-1, SirT-2, and CBP/p300 or acetylated lysine with FOXO-1 protein. Briefly, BxPC-3 cells were treated with DMSO or 150μM capsaicin for 24h and whole cell lysates were lysed using RIPA buffer and immunoprecipitated with anti-SirT-1, anti-SirT-2, anti-CBP/p300 or anti-acetylated lysine or FOXO-1 antibody as described by us previously (29). The samples were immunoblotted with respective antibody.
Electrophoretic Mobility Shift Assay (EMSA)
BxPC-3 cells were treated with DMSO or 150μM of capsaicin with or without transfection of WT-FOXO-1 for 24h. Nuclear fraction was isolated by nuclear extraction kit and FOXO-1 protein was captured using biotin labeled FOXO-1 binding site oligomer 5′-CAAAACAACAAAACAACAAAACAA-3′ and performed DNA binding activity using commercially available kit from Panomics (Santa Clara, CA).
Immunofluorescence Assay
BxPC-3 cells were plated on coverslips and allowed to attach overnight and then treated with 150μM capsaicin for 24h. Treated and untreated cells were fixed with acetone:methanol (1:1) mixture and blocked with goat-serum for 1h and incubated with FOXO-1 antibody overnight at 4°C. Immunofluorescence was detected by anti-rabbit immunoglobulin G (IgG) conjugated with alexa fluor 594 (Invitrogen) (red), alexa fluor 488 (green) and DAPI (blue). After four washing, coverslips were then mounted with antifade mounting reagents. Nuclei were stained with DAPI (blue) and the immunofluorescence was observed by a fluorescence microscope using oil immersion at 60X magnification.
Annexin-FITC apoptosis assay
Apoptosis induction by capsaicin was assessed by annexin V/FITC by flow cytometer. About 0.3 × 106 AsPC-1 or BxPC-3 cells were seeded in a 6-well plate and treated with 150μM of capsaicin for 24h after JNK inhibitor or BIM siRNA treatment. Apoptosis was determined using APOPTEST™-FITC kit according FITC-Annexin V apoptosis detection kit (BD Biosciences, San Jose, CA) to manufacturer’s instructions and analyzed by Accuri C6 flow cytometer.
Western blot analysis
Cells were exposed to various concentrations or 150μM capsaicin for 24h or different time points and lysed on ice as described by us previously (28, 29). In a separate experiment, cells were pre-treated with PEG-catalase (500U/ml), tiron (10mM) or LY294002 (10 μM) for 1h followed by treatment with 150μM capsaicin for 24h. Whole-cell extracts were prepared as mentioned above. The tumors from control and capsaicin treated mice were minced and lysed by the procedure described by us previously (28). The cell lysate was cleared by centrifugation at 14,000g for 30 min. Cell lysate containing 10–80 μg protein was resolved by 6–12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and the proteins were transferred onto polyvinylidene fluoride membrane. After blocking with 5% non-fat dry milk in Tris buffered saline, membrane was incubated with the desired primary antibody (1:1000 dilutions) overnight. Subsequently, the membrane was incubated with appropriate secondary antibody (1:2000 dilutions) and the antibody binding was detected by using enhanced chemiluminescence kit according to the manufacturer’s instructions. Each membrane was stripped and re-probed with antibody against actin (1:20000 dilutions) to ensure equal protein loading.
Statistical analysis
All statistical calculations were performed using GraphPad Prizm 5.0. Analysis of variance (ANOVA) was used to test the statistical significance of difference between control and treated groups followed by Bonferroni’s post-hoc analysis for multiple comparisons. P-values less than 0.05 were considered statistically significant.
Results
Capsaicin triggers apoptosis by activating JNK/FOXO/BIM cascade in pancreatic cancer cells
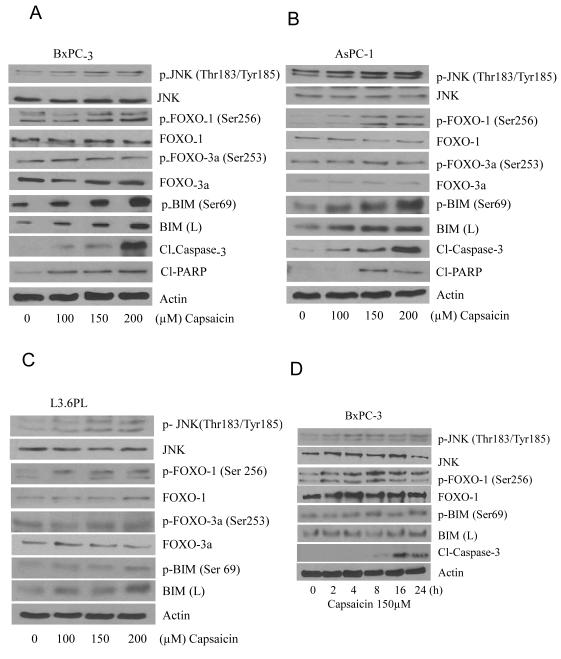
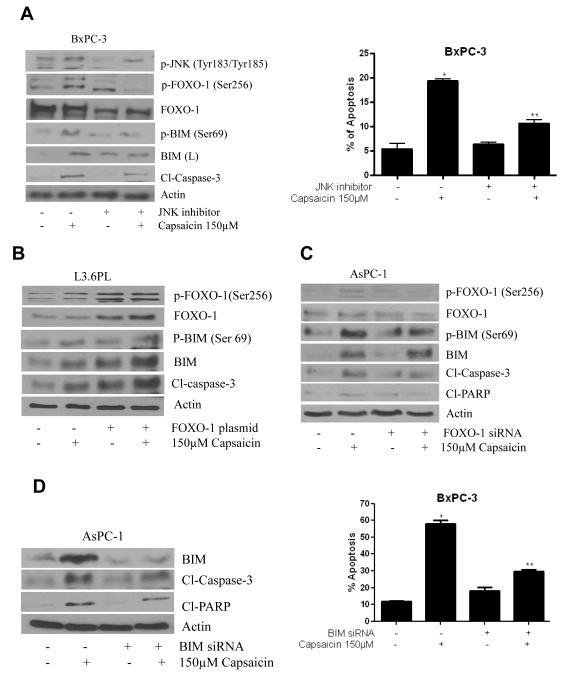
In our previous study, we have demonstrated that capsaicin induced apoptosis in pancreatic cancer cells by causing ROS generation through mitochondrial complex-I and complex-III (25). But the exact mechanism by which capsaicin induced apoptosis was not clear. Previous study reported that MAP-kinases get activated by oxidative stress and cause apoptosis by activating pro-apoptotic protein BIM in PC12 cells (15). Other studies reported the involvement of FOXO-3a in inducing cell death through BIM (2, 16-18). We hypothesized that capsaicin mediated ROS generation and apoptosis was through JNK/FOXO/BIM cascade. To determine the effect of capsaicin on JNK/FOXO/BIM cascade, BxPC-3, AsPC-1 and L3.6PL pancreatic cancer cells were treated with various concentrations of capsaicin for 24h or various time points. Our results reveal that capsaicin treatment increased the phosphorylation of JNK at Thr183/Tyr185, FOXO-1 at Ser256, and BIM at Ser69 but not FOXO-3a at Ser253 (Fig.1A, B&C). Capsaicin treatment also increased BIM protein levels. Further, cleavage of caspase-3 was observed by capsaicin treatment indicating apoptosis (Fig.1A, 1B&1C). In a time dependent study, our results demonstrated that capsaicin treatment increased the phosphorylation of JNK, FOXO-1 and BIM as early as 2h and cleavage of caspase-3 at 8h (Fig. 1D), indicating that cell death induced by capsaicin required early phosphorylation of JNK, FOXO-1 and BIM to initiate apoptosis.
Figure 1. Capsaicin triggers apoptosis by activating JNK/FOXO/BIM cascade in pancreatic cancer cells.
(A) BxPC-3, (B) AsPC-1 and (C) L3.6PL cells were treated with various concentrations of capsaicin for 24h. (D) BxPC-3 cells were treated with 150μM capsaicin in different time points. Cells were lysed and total lysate was prepared as described in materials and methods section. Representative immunoblots show the effect of capsaicin treatment on the phosphorylation of JNK at Thr183/Tyr185, FOXO-1 at Ser256, p-FOXO3a at Ser253, and BIM at Ser69 as well as protein levels of JNK, FOXO-1, FOXO-3a, BIM, Cl-PARP and Cl-caspase-3. The same blots were striped and reprobed for actin to ensure equal protein loading. These experiments were performed three times independently and similar results were obtained.
Capsaicin mediated ROS generation activates JNK/FOXO/BIM but not PI3K/AKT cascade
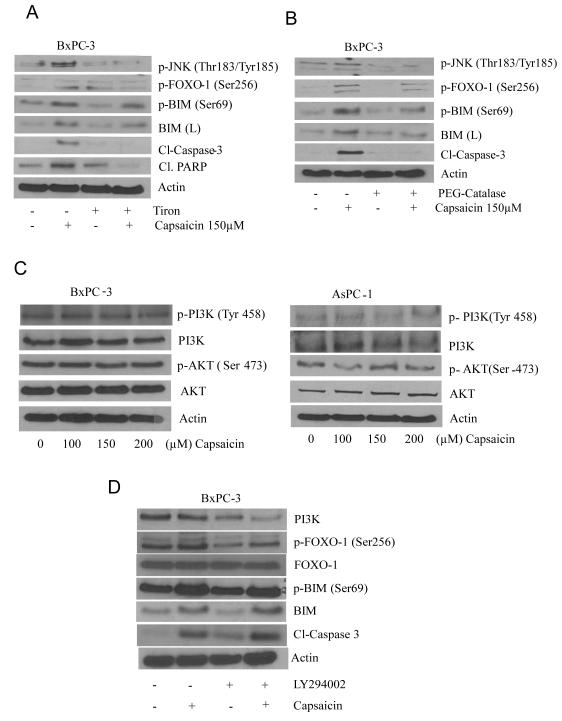
To test whether ROS were involved in the activation of JNK/FOXO/BIM cascade, cells were pretreated with antioxidants, tiron or PEG-catalase. Our results show that capsaicin failed to activate JNK/FOXO/BIM cascade and induce apoptosis when BxPC-3 cells were pre-treated with tiron or PEG- catalase (Fig. 2A&B). These results suggest that capsaicin induced apoptosis by JNK/FOXO/BIM cascade involved ROS as upstream regulator. Previous study reported that FOXO phosphorylation depends on two independent pathways. Phosphorylation of FOXO proteins in response to growth factor causes exclusion of FOXO protein from the nucleus by activated Akt (30). On the other hand, phosphorylation of FOXO protein in response to oxidative stress involving JNK imports FOXO into the nucleus. In our previous study, we have already reported that capsaicin mediated ROS generation induce apoptosis in pancreatic cancer cells (25). In order to see whether capsaicin modulates PI3-K/Akt pathway, we treated BxPC-3 and AsPC-1 cells with various concentration of capsaicin for 24h. Our results show that capsaicin neither affected the phosphorylation of PI3K and Akt nor the protein levels of PI3-K and Akt (Fig. 2C), indicating that PI3K/Akt pathway was not affected by capsaicin and not involved in the activation of FOXO-1. To further exclude the involvement of PI3K pathway in capsaicin mediated activation of FOXO-1, we treated BxPC-3 cells with PI3K specific inhibitor LY294002, prior to treatment with capsaicin. No significant change in the phosphorylation or expression of FOXO-1 or BIM was observed in the cells treated with LY-294002 and capsaicin together (Fig 2D). Nonetheless, LY294002 alone reduced the phosphorylation of FOXO-1 and protein level of BIM (Fig 2D). As shown in Fig 2D, the increase in the phosphorylation of FOXO-1 by capsaicin treatment was blocked by LY294002. However, as compared to capsaicin treatment alone, combined treatment of capsaicin and LY294002 increased the cleavage of caspase-3 (Fig 2D). These results indicate that LY294002 inhibits PI3K and further adds to capsaicin-induced apoptosis.
Figure 2. Capsaicin mediated ROS generation activates JNK/FOXO/BIM but not PI3K/Akt cascade.
BxPC-3 cells were treated with anti-oxidants (A) 10mM tiron and (B) 500 U/ml PEG-catalase for 1h followed by 150μM capsaicin for 24h and cells were lysed and immunoblotted with p-JNK (Thr 183/Tyr185), p-FOXO-1 (Ser256), p-BIM (ser69), BIM (L), Cl-PARP and Cl-caspase-3 respectively. (C) BxPC-3 and AsPC-1 cells were treated with various concentrations of capsaicin and representative immunoblotes show the effect of capsaicin on p-PI3K (Tyr 458), p-Akt (Ser 473), PI3-K and Akt. (D) BxPC-3 cells were pre-treated with LY294002 followed by treatment with 150 μM capsaicin for 24 h. Cells were lysed and immunoblotted with PI3-K, p-FOXO (Ser256), FOXO-1, p-BIM (Ser69), BIM and Cl-caspase-3. The same blots were stripped and reprobed for actin to ensure equal protein loading. These experiments were performed three times independently and similar results were obtained.
Capsaicin treatment causes nuclear retention of FOXO-1
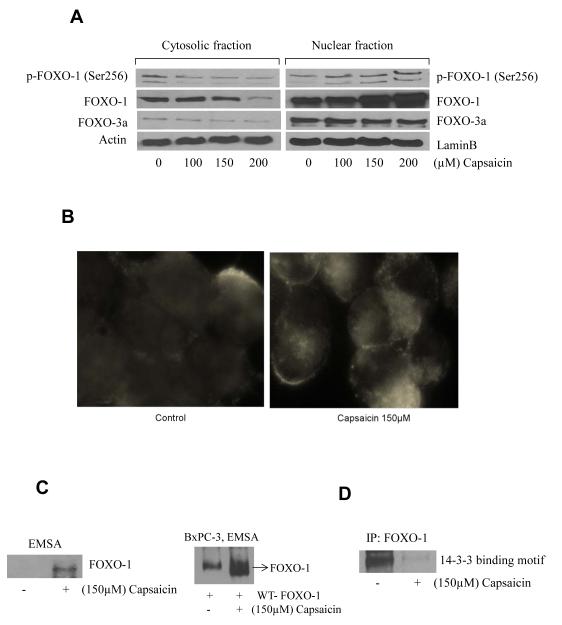
FOXO proteins if sequestered in the nucleus are involved in the transcription of BIM. Since we observed an increase in BIM expression by capsaicin treatment, we next wanted to see if FOXO was accumulated in the nucleus. Our results show that FOXO-1 levels steadily increased in the nuclear fraction of capsaicin treated cells (Fig. 3A). No change was observed in the expression of FOXO-3a by capsaicin treatment (Fig. 3A). The nuclear retention of FOXO-1 was further confirmed by immunofluorescence. Our results show intensified staining of FOXO-1 (red) in the nucleus in response to the capsaicin treatment (Fig. 3B). Next, we wanted to see if the retention of FOXO-1 in the nucleus led to increased DNA binding activity. Our Electrophoretic Mobility Shift Assay (EMSA) shows that capsaicin treatment significantly increased the DNA binding of FOXO-1 in the nucleus (Fig. 3C). In order to confirm the increased DNA binding activity of FOXO-1 in response to capsaicin treatment, BxPC-3 cells were transiently transfected with WTFOXO-1 plasmid and then treated with capsaicin. Our results demonstrate that capsaicin treatment substantially increased the DNA binding activity when compared to control (Fig. 3C). Previous studies suggested that once FOXO-1 is phosphorylated, it binds with 14-3-3 chaperone proteins, which export the complex from the nucleus resulting in the down regulation of BIM (28). In order to confirm the interaction of FOXO-1 and 14-3-3 proteins, we immunoprecipitated FOXO-1 and immunobloted for 14-3-3 proteins. As expected, capsaicin treatment drastically decreased 14-3-3 binding with FOXO-1 protein indicating its retention in the nucleus (Fig. 3D).
Figure 3. Capsaicin treatment causes nuclear retention of FOXO-1.
(A) BxPC-3 cells were treated with various concentrations of capsaicin and nuclear and cytosolic proteins from control and capsaicin treated cells were isolated and immunoblots were probed for p-FOXO-1 (Ser256), FOXO-1, FOXO-3a proteins. Same membrane was stripped and reprobed for actin or lamin B to ensure equal protein loading. (B) To confirm nuclear retention of FOXO-1, BxPC-3 cells were immunostained with FOXO-1 antibody (red) and actin (green) antibody and visualized under fluorescence microscope (Olympus Inc.). (C) BxPC-3 cells were treated with DMSO or 150μM of capsaicin and FOXO-1 DNA binding activity was determined by Electrophoretic Mobility Shift Assay (EMSA). In another experiment BxPC-3 cells were transiently transfected with WT-FOXO-1 plasmid and treated with 150μM of capsaicin and FOXO-1 DNA binding activity was determined by EMSA. (D) BxPC-3 cells were treated with DMSO or 150μM capsaicin for 24h and cell lysates were incubated with FOXO-1 antibody overnight. Cell lysates were resolved on SDS-PAGE and immunoblotted for 14-3-3 binding motif antibody. The experiments were repeated three times and similar results were observed.
CBP mediated FOXO-1 acetylation by capsaicin treatment and regulated by SirTs
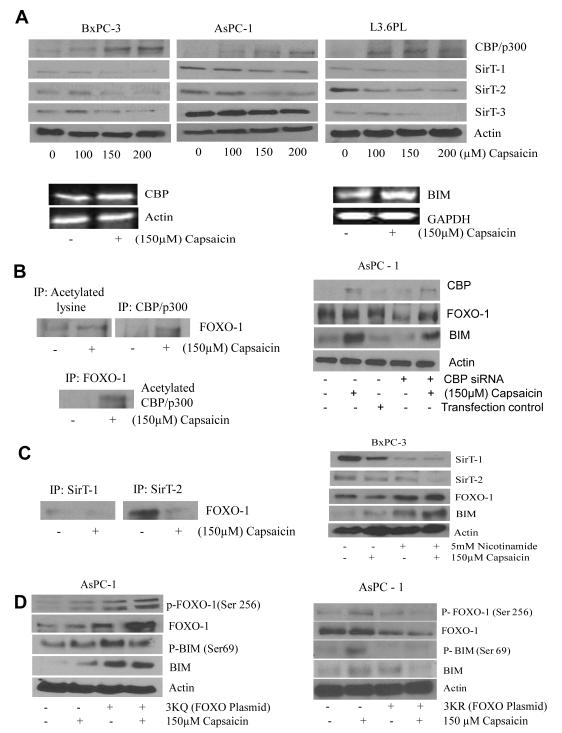
Stress-induced nuclear translocation initiates modification and regulation of FOXO proteins by acetylation. Transcription activity of FOXO is mediated through the acetylation of critical lysine residues by CREB binding proteins (CBP/p300) and SirTs respectively (1). In order to prove this, BxPC-3, AsPC-1 and L3.6PL cells were treated with various concentrations of capsaicin for 24h and analyzed by western blotting. Our results show that capsaicin treatment substantially increased the expression of CBP/p300 (Fig. 4A) in all the cell lines in a concentration dependent manner. On the other hand, expression of SirT-1, SirT-2 and SirT-3 which are the negative regulators of acetylation, were decreased (Fig. 4A) in all the cell lines. Furthermore, capsaicin treatment increased the mRNA levels of CBP and BIM (Fig. 4A), indicating that capsaicin mediated increased protein levels of BIM and CBP in pancreatic cancer cells were transcriptionally mediated.
Figure 4. CBP mediated FOXO-1 acetylation by capsaicin treatment is regulated by SirT’s.
(A) BxPC-3, AsPC-1, and L3.6PL cells were treated with various concentrations of capsaicin for 24h and immunoblotted with CBP/p300, SirT-1, SirT-2, SirT-3 antibodies. The same membrane was stripped and reprobed for actin to ensure equal protein loading. In a separate experiment, BxPC-3 cells were treated with DMSO or 150μM capsaicin and total RNA was isolated with Trizol and analyzed for the expression levels of BIM and CBP by RT-PCR. GAPDH and actin was used as internal control. (B) BxPC-3 cells were treated with DMSO or 150μM capsaicin for 24h and immunoprecipitated with acetylated lysine, CBP/p300 and FOXO-1 antibodies for overnight, resolved on SDS-PAGE and immunoblotted FOXO-1 and acetylated CBP/p300 antibody respectively. In another experiment BxPC-3 cells were transfected with 60 nM CBP siRNA for 48h and treated with 150μM capsaicin for 24h and immunoblotted with CBP/p300, FOXO-1, and BIM proteins. The same membrane was stripped and reprobed for actin to ensure equal protein loading. (C) BxPC-3 cells were treated with DMSO or 150μM capsaicin for 24h and immunoprecipitated with SirT-1 and SirT-2 antibodies for overnight, resolved on SDS-PAGE and immunoblotted with FOXO-1 anibody. In another experiment BxPC-3 cells were pre-treated with 5mM nicotinamide for 1h and after that 150μM capsaicin for 24h. Representative immunoblots show the effect of capsaicin on SirT-1, SirT-2, FOXO-1 and BIM. The same membrane was stripped and reprobed for actin to ensure equal protein loading. (D) AsPC-1 cells were transiently transfected with 2μg of 3KQ (acetylation mimic mutant) or 3KR (acetylation defective mutant) plasmid for 48h and treated with 150μM capsaicin for 24h, resolved on SDS-PAGE and immunoblotted with p-FOXO-1 (Ser 256), FOXO-1, p-BIM (Ser69) and BIM. The same membrane was stripped and reprobed for actin to ensure equal protein loading. Experiments were performed three times independently with similar observation made in each experiment.
To further confirm the acetylation of FOXO-1, control and capsaicin treated AsPC-1 cells were immunoprecipitated (IP) with anti-CBP/p300 or anti-acetylated lysine or anti-FOXO-1. Capsaicin treatment increased FOXO-1 acetylation (Fig. 4B). Our results also showed that capsaicin treatment significantly increased the levels of acetylated CBP/p300 in the cells immunoprecipitated with FOXO-1 (Fig. 4B), indicating that capsaicin caused FOXO-1 acetylation by recruiting CBP to FOXO-1. We next wanted to see the role of CBP-catalyzed acetylation of FOXO-1 in activating BIM protein levels. In order to prove this, we transfected BxPC-3 cells with CBP siRNA and treated with capsaicin. Our results revealed that CBP siRNA significantly decreased capsaicin- induced BIM expression, indicating that capsaicin-induced BIM expression was perhaps through CBP mediated FOXO-1 acetylation (Fig. 4B, right panel). Previous study reported that the transcriptional activities of FOXO proteins were regulated by CBP/p300-mediated acetylation and SirT-1-mediated deacetylation of FOXO (1). In order to confirm whether SirT-1 was involved in the acetylation of FOXO-1 in response to capsaicin treatment, AsPC-1 cells were treated with 150μM capsaicin for 24h and immunoprecipitated with anti-SirT-1, anti-SirT-2 or anti-FOXO-1 antibodies (Fig. 3C). Our results demonstrated that capsaicin treatment decreased the recruitment of SirT-1 and SirT-2 to FOXO-1 (Fig. 3C). These results indicated that capsaicin weakens the interaction of SirT-1/SirT-2 with FOXO-1 and increase the recruitment of CBP/p300, resulting in the acetylation of FOXO-1, which in turn cause induction of BIM. To confirm this hypothesis, BxPC-3 cells were treated with nicotinamide, which is a strong inhibitor of SirT.
Nicotinamide treatment increased capsaicin mediated BIM expression (Fig 4C). These results indicated that SirT inhibition by nicotinamide synergistically increased capsaicin mediated BIM expression. A significant increase in FOXO-1 was also observed in nicotinamide treated cells (Fig. 4C, right panel). These results showed the involvement of SirT-1 in regulating the acetylation of FOXO-1, resulting in the increased expression of BIM.
FOXO-1 acetylation plays critical role in FOXO-1 phosphorylation
Our next step was to elucidate the sequences of events related to FOXO-1 post-translational modification. In order to test this we transfected AsPC-1 cells with 3KQ plasmid. 3KQ is a FOXO-1 mutant (acetylation mimic) plasmid in which the Lys residues of all three CBP-dependent acetylation sites (Lys-242, Lys-245 and Lys-262) were replaced by glutamine. These substitutions mimic the constitutively acetylated form through neutralization of the positive charges (31, 32). Our results showed that 3KQ transfection increased the constitutive phosphorylation of FOXO-1 at Ser 256, which was further enhanced by capsaicin treatment (Fig 4D). The acetylation mimic (3KQ) further increased capsaicin mediated expression and phosphorylation of BIM at Ser 69 (Fig 4D). To confirm these observations, we then transfected AsPC-1 cells with 3KR plasmid. 3KR is acetylation defective mutant in which lysine residues (Lys-242, Lys-245, and Lys-262) were replaced by arginine residues (31). Our results revealed that 3KR (acetylation defective mutant) blocked FOXO-1 phosphorylation by capsaicin treatment (Fig 4D). BIM was not activated either by capsaicin treatment in AsPC-1 cells transfected with 3KR (Fig 4D). These results clearly suggest that acetylation precedes phosphorylation during post-translational modification of FOXO-1 by capsaicin treatment in pancreatic cancer cells.
Capsaicin-induced apoptosis and activation of JNK/FOXO/BIM cascade prevented by JNK inhibitor
To confirm, whether capsaicin mediated apoptosis was through the activation JNK/FOXO/BIM cascade, cells were treated with JNK inhibitor prior to capsaicin treatment. Our results showed that JNK inhibitor not only blocked the phosphorylation of JNK at Thr183/Tyr185 but also blocked the phosphorylation of FOXO-1 at Ser256 and BIM at Ser69 in BxPC-3 cells. In addition, JNK inhibitor also blocked capsaicin mediated BIM expression and cleavage of caspase-3 (Fig. 5A). These results indicated that capsaicin mediated activation of JNK/FOXO/BIM was responsible for inducing apoptosis in pancreatic cancer cells (Fig. 5A). These results were confirmed by apoptosis assay using flow cytometry, where JNK inhibitor significantly blocked capsaicin-induced apoptosis when compared with controls (Fig. 5A), indicating that JNK activation is required for capsaicin induced-apoptosis in pancreatic cancer cells.
Figure 5. Involvement of JNK, FOXO-1 and BIM in capsaicin induced apoptosis.
(A) BxPC-3 cells were treated with 20μM of JNK-inhibitor (SP60025) for 1h followed by 150μM capsaicin and cell lysates were immunoblotted with p-JNK (Thr183/Tyr185), p-FOXO-1 (Ser256), FOXO-1, p-BIM (Ser69), BIM and cl-caspase-3. The same membrane was stripped and reprobed for actin to ensure equal protein loading. In a separate experiment, BxPC-3 cells were treated with JNK inhibitor for 1h and 150μM capsaicin for 24h. Apoptosis assay was determined using annexin-V/FITC and propidium iodide and analyzed by flow-cytometry. (B) L3.6PL cells were transiently transfected with 2μg of FOXO-1 plasmid for 48h and treated with 150μM of capsaicin for 24h. Cell lysates were resolved on SDS-PAGE and immunobloted with p-FOXO-1 (Ser256), FOXO-1, p-BIM (Ser69) and BIM. The same membrane was stripped and reprobed for actin to ensure equal protein loading. (C) AsPC-1 cells were transfected with 60nM of FOXO-1 siRNA or BIM siRNA and treated with 150μM capsaicin for 24h. Cell lysates were resolved on SDS-PAGE and immunoblotted with p-FOXO-1 (Ser 256), FOXO-1, p-BIM (Ser69), BIM, Cl-caspase-3 and Cl-PARP. The same membrane was stripped and reprobed for actin to ensure equal protein loading. (D) BxPC-3 cells were transfected with 60 nM BIM siRNA for 48h and treated with 150μM capsaicin for 24h. Cell lysates were resolved on SDS-PAGE and immunobloted with BIM, Cl-caspase-3 and Cl-PARP. In an another experiment, BxPC-3 cells were transfected with 60 nM BIM siRNA for 48h and treated with 150μM capsaicin for 24h. Apoptosis assay was determined using annexin-V/FITC and propidium iodide and analyzed by flow-cytometry. Results are expressed as mean±SD (n=3) of three independent experiments. *Statistically different compared with control (P < 0.05), **statistically different when compared with capsaicin treatment (P < 0.05).
Involvement of FOXO-1 and BIM in capsaicin mediated apoptosis
To prove the involvement of FOXO-1 and BIM in capsaicin induced apoptosis, BxPC-3 and AsPC-1 cells were transiently transfected with WT-FOXO-1 plasmid or FOXO-1 siRNA respectively and treated with 150μM capsaicin for 24h. Our results demonstrated that transfection of WT-FOXO-1 increased the phosphorylation and protein level of FOXO-1 in response to capsaicin treatment (Fig. 5B). Our results also showed that WT-FOXO-1 transfection increased the phosphorylation and protein level of BIM, indicating that capsaicin mediated BIM activation was through FOXO-1 (Fig. 5B). On the other hand, FOXO-1 siRNA blocked the phosphorylation and protein levels of FOXO-1 and BIM in response to capsaicin treatment, indicating that activation of FOXO-1 regulates BIM and apoptosis (Fig. 5C). In order to prove the role of BIM in capsaicin induced apoptosis, AsPC-1 cells were transfected with BIM siRNA and treated with 150μM capsaicin. Our results revealed that BIM siRNA blocked capsaicin mediated increased expression of BIM and cleavage of PARP (Fig. 5D). These observations were confirmed by annexin V–FITC assay using flow-cytometer, where BIM siRNA significantly prevented capsaicin-induced apoptosis, indicating that capsaicin mediated activation of BIM cause apoptosis in pancreatic cancer cells. (Fig. 5D).
Capsaicin suppresses the growth of BxPC-3 human pancreatic tumor xenografts
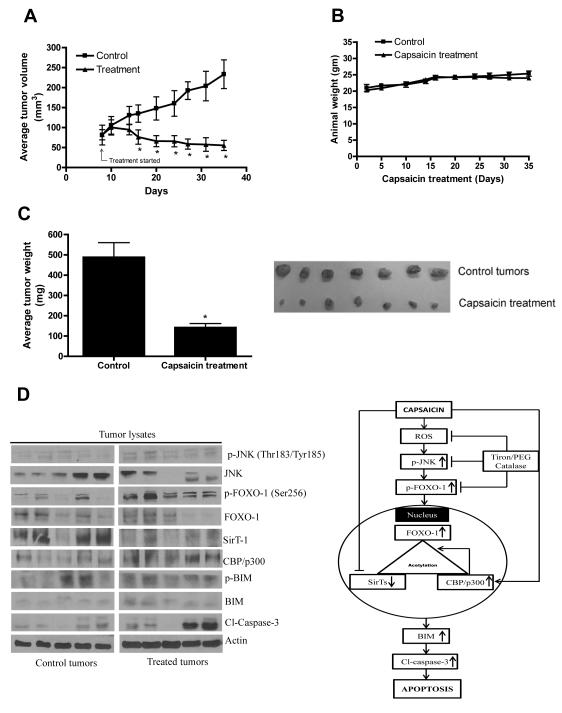
In order to test the possibility that capsaicin treatment would suppress pancreatic tumor growth, BxPC-3 tumor xenografts were implanted in athymic nude mice. Once each mouse had palpable tumors, mice were randomized into two groups and treated group of mice were fed 5mg/kg capsaicin every day and tumor growth was recorded periodically. Our results showed that oral gavage of 5mg/kg every day drastically reduced the growth of the tumors starting day 15 of the treatment and continued till the end of the experiment (Fig 6A). At day 35 of the treatment, tumor volume in the treated group was reduced by 76% as compared with control groups (233.48±35.82 mm3 versus 55.28±12.73 mm3, n=10, Fig 6A). The average body weight of the control and capsaicin treated mice did not change throughout the experiment, indicating no apparent systemic toxicity in capsaicin-treated mice (Fig. 6B). The average wet weight of the tumors dissected from capsaicin treated mice was approximately 71% less than the weight of the tumors from the control mice (Fig. 6C). Right side panel shows the size of control and capsaicin treated tumors to compare the efficacy of capsaicin.
Figure 6. Capsaicin suppresses the growth of BxPC-3 human pancreatic tumor xenograft by activating JNK/FOXO/BIM cascade.
About 1×106 BxPC-3 cells were injected subcutaneously on right flanks of each athymic nude mouse in PBS/Matrigel suspension. When tumors reached 70 mm3 in size, mice were randomly divided to two groups with 10 mice in each group. Mice were fed with 5mg/kg capsaicin everyday by oral gavage. Control mice received vehicle only. Tumors were measured by vernier calipers three times a week. Each mouse was weighed twice a week. Effect of capsaicin on (A) tumor volume, (B) body weight and (C) tumor weight were evaluated. Picture of control and capsaicin treated tumors (Fig. C) Right side panel. (D) Tumor lysates from control and capsaicin treated nude mice were immunoblotted and analysed for p-JNK (Thr183/Tyr185), JNK, p-FOXO-1 (Ser256), FOXO-1, SirT-1, CBP/p300, p-BIM (Ser69), BIM, Cl-caspase-3. The blots were stripped and reprobed for actin to ensure equal protein loading. Values are means ± SEM of 10 samples. *, P<0.05 statistically significant when compared with control. Each lane of blot corresponds to the tumor sample from one mouse. Fig 6D right side panel: Tentative mechanism of action of capsaicin in pancreatic cancers cells.
Capsaicin mediated tumor growth inhibition was associated with activation of JNK/FOXO/BIM cascade
We next aimed to investigate the mechanism and wanted to know whether capsaicin mediated pancreatic tumor growth was through JNK/FOXO/BIM cascade. To confirm this hypothesis, tumors from control and capsaicin treated mice were examined by western blotting. In agreement with our in vitro data in BxPC-3 cells, phosphorylation of JNK at Thr183/Tyr185, FOXO-1 at Ser256, BIM at Ser69 was found to be much higher in the tumors of capsaicin treated mice as compared with controls (Fig 6D). Our results also demonstrated that capsaicin significantly decreased SirT-1 and increased CBP/p300 and BIM protein level in the tumors (Fig. 6D). The cleavage caspase-3 was also observed in capsaicin treated tumors indicating apoptosis. Consistent with our in vitro results, we observed increased phosphorylation of JNK, FOXO-1 and BIM in the tumors from capsaicin treated mice as compared to control tumors, indicating that activation of JNK/FOXO/BIM cascade was associated with the overall capsaicin-mediated tumor growth suppression in vivo.
Discussion
In our previous study, we have shown that capsaicin treatment suppressed the growth of pancreatic cancer cells by generating ROS (25, 26). However, the exact mechanism by which capsaicin induced apoptosis in pancreatic cancer cells was not clear. Our current results demonstrate a novel mechanism by which capsaicin induced apoptosis in pancreatic cancer cells. Our study showed that capsaicin mediated ROS was associated with phosphorylation of JNK at Thr183/185 (26). Activation of JNK by stress signal has been shown to activate pro-apoptotic protein BIM (33). Other studies have shown apoptosis in cancer cells through the interaction of FOXO3a and BIM (2, 17, 18). FOXO4 is also reported to activate BIM and induce apoptosis (19). In the present study, our results clearly showed that capsaicin mediated JNK activation; FOXO-1 phosphorylation and acetylation were involved in BIM mediated apoptosis in pancreatic cancer cells. Furthermore, our results showed that capsaicin failed to activate JNK/FOXO/BIM cascade and apoptosis in the cells that were pre-treated with anti-oxidants tiron or PEG- catalase, suggesting the involvement of ROS in the activation of JNK/FOXO/BIM cascade. A previous study reported that phosphorylation and acetylation of FOXO-1 by HDAC inhibitor induce apoptosis through BIM (34). In agreement, our study revealed a novel mechanism where capsaicin mediated oxidative stress precedes acetylation and phosphorylation of FOXO-1 to activate BIM resulting in apoptosis.
Phosphorylation, ubiquitination, acetylation are the three main well-defined post-translational modifications of FOXO for regulating gene expression (8, 9, 35). In response to growth signals, FOXO proteins are phosphorylated by Akt. Akt mediated phosphorylated FOXO translocate to the nucleus, associates with 14-3-3 chaperon protein and get out of the nucleus (2, 9, 35, 36). On the other hand, stress-induced nuclear translocation of FOXO protein associates with histone acetylase proteins such as CBP/p300 and p300/CBP-associated factor (PCAF). This interaction not only is an initial step in assembling the transcriptional activation FOXO but it also leads to the acetylation of FOXO (1). Acetylated and phosphorylated FOXO-1 then recruits SirT-1 in the nucleus and this recruitment is dependent on oxidative stress (1). Akt mediated regulation of FOXO was not observed in our model as capsaicin failed to modulate PI3K/Akt. Nevertheless, capsaicin treatment elicited stress response in pancreatic cancer cells resulting in the activation of JNK by phosphorylation at Thr183/Tyr185. Activated JNK further phosphorylated FOXO-1 at Ser256 translocating FOXO-1 into the nucleus resulting in increased DNA binding. In another study, we have shown that FOXO-1 translocation into the nucleus increased DNA binding activity and transcription of pro-apoptotic gene BIM by benzyl isothiocyanate (28). Previous studies also reported that acetylation of FOXOs play a key role in activating downstream targets of FOXO-1 (4, 11). Our results indicated that capsaicin mediated FOXO-1 translocation into nucleus increased FOXO-1 DNA binding and expression of pro-apoptotic gene BIM. Our studies also demonstrated the involvement of FOXO-1 and BIM in capsaicin mediated apoptosis as FOXO-1 siRNA and BIM siRNA blocked apoptosis.
FOXOs can interact with protein such as CBP/ p300 in response to oxidative stress and thus increase the acetylation of FOXOs (4, 11). On the other hand, HDACs such as SirT-1 have been reported to interact with and deacetylate FOXO proteins (4, 11, 14). In agreement with these reports, our present studies indicated two possible pathways by which FOXO-1 get acetylated. First, FOXO-1 acetylation may have been induced by the recruitment of CBP to FOXO-1 directly. We provided several evidences to support the involvement of CBP in FOXO-1 acetylation by capsaicin treatment. Second, FOXO-1 acetylation could be mediated through weak interaction of SirT-1 with FOXO-1. In agreement, our results also demonstrated that capsaicin treatment decreased the interaction of SirT-1 with FOXO-1. Our results indicated the involvement of both CBP and SirT-1 in FOXO-1 acetylation by capsaicin treatment. The role of acetylated FOXO in BIM induced apoptosis was shown using CBP mediated acetylation site mimic. Using acetylation mimic and acetylation defective mutants, our study further established that the acetylation of FOXO-1 in fact regulated the phosphorylation of FOXO-1 leading to the activation of BIM in pancreatic cancer cells. Our results are in agreement with the other published studies where acetylation enhanced FOXO3 and BIM activation (11) and acetylation of FOXO-1 increased the sensitivity to phosphorylation (37).
Previous studies have shown 85-95% absorption of capsaicinoids from the gastrointestinal tract. Significant metabolism was observed in the liver resulting in relatively low Cmax and shorter half-life (38). Interestingly, other published reports suggests 0.5-4 mg/kg/day intake of capsaicinoids by humans (http://ec.europa.eu/food/fs/sc/scf/out120_en.pdf), which produces substantial local and circulating levels of capsaicin. Our oral dose of 5mg/kg/day capsaicin substantially suppressed the growth of established pancreatic tumors in athymic nude mice, without any noticeable side-effects. As per FDA guidelines, the human equivalent dose of 5mg/kg capsaicin used in mice would be 0.4mg/kg, which perhaps is possible to achieve clinically. Our laboratory is aggressively working towards determining the bioavailability and pharmacokinetics of capsaicin and its metabolites.
The tumors from capsaicin treated mice showed increased phosphorylation of JNK at Thr183/Tyr185, FOXO-1 at Ser256, BIM at Ser69 and protein levels of BIM and CBP/p300 and reduced SirT-1 levels. Capsaicin treated tumors showed cleavage of caspase-3 indicating apoptosis. Consistent with in vitro studies, our in vivo results demonstrated that the overall tumor growth suppression by capsaicin was associated with the activation JNK/FOXO/BIM pathway. A brief mechanism of capsaicin mediated apoptosis involving JNK/FOXO-1/BIM is shown in Fig. 6D (Right side panel). Taken together, our findings suggest a novel pathway where capsaicin mediated oxidative stress causes post-translational modification of FOXO-1 leading to the transcription BIM and apoptosis in pancreatic tumor cells in vitro and in vivo.
Acknowledgements
This work was supported in part by R01 grant CA129038 (to Sanjay K. Srivastava) awarded by the National Cancer Institute, NIH. Funds from Texas Tech University Health Science Center are also acknowledged. Kind gift of WT-FOXO-1, 3KR and 3KQ expression plasmids by Dr. Akiyoshi Fukamizu, University of Tsukuba, Japan is greatly appreciated.
Footnotes
Disclosure of Potential Conflict of interest Authors declare no conflict of interest.
References
- 1.Vogt PK, Jiang H, Aoki M. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle. 2005;4:908–13. doi: 10.4161/cc.4.7.1796. [DOI] [PubMed] [Google Scholar]
- 2.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 3.Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, et al. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. The Journal of cell biology. 2002;156:531–42. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 5.De Ruiter ND, Burgering BM, Bos JL. Regulation of the Forkhead transcription factor AFX by Ral-dependent phosphorylation of threonines 447 and 451. Molecular and cellular biology. 2001;21:8225–35. doi: 10.1128/MCB.21.23.8225-8235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. The EMBO journal. 2004;23:4802–12. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci. 2005;102:4494–9. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–9. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Tindall DJ. Dynamic FoxO transcription factors. Journal of cell science. 2007;120:2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 10.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci. 2004;101:10042–7. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 12.van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends in biochemical sciences. 2005;30:81–6. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 13.van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) The Journal of biological chemistry. 2004;279:28873–9. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. The EMBO journal. 2005;24:1021–32. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai B, Chang SH, Becker EB, Bonni A, Xia Z. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. The Journal of biological chemistry. 2006;281:25215–22. doi: 10.1074/jbc.M512627200. [DOI] [PubMed] [Google Scholar]
- 16.Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–29. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 17.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. The Journal of biological chemistry. 2007;282:27141–54. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 18.Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. The Journal of biological chemistry. 2003;278:49795–805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 19.Urbich C, Knau A, Fichtlscherer S, Walter DH, Bruhl T, Potente M, et al. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:974–6. doi: 10.1096/fj.04-2727fje. [DOI] [PubMed] [Google Scholar]
- 20.Inoue S, Riley J, Gant TW, Dyer MJ, Cohen GM. Apoptosis induced by histone deacetylase inhibitors in leukemic cells is mediated by Bim and Noxa. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2007;21:1773–82. doi: 10.1038/sj.leu.2404760. [DOI] [PubMed] [Google Scholar]
- 21.Govindarajan VS, Sathyanarayana MN. Capsicum--production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Critical reviews in food science and nutrition. 1991;29:435–74. doi: 10.1080/10408399109527536. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Carrillo L, Hernandez Avila M, Dubrow R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. American journal of epidemiology. 1994;139:263–71. doi: 10.1093/oxfordjournals.aje.a116993. [DOI] [PubMed] [Google Scholar]
- 23.Monsereenusorn Y. Subchronic toxicity studies of capsaicin and capsicum in rats. Research communications in chemical pathology and pharmacology. 1983;41:95–110. [PubMed] [Google Scholar]
- 24.Yun TK. Update from Asia. Asian studies on cancer chemoprevention. Annals of the New York Academy of Sciences. 1999;889:157–92. doi: 10.1111/j.1749-6632.1999.tb08734.x. [DOI] [PubMed] [Google Scholar]
- 25.Pramanik KC, Boreddy SR, Srivastava SK. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PloS one. 2011;6:e20151. doi: 10.1371/journal.pone.0020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Humphreys I, Sahu RP, Shi Y, Srivastava SK. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis : an international journal on programmed cell death. 2008;13:1465–78. doi: 10.1007/s10495-008-0278-6. [DOI] [PubMed] [Google Scholar]
- 27.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. Journal of the National Cancer Institute. 2009;101:176–93. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res. 2011;17:1784–95. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pramanik KC, Srivastava SK. Apoptosis Signal-Regulating Kinase 1-Thioredoxin Complex Dissociation by Capsaicin Causes Pancreatic Tumor Growth Suppression by Inducing Apoptosis. Antioxid Redox Signal. 2012;17:1417–32. doi: 10.1089/ars.2011.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glauser DA, Schlegel W. The emerging role of FOXO transcription factors in pancreatic beta cells. The Journal of endocrinology. 2007;193:195–207. doi: 10.1677/JOE-06-0191. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Luo J, Brooks CL, Gu W. Acetylation of p53 inhibits its ubiquitination by Mdm2. The Journal of biological chemistry. 2002;277:50607–11. doi: 10.1074/jbc.C200578200. [DOI] [PubMed] [Google Scholar]
- 32.Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci. 2004;101:2259–64. doi: 10.1073/pnas.0308762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Zhao Y, Liao W, Yang J, Wu L, Zheng Z, et al. Acetylation of FoxO1 activates Bim expression to induce apoptosis in response to histone deacetylase inhibitor depsipeptide treatment. Neoplasia. 2009;11:313–24. doi: 10.1593/neo.81358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nature reviews Molecular cell biology. 2007;8:440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 36.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 37.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci. 2005;102:11278–83. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaiyasit K, Khovidhunkit W, Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J Med Assoc Thai. 2009;92:108–13. [PubMed] [Google Scholar]