Abstract
INTRODUCTION
Chondral and osteochondral lesions of the knee are notoriously difficult to treat due to the poor healing capacity of articular cartilage and the hostile environment of moving joints, ultimately causing disabling pain and early osteoarthritis. There are many different reconstructive techniques used currently but few are proven to be of value. However, some have been shown to produce a better repair with hyaline-like cartilage rather than fibrocartilage.
METHODS
A systematic search of all available online databases including PubMed, MEDLINE® and Embase™ was undertaken using several keywords. All the multiple treatment options and methods available were considered. These were summarised and the evidence for and against them was scrutinised.
RESULTS
A total of 460 articles were identified after cross-referencing the database searches using the keywords. These revealed that autologous and matrix assisted chondrocyte implantation demonstrated both ‘good to excellent’ histological results and significant improvement in clinical outcomes.
CONCLUSIONS
Autologous and matrix assisted chondrocyte implantation have been shown to treat symptomatic lesions successfully with significant histological and clinical improvement. There is, however, still a need for further randomised clinical trials, perfecting the type of scaffold and the use of adjuncts such as growth factors. A list of recommendations for treatment and the potential future trends of managing these lesions are given.
Keywords: Autologous chondrocyte implantation, Osteochondral lesions, Chondral lesions, Mosaicplasty, Matrix assisted chondrocyte implantation, Scaffolds, Tissue engineering
Articular (hyaline) cartilage (AC) has two main functions: low friction movement and shock absorption. Its varying properties enable it to comply with these functions. With increasing activity in young people and the longevity of older people, the prevalence of disorders affecting AC is increasing.1 Normally, the repair of chondral and osteochondral lesions is by fibrocartilage from blood released from the bone marrow. This contains undifferentiated mesenchymal stem cells (MSCs), which produce fibrocartilage containing predominantly type I and III collagen with abnormal proteoglycans. These generally give inadequate mechanical properties, leading to cartilage breakdown and, often, early osteoarthritis.
Methods
All the available online databases including PubMed, MEDLINE® and Embase™ were searched for several keywords: autologous chondrocyte implantation, matrix assisted chondrocyte implantation, osteochondral and chondral defects, mosaicplasty and cartilage scaffolds as well as their common abbreviations. These were cross-referenced with ‘knee’. There was no restriction on publication date. This search yielded 460 articles on the management of knee cartilaginous defects, which were then reviewed.
Structure of articular cartilage
AC has a unique extracellular meshwork predominately of a type II collagen scaffold containing mainly water (approximately 70–80%) with the remainder being hydrophilic proteoglycans. The proteoglycans are made up of chondroitin sulphate and keratin sulphate and attach via a protein core to hyaluronan, forming a three-dimensional structure. These hold water within the collagen meshwork by their negative charge. The collagen meshwork is made up of 90% type II collagen with the remainder being type V, VI, IX and XI collagen.2,3 Type II collagen is strong in tension which, together with the contained water, gives the cartilage its toughness and resiliency (Fig 1).
Figure 1.
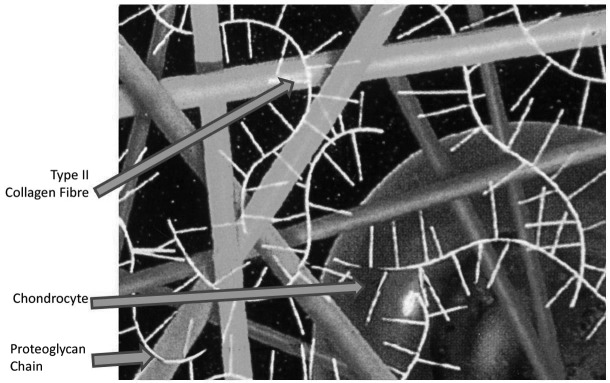
Spatial relations of collagen, proteoglycans and cells in cartilage
The chondrocytes are spread sparsely throughout the AC and have differing functions. In the superficial tangential zone they are small and flattened and produce lubricin, for boundary lubrication. In the middle zone they are round and arranged into columns giving resiliency for shock absorption. Thus, synovial joints have a coefficient of friction of 0.0053, far less than the lowest friction prosthetic joint.4
Metabolism and repair capability
AC is an avascular, aneural tissue with anaerobic metabolism and therefore has limited repair potential. In addition, chondrocytes have no significant migratory ability as they are embedded in the collagen matrix. Finally, the continuous use of the extremity with shearing and impact loading by the individual produces repetitive forces through any given lesion. This adds an unfavourable mechanical environment for spontaneous repair, eventually predisposing the individual to the development of osteoarthritis. The severity of damage is commonly graded using the Outerbridge classification (Table 1).5
Table 1.
Outerbridge classification of articular cartilage defects5
| Grade | Description |
| 0 | Normal |
| I | Cartilage with swelling and softening |
| II | Loss <50% cartilage thickness without exposure of subchondral bone |
| III | Loss <50% cartilage thickness without exposure of subchondral bone |
| IV | Complete loss of cartilage with subchondral bone exposure |
In a retrospective review of more than 31,000 arthroscopic procedures, Curl et al found a 63% prevalence of chondral lesions with an average of 2.7 lesions per knee.6 A prospective study of 1,000 consecutive arthroscopies demonstrated some type of chondral or osteochondral lesion (OCL) in 61% of the patients and focal chondral or osteochondral defects were found in 19%.7 In other studies, the prevalence of focal articular lesions has been reported to be as high as 22–50%.8
Osteoarthritis
Osteoarthritis is characterised by progressive AC loss, appositional new bone formation and sclerosis of the subchondral bone trabeculae, formation of marginal osteophytes and an imbalance between loss of cartilage resulting from matrix degradation and any attempt to repair this matrix. Despite major progress in the last few years, the aetiology, pathogenesis and progression of this disease are poorly understood. However, longstanding traumatic loss of AC is a well recognised predisposing risk factor for osteoarthritis.
Various methods have been used by orthopaedic surgeons to manage patients with severe and persistent pain caused by osteochondral injury. Many of these, like debridement, drilling, abrasion chondroplasty, microfracture and the insertion and use of carbon fibre pads, aim to induce only fibrocartilaginous reparative tissue, formed from primitive MSCs in the subchondral bone marrow. Other treatment strategies aim for repair with hyaline cartilage (AC cell autografting and osteochondral allografts).
In 1971 Bentley and Greer first showed that isolated chondrocytes could be used to repair articular surfaces of rabbit knees that had osteochondral defects or experimental arthritis.9 In 1982 Aston and Bentley showed that chondrocytes could be grown and multiplied by long-term culturing of cells at high density while maintaining the normal type II collagen and proteoglycans of the matrix.10 Cells were also grown and produced type II collagen and proteoglycans in a matrix of carbon fibre, leading to the potential for clinical application.11
Today, autologous chondrocyte implantation (ACI) is a treatment option for full-thickness chondral or osteochondral injuries that are painful and debilitating. Goals of surgery and rehabilitation include replacement of damaged cartilage with hyaline or hyaline-like cartilage, leading eventually to an improved level of function. Intermediate and long-term results are promising in terms of function and prevention of osteoarthritis.12–15
Indications for surgery
Patients with a symptomatic OCL in a joint (that is otherwise normal) of the femoral condyles/trochlea or the patella are recommended for ACI.16 For larger defects (1–12cm2) ACI should be considered.15 Smaller lesions (<1cm2) can be managed initially using mosaicplasty or microfracture. If this fails, then ACI should be considered.17,18
A well motivated and compliant patient between the ages of 15 and 55 years who has had an OCL for under a year and no previous surgery is the best candidate. A careful and comprehensive general and lower limb orthopaedic physical examination with standard weight bearing anteroposterior and patellofemoral x-rays are mandatory.19,20 Reciprocal (kissing) lesions are a contraindication to ACI.21 Malalignment of the tibiofemoral and patellofemoral joints with or without a cruciate ligament injury can be corrected at the time of surgery. Osteoarthritis and inflammatory arthritis are also contraindications.
Investigations
Until recently, the gold standard for investigation of the knee was arthroscopy. However, in 2008 von Endelhardt showed that high powered (>3T) magnetic resonance imaging (MRI) can be as reliable except in differentiating between grade II and III lesions (Fig 2).22 Furthermore, it can be used to assess the soft tissues around the knee as well as the surgical repair of defects.23 Cruciate ligament and meniscal pathology detected on MRI should be dealt with either before or at the time of dealing with the OCL.
Figure 2.
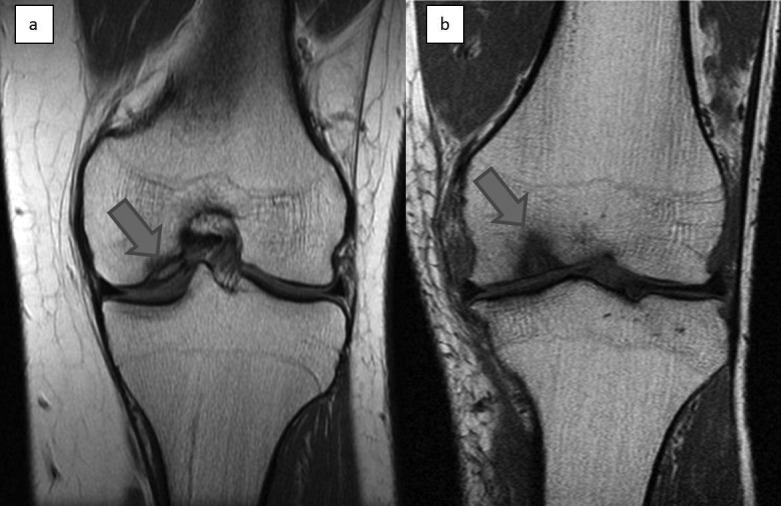
Coronal T1 weighted magnetic resonance imaging of the knee showing an area of osteochondritis dissecans affecting the medial femoral condyle (A) and an osteochondral lesion affecting the medial femoral condyle (B)
Choice of procedure
Abrasion and drilling
This is arthroscopic debridement or low speed drilling, using a fine (1–2mm) K-wire, of the OCL to directly stimulate release of stem cells from the underlying bone marrow. MSC stimulation results in 22% of type I collagen (fibrotic tissue), 30% of degenerated hyaline cartilage and 28% of fibrocartilage.24 This method is now used for very small lesions.
Microfracture and ‘marrow stimulating’ techniques
These techniques also rely on the stimulation of the underlying bone marrow, resulting in fibrocartilage growth. After curetting the OCL down to subchondral bone, a tapered awl can be used to produce the microfractures, approximately 3mm in depth and 3–5mm apart (Fig 3).25 The microfractures result in a blood clot containing mesenchymal cells that then form a fibrocartilaginous repair.
Figure 3.
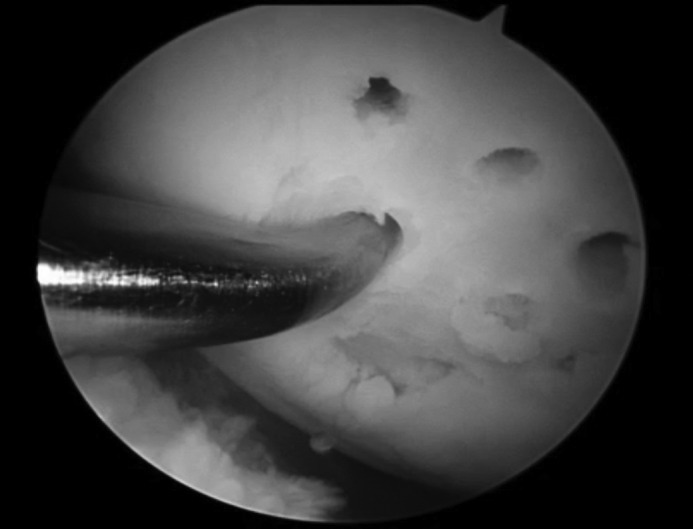
Arthroscopic view of the microfracture process
In a review article published in 2009, Mithoefer et al provided a systematic analysis of 28 studies of over 3,000 patients with an average follow-up duration of 41 months.26 It showed that microfracture provided effective short-term functional improvement of knee function but there were poor long-term results. The other shortcomings revealed included poor hyaline repair, variable cartilage volume and long-term functional deterioration.
Osteochondral autograft/allograft transfer (mosaicplasty)
Osteochondral autograft transfer (OAT) works by removing several plugs of hyaline cartilage and the underlying subchondral bone from an unaffected, non-weight bearing area of the knee. These are used as autograft implants and plugged into the chondral defect. There are several problems with the OAT procedure, the main one being that the topography of the donor site does not match the recipient site and will therefore change the biomechanics and loading. In 2010 Solheim et al published a long-term follow-up study on 69 patients with OAT showing good results up to 9 years after surgery.27 However, in a randomised controlled trial, Bentley et al showed that mosaicplasty was markedly inferior to ACI.12
Although very similar to OAT, osteochondral allograft transfer (OALT) does not rely on a donor site but on a cadaveric donor. OALT will theoretically have a like-for-like replacement with no donor site morbidity. It should be biomechanically and topographically similar. Results have shown good to excellent outcomes in up to 80% of cases in some reports and larger defects can be filled.28 Apart from having to be an open procedure, OALT carries the disadvantages of rejection, viral disease transmission and tissue availability. In 2009 Birman et al investigated the use of humeral heads for OALT on femoral condyles as they can be similar topographically.29 The drawback found in their research is that only small grafts can be harvested due to mismatching.
First generation ACI
In 1994 Brittberg et al described the use of ACI in treating full-thickness AC defects of the human knee.30 This was achieved with a two-stage procedure. Stage 1 involved arthroscopic biopsy of healthy AC and culture of the chondrocytes to produce between 5 and 10 million cells over a period of 4–6 weeks. Stage 2 involved debridement of the OCL and coverage by a periosteal flap followed by open implantation of these cells into the defect.
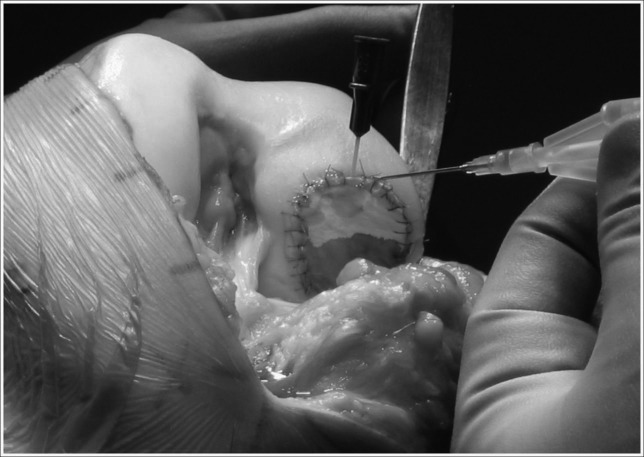
The periosteum is sutured with fine sutures and sealed with fibrin glue to make a watertight seal. The cultured cells are injected beneath it into the OCL (Fig 4). ACI has shown encouraging results. In 2002 Peterson et al examined the durability of ACI grafts, showing 84% had ‘good’ to ‘excellent’ results at 5–11 years.31 One year later, they evaluated treatment of osteochondritis dissecans with ACI, revealing a 90% successful clinical result.32
Figure 4.
Autologous chondrocyte implantation in a medial femoral condyle, demonstrating the injection of chondrocytes in suspension under a collagen type I/III membrane. The extent of the filling can be seen by the ‘tidemark’ on the membrane, produced by the liquid suspension.
Use of periosteum proved problematical as hypertrophy of the membrane producing painful clicking occurred in 25% of patients, who required arthroscopic resection. In a randomised controlled trial, Gooding et al demonstrated the superiority of a type I/III porcine collagen membrane matrix as a cover for the graft.33
In comparative studies, ACI has been evaluated against debridement, microfracture and mosaicplasty. Visna et al directly compared ACI and abrasive techniques, showing that even though early intervention is important, ACI significantly improved Lysholm and International Knee Documentation Committee scores over abrasion techniques.34 Browne et al demonstrated that those patients treated with ACI had a statistically significant improvement of symptoms compared with the microfracture group.35 By contrast, Knutsen et al showed no difference between microfracture and ACI at five years.36 Dozin et al compared mosaicplasty with ACI in a randomised controlled trial showing no significant difference.37
In a randomised controlled trial, Bentley et al compared ACI, using a collagen I/III membrane, with mosaicplasty and showed good or excellent results in 88% versus 69% in the mosaicplasty group.12 They also demonstrated superior International Cartilage Repair Society scores with 84% of patients in the ACI group having grade I or II compared with only 35% of the mosaicplasty group.
Matrix assisted chondrocyte implantation
Matrix assisted chondrocyte implantation (MACI) uses a scaffold, which is a type I/III collagen. It is also described as second generation ACI. In this process, the scaffold is used to provide a matrix preimplantation (Fig 5). This eliminates the need for a periosteal patch and all the morbidity associated with patching.33,38,39 The cells are cultured on the surface of the scaffold, which is then implanted into the defect and secured with fibrin glue. This speeds up the procedure greatly but has the potential disadvantage of a much lower number of implanted cells, up to five times fewer.13,15
Figure 5.
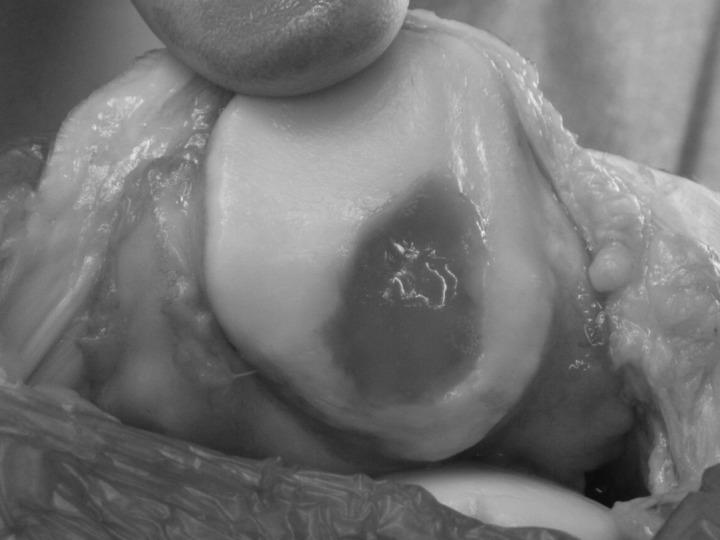
Matrix assisted chondrocyte implantation in a lateral patella facet. The scaffold is held in place with fibrin glue.
Saris et al used phenotypically selected chondrocytes optimised for their production of proteoglycans and presumed repair capacity.40 They showed that in the short term the clinical outcome between microfracture and ACI was similar but ACI showed superior tissue regeneration. In a randomised trial, this method showed a significantly better clinical outcome compared with microfracture41 but no comparison with standard ACI or MACI has been reported. Moreover, this technique costs approximately three times more than the standard technique.
Several papers have studied the differences between microfracture and MACI. In 2009 Kon et al compared second generation ACI, using a hyaluronan scaffold, and microfracture.42 They had 40 patients from each treatment with a minimum follow-up period of five years. Both groups showed a satisfactory outcome at five years but the MACI group showed improved outcome clinically and in terms of return to sporting activity.
Three-dimensional scaffolds
A three-dimensional scaffold mimicking cartilage structure (‘third generation ACI’) is now being used to provide an increased surface area-to-volume ratio for cellular migration, adhesion and differentiation. As seen in first generation ACI series, the results can be variable. Capito and Spector tried to improve on this by introducing a three-dimensional environment into which to seed the cultured chondrocytes.43 They can be made from fibres, sponges or gels and, after the integration of chondrocytes, can be used as a scaffold for implantation.44
The scaffolds can be made from either natural or synthetic materials and they generally have similar properties. They should have a degree of porosity to allow integration with the surrounding AC, which will also assist cell migration as well as nutrient and waste product passage, with the optimum pore size being 100–500µm.45,46 These products should also degrade non-toxically and keep their stability until the new AC is formed.47
Mesenchymal stem cells
MSCs are multipotent stem cells that can differentiate into a variety of cell types. They have been shown to differentiate into bone, cartilage, fat, marrow, muscle, skin and tendon. Any of these tissues are therefore potential sources of MSCs.48,49 Vidal et al showed that the chondrogenic potential of bone marrow is greater than that of adipose tissue.50 More recently, Fan et al described using synovial-derived MSCs for chondrogenesis.51 They demonstrated that these cells can be used in cartilage tissue engineering and they use growth factors, outlined below, to increase their potential.
Unfortunately, there is a lack of studies investigating the use of MSCs in the treatment of OCLs. Animal models have been employed and, more recently, Zscharnack et al have used an ovine model.52 They compared predifferentiated MSCs, undifferentiated MSCs, cell-free and controls using a MACI-like system of hydrogel scaffolds and MSCs instead of chondrocytes. After six months the predifferentiated MSCs showed significantly better histological scores with features of hyaline cartilage.
In an observational cohort study from 2010, Nejadnik et al showed that bone marrow-derived MSCs are as effective as chondrocytes for AC repair.53 The authors also stated that this method reduces the number of knee operations to one and removes donor site morbidity.
Growth factors
Growth factors have been a more recent addition to the management of OCLs and cartilage engineering. MSCs and chondrocytes are influenced by a number of different proteins including growth factors. MSCs have the ability to differentiate into a number of different tissue types. Growth factors, among other things, have the ability to influence this differentiation and are currently being investigated. Similarly to stem cells, growth factors are in the very early stages of investigation.
Conclusions
ACI has become a popular technique for treating isolated chondral defects of the knee and has now been performed on an estimated 35,000 patients worldwide.54 Most investigators have reported ‘good’ to ‘excellent’ clinical and histological results using this technique12,30,31,36,40,54–58 but there is still scepticism among some surgeons about its effectiveness, the type of repair produced and its durability. Which patients should receive ACI and the timing of its use in relation to other techniques such as microfracture remain somewhat unclear.
From a review of the available literature and our experience of the clinical outcome for isolated chondral defects of the knee in over 1,000 cases using ACI, we draw the following conclusions:
-
>
There is no current evidence to justify treatment in asymptomatic, very small (<1cm2) chondral defects of the knee.
-
>
Adult patients with symptomatic full-thickness defects have poor results if not treated.
-
>
Instability and malalignment require correction.
-
>
Smaller (<1cm2), well contained lesions may be suitable for microfracture but for patients who have larger defects, ACI is a satisfactory procedure in 70–80% of cases.
-
>
Motivated patients aged 15–55 years with a single lesion and a short (<1 year) history and no previous procedures have the best outcome.
-
>
ACI leads to a statistically significant improvement in objective and patient reported clinical outcome scores and produces a durable outcome for as long as ten years. The clinical results of the ACI and MACI techniques are comparable and the percentage of hyaline cartilage at biopsy appears to improve with time.
-
>
Lesions of the femoral condyles have superior results to those in the patellofemoral joint.59
Future trends
OCLs present a therapeutic challenge for a number of different reasons. The main difficulties are due to the poor healing potential of AC, its response to injury and constant mechanical loading, and potential disruption of any reconstruction by joint movement and load bearing stresses.
Research into OCLs over 40 years has progressed into multiple fields. The main issue with converting the research into clinical practice is the lack of long-term, evidence-based studies although there are a number of reasons for this. The rapid development of this field has proven sometimes to be detrimental to its own progression. There is such a variety of options giving similar reported outcomes with pain-free repair of OCLs over short periods that selection of patients can be problematic. The preliminary nature of this research also means that it is being spread over a large field. When several types of repair have been shown to be more statistically significant than others, the research can then be focused, leading to long-term validated studies.
Currently, primary arthroscopy is used to identify and document an OCL and to harvest chondrocytes for culture. If MSCs (from bone marrow) can be used to create chondrocytes and MRI continues to evolve into a more sensitive and specific investigation, this primary arthroscopic procedure can be avoided and replaced by a single arthroscopic procedure, reducing the overall morbidity of the reconstruction. MRI is also becoming more common in monitoring progress and repair of procedures so that this could negate the need for a follow-up arthroscopic biopsy.
Reviewing the latest tissue engineering studies has shown that there may not be one specific scaffold that has superior properties compared with the others. Many researchers are now concentrating on combining several different types of scaffolds and growth factors. This is designed to mimic the structural and environmental components of cartilage more accurately, theoretically providing a superior repair.
The future of autologous chondrocyte transplantation relies heavily on biomedical research. Once all the different elements of the scaffold can be optimised, they can be applied clinically. The ideal scaffold should have a three-layer, three-dimensional structure similar to AC, incorporating growth factors and chondrocytes. This should then be applied easily to an OCL and held in position in one simple procedure. Nevertheless, at this time, ACI and its modifications give the best chance of relieving painful osteochondral injuries and preventing ‘early onset’ osteoarthritis.
Acknowledgements
Permission obtained via RightsLink® to reproduce Figure 1 from the BMJ.
Permission sought for Figure 2 from Radiopaedia and learningradiology.com
References
- 1.Evans CH, Ghivizzani SC, Robbins PD. Orthopedic gene therapy in 2008. Mol Ther 2009; 17: 231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown DE, Neumann RD. Orthopaedic Secrets. 3rd edn.Philadelphia, PA: Hanley and Belfus; 2004 [Google Scholar]
- 3.Miller MD. Review of Orthopaedics. 5th edn.Philadelphia, PA: Saunders; 2008 [Google Scholar]
- 4.Linn FC. Lubrication of animal joints: I. The arthrotripsometer. J Bone Joint Surg Am 1967; 49: 1,079–1,098 [PubMed] [Google Scholar]
- 5.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br 1961; 43: 752–757 [DOI] [PubMed] [Google Scholar]
- 6.Curl WW, Krome J, Gordon ESet al.Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 1997; 13: 456–460 [DOI] [PubMed] [Google Scholar]
- 7.Hjelle K, Solheim E, Strand Tet al.Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 2002; 18: 730–734 [DOI] [PubMed] [Google Scholar]
- 8.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 2007; 14: 177–182 [DOI] [PubMed] [Google Scholar]
- 9.Bentley G, Greer RB. Homotransplantation of isolated epiphyseal and articular cartilage chondrocytes into joint surfaces of rabbits. Nature 1971; 230: 385–388 [DOI] [PubMed] [Google Scholar]
- 10.Aston JE, Bentley G. Culture of articular cartilage as a method of storage: assessment of maintenance of phenotype. J Bone Joint Surg Br 1982; 64: 384. [Google Scholar]
- 11.Hemmen B, Archer CW, Bentley G. Repair of articular cartilage defects by carbon fibre plugs loaded with chondrocytes. Trans Orthop Res Soc 1991; 16: 278. [Google Scholar]
- 12.Bentley G, Biant LC, Carrington RWet al.A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br 2003; 85: 223–230 [DOI] [PubMed] [Google Scholar]
- 13.Bartlett W, Skinner JA, Gooding CRet al.Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br 2005; 87: 640–645 [DOI] [PubMed] [Google Scholar]
- 14.Macmull S, Skinner JA, Bentley Get al.Treating articular cartilage injuries of the knee in young people. BMJ 2010; 340: c998. [DOI] [PubMed] [Google Scholar]
- 15.Gikas PD, Bayliss L, Bentley G, Briggs TW. An overview of autologous chondrocyte implantation. J Bone Joint Surg Br 2009; 91: 997–1,006 [DOI] [PubMed] [Google Scholar]
- 16.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 2003; 85(Suppl 2): 58–69 [DOI] [PubMed] [Google Scholar]
- 17.Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg 2002; 15: 170–176 [PubMed] [Google Scholar]
- 18.Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 2003; 85(Suppl 2): 25–32 [DOI] [PubMed] [Google Scholar]
- 19.Minas T. Chondrocyte implantation in the repair of chondral lesions of the knee: economics and quality of life. Am J Orthop 1998; 27: 739–744 [PubMed] [Google Scholar]
- 20.King PJ, Bryant T, Minas T. Autologous chondrocyte implantation for chondral defects of the knee: indications and technique. J Knee Surg 2002; 15: 177–184 [PubMed] [Google Scholar]
- 21.Jones DG, Peterson L. Autologous chondrocyte implantation. J Bone Joint Surg Am 2006; 88: 2,502–2,520 [DOI] [PubMed] [Google Scholar]
- 22.von Engelhardt LV, Schmitz A, Burian Bet al.3-Tesla MRI vs arthroscopy for diagnostics of degenerative knee cartilage diseases: preliminary clinical results. Orthopade 2008; 37: 914, 916–922 [DOI] [PubMed] [Google Scholar]
- 23.Potter HG, Chong le R, Sneag DB. Magnetic resonance imaging of cartilage repair. Sports Med Arthrosc 2008; 16: 236–245 [DOI] [PubMed] [Google Scholar]
- 24.Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res 1999; 365: 149–162 [DOI] [PubMed] [Google Scholar]
- 25.Mithoefer K, Steadman JR. The microfracture technique. Tech Knee Surg 2006; 5: 140–148 [Google Scholar]
- 26.Mithoefer K, McAdams T, Williams RJet al.Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 2009; 37: 2,053–2,063 [DOI] [PubMed] [Google Scholar]
- 27.Solheim E, Hegna J, Oyen Jet al.Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. Knee 2010; 17: 84–87 [DOI] [PubMed] [Google Scholar]
- 28.Görtz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am 2006; 88: 1,374–1,384 [DOI] [PubMed] [Google Scholar]
- 29.Birman MV, Le Dan T, Ismaily SK, Miller BS. The humeral head as a potential donor source for osteochondral allograft transfer to the knee. J Knee Surg 2009; 22: 99–105 [DOI] [PubMed] [Google Scholar]
- 30.Brittberg M, Lindahl A, Nilsson Aet al.Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994; 331: 889–895 [DOI] [PubMed] [Google Scholar]
- 31.Peterson L, Brittberg M, Kiviranta Iet al.Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 2002; 30: 2–12 [DOI] [PubMed] [Google Scholar]
- 32.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 2003; 85(Suppl 2): 17–24 [DOI] [PubMed] [Google Scholar]
- 33.Gooding CR, Bartlett W, Bentley Get al.A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee 2006; 13: 203–210 [DOI] [PubMed] [Google Scholar]
- 34.Visna P, Pasa L, Cizmár Iet al.Treatment of deep cartilage defects of the knee using autologous chondrocyte transplantation and by abrasive techniques – a randomized controlled study. Acta Chir Belg 2004; 104: 709–714 [DOI] [PubMed] [Google Scholar]
- 35.Browne JE, Erggelet C, Micheli LJet al.A controlled study of autologous chondrocyte implantation versus arthroscopic marrow stimulation techniques for full thickness articular cartilage lesions of the femur. Arthroscopy 2002; 18(Suppl): 19–20 [Google Scholar]
- 36.Knutsen G, Drogset JO, Engebretsen Let al.A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 2007; 89: 2,105–2,112 [DOI] [PubMed] [Google Scholar]
- 37.Dozin B, Malpeli M, Cancedda Ret al.Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med 2005; 15: 220–226 [DOI] [PubMed] [Google Scholar]
- 38.Peterson L, Minas T, Brittberg Met al.A two-to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 2000; 374: 212–234 [DOI] [PubMed] [Google Scholar]
- 39.Wood JJ, Malek A, Frassica FJet al.Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J Bone Joint Surg Am 2006; 88: 503–507 [DOI] [PubMed] [Google Scholar]
- 40.Saris DB, Vanlauwe J, Victor Jet al.Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med 2008; 36: 235–246 [DOI] [PubMed] [Google Scholar]
- 41.Saris DB, Vanlauwe J, Victor Jet al.Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med 2009; 37: 10S–19S [DOI] [PubMed] [Google Scholar]
- 42.Kon E, Gobbi A, Filardo Get al.Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med 2009; 37: 33–41 [DOI] [PubMed] [Google Scholar]
- 43.Capito RM, Spector M. Scaffold-based articular cartilage repair. IEEE Eng Med Biol Mag 2003; 22: 42–50 [DOI] [PubMed] [Google Scholar]
- 44.Ko HF, Sfeir C, Kumta PN. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Philos Transact A Math Phys Eng Sci 2010; 368: 1,981–1,997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005; 26: 433–441 [DOI] [PubMed] [Google Scholar]
- 46.Ikada Y. Challenges in tissue engineering. J R Soc Interface 2006; 3: 589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pieper JS, Hafmans T, Veerkamp JH, van Kuppevelt TH. Development of tailor-made collagen-glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials 2000; 21: 581–593 [DOI] [PubMed] [Google Scholar]
- 48.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 2002; 10: 432–463 [DOI] [PubMed] [Google Scholar]
- 49.Goepfert C, Slobodianski A, Schilling AFet al.Cartilage engineering from mesenchymal stem cells. Adv Biochem Eng Biotechnol 2010; 123: 163–200 [DOI] [PubMed] [Google Scholar]
- 50.Vidal MA, Robinson SO, Lopez MJet al.Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg 2008; 37: 713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan J, Gong Y, Ren Let al.In vitro engineered cartilage using synovium-derived mesenchymal stem cells with injectable gellan hydrogels. Acta Biomater 2010; 6: 1,178–1,185 [DOI] [PubMed] [Google Scholar]
- 52.Zscharnack M, Hepp P, Richter Ret al.Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am J Sports Med 2010; 38: 1,857–1,869 [DOI] [PubMed] [Google Scholar]
- 53.Nejadnik H, Hui JH, Feng Choong EPet al.Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med 2010; 38: 1,110–1,116 [DOI] [PubMed] [Google Scholar]
- 54.Henderson I, Gui J, Lavigne P. Autologous chondrocyte implantation: natural history of postimplantation periosteal hypertrophy and effects of repair-site debridement on outcome. Arthroscopy 2006; 22: 1,318–1,324 [DOI] [PubMed] [Google Scholar]
- 55.Micheli LJ, Browne JE, Erggelet Cet al.Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sport Med 2001; 11: 223–228 [DOI] [PubMed] [Google Scholar]
- 56.Horas U, Pelinkovic D, Herr Get al.Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am 2003; 85: 185–192 [DOI] [PubMed] [Google Scholar]
- 57.Browne JE, Anderson AF, Arciero Ret al.Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res 2005; 436: 237–245 [DOI] [PubMed] [Google Scholar]
- 58.Fu FH, Zurakowski D, Browne JEet al.Autologous chondrocyte implantation versus debridement for treatment of full-thickness chondral defects of the knee: an observational cohort study with 3-year follow-up. Am J Sports Med 2005; 33: 1,658–1,666 [DOI] [PubMed] [Google Scholar]
- 59.Bentley G. The Cellular Approach to Joint Cartilage Damage. Presented at: Annual meeting of the British Orthopaedic Association; September 2007; Manchester [Google Scholar]