Abstract
INTRODUCTION
The terms ‘enhanced recovery after surgery’, ‘enhanced recovery programme’ (ERP) and ‘fast track surgery’ refer to multimodal strategies aiming to streamline peri-operative care pathways, to maximise effectiveness and minimise costs. While the results of ERP in colorectal surgery are well reported, there have been no reviews examining if these concepts could be applied safely to hepatopancreatobiliary (HPB) surgery. The aim of this systematic review was to appraise the current evidence for ERP in HPB surgery.
METHODS
A MEDLINE® literature search was undertaken using the keywords ‘enhanced recovery’, ‘fast-track’, ‘peri-operative’, ‘surgery’, ‘pancreas’ and ‘liver’ and their derivatives such as ‘pancreatic’ or ‘hepatic’. The primary endpoint was length of post-operative hospital stay. Secondary endpoints were morbidity, mortality and readmission rate.
RESULTS
Ten articles were retrieved describing an ERP. ERP protocols varied slightly between studies. A reduction in length of stay was a consistent finding following the incorporation of ERP when compared with historical controls. This was not at the expense of increased rates of readmission, morbidity or mortality in any study.
CONCLUSIONS
The introduction of an ERP in HPB surgery appears safe and feasible. Currently, many of the principles of the multimodal pathway are derived from the colorectal ERP and distinct differences exist, which may impede its implementation in HPB surgery.
Keywords: Systematic review, Enhanced recovery, Fast track surgery, Hepatobiliary surgery, Pancreatic surgery, Liver surgery
‘Enhanced recovery after surgery’ or ‘fast track surgery’ pathways aim to streamline peri-operative care delivery and maximise effectiveness while minimising costs. They represent multimodal strategies that include patient education, optimal analgesic relief, stress reduction with regional anaesthesia, focused nursing and early mobilisation to augment the rapid return of functional recovery.1,2 They also represent a paradigm shift from traditional surgical philosophies and incorporate the use of minimally invasive methods and fewer or no surgical drains.
Enhanced recovery programmes (ERPs) have been the subject of numerous systematic reviews in colorectal surgery and most have demonstrated reduced post-operative stay, lower complication rates and reduced hospital costs, leading to their increasing use.2–5 There are also reports demonstrating improved outcomes with the use of similar pathways in vascular6,7 and urological8,9 procedures. However, peri-operative strategies with a strong evidence base supporting its use are not yet implemented widely in hepatopancreatobiliary (HPB) surgery.
Post-operative stay after pancreatic or liver resection is usually 12–17 and 8–14 days respectively at high volume centres.10–14 Pancreatic resection has always been considered a high risk procedure with an associated morbidity and mortality of 30–60% and 5% respectively.10,11 Liver resection too is considered high risk, and has an associated morbidity and mortality of 38–45% and 2.7–3.1% respectively.13,14
Controversy exists over the role of an ERP in HPB surgery. There have been no previous systematic reviews conclusively proving whether such concepts could be applied safely to such complex and major abdominal surgery. The aim of this systematic review was to appraise the current evidence for the incorporation of an ERP for major pancreatic and hepatic resections.
Methods
A MEDLINE® literature search was undertaken using the keywords ‘enhanced recovery’, ‘fast-track’, ‘peri-operative’, ‘surgery’, ‘pancreas’ and ‘liver’ and their derivatives such as ‘pancreatic’ or ‘hepatic’. The inclusion criteria were studies examining the impact of fast track surgery on outcomes in any HPB surgery. Studies were included if they incorporated a sufficient description of the multimodal clinical ERP together with the required outcome measures. Studies were excluded if they examined only a single intervention in peri-operative management outside the context of an ERP. The search was limited to English language manuscripts only. All articles retrieved had the references cross-checked to ensure capture of cited pertinent articles. The primary endpoint was length of post-operative hospital stay. Secondary endpoints were morbidity, mortality and readmission rate. The evidence that established each element of the pathway was not the purpose of this review and is not discussed further.
Results
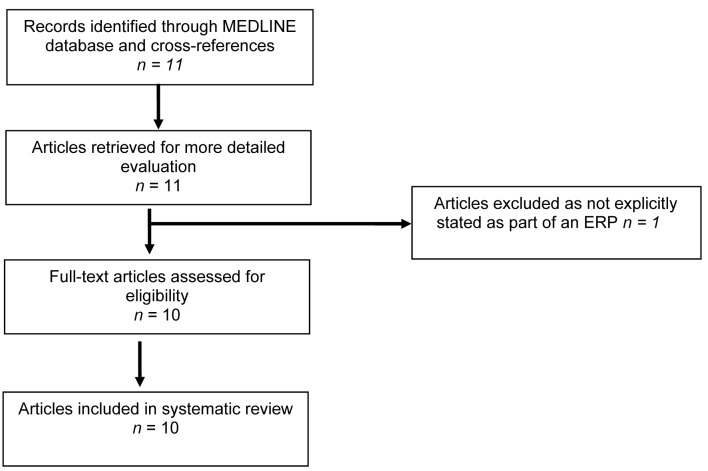
A total of 11 articles, published between 2007–2011, were retrieved that met the inclusion criteria.15–25 One article that described a randomised controlled trial (RCT) of early enteral nutrition in patients undergoing major upper gastrointestinal surgical resection was excluded as the patients were not explicitly described as being part of an ERP (Fig 1).15 Table 1 shows a summary of the remaining ten articles. Two articles describing a single intervention in one parameter of peri-operative care but within an ERP were included.21,22 One of these studies comprised an RCT of laxatives and oral nutritional supplements following liver resection.22 The other investigated the effects of analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia.21
Figure 1.
Flow diagram for the systematic review
Table 1.
Articles describing the enhanced recovery programme in hepatobiliary and pancreatic surgery
| Authors | Year | Surgery (liver / pancreas) | Study design | Surgery type (ERP cohort where applicable) | Patients in ERP | Significant study findings compared with historical control |
| Berberat et al16 | 2007 | Pancreas | Prospective historical comparison study | Pancreatic head resection 70.6%; distal 20%; total 5.9%; segmental 3.5% | 255 | |
| Balzano et al17 | 2008 | Pancreas | Prospective historical comparison study | PD | 252 | More rapid time to passing first stool (5 vs 6 days, p<0.001); shorter length of stay (13 vs 15 days, p<0.001); less morbidity (47.2% vs 58.7%, p=0.014) with no difference in readmission rate (7.1% vs 6.3%, p=0.865) |
| van Dam et al18 | 2008 | Liver | Prospective case series comparing with a historical control | Hemihepatectomy 33%; hemihepatectomy + metastasectomy 10%; extended hemihepatectomy 11%; multisegmental 28%; central resection 2%; metastasectomy 16%; repeat hepatectomy 11% | 61 | Reduced length of stay (6 vs 8 days, p<0.001); no significant difference in morbidity (41% vs 31%, p=0.197) or readmission rate (13% vs 10%, p=0.61) |
| MacKay et al19 | 2008 | Liver | Prospective case series | 1 lobectomy; 2 trisegmentectomy; 3 bisegmentectomy; 6 segment | 12 | |
| Stoot et al20 | 2009 | Liver | Prospective multlcentre comparison study | Laparoscopic lateral resection, 1 segment IV | 13 | No significant reductions in length of stay (5 vs 7 days, p=0.305) or morbidity/mortality; significantly less intra-operative blood loss (50ml vs 250ml, p=0.002) |
| Koea et al21 | 2009 | Liver | Consecutive patients in an ERP comparing analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia | Hemihepatectomy 36%; extended hepatectomy 4%; multisegementectomy 18%; monosegmentectomy 5%; metastasectomy 22% | 50 | |
| Hendry et al22 | 2010 | Liver | Randomised controlled trial of laxatives and oral nutrition supplements within an ERP | Major resection 77.9%; minor resection 22.1% | 68 | |
| Montiel Casado et al23 | 2010 | Pancreas | Retrospective historical comparison study | Classic PD | 82 | |
| di Sebastiano et al24 | 2011 | Pancreas | Prospective historical comparison study | Pylorus preserving PD 62.1%; PD 2.7%; duodenum preserving pancreatic head resection 2.7%; distal pancreatectomy 13.8%; central pancreatectomy 2.1%; total pancreatectomy 6.9%; completion pancreatectomy 1.4%; other 8.3% | 145 | |
| Lin et al25 | 2011 | Liver | Prospective comparison study at same site before and after introduction of ERP | Blsegmentectomy 30.4%; segmentectomy 23.2%; hemihepatectomy 16.1%; non- anatomlcal resection 12.5%; central resection 10.7%; extended hemlhepatectomy 7.1% | 61 | Reduced length of stay (7 vs 11 days, p<0.01); no difference In morbidity (37.7% vs 37.5%, p=0.982), mortality (1.8% vs 1.6%, p=0.706) or readmission rate (7.1% vs 3.3%, p=0.424) |
ERP = enhanced recovery programme; PD = pancreaticoduodenectomy
Fast track surgery was described in six articles in liver resections18–22,25 and in four articles in pancreatic surgery.16,17,23,24 A total of 734 patients were included having had pancreatic surgery and a total of 265 patients after liver resection.
Six studies were prospective case series that compared outcomes of the ERP with historical controls, not necessarily in the authors’ institution.16–19,24,25 One study was a retrospective case series that compared outcomes with historical controls.23 The article by Stoot et al was a multicentre study comparing the ERP with both historical controls in the same centres before the introduction of the ERP or during the same period in other centres using traditional care.20 The two studies that described single interventions in one parameter but within an ERP compared outcomes in the study cohorts.21,22
The ERP protocol did vary between studies. However, all described a multimodal clinical pathway incorporating patient education, regional anaesthesia, optimal pain relief, judicious use of surgical drains (including nasogastric tubes and urinary catheters), early mobilisation and early introduction of oral liquids post-operatively (Tables 2 and 3).
Table 2.
Summary of fast track multimodal elements in each study in pancreatic resectional surgery
| Berberat et al16 | Balzano et al17 | Montiel Casado et al23 | di Sebastiano et al24 | |
| Pre-operatively | Information given to patient about fast track rehabilitation | Information given to patient; LMWH | Oral nutrition until 10pm; no premedication | |
| Day 0 | LMWH; octreotide; NG tube and drains used routinely; ICU stay; epidural or PCA | Thoracic epidural (T7-9; bupivacaine 0.125% and fentanyl 2µg/ml) plus IV paracetamol and NSAIDs | Epidural analgesia; removal of NG tube after surgery; ICU stay; liquids; prokinetic and octreotide | Analgesia by elastomeric pump*; remove NG tube on extubation; warm IV fluids; ICU stay; CVP <5mmHg |
| Day 1 | Metoclopramide, lactulose and magnesium until first stool; oral fluids within 6h post-operatively | Remove NG tube if draining <300ml; mobilise out of bed; IV fluids until adequate oral intake | Move to ward; moving patient to chair; inhalation; liquid diet | Move to ward; mobilise four times daily; clear oral fluids within 4h post-operatively; metoclopramide and paracetamol |
| Day 2 | Stepwise reduction in analgesia to non-opioids | Enhanced mobilisation (>2h out of bed) | Light diet; continue as per day 1 | |
| Day 3 | Removal of drains between days 1 and 3; gradual increase in diet | Enhanced mobilisation (>4h out of bed); clear free fluids | Remove epidural; semiliquid diet; remove Foley catheter | Stop elastomeric pump; start NSAIDs; remove catheter; soft diet |
| Day 4 | Solid food intake | Soft diet | Normal diet | |
| Day 5 | Diet increased daily until 1,000kcal on day 8; remove drain (if <200 ml); remove epidural | Discharge if no fever; good pain control and tolerance of oral analgesics | Discharged if no fever, pain control with oral analgesics, solid foods >1,000kcal/day; adequate mobilisation and willingness for discharge | Plan for discharge on day 7 if pain control with oral analgesics, no nausea, solid food; adequate mobilisation and willingness for discharge |
LMWH = low molecular weight heparin; NG = nasogastric; ICU = intensive care unit; PCA = patient controlled analgesia; IV = intravenous; NSAID = non-steroidal anti-inflammatory drug; CVP = central venous pressure
ketoprofen 960mg, tramadol 600mg, ranitidine 450mg, metoclopramide 90mg, morphine 15–30mg dissolved in 300ml saline solution
Table 3.
Summary of fast track multimodal elements in each study in liver resectional surgery
| van Dam et al18 | MacKay et al19 | Stoot et al20 | Koea et al21 | Hendry et al22 | Lin et al25 | |
| Pre-operatively | Oral nutrition until midnight; no premedication | Information given to patient about fast track rehabilitation | Information given to patient; no premedication; carbohydrate drink until 2h pre-operatively | Nil by mouth for 4h pre-operatively | Oral nutrition until midnight; no premedication | Information given to patient; no premedication or bowel preparation |
| Day 0 | Thoracic epidural; remove NG post-operatively; no routine drains; oral fluids post-operatively; CVP <5mmHg | Oral fluids until 2h pre-operatively; no routine use of drains; oral fluids and supplementary drinks; PCA | Thoracic epidural catheter; no routine NG tube; oral liquid diet 6h post-operatively; laxatives and prokinetics; CVP <5mmHg | No routine use of NG tubes or surgical drains; liquid/light diet on waking | Thoracic epidural; remove NG post-operatively; no routine drains; free clear fluids post-operatively; out of bed for 2h | Thoracic epidural catheter; no routine drains or NG tube; oral liquid diet 6h post- operatively |
| Day 1 | Mobilise; IV fluids stopped; normal diet; paracetamol and magnesium oxide | Diet If tolerated; small Gelofusine® boluses If hypovolaemic (stopped after 24h) | Mobilise; IV fluids stopped; normal diet; paracetamol and magnesium oxide | Remove arterial line and catheter; unrestricted diet; mobilise; routine blood tests | Mobilise; IV fluids stopped; normal diet; paracetamol | Mobilise >2h; reduce IV fluids; 1l liquid diet; catheter out |
| Day 2 | As above | Remove PCA; step-down analgesia; remove catheter; mobilise | As above | Mobilise; continue diet; repeat blood tests | As above | Mobilise four times daily; epidural removed; NSAIDs |
| Day 3 | Stop epidural; start NSAIDs; remove catheter; full oral intake | Mobilise; continue diet; repeat blood tests | Stop epidural; start NSAIDs; remove catheter; full oral intake | As above; first surgical dressing change | Stop epidural; start NSAIDs; remove catheter; full oral intake | Mobilise four times daily <6h; 21 light diet |
| Day 4 | Review discharge criteria | Review discharge criteria | Review discharge criteria | Review discharge criteria | Oral medication; stop IV fluids; mobilise >6h | |
| Day 5 | Check blood tests; remove central venous line; discharge | |||||
| Normal diet; give discharge instructions; mobilise four times daily >6h | Discharged If pain control with oral analgesics and solid foods; adequate mobilisation | Discharge when normal or decreasing bilirubin, good pain control, normal diet tolerated and mobilising to pre-operative level | Discharge on day 6 when fully mobile, pain control adequate and normal organ function; follow-up In outpatients clinic on days 10, 15 and 30 |
NG = nasogastric; CVP = central venous pressure; IV = Intravenous; NSAID = non-steroidal anti-inflammatory drug; PCA = patient controlled analgesia
The demographics and study outcomes of individual articles are shown in Tables 1,4 and 5. The two studies that described single interventions in one parameter but within an ERP have an overall value described that includes all investigational cohorts of patients with the ERP. Tables 4 (pancreatic resections) and 5 (liver resections) demonstrate consistently reduced length of post-operative stay in both liver and pancreatic resectional surgery with the incorporation of an ERP. This reduced length of stay is in comparison to both the studies’ controls and historical controls.10–14 In the studies involving liver resections, two articles specified intra-operative blood loss ranging between a mean of 50ml and 760ml.20,25 This was significantly less than with traditional care in one study.20 In the pancreatic resection studies, intra-operative blood loss ranged between 300ml and 700ml and was specified in 3 out of 4 articles.17,24 In the study by Balzano et al, this blood loss was not significantly reduced when compared with a historical control.17
Table 4.
Outcomes of studies implementing fast track pancreatic resectional surgery
| Authors | NG tube removed | Feeding | Gastrointestinal function | Abdominal drains | Urinary catheter removed | Length of post-operative stay | Morbidity | Mortality | Readmission rate |
| Berberat et al17 | 80.4% removed post-operatively; 13.3% removed on day 1; reinsertion rate 11.4% | First liquid 1 day (0–6 days); complete oralisation 5 days (1–24 days) | First stool 4 days (1–9 days) | 3 days (0–19 days) | 5 days (1–49 days) | 10 days (4–115 days) | 41.2% | 2% | 3.5% |
| Balzano et al17 | 92.9% removed on day 1; 84.2% did not require reinser-tion | All patients without NG tube In situ commenced liquid diet on day 3 and food on day 4 | First flatus 3 days (1–6 days); first stool 5 days (1–9 days) | Not stated; percutaneous drainage required In 3.6% | Not stated | 13 days (7–110 days) | 47.2% | 3.6% | 7.1% |
| Montiel Casado et al23 | Removed after surgery; not stated if needed reinser-tion; delayed gastric emptying in 2.4% | Actual outcomes not stated | Not stated | Not stated | Not stated | 11 days (4–18 days) | 47.6% | 4.9% | 14.6% |
| di Sebastiano et al24 | Removed within a few hours of surgery in 24.1%; on day 1 in 42.1%; later in 33.8% | First liquid 1 day (0–8 days); complete oralisation 5 days (3–11 days) | First flatus 3 days (1–7 days); first stool 5 days (2–9 days) | 5 days (3–23 days) for right drain and 6 days (3–29 days) for left drain; 7 patients discharged with drain | 3 days (1–9 days) | 10 days (6–69 days) | 38.6% | 2.7% | 30-day rate: 6.2% |
NG = nasogastric
Table 5.
Outcomes of studies implementing fast track liver resectional surgery
| NG tube removed | Feeding | Gastrointestinal function | Abdominal drains | Urinary catheter removed | Length of post-operative stay | Morbidity | Mortality | Readmission rate | |
| van Dam et al18 | NG tube inserted at induction in 78.7%; removed within 4h of surgery; reinserted in 3.28% | 92% had oral intake within 4h post- operatively; normal diet In 1 day (0–3 days) | Not stated; 5% constipated after day 3 | 2% had Intra-operative drains; not stated when removed | Not stated | 6 days (3–82 days) | 41% | 0% | 13% |
| MacKay et al19 | Not stated | Not stated | First stool 4.5 days (data only available In 83.3% of patients); flatus not stated | Not used in any patient | Not stated | 4 days (2–7 days) | 25% | 0% | Not stated |
| Stoot et al20 | No NG tubes inserted | Normal diet in 1 day (1–2 days) | Not stated | No drains Intra-operatively | Not stated | 5 days (3–10 days) | 1% | 0% | 0% |
| Koea et al21 | Not stated | Regular diet on day 1 in 26% | First flatus passed on day 1 in 25% | 2% had drains Intra-operatively; not stated when removed | Not stated | 4.6–7.2 days | 16–22% | 0% | 3% |
| Hendry et al22 | Aimed to remove all post-operatively; not stated number reinserted | First liquid on day 0 in 94%; diet on day 1 in 37% and day 2 in 91% | First flatus 3 days (2–4 days); first stool 5 days (4–6 days) | 13% had intra-operative drains | Not stated | 6 days (4–7 days) | 30-day rate: 25% | 30-day rate: 3% | 7% |
| Lin et al25 | Not Inserted pre-operatively; inserted for complications in 3.57% | Not stated | Not stated | Not stated | Not stated; one patient required intra-abdominal drain for bile leak | 7 days (3–26 days) | 46.4% | 1.8% | 7.1% |
NG = nasogastric
Six studies described the return of gut function after surgery within an ERP.16,17,19,21,22,24 In the liver resection group, the first flatus passage was at days 3–5 and the first stool at days 4–5.16,17,19 One article compared first stool with a historical control and found a more rapid return of gut function of 1 day (p<0.001).17 In the pancreatic resection group, stool was passed at days 4.5–519,22 and flatus on day 3 post-operatively.22 In the article by Koea et al, which investigated different analgesics within an ERP, all patients receiving intrathecal morphine passed flatus on post-operative day 1 (n=50).21 In the epidural group, 12 passed flatus on day 1, 28 on day 2 and 10 on day 3 (n=50). Stool passage was not documented.
Discussion
This article aimed to review the current evidence for implementing an ERP in HPB surgery. It demonstrates that the incorporation of such protocols appears feasible and safe. Most notably, the length of post-operative stay can be reduced significantly. However, whether this is at the expense of increased rates of readmission is unknown at present due to the limited number of trials. While the ERP has been the topic of numerous trials in colorectal surgery, scanty reports exist for its efficacy in HPB surgery.
Many of the principles of the ERP have been extracted from ERPs in colorectal surgery. As a result, it is possible that these principles cannot be transcribed so easily to HPB surgery. Procedures may be more complicated and may involve longer lengths of post-operative stay because of this. Differences exist, for example, in pre-operative fluids. In liver surgery a relative hypovolaemia, low central venous pressure and avoidance of excessive pre-operative fluids is preferred to minimise intra-operative blood loss.
Minimally invasive surgery is often included as part of an ERP in colorectal surgery although its positive effects are yet to be proved conclusively.5 Laparoscopic liver resection is under investigation and currently the topic of many reviews.20,26 Hospital stays of five days have been reported following major resections for benign disease.27 While minimally invasive surgery does reduce morbidity secondary to large upper abdominal incisions, the application of regional anaesthetic techniques and optimum analgesic control in open surgery can also reduce hospital stay. Indeed, in colorectal surgery, laparoscopic resection is being challenged by open surgery in the setting of an ERP,28 with one RCT demonstrating no difference in mortality, morbidity, readmission rate or hospital stay.29
Concerns over the safety of laparoscopic HPB surgery remain due to reported rates of conversion of 8–15% secondary to haemorrhage and margin positive rates of 2%.30 In addition, there are the concerns of pneumoperitoneum increasing the risks of tumour dissemination and the additional incisions needed to remove large specimens.31
While laparoscopic liver resection is now used widely in most HPB centres, especially for atypical or wedge resections, the adoption of laparoscopic surgery for major pancreatic resections has not advanced at an equivalent rate. In particular, the application of laparoscopic surgery for complex procedures such as pancreaticoduodenectomy, even in leading institutions for robotic surgery, has not demonstrated an improvement in length of stay or morbidity, which would justify the widescale adoption of these techniques.32
Another contentious issue to many pancreatic surgeons will be the ERP’s minimal use of intra-operative abdominal drains. Many see correctly positioned drains as essential in recognising life threatening post-operative complications such as anastomotic breakdown and haemorrhage. Perhaps this principle of ‘no abdominal drain’ use, transcribed from the ERP in colorectal surgery, cannot be applied so easily in HPB surgery. While it is not the purpose of this review to appraise evidence for individual parameters of the ERP, this again serves to highlight the differences from colorectal fast track surgery. Perhaps of greater importance to the pancreatic surgeon is a protocol for early versus late drain removal or even no drain placement for patients deemed lower risk for an anastomotic leak. This issue has been the subject of several publications.33–35
A further contentious issue is that of post-operative feeding. Concerns in particular surround protecting the pancreatic anastomosis following pancreatic resection. The articles in this review implementing early oralisation (some combined with octreotide) as part of an ERP have not shown any increase in complication rate.16,17,24,23 Many surgeons nevertheless remain committed to a post-operative period of ‘bowel rest’, with the theory that it will reduce the risk of anastomotic leak.
Of concern in studies evaluating the efficacy of implementing the ERP is the choice of primary outcome. Frequently, studies used length of hospital stay. This may not, however, best reflect the quality of functional recovery. The Cochrane review of the ERP in colorectal surgery concluded that there was no proof that the use of this endpoint was a medically important parameter and that complication rates may be a better quantitative measure of safety.5 We therefore propose that the implementation of a standardised multimodal protocol in HPB surgery that increases awareness of goals that improve safety and clinical outcomes is of greater importance.
As evidenced by the Cochrane meta-analysis, simply implementing an ERP does not ensure improved results.5 What is more important is that there is stringent overseeing of protocol adherence by all members of the multidisciplinary team together with continued alertness for decreasing compliance. Implementing and auditing such protocols tailored for the HPB surgeon has been demonstrated to be safe. Emphasis must surely now be placed on any attempt to reduce morbidity from such high risk intervention by the introduction of standardised care protocols.
Conclusions
The introduction of an ERP in HPB surgery appears safe and feasible. Currently, many of the principles of the multimodal pathway are derived from the colorectal ERP and distinct differences exist that may inhibit its uptake among HPB surgeons. RCTs are needed to clearly define evidence-based parameters in this complex group of patients.
References
- 1.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003; 362: 1,921–1,928 [DOI] [PubMed] [Google Scholar]
- 2.Lassen K, Soop M, Nygren Jet al Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009; 144: 961–969 [DOI] [PubMed] [Google Scholar]
- 3.Stephen AE, Berger DL. Shortened length of stay and hospital cost reduction with implementation of an accelerated clinical care pathway after elective colon resection. Surgery 2003; 133: 277–282 [DOI] [PubMed] [Google Scholar]
- 4.Adamina M, Kehlet H, Tomlinson GAet al Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 2011; 149: 830–840 [DOI] [PubMed] [Google Scholar]
- 5.Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011; 2: CD007635. [DOI] [PubMed] [Google Scholar]
- 6.Huber TS, Carlton LM, Harward TRet al Impact of a clinical pathway for elective infrarenal aortic reconstructions. Ann Surg 1998; 227: 691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niino T, Hata M, Sezai Aet al Optimal clinical pathway for the patient with type B acute aortic dissection. Circ J 2009; 73: 264–268 [DOI] [PubMed] [Google Scholar]
- 8.Koch MO, Smith JA. Influence of patient age and co-morbidity on outcome of a collaborative care pathway after radical prostatectomy and cystoprostatectomy. J Urol 1996; 155: 1,681–1,684 [PubMed] [Google Scholar]
- 9.Leibman BD, Dillioglugil O, Abbas Fet al Impact of a clinical pathway for radical retropubic prostatectomy. Urology 1998; 52: 94–99 [DOI] [PubMed] [Google Scholar]
- 10.Büchler MW, Wagner M, Schmied BMet al Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg 2003; 138: 1,310–1,314 [DOI] [PubMed] [Google Scholar]
- 11.Gouma DJ, van Geenen RC, van Gulik TMet al Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 2000; 232: 786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimick JB, Wainess RM, Cowan JAet al National trends in the use and outcomes of hepatic resection. J Am Coll Surg 2004; 199: 31–38 [DOI] [PubMed] [Google Scholar]
- 13.Jarnagin WR, Gonen M, Fong Yet al Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002; 236: 397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrowsky H, McCormack L, Trujillo Met al A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg 2006; 244: 921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow R, Price P, Reid TDet al Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clin Nutr 2011; 30: 560–566 [DOI] [PubMed] [Google Scholar]
- 16.Berberat PO, Ingold H, Gulbinas Aet al Fast track – different implications in pancreatic surgery. J Gastrointest Surg 2007; 11: 880–887 [DOI] [PubMed] [Google Scholar]
- 17.Balzano G, Zerbi A, Braga Met al Fast-track recovery programme after pancreatico-duodenectomy reduces delayed gastric emptying. Br J Surg 2008; 95: 1,387–1,393 [DOI] [PubMed] [Google Scholar]
- 18.van Dam RM, Hendry PO, Coolsen MMet al Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg 2008; 95: 969–975 [DOI] [PubMed] [Google Scholar]
- 19.MacKay G, O’Dwyer PJ. Early discharge following liver resection for colorectal metastases. Scott Med J 2008; 53: 22–24 [DOI] [PubMed] [Google Scholar]
- 20.Stoot JH, van Dam RM, Busch ORet al The effect of a multimodal fast-track programme on outcomes in laparoscopic liver surgery: a multicentre pilot study. HPB 2009; 11: 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koea JB, Young Y, Gunn K. Fast track liver resection: the effect of a comprehensive care package and analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg 2009; 271986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendry PO, van Dam RM, Bukkems SFet al Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg 2010; 97: 1,198–1,206 [DOI] [PubMed] [Google Scholar]
- 23.Montiel Casado MC, Pardo Sánchez F, Rotellar Sastre Fet al Experience of a cephalic pancreatoduodenectomy fast-track program. Cir Esp 2010; 87: 378–384 [DOI] [PubMed] [Google Scholar]
- 24.di Sebastiano P, Festa L, De Bonis Aet al A modified fast-track program for pancreatic surgery: a prospective single-center experience. Langenbecks Arch Surg 2011; 396: 345–351 [DOI] [PubMed] [Google Scholar]
- 25.Lin DX, Li X, Ye QWet al Implementation of a fast-track clinical pathway decreases postoperative length of stay and hospital charges for liver resection. Cell Biochem Biophys 2011; 61: 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Gulik T. Open versus laparoscopic resection for liver tumours. HPB 2009; 11: 465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalil AN, Mastalir ET. Laparoscopic hepatectomy for benign liver tumors. Hepatogastroenterology 2002; 49: 803–805 [PubMed] [Google Scholar]
- 28.Kumar A, Hewett PJ. Fast-track or laparoscopic colorectal surgery? ANZ J Surg 2007; 77: 517–518 [DOI] [PubMed] [Google Scholar]
- 29.Basse L, Jakobsen DH, Bardram Let al Functional recovery after open versus laparoscopic colonic resection: a randomized, blinded study. Ann Surg 2005; 241: 416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurence JM, Lam VW, Langcake MEet al Laparoscopic hepatectomy, a systematic review. ANZ J Surg 2007; 77: 948–953 [DOI] [PubMed] [Google Scholar]
- 31.Koea JB. Laparoscopic hepatectomy: fad, foolhardy or future. ANZ J Surg 2007; 77: 921. [DOI] [PubMed] [Google Scholar]
- 32.Giulianotti PC, Sbrana F, Bianco FMet al Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010; 24: 1,646–1,657 [DOI] [PubMed] [Google Scholar]
- 33.Molinari E, Bassi C, Salvia Ret al Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg 2007; 246: 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassi C, Molinari E, Malleo Get al Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg 2010; 252: 207–214 [DOI] [PubMed] [Google Scholar]
- 35.Conlon KC, Labow D, Leung Det al Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg 2001; 234: 487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]