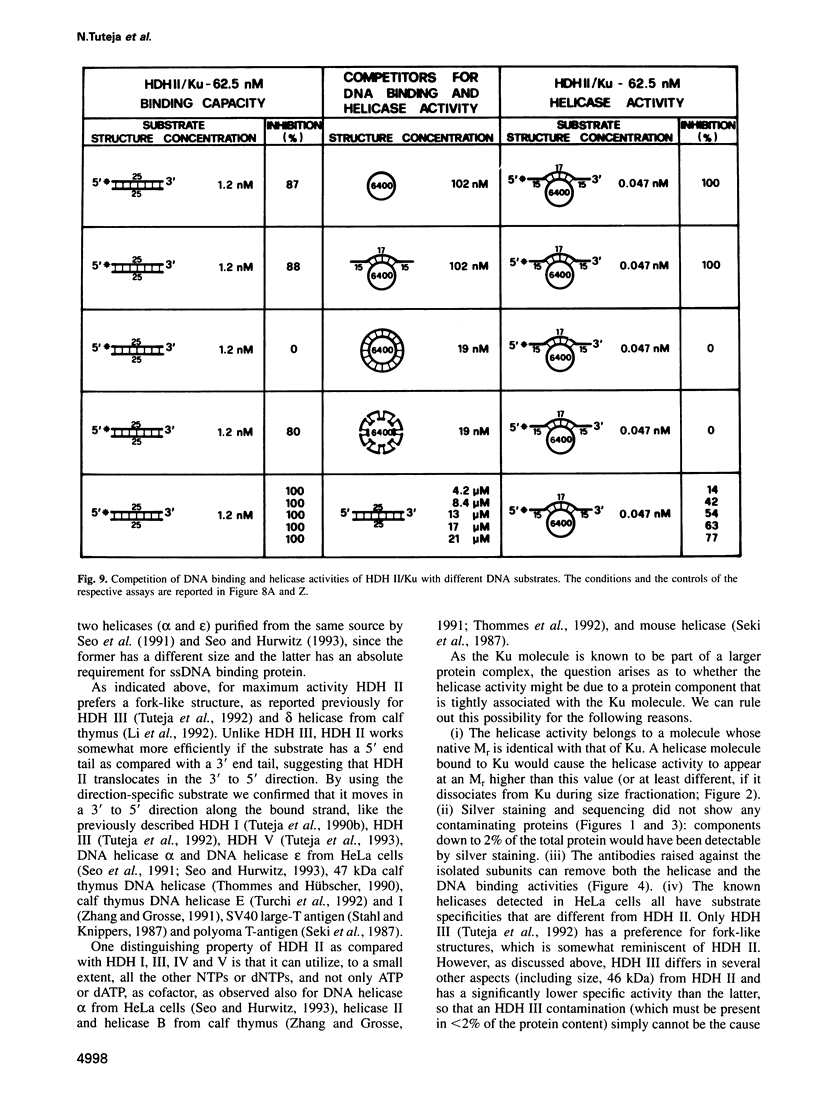
Abstract
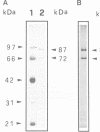
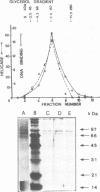
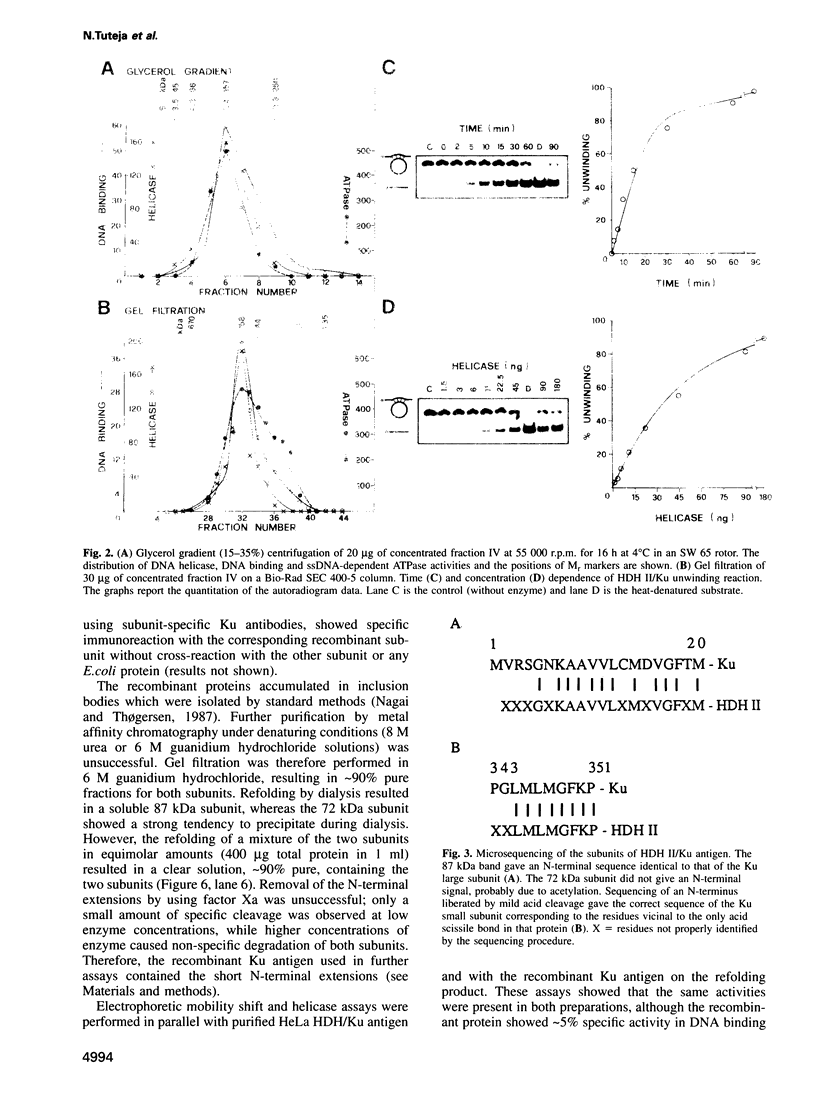
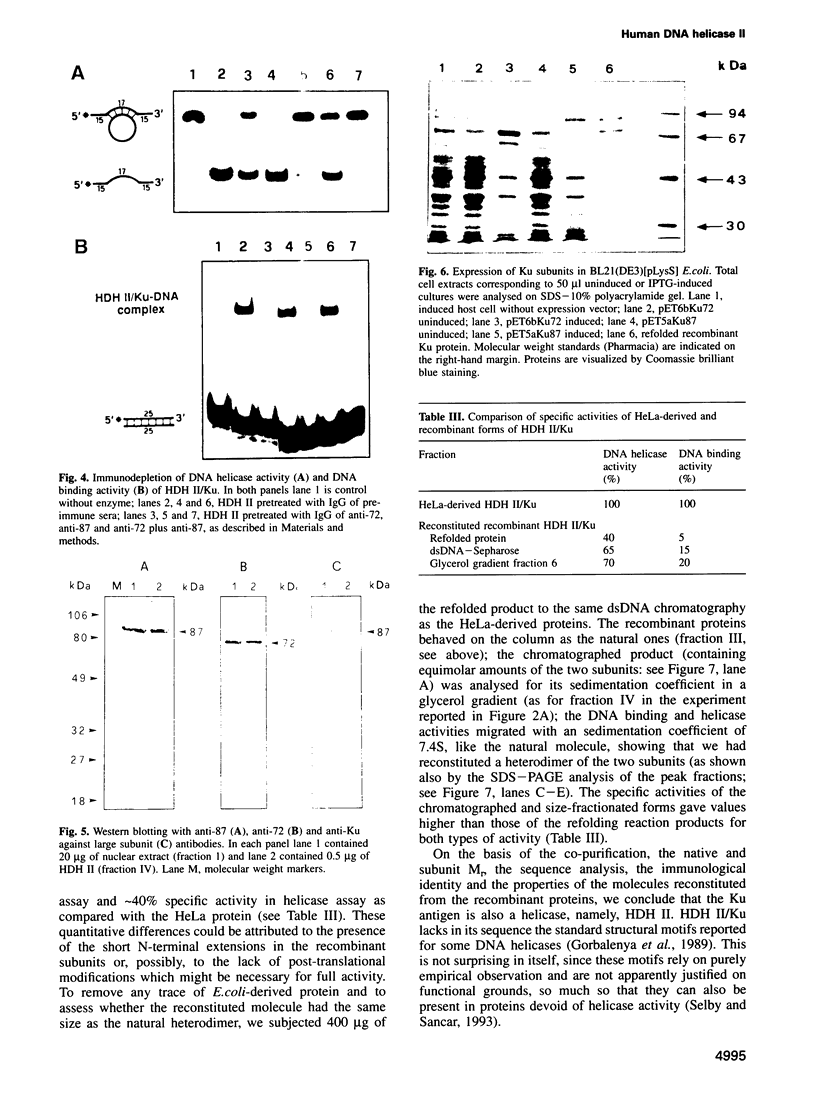
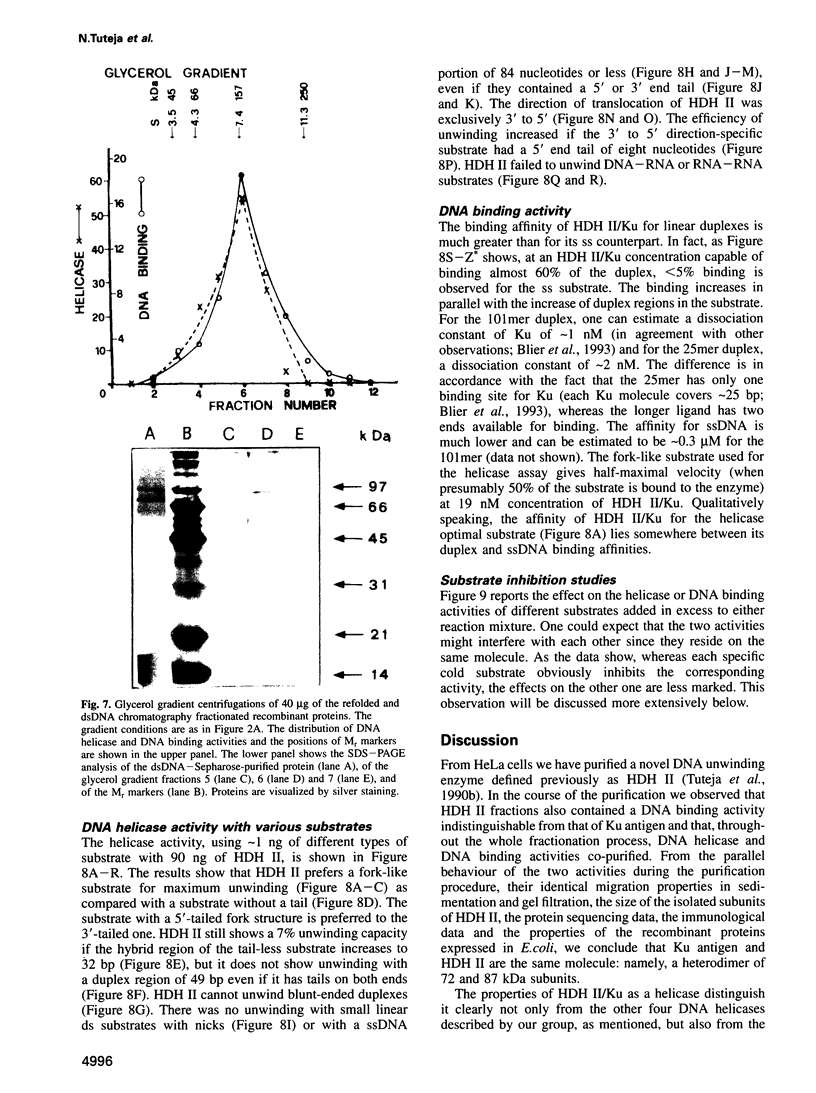
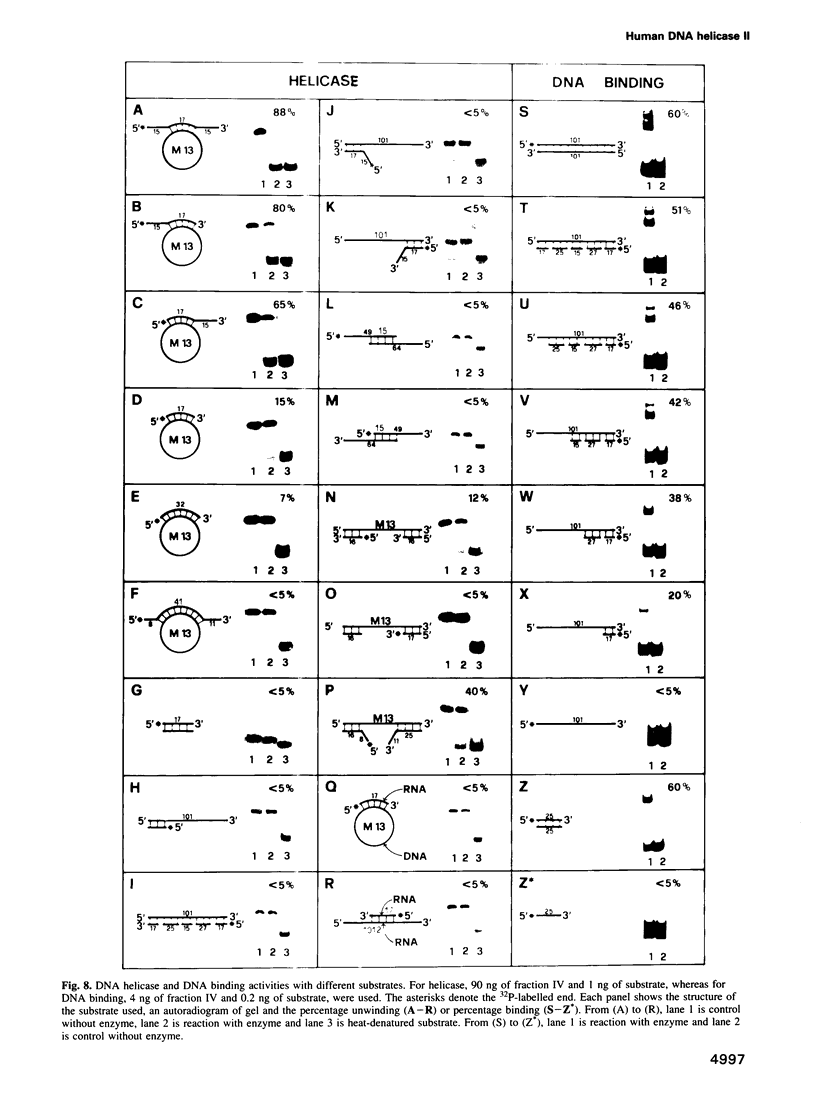
Human DNA helicase II (HDH II) is a novel ATP-dependent DNA unwinding enzyme, purified to apparent homogeneity from HeLa cells, which (i) unwinds exclusively DNA duplexes, (ii) prefers partially unwound substrates and (iii) proceeds in the 3' to 5' direction on the bound strand. HDH II is a heterodimer of 72 and 87 kDa polypeptides. It shows single-stranded DNA-dependent ATPase activity, as well as double-stranded DNA binding capacity. All these activities comigrate in gel filtration and glycerol gradients, giving a sedimentation coefficient of 7.4S and a Stokes radius of approximately 46 A, corresponding to a native molecular weight of 158 kDa. The antibodies raised in rabbit against either polypeptide can remove from the solution all the activities of HDH II. Photoaffinity labelling with [alpha-32P]ATP labelled both polypeptides. Microsequencing of the separate polypeptides of HDH II and cross-reaction with specific antibodies showed that this enzyme is identical to Ku, an autoantigen recognized by the sera of scleroderma and lupus erythematosus patients, which binds specifically to duplex DNA ends and is regulator of a DNA-dependent protein kinase. Recombinant HDH II/Ku protein expressed in and purified from Escherichia coli cells showed DNA binding and helicase activities indistinguishable from those of the isolated protein. The exclusively nuclear location of HDH II/Ku antigen, its highly specific affinity for double-stranded DNA, its abundance and its newly demonstrated ability to unwind exclusively DNA duplexes, point to an additional, if still unclear, role for this molecule in DNA metabolism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blier P. R., Griffith A. J., Craft J., Hardin J. A. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J Biol Chem. 1993 Apr 5;268(10):7594–7601. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dvir A., Peterson S. R., Knuth M. W., Lu H., Dynan W. S. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11920–11924. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir A., Stein L. Y., Calore B. L., Dynan W. S. Purification and characterization of a template-associated protein kinase that phosphorylates RNA polymerase II. J Biol Chem. 1993 May 15;268(14):10440–10447. [PubMed] [Google Scholar]
- Falzon M., Fewell J. W., Kuff E. L. EBP-80, a transcription factor closely resembling the human autoantigen Ku, recognizes single- to double-strand transitions in DNA. J Biol Chem. 1993 May 15;268(14):10546–10552. [PubMed] [Google Scholar]
- Geider K., Hoffmann-Berling H. Proteins controlling the helical structure of DNA. Annu Rev Biochem. 1981;50:233–260. doi: 10.1146/annurev.bi.50.070181.001313. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989 Jun 26;17(12):4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Griffith A. J., Craft J., Evans J., Mimori T., Hardin J. A. Nucleotide sequence and genomic structure analyses of the p70 subunit of the human Ku autoantigen: evidence for a family of genes encoding Ku (p70)-related polypeptides. Mol Biol Rep. 1992 May;16(2):91–97. doi: 10.1007/BF00419754. [DOI] [PubMed] [Google Scholar]
- Hughes M. J., Liang H. M., Jiricny J., Jost J. P. Purification and characterization of a protein from HeLa cells that binds with high affinity to the estrogen response element, GGTCAGCGTGACC. Biochemistry. 1989 Nov 14;28(23):9137–9142. doi: 10.1021/bi00449a027. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserman H. B., Ingebritsen T. S., Benbow R. M. Regulation of Xenopus laevis DNA topoisomerase I activity by phosphorylation in vitro. Biochemistry. 1988 May 3;27(9):3216–3222. doi: 10.1021/bi00409a014. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees-Miller S. P., Chen Y. R., Anderson C. W. Human cells contain a DNA-activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53, and the human Ku autoantigen. Mol Cell Biol. 1990 Dec;10(12):6472–6481. doi: 10.1128/mcb.10.12.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Tan C. K., So A. G., Downey K. M. Purification and characterization of delta helicase from fetal calf thymus. Biochemistry. 1992 Apr 7;31(13):3507–3513. doi: 10.1021/bi00128a027. [DOI] [PubMed] [Google Scholar]
- Lohman T. M. Escherichia coli DNA helicases: mechanisms of DNA unwinding. Mol Microbiol. 1992 Jan;6(1):5–14. doi: 10.1111/j.1365-2958.1992.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Lohman T. M. Helicase-catalyzed DNA unwinding. J Biol Chem. 1993 Feb 5;268(4):2269–2272. [PubMed] [Google Scholar]
- Matson S. W., Kaiser-Rogers K. A. DNA helicases. Annu Rev Biochem. 1990;59:289–329. doi: 10.1146/annurev.bi.59.070190.001445. [DOI] [PubMed] [Google Scholar]
- May G., Sutton C., Gould H. Purification and characterization of Ku-2, an octamer-binding protein related to the autoantigen Ku. J Biol Chem. 1991 Feb 15;266(5):3052–3059. [PubMed] [Google Scholar]
- Mimori T., Hardin J. A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986 Aug 5;261(22):10375–10379. [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Ottiger H. P., Hübscher U. Mammalian DNA polymerase alpha holoenzymes with possible functions at the leading and lagging strand of the replication fork. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3993–3997. doi: 10.1073/pnas.81.13.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W. H., Sthoeger Z. M. Molecular cloning of cDNA encoding the p70 (Ku) lupus autoantigen. J Biol Chem. 1989 Mar 25;264(9):5047–5052. [PubMed] [Google Scholar]
- Schoepfer R. The pRSET family of T7 promoter expression vectors for Escherichia coli. Gene. 1993 Feb 14;124(1):83–85. doi: 10.1016/0378-1119(93)90764-t. [DOI] [PubMed] [Google Scholar]
- Seki M., Enomoto T., Hanaoka F., Yamada M. DNA-dependent adenosinetriphosphatase B from mouse FM3A cells has DNA helicase activity. Biochemistry. 1987 May 19;26(10):2924–2928. doi: 10.1021/bi00384a038. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993 Apr 2;260(5104):53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- Seo Y. S., Hurwitz J. Isolation of helicase alpha, a DNA helicase from HeLa cells stimulated by a fork structure and signal-stranded DNA-binding proteins. J Biol Chem. 1993 May 15;268(14):10282–10295. [PubMed] [Google Scholar]
- Seo Y. S., Lee S. H., Hurwitz J. Isolation of a DNA helicase from HeLa cells requiring the multisubunit human single-stranded DNA-binding protein for activity. J Biol Chem. 1991 Jul 15;266(20):13161–13170. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Stahl H., Knippers R. The simian virus 40 large tumor antigen. Biochim Biophys Acta. 1987 Oct 9;910(1):1–10. doi: 10.1016/0167-4781(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Thömmes P., Ferrari E., Jessberger R., Hübscher U. Four different DNA helicases from calf thymus. J Biol Chem. 1992 Mar 25;267(9):6063–6073. [PubMed] [Google Scholar]
- Thömmes P., Hübscher U. DNA helicase from calf thymus. Purification to apparent homogeneity and biochemical characterization of the enzyme. J Biol Chem. 1990 Aug 25;265(24):14347–14354. [PubMed] [Google Scholar]
- Thömmes P., Hübscher U. Eukaryotic DNA helicases: essential enzymes for DNA transactions. Chromosoma. 1992 Jun;101(8):467–473. doi: 10.1007/BF00352468. [DOI] [PubMed] [Google Scholar]
- Turchi J. J., Siegal G., Bambara R. A. DNA helicase E and DNA polymerase epsilon functionally interact for displacement synthesis. Biochemistry. 1992 Sep 22;31(37):9008–9015. doi: 10.1021/bi00152a043. [DOI] [PubMed] [Google Scholar]
- Tuteja N., Danciger M., Klisak I., Tuteja R., Inana G., Mohandas T., Sparkes R. S., Farber D. B. Isolation and characterization of cDNA encoding the gamma-subunit of cGMP phosphodiesterase in human retina. Gene. 1990 Apr 16;88(2):227–232. doi: 10.1016/0378-1119(90)90035-p. [DOI] [PubMed] [Google Scholar]
- Tuteja N., Rahman K., Tuteja R., Falaschi A. DNA helicase IV from HeLa cells. Nucleic Acids Res. 1991 Jul 11;19(13):3613–3618. doi: 10.1093/nar/19.13.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N., Rahman K., Tuteja R., Falaschi A. Human DNA helicase V, a novel DNA unwinding enzyme from HeLa cells. Nucleic Acids Res. 1993 May 25;21(10):2323–2329. doi: 10.1093/nar/21.10.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N., Rahman K., Tuteja R., Ochem A., Skopac D., Falaschi A. DNA helicase III from HeLa cells: an enzyme that acts preferentially on partially unwound DNA duplexes. Nucleic Acids Res. 1992 Oct 25;20(20):5329–5337. doi: 10.1093/nar/20.20.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N., Tuteja R., Rahman K., Kang L. Y., Falaschi A. A DNA helicase from human cells. Nucleic Acids Res. 1990 Dec 11;18(23):6785–6792. doi: 10.1093/nar/18.23.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth E. C., Marusic L., Ochem A., Patthy A., Pongor S., Giacca M., Falaschi A. Interactions of USF and Ku antigen with a human DNA region containing a replication origin. Nucleic Acids Res. 1993 Jul 11;21(14):3257–3263. doi: 10.1093/nar/21.14.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R. A kinase with Ku-dos. Curr Biol. 1993 Jul 1;3(7):449–450. doi: 10.1016/0960-9822(93)90353-p. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Wen J., Ayala A., Cook R. cDNA-derived amino acid sequence of the 86-kDa subunit of the Ku antigen. J Biol Chem. 1989 Aug 15;264(23):13407–13411. [PubMed] [Google Scholar]
- Zhang S. S., Grosse F. Purification and characterization of two DNA helicases from calf thymus nuclei. J Biol Chem. 1991 Oct 25;266(30):20483–20490. [PubMed] [Google Scholar]