Abstract
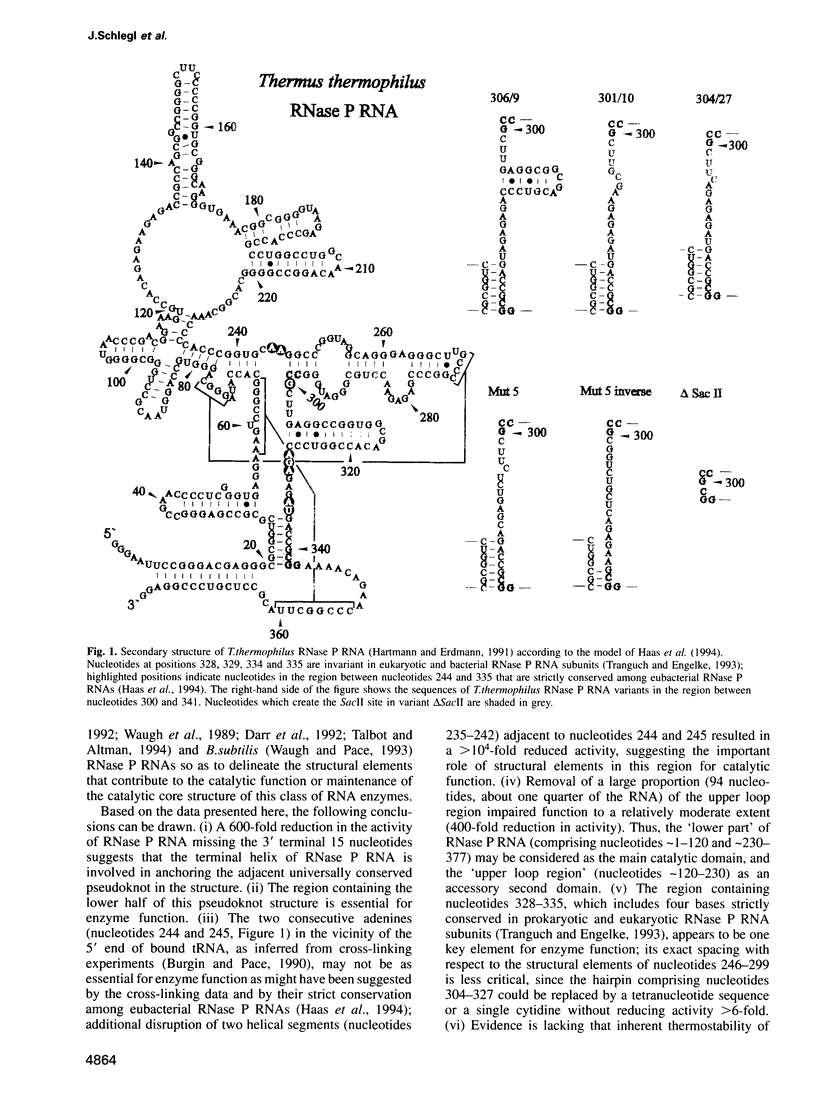
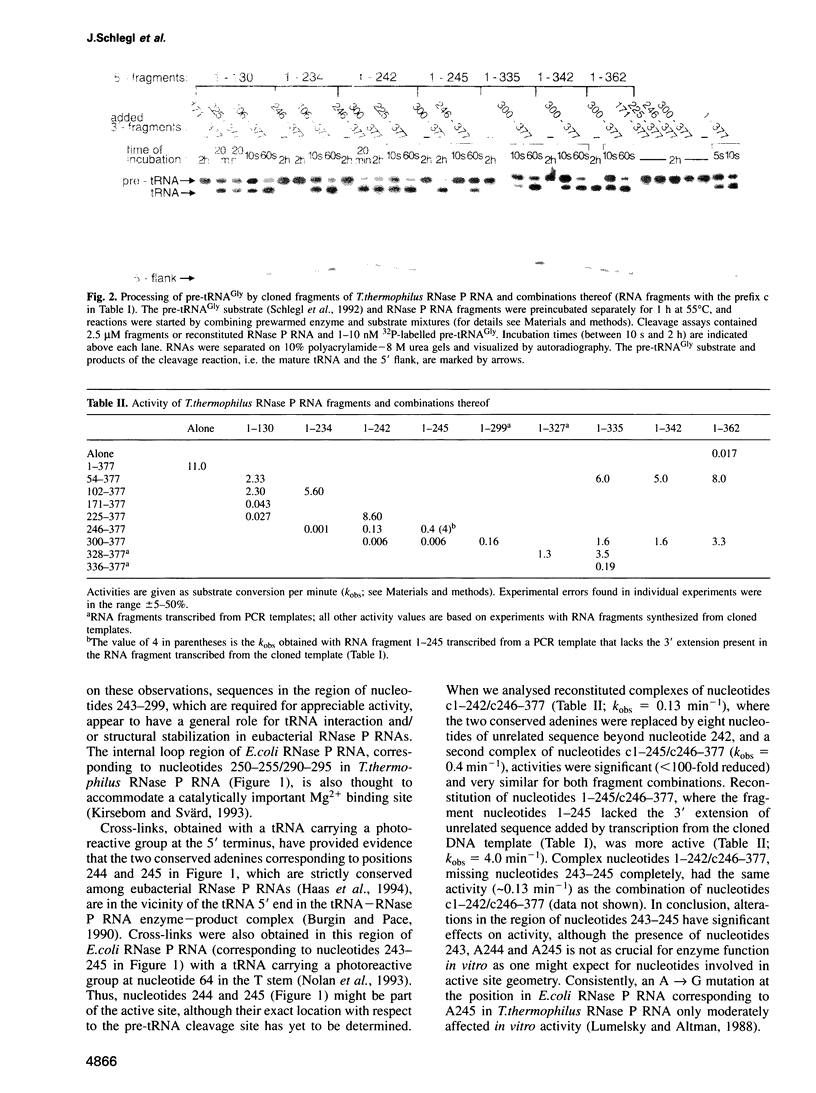
We have performed a deletion and mutational analysis of the catalytic ribonuclease (RNase) P RNA subunit from the extreme thermophilic eubacterium Thermus thermophilus HB8. Catalytic activity was reduced 600-fold when the terminal helix, connecting the 5' and 3' ends of the molecule, was destroyed by deleting 15 nucleotides from the 3' end. In comparison, the removal of a large portion (94 nucleotides, about one quarter of the RNA) of the upper loop region impaired function only to a relatively moderate extent (400-fold reduction in activity). The terminal helix appears to be crucial for the proper folding of RNase P RNA, possibly by orientating the adjacent universally conserved pseudoknot structure. The region containing the lower half of the pseudoknot structure was shown to be a key element for enzyme function, as was the region of nucleotides 328-335. Deleting a conserved hairpin (nucleotides 304-327) adjacent to this region and replacing the hairpin by a tetranucleotide sequence or a single cytidine reduced catalytic activity only 6-fold, whereas a simultaneous mutation of the five highly conserved nucleotides in the region of nucleotides 328-335 reduced catalytic activity by > 10(5)-fold. The two strictly conserved adenines 244 and 245 (nucleotides 248/249 in Escherichia coli RNase P RNA) were not as essential for enzyme function as suggested by previous data. However, additional disruption of two helical segments (nucleotides 235-242) adjacent to nucleotides 244 and 245 reduced activity by > 10(4)-fold, supporting the notion that nucleotides in this region are also part of the active core structure.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer M. F., Reilly R. M., McCorkle G. M., Hai T. Y., Altman S., RajBhandary U. L. The recognition by RNase P of precursor tRNAs. J Biol Chem. 1988 Feb 15;263(5):2344–2351. [PubMed] [Google Scholar]
- Been M. D., Perrotta A. T., Rosenstein S. P. Secondary structure of the self-cleaving RNA of hepatitis delta virus: applications to catalytic RNA design. Biochemistry. 1992 Dec 1;31(47):11843–11852. doi: 10.1021/bi00162a024. [DOI] [PubMed] [Google Scholar]
- Brown J. W., Haas E. S., Pace N. R. Characterization of ribonuclease P RNAs from thermophilic bacteria. Nucleic Acids Res. 1993 Feb 11;21(3):671–679. doi: 10.1093/nar/21.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W., Pace N. R. Ribonuclease P RNA and protein subunits from bacteria. Nucleic Acids Res. 1992 Apr 11;20(7):1451–1456. doi: 10.1093/nar/20.7.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin A. B., Pace N. R. Mapping the active site of ribonuclease P RNA using a substrate containing a photoaffinity agent. EMBO J. 1990 Dec;9(12):4111–4118. doi: 10.1002/j.1460-2075.1990.tb07633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesiolka J., Hardt W. D., Schlegl J., Erdmann V. A., Hartmann R. K. Lead-ion-induced cleavage of RNase P RNA. Eur J Biochem. 1994 Jan 15;219(1-2):49–56. doi: 10.1111/j.1432-1033.1994.tb19913.x. [DOI] [PubMed] [Google Scholar]
- Darr S. C., Zito K., Smith D., Pace N. R. Contributions of phylogenetically variable structural elements to the function of the ribozyme ribonuclease P. Biochemistry. 1992 Jan 21;31(2):328–333. doi: 10.1021/bi00117a003. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Altman S. Similar cage-shaped structures for the RNA components of all ribonuclease P and ribonuclease MRP enzymes. Cell. 1990 Aug 10;62(3):407–409. doi: 10.1016/0092-8674(90)90003-w. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Altman S. M1 RNA with large terminal deletions retains its catalytic activity. Cell. 1986 Apr 25;45(2):177–183. doi: 10.1016/0092-8674(86)90381-8. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Altman S. Reconstitution of enzymatic activity from fragments of M1 RNA. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1266–1270. doi: 10.1073/pnas.89.4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Haas E. S., Morse D. P., Brown J. W., Schmidt F. J., Pace N. R. Long-range structure in ribonuclease P RNA. Science. 1991 Nov 8;254(5033):853–856. doi: 10.1126/science.1719634. [DOI] [PubMed] [Google Scholar]
- Hardt W. D., Schlegl J., Erdmann V. A., Hartmann R. K. Gel retardation analysis of E. coli M1 RNA-tRNA complexes. Nucleic Acids Res. 1993 Jul 25;21(15):3521–3527. doi: 10.1093/nar/21.15.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R. K., Erdmann V. A. Analysis of the gene encoding the RNA subunit of ribonuclease P from T. thermophilus HB8. Nucleic Acids Res. 1991 Nov 11;19(21):5957–5964. doi: 10.1093/nar/19.21.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsebom L. A., Svärd S. G. Identification of a region within M1 RNA of Escherichia coli RNase P important for the location of the cleavage site on a wild-type tRNA precursor. J Mol Biol. 1993 Jun 5;231(3):594–604. doi: 10.1006/jmbi.1993.1312. [DOI] [PubMed] [Google Scholar]
- Lumelsky N., Altman S. Selection and characterization of randomly produced mutants in the gene coding for M1 RNA. J Mol Biol. 1988 Aug 5;202(3):443–454. doi: 10.1016/0022-2836(88)90277-x. [DOI] [PubMed] [Google Scholar]
- Nolan J. M., Burke D. H., Pace N. R. Circularly permuted tRNAs as specific photoaffinity probes of ribonuclease P RNA structure. Science. 1993 Aug 6;261(5122):762–765. doi: 10.1126/science.7688143. [DOI] [PubMed] [Google Scholar]
- Schlegl J., Fürste J. P., Bald R., Erdmann V. A., Hartmann R. K. Cleavage efficiencies of model substrates for ribonuclease P from Escherichia coli and Thermus thermophilus. Nucleic Acids Res. 1992 Nov 25;20(22):5963–5970. doi: 10.1093/nar/20.22.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi H., Shimura Y. Functional domains of the RNA component of ribonuclease P revealed by chemical probing of mutant RNAs. EMBO J. 1988 Dec 1;7(12):3817–3821. doi: 10.1002/j.1460-2075.1988.tb03266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D., Pace N. R. Multiple magnesium ions in the ribonuclease P reaction mechanism. Biochemistry. 1993 May 25;32(20):5273–5281. doi: 10.1021/bi00071a001. [DOI] [PubMed] [Google Scholar]
- Talbot S. J., Altman S. Gel retardation analysis of the interaction between C5 protein and M1 RNA in the formation of the ribonuclease P holoenzyme from Escherichia coli. Biochemistry. 1994 Feb 15;33(6):1399–1405. doi: 10.1021/bi00172a016. [DOI] [PubMed] [Google Scholar]
- Tranguch A. J., Engelke D. R. Comparative structural analysis of nuclear RNase P RNAs from yeast. J Biol Chem. 1993 Jul 5;268(19):14045–14055. [PubMed] [Google Scholar]
- Waugh D. S., Green C. J., Pace N. R. The design and catalytic properties of a simplified ribonuclease P RNA. Science. 1989 Jun 30;244(4912):1569–1571. doi: 10.1126/science.2472671. [DOI] [PubMed] [Google Scholar]
- Waugh D. S., Pace N. R. Gap-scan deletion analysis of Bacillus subtilis RNase P RNA. FASEB J. 1993 Jan;7(1):188–195. doi: 10.1096/fasebj.7.1.7678561. [DOI] [PubMed] [Google Scholar]