Abstract
Purpose.
To determine the signaling pathway involved in expanding contact-inhibited human corneal endothelial cells (HCECs) using p120 and Kaiso small interfering RNAs (siRNAs).
Methods.
Expansion of HCEC monolayers on collagen IV in SHEM using p120 siRNA was optimized regarding various dosage, frequency, and starting date before being added Kaiso siRNA or various inhibitors of Rho, ROCK, NFκB, and TAK1. Phase contrast micrographs were used for monitoring cell shape, monolayer size, and cell density. Immunostaining was used to determine cytolocalization of BrdU, p120, pNFkB, F-actin, α-catenin, β-catenin, LEF1, Na+/K+-ATPase, N-cadherin, ZO-1, and S100A4. Western blotting was used to determine the protein level of RhoA and RhoA-guanosine-5′-triphosphate (GTP).
Results.
The HCEC monolayer size in diameter was expanded from 2.1 ± 0.4 mm to 4.3 ± 0.3 mm (P < 0.05) by increasing p120 siRNA from 40 nM to 100 nM starting at day 7, to 5.0 ± 0.4 mm (P < 0.05) by adding 100 nM Kaiso siRNA, to 6.8 ± 0.3 mm by using one-fourth corneoscleral rim (P < 0.05), and to 8.1 ± 0.5 mm by using one-half corneoscleral rim (P < 0.05). Such proliferative effect required activation of RhoA-ROCK-noncanonical bone morphogenic protein (BMP) signaling and nuclear translocation of phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells (pNFκB). After withdrawal of siRNAs for 1 week, the resultant HCEC monolayer maintained a hexagonal shape, the average cell density of 2254 ± 87 mm2 (n = 3), and normal expression patterns of F-actin, α-catenin, β-catenin, N-cadherin, ZO-1, and Na+/K+-ATPase without S100A4 and alpha-smooth muscle actin (α-SMA).
Conclusions.
The optimized knockdown with p120 and Kaiso siRNAs further expands the size of HCEC monolayers without endothelial mesenchymal transition (EMT) via selective activation of p120/Kaiso signaling that requires the RhoA-ROCK-noncanonical BMP-NFkB signaling.
Keywords: cornea, endothelium, p120 catenin, Kaiso, siRNA, proliferation, tissue engineering
The optimized knockdown with p120 and Kaiso small interfering RNAs (siRNAs) further expands the size of human corneal endothelial cell monolayers without endothelial mesenchymal transition via selective activation of RhoA-ROCK-noncanonical BMP signaling.
Introduction
Human corneal endothelial cells (HCECs) play a pivotal role in regulating corneal stromal hydration and hence corneal transparency, in part through expression of tight junction component ZO-1 to yield its barrier function, and in part through expression of Na-K-ATPase to exert its pump function.1,2 Human corneal endothelial cells are notorious for their limited proliferative capacity in vivo,3 which is presumably caused by the mitotic arrest at the G1 phase of the cell cycle.4 A similar mitotic block due to contact inhibition is also noted in cat5 and human6 corneal explants, as well as confluent rat corneal endothelial cultures.7 Although a number of methods have been attempted to unlock such mitotic block of HCECs in cultures8–15 using stripped Descemet's membrane, none has consistently produced functional HCEC monolayers suitable for transplantation using a donor source that is practically available.
The conventional approach of expanding HCECs in vitro is to disrupt cell junctions by EDTA, followed by culturing them in a medium supplemented with bFGF.16 Nevertheless, such a culturing method casts the concern of activating endothelial-mesenchymal transformation (EMT).17 Recently, we have demonstrated that such a pathologic process is mediated by the canonical Wnt signaling resulting, especially if TGF-β1 is added.18 A similar phenomenon is also observed in human retinal pigment epithelial cells (ARPE-19), in which cell proliferation is also restricted by contact inhibition upon confluence.19,20 In contrast, knockdown by small interfering RNA (siRNA) to p120 catenin (hereafter termed p120) uniquely promotes proliferation of contact-inhibited HCEC monolayers without disrupting the intercellular junction.18 Consequently, the expanded HCEC monolayers maintain a hexagonal cell shape, an in vivo density, and junctional expression of N-cadherin, ZO-1, and Na-K-ATPase.18 A similar result has also been confirmed in confluent ARPE-19.20 Using this novel approach, we have successfully expanded the average size of HCEC monolayers from 0.4 ± 0.2 mm to 2.1 ± 0.4 mm in diameter from Descemet's membrane stripped from one-eighth of the corneoscleral rim normally discarded after conventional corneal transplantation.18
We have reported that this novel tissue engineering strategy involves the activation of the p120/Kaiso signaling, which is evidenced by nuclear translocation of p120 to release nuclear suppressor Kaiso and nuclear colocalization of p120 and BrdU.18,20 Furthermore, we have noted that this p120/Kaiso signaling is associated with activation of RhoA–ROCK signaling, destabilization of microtubules, and inhibition of Hippo signaling, but not with activation of canonical Wnt signaling.18 In the p120 null mouse epidermis, there is nuclear localization of NFκB partly controlled by Rho and GTPases and many cycling cells in the basal layer.21 Herein, we have further explored and identified the noncanonical bone morphogenic protein (BMP) signaling as the downstream RhoA–ROCK signaling target to activate nuclear translocation of NFκB to unlock the mitotic block in contact-inhibited HCEC monolayers.
Materials and Methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), Ham's/F12 medium, human epidermal growth factor (hEGF), HEPES buffer, Hanks' balanced salt solution (HBSS), PBS, gentamicin, fetal bovine serum (FBS), Texas-Red-X phalloidin, Alexa-Fluor-conjugated secondary IgG, and all real-time PCR primers and probes were purchased from Invitrogen (Carlsbad, CA). Collagenase A and insulin-transferrin-sodium selenite media supplement were obtained from Roche Applied Science (Indianapolis, IN). Hydrocortisone, dimethyl sulfoxide, cholera toxin, bovine serum albumin, paraformaldehyde, methanol, Triton X-100, Hoechst 33342 dye, CAY10512, 5Z-7-Oxozeaenol, noggin, and nocodazole were purchased from Sigma-Aldrich (St. Louis, MO). Collagen IV coated 24-well dishes and monoclonal antibody against β-catenin was obtained from BD Biosciences (Franklin Lakes, NJ). Monoclonal antibodies against BrdU and Na+/K+-ATPase were purchased from Millipore (Billerica, MA). Monoclonal antibody against Kaiso and polyclonal antibodies against α-catenin, N-cadherin (type I), pNFκB (pSer276NFkB), and S100A4 were obtained from Abcam (La Jolla, CA). Polyclonal antibody against p120 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). RNeasy Mini Kit was purchased from Qiagen (Valencia, CA). HiPerFect siRNA transfection reagents were obtained from Qiagen. Kaiso siRNA was obtained from Invitrogen. p120 siRNA was designed by us and obtained from Invitrogen with the target sequence of 59-CAGAGGTGATCGCCATGCTTGGATT-39.
HCEC Isolation and Culture
A total of 40 human corneas from individuals aged 18 to 78 years and maintained at 4°C in Optisol (Chiron Vision, Irvine, CA) for less than 7 days after death were obtained from the Florida Lions Eye Bank (Miami, FL) and handled according to the Declaration of Helsinki. Human corneal epithelial cells were isolated and cultured as previously reported.8,22 In short, after central corneal buttons had been used for corneal transplantation, the remaining corneoscleral rims were rinsed three times with DMEM containing 50 μg/mL gentamicin and 1.25 μg/mL amphotericin B. Under a dissecting microscope, the trabecular meshwork was cleaned up to the Schwalbe's line, and Descemet's membranes were stripped from different sizes of the rim. After digestion at 37°C for 16 hours with 1 mg/mL collagenase A in SHEM, which was made of an equal volume of HEPES-buffered DMEM and Ham's F12 supplemented with 5% FBS, 0.5% dimethyl sulfoxide, 2 ng/mL hEGF, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 0.5 μg/mL hydrocortisone, 1 nM cholera toxin, 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin B, HCEC aggregates were collected by centrifugation at 300g for 3 minutes to remove the digestion solution, and were cultured in 24-well dishes coated with collagen IV in SHEM. Cultures were monitored by phase contrast micrography, and the size of monolayers was determined by digitizing the surface area using ImageJ (National Institutes of Health, Bethesda, MD) and the cell counting was analyzed using Axio Vision software (Carl Zeiss, Thornhood, NY). The cultures from the same corneoscleral rims were used for control and treatment groups, respectively, in each experiment.
siRNA Transfection
For the pulse siRNA knockdown, parallel HCEC monolayers were subjected to small cytoplasmic RNA (scRNA) or siRNA transfection by mixing 50 μL of serum-free, antibiotic-free SHEM with 1 μL of HiPerFect siRNA transfection reagent (final dilution, 1:300) and 3 μL of 20 μM of scRNA or siRNA to p120 alone or p120 and Kaiso (p120-Kaiso) drop-wise to achieve various final concentrations, followed by culturing in 250 μL fresh SHEM at 37°C using different regimens. Extended culture of HCEC monolayers in SHEM was treated with weekly transfection of scrambled (sc)RNA, p120 siRNA, or p120-Kaiso siRNA. BrdU was added at a final concentration of 10 μM in the culture medium for 24 hours before termination. For each culture, at least 2000 total nuclei were counted, and the BrdU labeling index, defined as the number of BrdU-labeled nuclei divided by the total number of labeled and unlabeled nuclei, was calculated. Some cultures were treated with 5 μg/mL nocodazole in the culture medium to facilitate p120 nuclear translocation18,23,24 in conjunction with p120 siRNA transfection.
RNA Extraction, Reverse Transcription, and Real-Time PCR
Total RNAs were extracted using RNeasy Mini Kit (Qiagen) and were reverse-transcribed using High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Complementary DNA of each cell junction component was amplified by real-time RT-PCR using specific primer-probe mixtures, glyceraldehyde 3-phosphate dehydrogenase as the internal control and DNA polymerase in 7000 Real-time PCR System (Applied Biosystems). Real-time RT-PCR profile consisted of 10 minutes of initial activation at 95°C, followed by 40 cycles of 15 seconds denaturation at 95°C, and 1 minute annealing and extension at 60°C. The genuine identity of each PCR product was confirmed by the size determination using 2% agarose gels followed by ethidium bromide staining together with PCR marker according to EC3 Imaging System (BioImaging System, Upland, CA).
Immunostaining
Human corneal epithelial cell monolayer cultures were air-dried and fixed in 4% formaldehyde, pH 7.0, for 15 minutes at room temperature, rehydrated in PBS, incubated with 0.2% Triton X-100 for 15 minutes, and rinsed three times with PBS for 5 minutes each. For single immunostaining of Kaiso or double immunostaining to both BrdU and p120, samples were fixed with 75% methanol plus 25% acetic acid for 15 minutes, denatured with 2 M HCl for 30 minutes at 37°C and neutralized by 0.1 M borate buffer, pH 8.5 for 5 minutes three times. After incubation with 2% BSA to block nonspecific staining for 30 minutes, they were incubated with the desired first antibody (all at 1:50 dilution) for 16 hours at 4°C. After three washes with PBS, they were incubated with corresponding Alexa-Fluor-conjugated secondary IgG (all 1:100 dilution) for 60 minutes. The samples were then counterstained with Hoechst 33342 and analyzed with Zeiss LSM 700 confocal microscope (Carl Zeiss). Corresponding mouse and rabbit sera were used as negative controls for primary monoclonal and polyclonal antibodies, respectively.
RhoA Activity Assay
The assay of Rho activation was performed in 10 to 50 μg of protein of cell lysates using RhoA Activation Assay Biochem Kit (Cytoskeleton, Denver, CO) to pull down the guanosine-5′-triphosphate (GTP)-bound form of RhoA by a GST fusion protein containing rhotekin (7–89 residues) and RBD protein using brightly colored glutathione affinity beads. The amount of activated RhoA pulled down was quantitatively determined by Western blotting using anti-RhoA antibody.
Western Blotting
Cell lysates were prepared in radio-immunoprecipitation assay buffer and resolved on 4% to 15% (wt/vol) gradient acrylamide gels under denaturing and reducing conditions for Western blotting. The protein extracts were transferred to a nitrocellulose membrane, which was then blocked with 5% (wt/vol) fat-free milk in Tris-buffered saline with Tween 20 (50 mM Tris-HCl, pH 7.5, 150 mM NaCl (wt/vol) 0.05% Tween-20 (vol/vol)), followed by sequential incubation with specific primary antibody against RhoA and their respective secondary antibodies using α-tubulin as the loading control. Immunoreactive proteins were detected with Western Lighting Chemiluminesence (PerkinElmer, Waltham, MA).
Statistical Analysis
All summary data were reported as means ± SD. calculated for each group and compared using the Student's unpaired t-test by Microsoft Excel (Microsoft, Redmond, WA). Test results were reported as two-tailed P values, where P less than 0.05 was considered statistically significant.
Results
Expansion of HCEC Monolayers by Optimizing p120 siRNA KnockDown Regimen
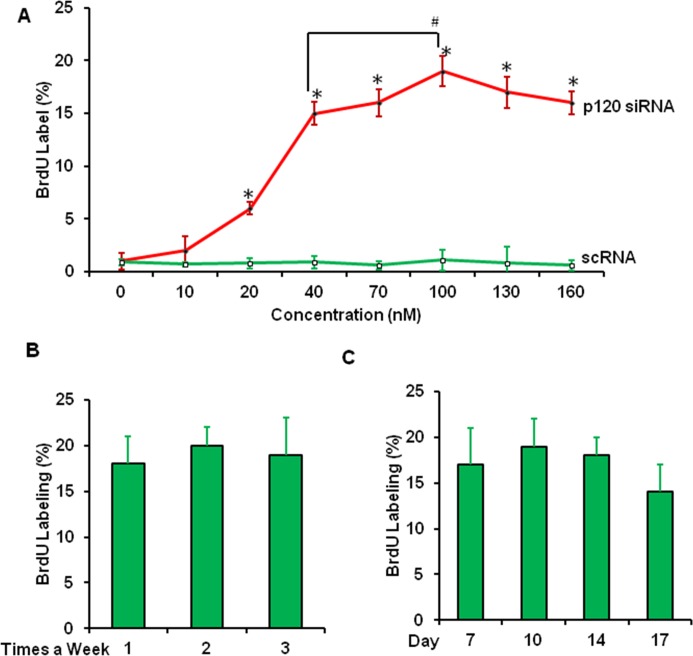
Using the knockdown regimen with weekly application of 40 nM of p120 siRNA starting from day 14 after initiation of the HCEC culture, we have successfully expanded the average size of HCEC monolayers in diameter from 0.4 ± 0.2 mm to 2.1 ± 0.4 mm using Descemet's membrane stripped from one-eighth of the corneoscleral rim that is normally discarded after conventional corneal transplantation.18 We first optimized this knockdown regimen by varying the concentration of p120 siRNA from 10 to 160 nM starting from day 14 after initiation of HCEC cultures, and analyzed HCEC proliferation by BrdU labeling 48 hours after transfection. The BrdU labeling was dose-dependently promoted by p120 siRNA knockdown and reached the peak at 100 nM, which promoted significantly higher BrdU labeling than that from 40 nM previously reported (Fig. 1A). The BrdU labeling was not significantly changed by p120 siRNA knockdown when applied at the frequency of once, twice, and three times a week (Fig. 1B), or by starting p120 siRNA knockdown from day 7, 10, 14, or 17 for 48 hours (Fig. 1C).
Figure 1.
Optimization of p120 siRNA knockdown regimen based on BrdU labeling. BrdU labeling was performed in HCEC cultures treated with scRNA (○) or p120 siRNA (•) at concentrations from 10 to 160 nM starting from day 14 of culturing ([A], *P < 0.05 for each concentration between scRNA and p120 siRNA, and #P < 0.05 between 40–100 nM p120 siRNA, n = 3), or with 100 nM of p120 siRNA starting from day 14 of culturing once, twice, or three times a week ([B], P > 0.05 between groups, n = 3), or starting from day 7, 10, 14, and 17 of culturing ([C], P > 0.05 between each group, n = 3). All cultures were treated for 48 hours.
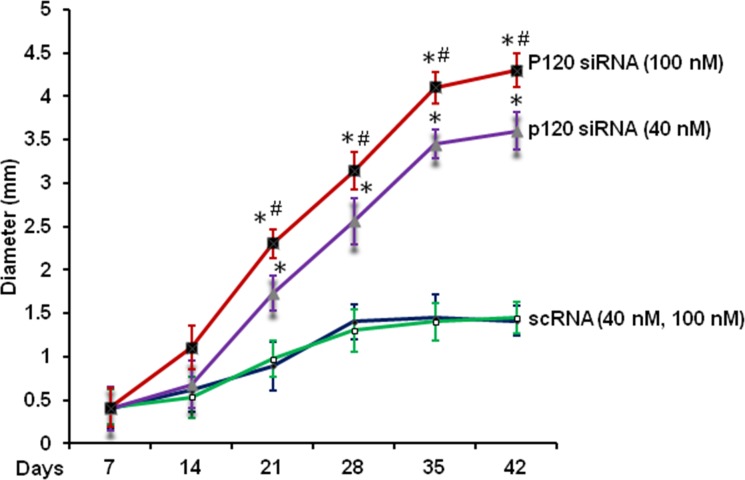
We then determined whether the above optimized knockdown regimen could further expand the HCEC monolayer size. Compared with the control with 40 nM scRNA, weekly application of 40 nM p120 siRNA starting from day 14 promoted the size of HCEC monolayers in diameter from 1.4 ± 0.2 mm to 3.2 ± 0.3 mm after 5 weeks of culturing (Fig. 2, P < 0.05). Indeed, weekly application of 100 nM of p120 siRNA starting from day 7 further expanded the size to 4.1 ± 0.4 mm in diameter (Fig. 2, P < 0.05). Similar to what we have reported,18 some control HCEC monolayers exhibited disintegration after 4 weeks of culturing (not shown). Although no such disintegration was noted in the group treated with p120 siRNA, the expansion of the HCEC monolayer slowed down after 5 weeks of culturing.
Figure 2.
Human corneal epithelial cell monolayer sizes expanded by optimized p120 siRNA knockdown regimen. Human corneal epithelial cell cultures were treated weekly with 40 nM of scRNA (○) or p120 siRNA ( ) starting from day 14 or with 100 nM of scRNA (•) or p120 siRNA (▪) starting from day 7 for a total of 5 weeks of culturing. (*P < 0.05, n = 3, at each time point between scRNA and p120 siRNA; #P < 0.05 between 40 nM p120 siRNA and 100 nM p120 siRNA, n = 3).
) starting from day 14 or with 100 nM of scRNA (•) or p120 siRNA (▪) starting from day 7 for a total of 5 weeks of culturing. (*P < 0.05, n = 3, at each time point between scRNA and p120 siRNA; #P < 0.05 between 40 nM p120 siRNA and 100 nM p120 siRNA, n = 3).
Further Optimization of p120 siRNA KnockDown by Adding Kaiso siRNA
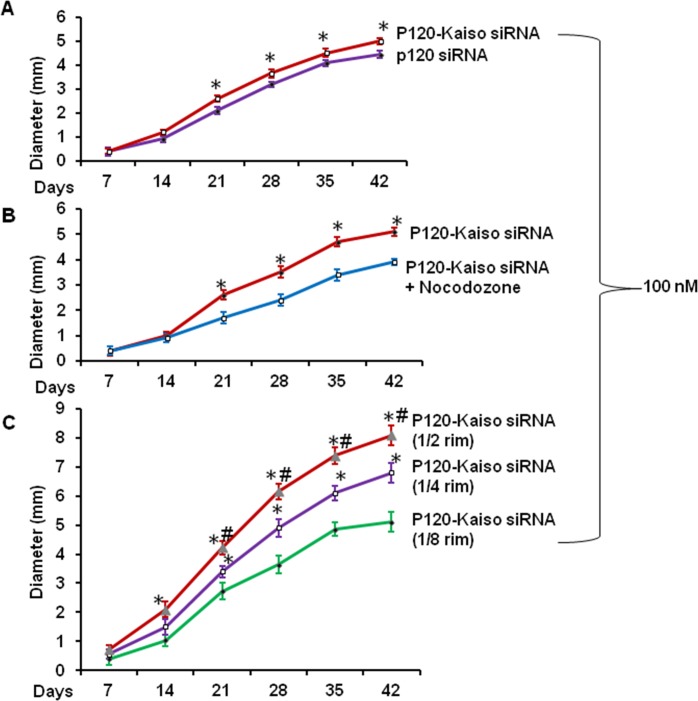
Although p120 siRNA knockdown reduced both p120 and Kaiso transcripts, Kaiso siRNA knockdown alone did not activate p120/Kaiso signaling as judged by the lack of release of nuclear Kaiso, while knockdown by both p120 and Kaiso siRNA released more nuclear Kaiso as judged by the further decline of nuclear Kaiso staining.18 Hence, we would like to examine whether the size of HCEC monolayers could further be expanded by p120-Kaiso knockdown. Compared with the control receiving 100 nM of p120 siRNA alone from day 14, knockdown with additional 100 nM of Kaiso siRNA starting from day 7 indeed further expanded the HCEC monolayer size to 5.0 ± 0.4 mm in diameter after 5 weeks of culturing (P < 0.05, Fig. 3A). Although the expansion slowed down without BrdU labeling thereafter, the resultant monolayers still exhibited a hexagonal shape.
Figure 3.
Further expansion of HCEC monolayer size by Kaiso siRNA, nocodazole, or increasing the initial size. The HCEC monolayer size in diameter was measured weekly when treated with the optimized p120 siRNA knockdown regimen with (○) or without (•) 100 nM Kaiso siRNA ([A], *P < 0.05, n = 3). The size achieved by knockdown with both p120 siRNA and Kaiso siRNA (•) was inhibited by 5 μg/mL nocodazole (○; [B], *P < 0.05 n = 3) but promoted without nocodazole by increasing initial HCEC aggregates from one-eighth (•) to one-fourth (○) or one-half ( ) of the corneoscleral rim ([C], *P < 0.05 when compared with one-eighth corneoscleral group, #P < 0.05 when compared to one-fourth corneoscleral group, n = 3).
) of the corneoscleral rim ([C], *P < 0.05 when compared with one-eighth corneoscleral group, #P < 0.05 when compared to one-fourth corneoscleral group, n = 3).
Previous studies have shown that nocodazole destabilizes microtubules to facilitate p120 nuclear translocation.23,25 We have also reported that nocodazole, which destabilizes microtubules, synergistically promotes BrdU labeling when added with p120 siRNA knockdown in a short term, but taxol, which stabilizes microtubules, abolishes such an effect.18 Hence, we wondered whether addition of nocodazole could further help expand HCEC monolayers that had been optimized by the aforementioned p120-Kaiso siRNA knockdown. Our result showed that addition of nocodazole did not promote and inhibited the expansion of HCEC monolayers to 3.9 ± 0.2 mm in diameter (P < 0.05, Fig. 3B). Using the aforementioned optimized knockdown regiment with p120-Kaiso siRNAs, we noted that the HCEC monlayer size in diameter could further be expanded to 6.8 ± 0.3 mm and 8.1 ± 0.5 mm by using Descemet's membrane stripped from one-fourth and one-half of the remaining corneoscleral rim, respectively (both P < 0.05, Fig. 3C).
RhoA-ROCK Activated by p120-Kaiso siRNas Leads to Noncanonical BMP Signaling to Promote Proliferation of HCEC Monolayers
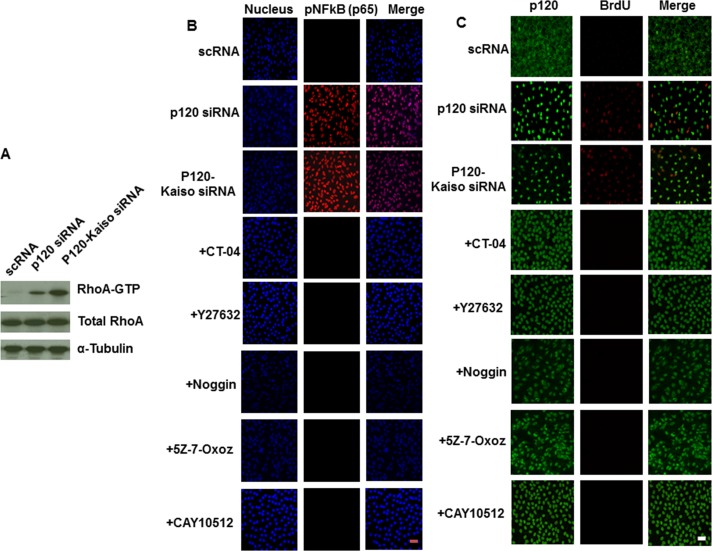
We have reported that selective activation of p120/Kaiso signaling by knockdown with p120 siRNA in SHEM also activates Rho–ROCK signaling, which is required for promoting proliferation of contact-inhibited HCEC monolayers.18 Herein, we learned that knockdown by p120-Kaiso siRNAs further activated Rho more so than p120 siRNA as demonstrated by a 4-fold increase of RhoA-GTP (Fig. 4A). Since we did not observed any beneficial effect by destabilizing microtubules to further promote HCEC proliferation (Fig. 3), we searched for other potential downstream candidates of the RhoA–ROCK signaling that might be linked to proliferation promoted by p120-Kaiso siRNAs. In the p120 null mouse epidermis, there is nuclear localization of NFκB partly controlled by Rho and GTPases and many cycling cells in the basal layer.21 Using HCEC monolayers treated by different knockdown regimens for 1 week, we noted that the NFκB signaling was involved because pNFκB (p65) was translocated to the nucleus after knockdown by p120 siRNA and p120-Kaiso siRNAs, but not by scRNA. Furthermore, such nuclear translocation was completely blocked by Rho and ROCK inhibitors, CT-04 and Y27632, respectively, similar to the finding of the positive control treated with CAY10512, an inhibitor of NFκB18 (Fig. 4B).
Figure 4.
RhoA-ROCK-pNFkB signaling involved in proliferation activated by p120-Kaiso siRNAs. Human corneal epithelial cell monolayers cultured at 1 week were treated by 2 days knockdown by scRNA, p120 siRNA, or p120-Kaiso siRNAs, The RhoA-GTP level was measured in respective cell extracts by Western blot ([A], P < 0.05 and P < 0.01 for p120 siRNA and p120-kaisos, respectively, n = 3 when compared with scRNA and normalized by α-tubulin). In HCEC monolayers cultured for 2 weeks, nuclear translocation of pNFκB (p65) was induced only p120 siRNA and p120-Kaiso siRNAs, of which the latter was abolished by simultaneous addition for 2 days of 5 μg/mL CT-04, 20 μM Y27632, 1 μM CAY 10512, 5 μg/mL noggin, or 1 μM 5Z-7-Oxozeaenol (B). Double immunostaining of p120 and BrdU also revealed nuclear colocalization of p120 and BrdU induced only both p120 siRNA and p120-Kaiso siRNAs, of which the latter was abolished by simultaneous addition of 5 μg/mL CT-04, 20 μM Y27632, 1 μM CAY 10512, 5 μg/mL Noggin, or 1 μM 5Z-7-Oxozeaenol (C). Scale bar: 100 μm.
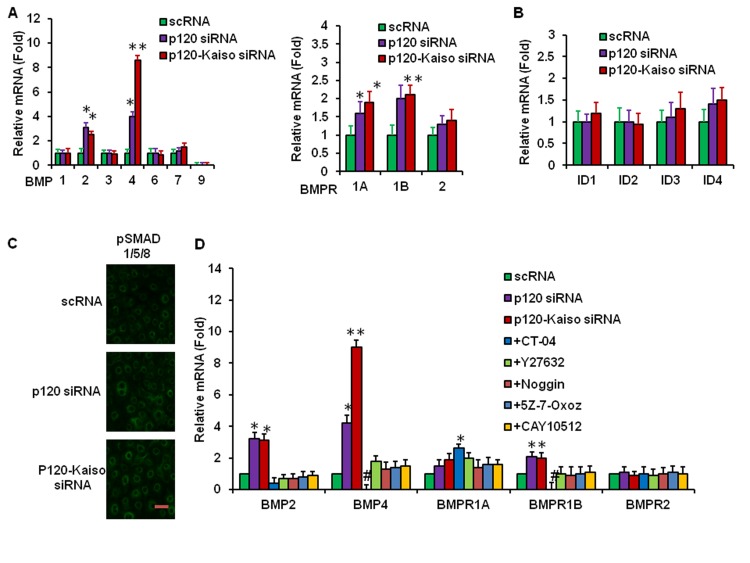
Consistent with the finding noted by p120 siRNA in our previous report,18 p120-Kaiso siRNAs triggered nuclear translocation of p120 and nuclear colocalization of BrdU (Fig. 4C), indicating that p120-Kaiso siRNAs also unlocked the mitotic block in the contact-inhibited HCEC monolayer. Herein, we noted that nuclear localization of pNFκB (p65), p120, and BrdU was also aborted by simultaneous addition for 2 days of noggin, an extracellular BMP inhibitor,26 and 5Z-7-Oxozeaenol, a small molecular weight inhibitor of the mitogen-activated protein kinase kinase kinase TAK1,27 which is involved in noncanonical BMP signaling to generate pNFκB.28–30 Thus, we investigated whether knockdown by p120-Kaiso siRNAs elicited BMP signaling. Quantitative RT-PCR analyses revealed upregulation of BMP2, BMP4, and BMPR1B transcripts by both p120 siRNA and p120-Kaiso siRNAs, with the latter more so than the former, when compared with the corresponding scRNA control (Fig. 5A, *P < 0.05 and **P < 0.01) However, transcript expression of ID1, ID, ID3, and ID4 was not affected (Fig. 5B, all P > 0.05) and nuclear staining of pSMAD1/5/8 was not observed by knockdown with both p120 siRNA and p120-Kaiso siRNAs (Fig. 5C), suggesting that the canonical BMP signaling was not activated. Upregulation of BMP2, BMP4, and BMPR1B was markedly attenuated by the Rho inhibitor CT-04 and to a lesser extent by the ROCK inhibitor Y27632, NFkB inhibitor CAY10512, the BMP inhibitor noggin, and the TAK1 inhibitor 5Z-7-Oxozeaenol (Fig. 5D, all P < 0.05). The downregulation of BMP4 and BMPR1A transcript by CT-04 was even less than the control level achieved by scRNA (Fig. 5D, P < 0.05). These results collectively suggested that the signaling of RhoA activation triggered by knockdown with p120-Kaiso siRNAs led to downstream noncanonical BMP signaling to generate nuclear translocation of pNFκB and colocalization of p120 and BrdU.
Figure 5.
Activation of the Noncanonical BMP signaling by p120-Kaiso siRNAs. Human corneal epithelial cell monolayers treated by different knockdown regimens for 2 days before qRT-PCR analysis of transcript expression of BMP ligands and bone morphogenic protein receptors ([A], *P < 0.05 and **P < 0.01 compared with the scRNA control), ID1-4 ([B], all P > 0.05 compared with the scRNA control), and immunostaining of pSMAD1/5/8 [C], scale bar: 100 μm). Over-expression of BMP2, BMP4, and BMPR1B transcript by p120-Kaiso siRNAs was challenged by various small molecular weight inhibitors and Noggin ([D], *P < 0.05 and **P < 0.01 compared with the scRNA control).
Maintenance of HCEC Morphology, Density, and Phenotype 1 Week After Withdrawal of p120-Kaiso siRNA
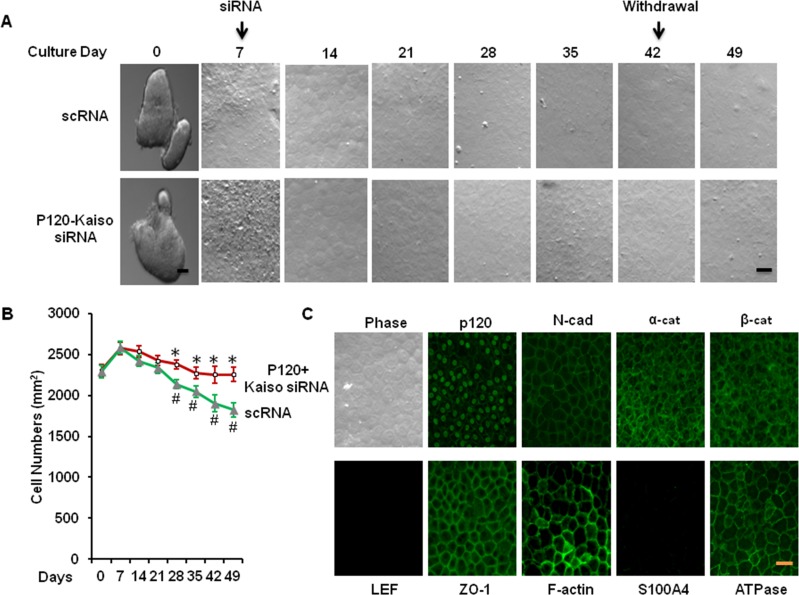
During the entire experiment, we have noted that HCEC monolayers retained their hexagonal shape after extending p120 siRNA knockdown for a total of 5 weeks of culturing. In contrast, HCEC monolayers treated with scRNA enlarged the cell size resulting in a reduced density (Figs. 6A, 6B). Compared with the density of in vivo HCEC monolayers (2254 ± 87 mm2), that of HCEC monolayers maintained the same range during 4 weeks of p120-Kaiso siRNA knockdown and reached 2289 ± 68 /mm2 1 week after withdrawal (P < 0.05, n = 3). In contrast, the density of HCEC monolayers treated with scRNA dropped to 1827 ± 70 /mm2 (P < 0.05, n = 3). Similar to what we have reported,18 the normal HCEC phenotype, judged by the immunostaining staining to reveal junctional cytolocalization of p120, β-catenin, N-cadherin, ZO-1, and Na+/K+-ATPase as well as perimembranous localization of F-actin, was maintained in the control treated with scRNA (Fig. 6C). Prolonged treatment with p120 siRNA for 2 weeks resulted in a significant reduction of p120 junctional staining and an increase in p120 nuclear staining, which is colocalized with BrdU labeling.18 Similar to what has been reported, 1 week after withdrawal of p120-Kaiso siRNAs, the immunostaining pattern was completely reverted to that of the control with the same cytolocalization of p120, N-cadherin, α-catenin, β-catenin, ZO-1, F-actin, and Na-K-ATPase was all at the cell junction (Fig. 6C). The absence of LEF1 and S100A4 suggested that there was neither activation of the canonical Wnt signaling nor endothelial mesenchymal transition (Fig. 6C).
Figure 6.
Maintenance of normal hexagonal shape, density, and phenotype of HCEC monolayers 1 week after withdrawal of p120-Kaiso siRNA. Human corneal epithelial cell cultures were treated with the optimized knockdown regimen of p120-Kaiso siRNAs weekly starting from day 7 for a total of 5 weeks of culturing. One week after withdrawal of p120 siRNA, the resultant HCEC monolayers retained the normal hexagonal shape (A), the in vivo cell density ( , scRNA, ○, p120-Kaiso siRNA; [B], P > 0.05 when compared with the size of in vivo HCEC, *P < 0.05 when compared with the size of HCEC treated with scRNA; #P < 0.05 when compared with the size of in vivo HCEC) and the in vivo immunosfluorescence positive staining patterns of p120, N-cadherin, α-catenin, β-catenin, ZO-1, F-actin, and Na-K-ATPase, and negative staining patterns of LEF1 and S100A4 ([C], scale bar: 100 μm for [A, C]). Scale bar: 100 μm.
, scRNA, ○, p120-Kaiso siRNA; [B], P > 0.05 when compared with the size of in vivo HCEC, *P < 0.05 when compared with the size of HCEC treated with scRNA; #P < 0.05 when compared with the size of in vivo HCEC) and the in vivo immunosfluorescence positive staining patterns of p120, N-cadherin, α-catenin, β-catenin, ZO-1, F-actin, and Na-K-ATPase, and negative staining patterns of LEF1 and S100A4 ([C], scale bar: 100 μm for [A, C]). Scale bar: 100 μm.
Discussion
We chose to start the knockdown regimen with 40 nM p120 siRNA from 14 days of culturing because HCEC became well-adherent as single monolayers of hexagonal cells.18,22 We have reported that the HCEC monolayer size in diameter can be expanded from 1.4 ± 0.3 mm to 2.1 ± 0.4 mm using the Descemet's membrane stripped from one-eighth of the corneoscleral rim based on the regimen of weekly application of 40 nM p120 siRNA starting from day 14 for 2 weeks.18 This novel tissue engineering technique selectively activates the p120/Kaiso signaling to unlock the mitotic block governed by contact inhibition, but is not accompanied by activation of the canonical Wnt signaling.18 Because the Kaiso nuclear level dictates the proliferative activity and can only be lowered by nuclear translocation of p120,18 knockdown with p120-Kaiso siRNA exhibited a synergistic action to further unlock the mitotic block mediated by contact inhibition. Because this engineering approach can be applied directly on contact-inhibited HCEC monolayers, it circumvents the need of using trypsin and EDTA to obtain single cells before expansion. Consequently, it also prevents the complication of endothelial mesenchymal transition that is mediated by activation of canonical Wnt signaling and Smad/Zeb signaling when the adherin-mediated intercellular junction is disrupted by trypsin and/or EDTA.18–20 We have used the same technique to unlock the mitotic block mediated by contact inhibition in RPE cells.20
In this study, using the BrdU labeling, we have optimized the knockdown regimen of p120 siRNA regarding the frequency (time per week), the concentration and the starting date. We noted that the BrdU labeling was not affected by the frequency and the starting date of p120 siRNA knockdown, but was dose-dependently increased (Fig. 1). Consequently, the HCEC monolayer size in diameter was significantly promoted from 3.2 ± 0.3 mm to 4.1 ± 0.4 mm by changing the knockdown regimen from the reported weekly application of 40 nM p120 siRNA starting from day 1418 to weekly application of 100 nM p120 siRNA starting from day 7 for a total of 5 weeks (P < 0.05, Fig. 2). Consistent with the notion that p120 siRNA knockdown activates p120/Kaiso signaling with p120-catenin nuclear translocation that is correlated with nuclear release of Kaiso and nuclear BrdU labeling,18 we also noted that addition of 100 nM of Kaiso siRNA to the above optimized p120 knockdown regimen further expanding the size of HCEC monolayer to 5.0 ± 0.4 mm in diameter after 5 weeks of culture (P < 0.05, Fig. 3A) via the same mechanism (Fig. 4). Although nucleocytoplasmic shuttling of p120 via microtubules can be modulated by destabilizing microtubules using nocodazole to promote proliferation of HCEC monolayers induced by p120 siRNA but counteracted by taxol,18 simultaneous addition of nocodazole actually inhibited the resultant size achieved by the aforementioned optimized knockdown with p120-Kaiso siRNAs (P < 0.05, Fig. 3B). Such a negative influence by nocodazole might be explained by the finding that nocodazole prevents proliferating cells from passing though G2- or M-phase because it disrupts the polymerization of microtubules during extended culturing.31 By increasing the size of the stripped Descemet's membrane from one-eighth to one-fourth and one-half of the remaining corneoscleral rim, we were able to achieve the size of 6.8 ± 0.3 mm and 8.1 ± 0.5 mm in diameter, respectively (both P < 0.05, Fig. 3C) (i.e., close to the size amenable for endothelial transplantation in human eyes).
Previously, we have reported that activation of RhoA–ROCK signaling following knockdown by p120 siRNA is required to promote the aforementioned expansion of contact-inhibited HCEC monolayers.18 Herein, we re-affirmed this finding in HCEC monolayers treated with p120-Kaiso siRNA as evidenced by a higher level of GTP-RhoA, nuclear translocation of p120, and colocalization of BrdU (Fig. 4). Furthermore, both nuclear translocation of p120 and nuclear colocalization of BrdU were abolished by simultaneous addition of the RhoA inhibitor, CT-04, or the ROCK inhibitor, Y27632, suggesting that RhoA–ROCK signaling is required to aid p120-Kaiso signaling to promote proliferation induced by p120-Kaiso siRNAs. In the p120 null mouse epidermis, there is nuclear localization of NFκB partly controlled by Rho and GTPases and many cycling cells in the basal layer.21 Our study showed that one downstream target of the RhoA–ROCK signaling turned out to be the noncanonical BMP signaling that led to activation of NFκB signaling. This conclusion was based on the finding that nuclear translocation and colocalization of BrdU were also correlated with nuclear translocation of pNFκB (p65; Fig. 4), which could be abolished by a small molecular weight inhibitor of NFκB (i.e., CAY10512).18 Because both nuclear translocation of pNFκB (p65) and p120 were aborted by noggin, a potent extracellular inhibitor of BMPs26 and by 5Z-7-Oxozeaenol, a small molecular weight inhibitor of Tak1,27 we speculated that the canonical pNFκB pathway might be linked to the noncanonical BMP pathway via the formation of the XIAP-Tab1-Tak1 complex.28–30 This hypothesis was supported by upregulation of BMP2, BMP4, and BMPR1B transcript by p120-Kaiso siRNAs, which was aborted by CT-04 and Y27632 (Fig. 5). The notion that the canonical BMP signaling was not involved was supported by the lack of nuclear translocation of pSmad1/5/8 and the lack of upregulation of ID1-4 transcripts, (i.e., known downstream targets of the canonical BMP signaling). Because upregulation of BMP2, BMP4, and BMPR1B was also aborted by CAY10512 and noggin, it is likely that expression of the noncanonical BMP signaling via NFκB has a positive influence on expression of BMP ligands and receptors. Future studies are needed to determine how nuclear translocation of pNFκB controls nuclear translocation of p120, and whether two together facilitates the G1-S transition.
Consistent with what we have reported,18 the normal HCEC monolayer phenotype could be restored 1 week after discontinuation of the knockdown with p120A-Kaiso siRNAs (Fig. 6). This was supported by the maintenance of a hexagonal cell shape, a similar in vivo density, and a normal HCEC phenotype as evidenced by the normal cytolocalization of p120, N-cadherin, α-catenin, β-catenin, ZO-1, Na-K-ATPase, and F-actin all at the cell junction (Fig. 6). The negative expression of LEF1 and S100A4 confirmed the lack activation of endothelial mesenchymal transition. The lack of further proliferation and BrdU labeling of HCEC monolayers after 5 weeks of culturing suggested that the mitotic block mediated by contact inhibition eventually prevails. Further investigation into the mechanism leading to this limitation might further improve engineering of HCEC monolayers as surgical grafts reaching the transplantable size of 8 mm in diameter from one-eighth or smaller corneoscleral rim.
Acknowledgments
Supported by Grant R43 EY022502 from the National Eye Institute, National Institutes of Health, Bethesda, Maryland (Y-TZ, and SCGT), and a research grant from TissueTech, Inc., Miami, Florida.
Disclosure: Y.-T. Zhu, None; B. Han, None; F. Li, None; S.-Y. Chen, None; S. Tighe, None; S. Zhang, None; S.C.G. Tseng, TissueTech, Inc. (F)
References
- 1. Bonanno JA. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog Retin Eye Res. 2003; 22: 69–94 [DOI] [PubMed] [Google Scholar]
- 2. Fischbarg J, Maurice DM. An update on corneal hydration control. Exp Eye Res. 2004; 78: 537–541 [DOI] [PubMed] [Google Scholar]
- 3. Laing RA, Neubauer L, Oak SS, Kayne HL, Leibowitz HM. Evidence for mitosis in the adult corneal endothelium. Ophthalmology. 1984; 91: 1129–1134 [DOI] [PubMed] [Google Scholar]
- 4. Joyce NC. Cell cycle status in human corneal endothelium. Exp Eye Res. 2005; 81: 629–638 [DOI] [PubMed] [Google Scholar]
- 5. Petroll WM, Jester JV, Bean JJ, Cavanagh HD. Myofibroblast transformation of cat corneal endothelium by transforming growth factor-beta1, -beta2, and -beta3. Invest Ophthalmol Vis Sci. 1998; 39: 2018–2032 [PubMed] [Google Scholar]
- 6. Senoo T, Obara Y, Joyce NC. EDTA: a promoter of proliferation in human corneal endothelium. Invest Ophthalmol Vis Sci. 2000; 41: 2930–2935 [PubMed] [Google Scholar]
- 7. Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: contact inhibition and TGF-beta2. Invest Ophthalmol Vis Sci. 2002; 43: 2152–2159 [PubMed] [Google Scholar]
- 8. Li W, Sabater AL, Chen YT, et al. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007; 48: 614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen KH, Azar D, Joyce NC. Transplantation of adult human corneal endothelium ex vivo: a morphologic study. Cornea. 2001; 20: 731–737 [DOI] [PubMed] [Google Scholar]
- 10. Ishino Y, Sano Y, Nakamura T, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci. 2004; 45: 800–806 [DOI] [PubMed] [Google Scholar]
- 11. Mimura T, Yamagami S, Yokoo S, et al. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004; 45: 2992–2997 [DOI] [PubMed] [Google Scholar]
- 12. Yokoo S, Yamagami S, Yanagi Y, et al. Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest Ophthalmol Vis Sci. 2005; 46: 1626–1631 [DOI] [PubMed] [Google Scholar]
- 13. Hsiue GH, Lai JY, Chen KH, Hsu WM. A novel strategy for corneal endothelial reconstruction with a bioengineered cell sheet. Transplantation. 2006; 81: 473–476 [DOI] [PubMed] [Google Scholar]
- 14. Sumide T, Nishida K, Yamato M, et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J. 2006; 20: 392–394 [DOI] [PubMed] [Google Scholar]
- 15. Hatou S, Yoshida S, Higa K, et al. Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/beta-catenin signaling. Stem Cells Dev. 2013; 22: 828–839 [DOI] [PubMed] [Google Scholar]
- 16. Engelmann K, Bohnke M, Friedl P. Isolation and long-term cultivation of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 1988; 29: 1656–1662 [PubMed] [Google Scholar]
- 17. Lee JG, Kay EP. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp Eye Res. 2006; 83: 1309–1316 [DOI] [PubMed] [Google Scholar]
- 18. Zhu YT, Chen HC, Chen SY, Tseng SC. Nuclear p120-catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junction. J Cell Sci. 2012; 125 (pt 15): 3636–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen HC, Zhu YT, Chen SY, Tseng SC. Wnt signaling induces epithelial-mesenchymal transition with proliferation in ARPE-19 cells upon loss of contact inhibition. Lab Invest. 2012; 92: 676–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen HC, Zhu YT, Chen SY, Tseng SC. Selective activation of p120(ctn)-kaiso signaling to unlock contact inhibition of ARPE-19 cells without epithelial-mesenchymal transition. PLoS One. 2012; 7: e36864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell. 2006; 124: 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu YT, Hayashida Y, Kheirkhah A, He H, Chen SY, Tseng SC. Characterization and comparison of intercellular adherent junctions expressed by human corneal endothelial cells in vivo and in vitro. Invest Ophthalmol Vis Sci. 2008; 49: 3879–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roczniak-Ferguson A, Reynolds AB. Regulation of p120-catenin nucleocytoplasmic shuttling activity. J Cell Sci. 2003; 116: 4201–4212 [DOI] [PubMed] [Google Scholar]
- 24. Yanagawa R, Furukawa Y, Tsunoda T, et al. Genome-wide screening of genes showing altered expression in liver metastases of human colorectal cancers by cDNA microarray. Neoplasia. 2001; 3: 395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. J Biol Chem. 2004; 279: 9512–9521 [DOI] [PubMed] [Google Scholar]
- 26. Dummula K, Vinukonda G, Chu P, et al. Bone morphogenetic protein inhibition promotes neurological recovery after intraventricular hemorrhage. J Neurosci. 2011; 31: 12068–12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ninomiya-Tsuji J, Kajino T, Ono K, et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase. J Biol Chem. 2003; 278: 18485–18490 [DOI] [PubMed] [Google Scholar]
- 28. Matluk N, Rochira JA, Karaczyn A, Adams T, Verdi JM. A role for NRAGE in NF-kappaB activation through the non-canonical BMP pathway. BMC Biol. 2010; 8: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu M, Lin SC, Huang Y, et al. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007; 26: 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofer-Warbinek R, Schmid JA, Stehlik C, Binder BR, Lipp J, de Martin R. Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J Biol Chem. 2000; 275: 22064–22068 [DOI] [PubMed] [Google Scholar]
- 31. Kallas A, Pook M, Maimets M, Zimmermann K, Maimets T. Nocodazole treatment decreases expression of pluripotency markers Nanog and Oct4 in human embryonic stem cells. PLoS One. 2011; 6: e19114 [DOI] [PMC free article] [PubMed] [Google Scholar]