Abstract
Introduction:
The Heaviness of Smoking Index (HSI) is the measure of dependence most strongly predictive of relapse. However, recent research suggests it may not be predictive of longer-term relapse. Our aim was to examine its predictive power over the first 2 years after quitting and explore whether use of stop-smoking medications is a moderator.
Methods:
Data (n = 7,093) came from the first 7 waves (2002–2009) of the International Tobacco Control Four-Country Survey, an annual cohort survey of smokers in Canada, the United States, the United Kingdom, and Australia. HSI and its 2 components (cigarettes per day [CPD] and time to first cigarette [TTFC]) were used to predict smoking relapse risk in the 2 years after the start of a quit attempt.
Results:
Scores on HSI and its components all strongly predicted relapse, but there was an interaction with time (p < .001). These measures were strong predictors of relapse within the first week of quitting (hazard ratios [HR] = 1.17, 1.24, and 1.30 for HSI, CPD, and TTFC, respectively; all p < .001), less predictive of relapse occurring between 1 week and 1 month, and not clearly predictive beyond 1 month. Among those using medication to quit, hazard ratio for HSI (HR = 1.11, p < .001) was significantly lower than for those not using (HR = 1.24, p < .001) in the first week but not beyond.
Conclusions:
HSI and its 2 components are strong predictors of short-term smoking relapse, but they rapidly lose predictive power over the first weeks of an attempt, becoming marginally significant at around 1 month and not clearly predictive beyond then.
INTRODUCTION
Addictions are characterized by high rates of relapse, with relapse still common even after prolonged periods of abstinence. For nicotine addiction, at least to cigarettes, the measure of dependence most strongly associated with reduced likelihood of quitting success is the Heaviness of Smoking Index (HSI; Borland, Yong, O’Connor, Hyland, & Thompson, 2010; Hyland et al., 2006). This measure, first developed by Heatherton, Kozlowski, Frecker, Rickert, and Robinson (1989), consists of two items, cigarettes per day (CPD) and time to first cigarette (TTFC), a subset taken from the six-item Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). The HSI is most commonly scored using four-level categorizations of each measure but can be scored to give a functionally equivalent continuous measure (Borland et al., 2010). The HSI has been shown to predict cessation outcomes (Courvoisier & Etter, 2010; de Leon et al., 2003; Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994), including two International Tobacco Control Four-Country (ITC-4) studies (Borland et al., 2010; Hyland et al., 2006). Of the two component measures of the HSI (i.e., CPD and TTFC), some studies (e.g., Baker et al., 2007) have found TTFC to account for most of the observed effect, but others (Borland et al. 2010) have shown both to contribute independently. However, Herd, Borland, and Hyland (2009) failed to find any predictive power of HSI measured in the previous wave when examining relapse among those quit in the subsequent survey wave (typically about a year later). Herd et al. (2009) argued that the disparity between their findings and those of other studies may have to do with the different time intervals for prediction of relapse as their study had relatively few who had quit very recently, suggesting that HSI may only predict relapse in the early days of a quit attempt. This hypothesis is supported by the finding of Zhou et al. (2009) of a significant interaction between baseline FTND scores and relapse using an Internet panel sample recruited from multiple countries and followed up every 3 months. Specifically, Zhou et al. (2009) found that higher baseline FTND scores were associated with higher likelihood of relapse in the first 3 months, but not in the second 3 months after cessation. These observations raise important questions as to what these indices are measuring and of the possibility that the determinants of longer term maintenance are fundamentally different from those for the short term. The use of cessation medication is known to reduce short-term relapse (Cahill, Stevens, Perera, & Lancaster, 2013), so one would expect that the effect of measures of dependence on relapse would be attenuated among users, at least during the period of use.
The study aims were to examine, among a sample of daily smokers who had made a quit attempt, (a) the relationship between their HSI scores recorded at the wave before the target quit attempt and relapse risk over a 2-year period, predicting an interaction, such that the predictive power was expected to be greater in the early stages of the attempt; (b) the predictive utility of the component measures (CPD and TTFC) compared with the combined HSI scale; and (c) whether use of stop-smoking medications moderated the predictive relationship between HSI and relapse.
METHODS
Data Source and Sample
Data come from the first seven waves of the ITC-4 survey conducted in the United States, United Kingdom, Canada, and Australia. The ITC-4 is a longitudinal cohort study of adult smokers in each of the four countries. The ITC cohort was constructed with probability sampling methods (random-digit dialing methods from list-assisted phone numbers) from the population of each country within strata defined by geographic region and community size. It was, therefore, designed to be broadly representative of its respective populations. The cohort was followed up yearly, and a replenishment sample was obtained at each subsequent wave to replace those lost due to attrition, using the same sampling protocol as in Wave 1. The broad aim of the ITC-4 was to evaluate the psychosocial and behavioral impact of tobacco control policies on smokers. A full description of the ITC conceptual framework and methodology can be found elsewhere (Fong et al., 2006; Thompson et al., 2006).
Respondents were recruited into the study as smokers who met the following criteria: aged 18+ years, had smoked at least 100 cigarettes in their lifetime, and smoked at least once in the past 30 days. Participants who subsequently quit smoking were retained in the study. To be eligible for the study, respondents had to be a daily smoker at a baseline wave (Wave T), to have provided valid HSI data at this wave, to have made a quit attempt lasting at least 1 day by the next survey wave (Wave T+1), and to have provided outcome data on that attempt either at that wave, or for those still quit at subsequent waves, up until 2 years after the target attempt. Based on the data available across the seven waves (n = 20,417), we derived a study sample of 7,093 who met our inclusion criteria. Table 1 presents the characteristics of the sample.
Table 1.
Sample Characteristics
| Smokers (n = 7,093) | |
|---|---|
| Age at recruitment in years (%) | |
| 18–24 | 9.8 |
| 25–39 | 29.6 |
| 40–54 | 35.8 |
| 55+ | 24.8 |
| Sex: male (%) | 42.4 |
| Education levels (%) | |
| Low | 50.1 |
| Medium | 33.1 |
| High | 16.8 |
| Household income (%) | |
| Low | 27.9 |
| Medium | 33.9 |
| High | 32.1 |
| No information | 6.1 |
| Country (%) | |
| Canada | 25.6 |
| United States | 21.1 |
| United Kingdom | 25.4 |
| Australia | 27.9 |
| Stop-smoking medications (%) | |
| Reported using any for quit attempts | 40.6 |
| Cohort/year of recruitment (%) | |
| Wave 1 (2002) | 54.2 |
| Wave 2 (2003) | 9.2 |
| Wave 3 (2004) | 13.5 |
| Wave 4 (2005/2006) | 9.6 |
| Wave 5 (2006/2007) | 7.4 |
| Wave 6 (2007/2008) | 6.1 |
Measures
Nicotine Dependence
This was assessed by the two questions from the HSI, CPD (coded: 0, 0–10 CPD; 1, 11–20 CPD; 2, 21–30 CPD; 3, 31+ CPD) and TTFC after waking in minutes (coded: 0, 61+ min; 1, 31–60min; 2, 6–30min; 3, 5min or less; and the composite, 0–6; Heatherton et al., 1989).
Outcome Variable
Time to relapse was the main outcome. At each follow-up, respondents were asked their smoking status. If quit, they were coded as having made a quit attempt since the last survey and were asked how long ago they quit. If smoking, they were asked whether they had tried to quit since the previous wave, and if so, they were asked the duration of the quit attempt. For those who made multiple attempts since the previous wave, they were asked the duration of their most recent attempt. For those who were quit at two or more successive waves, their quit duration was computed by adding on the exact interwave interval, plus any reported durations within interwave intervals, up to 2 years. The quit length information was then used to derive time to relapse in days for each individual. Relapse was defined as any quit attempt that ended in failure (i.e., resumed smoking) identified at a given follow-up assessment. Data were censored for those whose quit attempt lasted beyond 2 years and at the last confirmed point of abstinence if quit attempt lasted less than 2 years for those who had dropped out of the study.
Other Variables
Data were also collected on a range of sociodemographic variables, including age (18–24, 25–39, 40–54, and 55+ years), sex, annual household income, highest level of education, and country of residence. Household income and highest level of education attained were not based on equivalent systems across the four countries, and hence, as per our previous study (Hyland et al., 2006), they were equated across the four countries by coding them into low, medium, and high. Income has an additional category of “no information” to capture those who did not provide or refused to provide data on income so that this subgroup can be included in multivariate analysis. At each follow-up, respondents who had made a quit attempt since the previous wave were asked about whether they used any stop-smoking medication for their last/current quit attempts.
Statistical Analyses
We used survival analyses to model the association between HSI and its two components (CPD and TTFC) and time to relapse. Our modeling strategy used a fully parametric approach, parameterized as a proportional hazard model. We modeled the underlying distribution of time using the Weibull distribution. We fitted separate models for CPD, TTFC, and HSI. Because our hypothesis was that the effect of HSI and its components was not constant over time (time-varying coefficient), we fitted interactions between time and the HSI predictor. To this end, we expanded our data set into intervals (1–7 days, 8–14 days, 15–30 days, 31–90 days, 91–180 days, 181–270 days, 271–420 days, and 421–730 days) and allowed an interaction between each of these intervals and time, where the intervals were entered as indicator variables and time was entered as a continuous variable. All models controlled for potential confounders: sociodemographics, any use of stop-smoking medications, country, survey year, and year recruited into the study. In a separate model, we also tested for the possible interaction effect between HSI and medication use to determine the extent to which the effect of HSI on relapse was moderated by medication use.
For the purpose of sensitivity analysis, we repeated the above analyses using a modified version of the categorical CPD (recoded as 0: 0–9 CPD, 1: 10–15 CPD, 2:16–20 CPD, 3: 21+ CPD) to yield a better distribution of scores because of the small number in the 31+ CPD category (see Supplementary Table 1 for distribution comparison). This version was then combined with TTFC to compute the scores for a modified version of the HSI.
Hazard ratios are reported as an index of the strength of the association between independent variables and time to relapse. The hazard ratio is defined as the multiplicative change in the hazard rate (i.e., the rate of relapse at time T, conditional upon maintenance of abstinence to time T) that occurs when the predictor variable changes by one unit. We report the time-varying coefficients as hazard ratios over the eight intervals of interest. They are interpreted as the effect of a 1 unit change in, for example, HSI over the interval of interest on the risk of relapse.
All analyses were conducted in Stata 12.1 using the specialist commands manipulating and analyzing survival data (e.g., stset, stsplit, streg) and also using clustered sandwich estimators to account for any within-subjects clustering due to individuals providing data for more than one quit attempt during the study period.
RESULTS
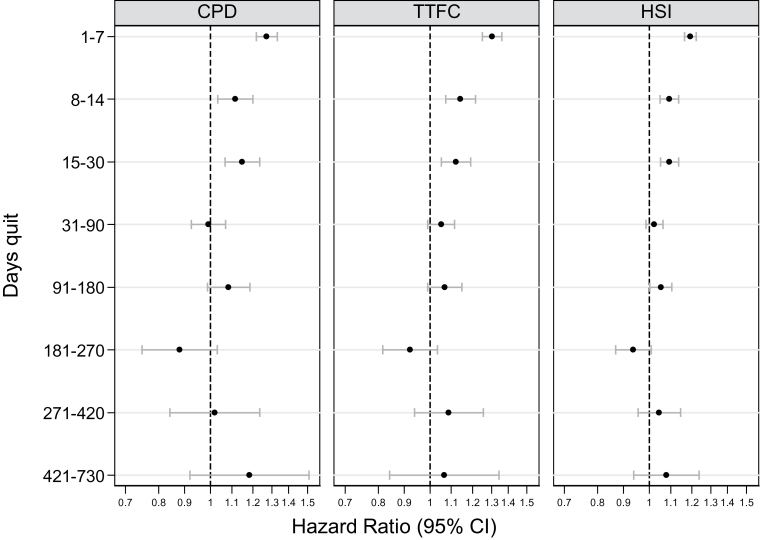
The analysis showed an overall significant relationship between the main measure (HSI) and its both components (CPD and TTFC), and relapse, with higher scores associated with shorter latency to relapse. As expected, there was a significant interaction on relapse risk between all three dependence measures and time (all p < .001). Figure 1 shows that the predictive effects of all three measures were strong in the first week, then declined, and were largely absent beyond 1 month (except for a marginal effect for HSI from 3 to 6 months). There was also a significant interaction between use of stop-smoking medications and all three measures of dependence (all p < .001). Results stratified by medication use indicated that this effect was most pronounced in the first week, where the predictive power of the dependence measures was considerably lower for the subgroup reporting use of stop-smoking medications than those who reported not using any (see Table 2, where CIs of the estimates for HSI and TTFC do not overlap in the first week but not beyond, and a marginal effect is found for CPD).
Figure 1.
Hazard ratios for relapse prediction of dependence measures as a function of time quit.
Table 2.
Results Showing Relationship Between Baseline Dependence Measures and Relapse by Stop-Smoking Medication Use
| Predicting relapse risk over different quit periods among daily smokers who made a quit attempt, HR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1–7 | 8–14 | 15–30 | 31–90 | 91–180 | 181–270 | 271–420 | 421–730 | |
| Dependence measures | ||||||||
| HSI: med users | 1.12 (1.08–1.18)*** | 1.11 (1.04–1.19)** | 1.04 (0.98–1.11) | 1.00 (0.95–1.06) | 1.07 (0.99–1.16) | 0.88 (0.77–0.99)* | 1.03 (0.87–1.22) | 0.95 (0.73–1.24) |
| HSI: med nonusers | 1.26 (1.22–1.30)*** | 1.09 (1.04–1.15)*** | 1.11 (1.06–1.17)*** | 1.01 (0.96–1.06) | 0.98 (0.92–1.05) | 0.93 (0.84–1.03) | 1.02 (0.92–1.15) | 1.07 (0.91–1.27) |
| CPD: med users | 1.20 (1.11–1.29)*** | 1.10 (0.97–1.24) | 1.11 (0.99–1.24) | 0.99 (0.89–1.11) | 1.16 (1.01–1.32)* | 0.78 (0.61–0.99)* | 0.89 (0.63–1.27) | 0.82 (0.49–1.37) |
| CPD: med nonusers | 1.37 (1.29–1.45)*** | 1.16 (1.06–1.28)** | 1.15 (1.04–1.28)** | 0.96 (0.87–1.06) | 0.93 (0.81–1.07) | 0.90 (0.73–1.12) | 1.05 (0.83–1.32) | 1.24 (0.92–1.66) |
| TTFC: med users | 1.17 (1.09–1.26)*** | 1.22 (1.09–1.38)** | 1.01 (0.91–1.12) | 1.02 (0.93–1.11) | 1.06 (0.94–1.19) | 0.87 (0.72–1.06) | 1.17 (0.90–1.53) | 1.00 (0.66–1.53) |
| TTFC: med nonusers | 1.43 (1.36–1.50)*** | 1.13 (1.04–1.22)** | 1.17 (1.08–1.27)*** | 1.04 (0.96–1.12) | 0.99 (0.89–1.09) | 0.89 (0.76–1.03) | 1.02 (0.85–1.22) | 1.03 (0.77–1.36) |
Note. CPD = cigarette per day; HR = hazard ratios were adjusted for age, sex, income, education, country, survey year, and year of recruitment; HSI = Heaviness of Smoking Index; med = medication; TTFC = time to first cigarette upon waking.
*p < .05; **p < .01; ***p < .001.
When the above analyses were repeated using the modified version of the CPD and HSI, the results were very similar although the 95% CIs for the estimates were wider particularly for the longer term quit periods (see Supplementary Table 2).
Discussion
This study shows that HSI is predictive of relapse in the first month of a quit attempt and particularly so in the first week. Our analyses have confirmed the interaction with time of the predictive effect of the HSI as found by Zhou et al. (2009) for FTND, thus providing support for speculation by Herd et al. (2009) that the reason for their failure to find an effect was because of the much longer mean time of relapse than in studies that have found effects. As expected, our analyses also show that the relapse predictive utility of the dependence measures is moderated by use of stop-smoking medications, with use of medication reducing their predictive power especially during the first week of an attempt.
The diminishing relapse predictive utility of the HSI with time quit is the mirror image of what Herd et al. (2009) found for reported frequency of strong urges to smoke, where there was no clear effect on relapse in the first month, but a strong relationship thereafter. Taken together, these findings suggest that there are two (at least) functionally distinct aspects of dependence: one measured by habit strength (e.g., HSI), which is important in the early days of a quit attempt, and the other indexed by the frequency of strong urges to smoke experienced after quitting. As frequency of urges changes dynamically over time and was measured at varying times postquitting (Herd & Borland, 2009), it is not clear whether the key determinant is some stable characteristic of reporting cravings or something that emerges. Fidler, Shahab, and West (2011) showed that craving intensity experienced during a normal smoking day can predict short-term relapse risk, suggesting that cravings before quitting may be measuring something different from those after quitting. We clearly need a better understanding of what determines the persistence of strong cravings, as this appears to be at least one factor influencing longer term maintenance/relapse.
The idea that there might be more than one process involved in maintenance of change is not new. For example, Piasecki, Fiore, McCarthy, and Baker (2002) theorized three sets of forces, namely, withdrawal, self-control, and the exhaustion of self-control, as co-determinants of relapse. These authors postulate that in an early period of a quit attempt, self-control is critical for preventing relapse, but later in the period, relapse is more under the influence of the residual strength of cue-specific factors. Such an interpretation would imply that cravings measured later in a quit attempt are more strongly related to cue-specific factors, whereas those early in an attempt, or when smoking, may reflect more internally generated determinants.
The findings for the two component measures of the HSI were largely similar to that of the composite scale, thus lending further support for the predictive validity of these two measures, consistent with our previous work, albeit on an overlapping sample (Borland et al., 2010). The revised scoring of CPD appears to have resulted in both a better distribution of scores and a slightly more precise overall prediction, suggesting that a change to take into account the reduction in consumption associated with ubiquitous smoke-free places should be considered for more general use.
The finding of a moderating effect of medication use only for the first week of quitting but not beyond suggests that most of the benefit of stop-smoking medication use is in helping more highly addicted smokers to overcome the challenges of nicotine withdrawal in the early days of quitting. The reasons for the lack of a differential effect beyond the first week are unclear, and future research is needed to explore type of medication used, adherence, dosage, timing, and duration of use, all of which could affect the effectiveness of medication use. Nevertheless, quitters tend to continue to use quit medications until they feel they no longer need to or the recommended interval for use ends (Balmford, Borland, Hammond, & Cummings, 2011). We note that there was no evidence of any rebound effect associated with stopping medication use, but this study had limited capacity to find such an effect, particularly given that a detailed analysis would require knowing the timing of medication use among those still quit at that time.
Strengths and Limitations
The strengths of this study include its prospective cohort design, multicountry data, large sample for analysis, and the use of survival analysis. One limitation of our study is the use of self-reported data. Reports on when relapse took place are subject to recall bias due to telescoping effects meaning we should exercise caution in making too precise estimates of the timing of the loss of predictive power. Somewhere around 1 month is as accurate as our measures will allow. Also, given the follow-up intervals were about a year apart, brief quit attempts and any associated medication use, particularly ones that occurred a long time prior to the survey interview, are underreported (Berg et al., 2010; Borland, Partos, & Cummings, 2012). However, this should not affect the internal validity of this study as it is inconceivable that short quit attempts that happen shortly after one interview and a long time from the next interview where they are to be reported on differ systematically from those closer to the survey interview. It could also be argued that our relapse estimates might be biased without biochemical validation. However, self-reported relapses have been shown to be generally reliable in large nonintervention population-based surveys (SRNT Subcommittee on biochemical verification, 2002). Another limitation is the potential threat to external validity of the study due to sample attrition, but again we can think of no mechanism by which those reluctant to participate in surveys or those harder to contact would differ in the relationship between these aspects of their smoking habit and quitting outcomes. The ITC study has higher attrition rates for those quit at the previous contact (Thompson et al., 2006), but this really is likely only to affect estimates of relapse rates, not their determinants.
An important implication of this study is that neither researchers nor clinicians should rely exclusively on measures of habit strength such as the HSI to characterize levels of nicotine dependence, if by dependence is meant difficulty in successfully quitting smoking, something that relates to both short-term and long-term relapse propensity. Our findings suggest that the HSI is only a useful indicator of that aspect of dependence that relates to short-term success. Measures of propensity to long-term relapse are needed. Others have suggested a more prominent role for the strength of urges to smoke on typical smoking days (Baker, Breslau, Covey, & Shiffman, 2012), but we do not know when might measuring this indicate a propensity to remain quit long term. If Piasecki et al. (2002) are correct, then measures that focus on cue-specific cravings might provide useful information, in particular, cues that are associated with negative affect, as negative affect can be both a specific trigger and a reason for smoking (Borland, 2014). Residual beliefs about the value of smoking have also been associated with long-term relapse (Dijkstra & Borland, 2003). It is possible that environmental factors are more important at this stage. Herd et al. (2009) found that number of smokers in the person’s social network is predictive of long-term relapse, but given that social norms toward smoking are now much less positive, environmental factors are unlikely to be the main explanation for the continuing high rates of relapse.
CONCLUSIONS
In conclusion, HSI and its two component measures are strong predictors of short-term relapse. Beyond the first week, they start to lose their predictive power, suggesting that aspects of nicotine dependence other than those related to habit strength may become more important in determining long-term quit maintenance, that is, beyond around 1 month. We should stop thinking of the HSI (or its components) as generic measures of dependence and begin to think of them as indicators of that aspect of dependence, which is associated with early relapse from quit attempts. Research is needed to identify determinants of later relapse and to ascertain whether differential associates of early and late relapse also apply to other addictions.
SUPPLEMENTARY MATERIAL
Supplementary Tables 1 and 2 can be found online at http://www.ntr.oxfordjournals.org.
FUNDING
The ITC Four-Country Survey is supported by multiple grants from Roswell Park Transdisciplinary Tobacco Use Research Center (R01 CA 100362, P50 CA111236) and also in part from Roswell Park Cancer Institute, Buffalo, New York (P01 CA138389), all funded by the National Cancer Institute of the United States, Robert Wood Johnson Foundation (045734), Canadian Institutes of Health Research (57897, 79551), National Health and Medical Research Council of Australia (265903, 450110, APP1005922), Cancer Research UK (C312/A3726), Canadian Tobacco Control Research Initiative (014578); Centre for Behavioural Research and Program Evaluation, National Cancer Institute of Canada/Canadian Cancer Society.
DECLARATION OF INTERESTS
All waves of the study have received ethical approval from the relevant institutional review or research ethics committee at Cancer Council Victoria (Australia), Roswell Park Cancer Institute (USA), University of Waterloo (Canada), and University of Strathclyde (UK).
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank members of the Data Management Core at the University of Waterloo for assistance in preparing the data for this analysis.
REFERENCES
- Baker T. B., Breslau N., Covey L., Shiffman S. (2012). DSM criteria for tobacco use disorder and tobacco withdrawal: A critique and proposed revisions for DSM-5. Addiction, 107, 263–275. 10.1111/j.1360-0443.2011.0357.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. B., Piper M. E., McCarthy D. E., Bolt D. M., Smith S. S., Kim S. Y. … Toll B. A. (2007). Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research, 9(Suppl. 4), S555–S570. 10.1080/ 14622200701673480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmford J., Borland R., Hammond D., Cummings K. M. (2011). Adherence to and reasons for premature discontinuation from stop-smoking medications: Data from the ITC Four-Country Survey. Nicotine & Tobacco Research, 13, 94–102. 10.1093/ntr/ntq215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C. J., An L. C., Kirch M., Guo H., Thomas J. L., Patten C. A. … West R. (2010). Failure to report attempts to quit smoking. Addictive Behaviors, 35, 900–904. 10.1016/j.addbeh.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Borland R. (2014). Understanding hard to maintain behaviour change: A dual-process approach. Oxford: Wiley-Blackwell [Google Scholar]
- Borland R., Partos T. R., Cummings K. M. (2012). Systematic biases in cross-sectional community studies may underestimate the effectiveness of stop-smoking medications. Nicotine & Tobacco Research, 14, 1483–1487. 10.1093/ntr/nts002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R., Yong H. H., O’Connor R. J., Hyland A., Thompson M. E. (2010). The reliability and predictive validity of the Heaviness of Smoking Index and its two components: Findings from the International Tobacco Control Four-Country study. Nicotine & Tobacco Research, 12(Suppl. 1), S45–S50. 10.1093/ntr/ntq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K., Stevens S., Perera R., Lancaster T. (2013). Pharmacological interventions for smoking cessation: An overview and network meta-analysis. The Cochrane Database of Systematic Reviews, 5, CD009329.10.1002/14651858.CD009329.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvoisier D. S., Etter J. F. (2010). Comparing the predictive validity of five cigarette dependence questionnaires. Drug and Alcohol Dependence, 107, 128–133. 10.1016/j.drugalcdep.2009.09.011 [DOI] [PubMed] [Google Scholar]
- de Leon J., Diaz F. J., Becoña E., Gurpegui M., Jurado D., Gonzalez-Pinto A. (2003). Exploring brief measures of nicotine dependence for epidemiological surveys. Addictive Behaviors, 28, 1481–1486. 10.1016/S0306-4603(02)00264-2 [DOI] [PubMed] [Google Scholar]
- Dijkstra A., Borland R. (2003). Residual outcome expectations and relapse in ex-smokers. Health Psychology, 22, 340–346. 10.1037/0278-6133.22.4.340 [DOI] [PubMed] [Google Scholar]
- Fidler J. A., Shahab L., West R. (2011). Strength of urges to smoke as a measure of severity of cigarette dependence: Comparison with the Fagerström Test for Nicotine Dependence and its components. Addiction, 106, 631–638. 10.1111/j.1360-0443.2010.03226.x [DOI] [PubMed] [Google Scholar]
- Fong G. T., Cummings K. M., Borland R., Hastings G., Hyland A., Giovino G. … Thompson M. E. (2006). The conceptual framework of the International Tobacco Control (ITC) Policy Evaluation project. Tobacco Control, 15(Suppl. 3), 3–11. 10.1136/tc.2005.015438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Rickert W., Robinson J. (1989). Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction, 84, 791–799. 10.1111/j.1360-0443.1989.tb03059.x [DOI] [PubMed] [Google Scholar]
- Herd N., Borland R. (2009). The natural history of quitting smoking: Findings from the International Tobacco Control (ITC) Four Country Survey. Addiction, 104, 2075–2087. 10.1111/j.1360-0443.2009.02731.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd N., Borland R., Hyland A. (2009). Predictors of smoking relapse by duration of abstinence: Findings from the International Tobacco Control (ITC) Four Country Survey. Addiction, 104, 2088–2099. 10.1111/j.1360-0443.2009.02732.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A., Borland R., Yong H. H., McNeill A., Fong G. T., O’Connor R. J., Cummings K. M. (2006). Individual level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four-Country Survey. Tobacco Control, 15(Suppl. 3), 83–94. 10.1136/tc.2005.013516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski L. T., Porter C. Q., Orleans C. T., Pope M. A., Heatherton T. (1994). Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug and Alcohol Dependence, 34, 211–216 [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Fiore M. C., McCarthy D. E., Baker T. B. (2002). Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction, 97, 1093–1108. 10.1046/j.1360-0443.2002.00216.x [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on biochemical verification (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4, 149–159. 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- Thompson M. E., Fong G. T., Hastings G., Boudreau C., Driezen P., Hyland A. … Laux F. L. (2006). The methodology of the International Tobacco Control (ITC) Four-Country Survey. Tobacco Control, 15(Suppl. 3), 12–18. 10.1136/tc.2005.013870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Nonnemaker J., Sherrill B., Gilsenan A. W., Coste F., West R. (2009). Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study. Addictive Behaviors, 34, 365–373. 10.1016/j.addbeh.2008.11.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.