Abstract
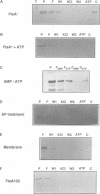
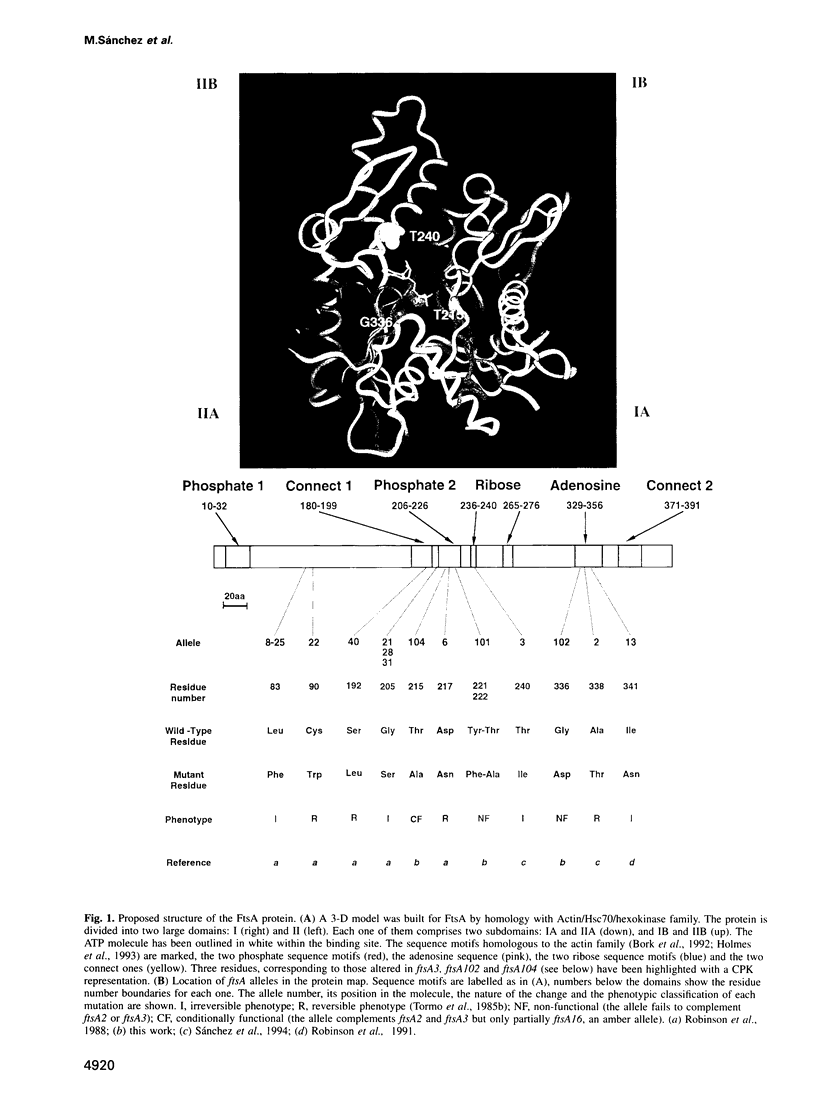
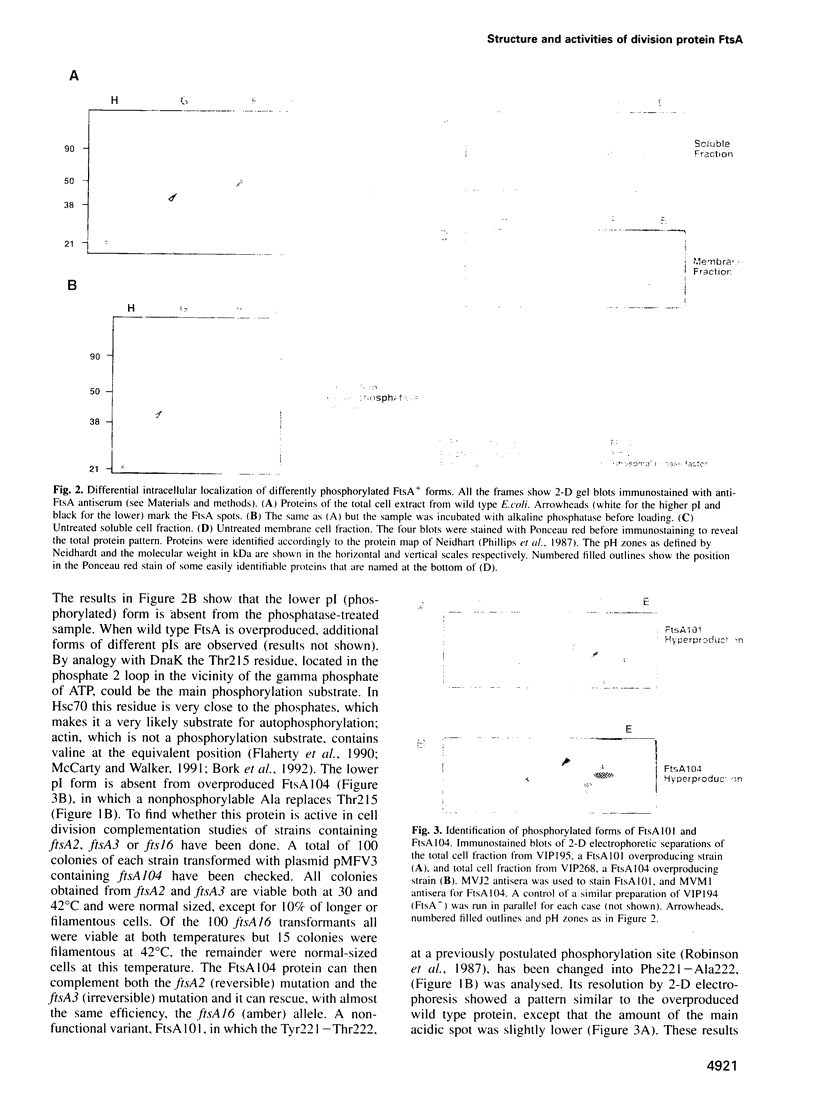
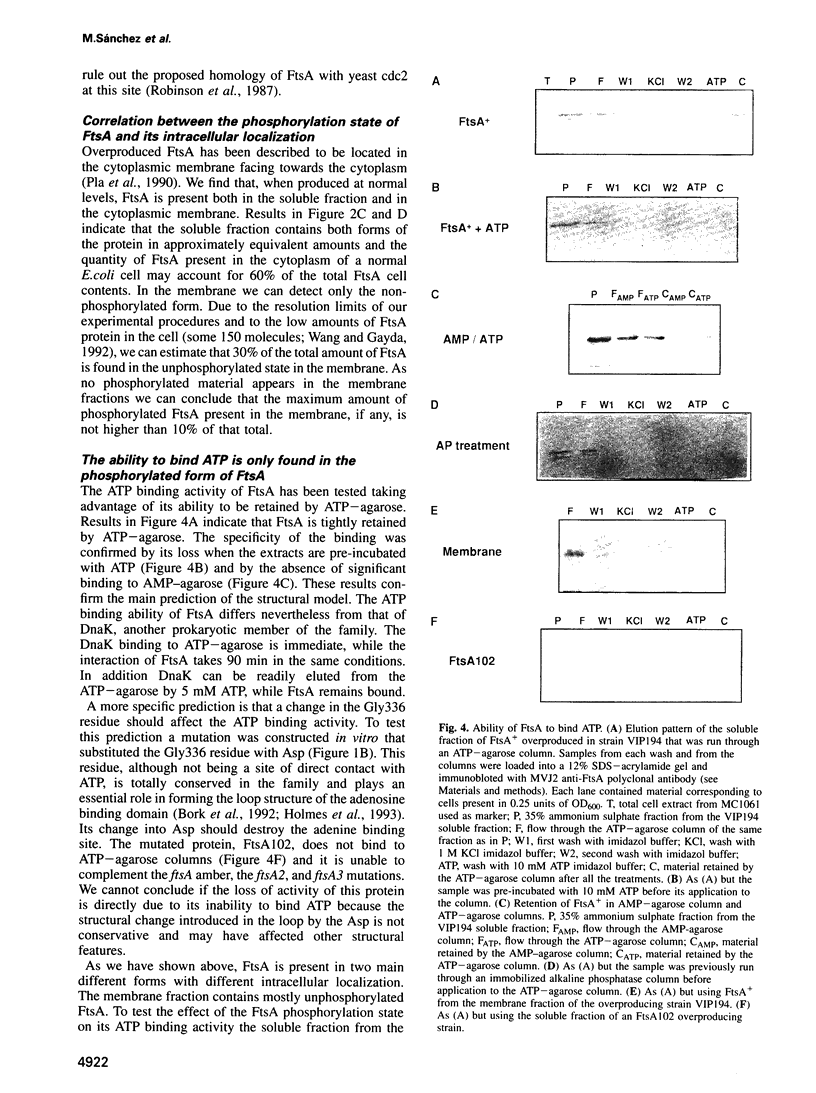
Cell division protein FtsA, predicted to belong to the actin family, is present in different cell compartments depending on its phosphorylation state. The FtsA fraction isolated from the cytoplasm is phosphorylated and capable of binding ATP, while the membrane-bound form is unphosphorylated and does not bind ATP. A variant of the protein FtsA102, in which the nucleotide binding site was destroyed by mutagenesis of a highly conserved residue predicted to be needed for the binding, does not bind ATP. Another variant, FtsA104, cannot be phosphorylated because the predicted phosphorylatable residue has been replaced by a non-phosphorylatable one. This protein although unable to bind ATP in vitro, is able to rescue the reversible ftsA2, the irreversible ftsA3 and, almost with the same efficiency, the ftsA16 amber alleles. Consequently, phosphorylation and ATP binding may not be essential for the function of FtsA. Alternatively they may have a regulatory role on the action of FtsA in the septator.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amster-Choder O., Wright A. Regulation of activity of a transcriptional anti-terminator in E. coli by phosphorylation in vivo. Science. 1990 Aug 3;249(4968):540–542. doi: 10.1126/science.2200123. [DOI] [PubMed] [Google Scholar]
- Beall B., Lutkenhaus J. Impaired cell division and sporulation of a Bacillus subtilis strain with the ftsA gene deleted. J Bacteriol. 1992 Apr;174(7):2398–2403. doi: 10.1128/jb.174.7.2398-2403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P., Sander C., Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7290–7294. doi: 10.1073/pnas.89.16.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S., Aiba H., de Crombrugghe B. Mutations in the Escherichia coli operon that define two promoters and the binding site of the cyclic AMP receptor protein. J Mol Biol. 1982 Jan 15;154(2):211–227. doi: 10.1016/0022-2836(82)90061-4. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Lutkenhaus J. F., Salmond G. P., Martinez-Salas E., Vincente M. Role of the ftsA gene product in control of Escherichia coli cell division. J Bacteriol. 1979 Nov;140(2):388–394. doi: 10.1128/jb.140.2.388-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Vicente M. Cell length, cell growth and cell division. Nature. 1976 Nov 25;264(5584):328–333. doi: 10.1038/264328a0. [DOI] [PubMed] [Google Scholar]
- Dopazo A., Tormo A., Aldea M., Vicente M. Structural inhibition and reactivation of Escherichia coli septation by elements of the SOS and TER pathways. J Bacteriol. 1987 Apr;169(4):1772–1776. doi: 10.1128/jb.169.4.1772-1776.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Garrido T., Sánchez M., Palacios P., Aldea M., Vicente M. Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J. 1993 Oct;12(10):3957–3965. doi: 10.1002/j.1460-2075.1993.tb06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. C., Sander C., Valencia A. A new ATP-binding fold in actin, hexokinase and Hsc70. Trends Cell Biol. 1993 Feb;3(2):53–59. doi: 10.1016/0962-8924(93)90161-s. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- McCarty J. S., Walker G. C. DnaK as a thermometer: threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9513–9517. doi: 10.1073/pnas.88.21.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Dai K., Lutkenhaus J. Escherichia coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1053–1057. doi: 10.1073/pnas.90.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pla J., Dopazo A., Vicente M. The native form of FtsA, a septal protein of Escherichia coli, is located in the cytoplasmic membrane. J Bacteriol. 1990 Sep;172(9):5097–5102. doi: 10.1128/jb.172.9.5097-5102.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RayChaudhuri D., Park J. T. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992 Sep 17;359(6392):251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- Rieul C., Cortay J. C., Bleicher F., Cozzone A. J. Effect of bacteriophage M13 infection on phosphorylation of dnaK protein and other Escherichia coli proteins. Eur J Biochem. 1987 Nov 2;168(3):621–627. doi: 10.1111/j.1432-1033.1987.tb13461.x. [DOI] [PubMed] [Google Scholar]
- Robinson A. C., Begg K. J., MacArthur E. Isolation and characterization of intragenic suppressors of an Escherichia coli ftsA mutation. Res Microbiol. 1991 Jul-Aug;142(6):623–631. doi: 10.1016/0923-2508(91)90075-l. [DOI] [PubMed] [Google Scholar]
- Robinson A. C., Begg K. J., Sweeney J., Condie A., Donachie W. D. Mapping and characterization of mutants of the Escherichia coli cell division gene, ftsA. Mol Microbiol. 1988 Sep;2(5):581–588. doi: 10.1111/j.1365-2958.1988.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Robinson A. C., Collins J. F., Donachie W. D. Prokaryotic and eukaryotic cell-cycle proteins. 1987 Aug 27-Sep 2Nature. 328(6133):766–766. doi: 10.1038/328766a0. [DOI] [PubMed] [Google Scholar]
- Shapiro L. Protein localization and asymmetry in the bacterial cell. Cell. 1993 Jun 4;73(5):841–855. doi: 10.1016/0092-8674(93)90266-s. [DOI] [PubMed] [Google Scholar]
- Sherman MYu, Goldberg A. L. Involvement of the chaperonin dnaK in the rapid degradation of a mutant protein in Escherichia coli. EMBO J. 1992 Jan;11(1):71–77. doi: 10.1002/j.1460-2075.1992.tb05029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirion M. M., ben-Avraham D. Normal mode analysis of G-actin. J Mol Biol. 1993 Mar 5;230(1):186–195. doi: 10.1006/jmbi.1993.1135. [DOI] [PubMed] [Google Scholar]
- Tormo A., Ayala J. A., de Pedro M. A., Aldea M., Vicente M. Interaction of FtsA and PBP3 proteins in the Escherichia coli septum. J Bacteriol. 1986 Jun;166(3):985–992. doi: 10.1128/jb.166.3.985-992.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Dopazo A., de la Campa A. G., Aldea M., Vicente M. Coupling between DNA replication and cell division mediated by the FtsA protein in Escherichia coli: a pathway independent of the SOS response, the "TER" pathway. J Bacteriol. 1985 Nov;164(2):950–953. doi: 10.1128/jb.164.2.950-953.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Fernández-Cabrera C., Vicente M. The ftsA gene product: a possible connection between DNA replication and septation in Escherichia coli. J Gen Microbiol. 1985 Feb;131(2):239–244. doi: 10.1099/00221287-131-2-239. [DOI] [PubMed] [Google Scholar]
- Tormo A., Martínez-Salas E., Vicente M. Involvement of the ftsA gene product in late stages of the Escherichia coli cell cycle. J Bacteriol. 1980 Feb;141(2):806–813. doi: 10.1128/jb.141.2.806-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Vicente M. The ftsA gene product participates in formation of the Escherichia coli septum structure. J Bacteriol. 1984 Mar;157(3):779–784. doi: 10.1128/jb.157.3.779-784.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente M., Palacios P., Dopazo A., Garrido T., Pla J., Aldea M. On the chronology and topography of bacterial cell division. Res Microbiol. 1991 Feb-Apr;142(2-3):253–257. doi: 10.1016/0923-2508(91)90038-c. [DOI] [PubMed] [Google Scholar]
- Wang H., Gayda R. C. Quantitative determination of FtsA at different growth rates in Escherichia coli using monoclonal antibodies. Mol Microbiol. 1992 Sep;6(17):2517–2524. doi: 10.1111/j.1365-2958.1992.tb01428.x. [DOI] [PubMed] [Google Scholar]
- Ward J. E., Jr, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in E. coli. Cell. 1985 Oct;42(3):941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- de Boer P., Crossley R., Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature. 1992 Sep 17;359(6392):254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]