Abstract
Introduction:
Despite decades of tobacco use decline among the general population in the United States, tobacco use among low-income populations continues to be a major public health concern. Smoking rates are higher among individuals with less than a high school education, those with no health insurance, and among individuals living below the federal poverty level. Despite these disparities, smoking cessation treatments for low-income populations have not been extensively tested. In the current study, the efficacy of 2 adjunctive smoking cessation interventions was evaluated among low-income smokers who were seen in a primary care setting.
Methods:
A total of 846 participants were randomly assigned either to motivational enhancement treatment plus brief physician advice and 8 weeks of nicotine replacement therapy (NRT) or to standard care, which consisted of brief physician advice and 8 weeks of NRT. Tobacco smoking abstinence was at 1, 2, 6, and 12 months following baseline.
Results:
The use of the nicotine patch, telephone counseling, and positive decisional balance were predictive of increased abstinence rates, and elevated stress levels and temptation to smoke in both social/habit and negative affect situations decreased abstinence rates across time. Analyses showed intervention effects on smoking temptations, length of patch use, and number of telephone contacts. Direct intervention effects on abstinence rates were not significant, after adjusting for model predictors and selection bias due to perirandomization attrition.
Conclusions:
Integrating therapeutic approaches that promote use of and adherence to medications for quitting smoking and that target stress management and reducing negative affect may enhance smoking cessation among low-income smokers.
BACKGROUND
Tobacco use is associated with approximately 438,000 deaths annually and is the leading cause of preventable morbidity, mortality, and health expense in the United States (CDC, 2012). The decline in tobacco use has slowed in recent years (declining from 20.9% in 2005 to 19.3% in 2010) and did not meet the Healthy People 2010 objective to reduce cigarette smoking among adults to ≤12% (CDC, 2011a). There is a broad gap between socioeconomic status (SES) groups with respect to tobacco use. Smoking prevalence remains highest among those with less than a high school education (28.4%), those with no health insurance (28.6%), and those living below the federal poverty level (27.7%; CDC, 2011a, 2011b, 2012).
In response to these disparities, public policy efforts have been made to increase state Medicaid insurance coverage for evidence-based tobacco cessation treatment (CDC, 2008). As of 2009, 46 states and the District of Columbia offer insurance coverage for tobacco cessation treatment to Medicaid recipients (CDC, 2010), although the degree of coverage for services varies widely and is typically underutilized (Coates et al., 2012). Interventions delivered in health care settings by physicians have the potential to reach a wide range of smokers, considering that more than 70% of smokers see their physician each year (Goldstein et al., 1998; Wadland, Stoffelmayr, & Ives, 2001). Moreover, such interventions can combine brief behavioral interventions with effective pharmacological agents such as nicotine replacement therapy (NRT) while addressing existing medical conditions and lifestyle-related factors (Wadland et al., 2001).
Physician-delivered smoking cessation interventions, even when brief, can be effective and can significantly increase smoking abstinence rates (Aveyard, Begh, Parsons & West, 2012). However, only 20% of smokers report that they have received smoking cessation assistance by their physician (Ferketich, Khan, & Wewers, 2006). One barrier often reported by physicians is the lack of motivation to quit smoking by patients. Despite the fact that over 60% of smokers report a desire to quit, most are unwilling to make a serious quit attempt within the next 6 months, and motivation is lowest among low-SES groups (Reid, Hammond, Boudreau, Fong, & Siahpush, 2010; Smit, Fidler, & West, 2011). Motivational enhancement (ME) interventions have been shown to be moderately effective in substance abuse–related treatment (Smedslund et al., 2011). Although meta-analytic reviews have found that, overall, ME interventions for smoking cessation are effective (Heckman, Egleston & Hofmann, 2010; Hettema & Hendricks, 2010) results vary widely depending on the population studied. For example, Persson and Hjalmarson (2006) found significantly higher quit rates at 12-month follow-up among diabetic smokers in a primary care setting, who were given an ME intervention compared with those given brief physician advice. Similarly, smokers given ME plus bupropion were more likely to be abstinent at both 6- and 12-month follow-ups compared with those given bupropion and brief physician advice (Soria, Legido, Escolano, López Yeste, & Montoya, 2006). However, among pregnant smokers and young parents, there is no evidence for ME’s effectiveness over other interventions (Lumley et al., 2009), and the empirical support for ME smoking cessation interventions with psychiatric populations (Baker et al., 2006), substance users (Haug, Svikis, & Diclemente, 2004; Stein et al., 2006), and general samples of primary care and hospital patients (Heckman et al., 2010) has not been established. To date, there are only a limited number of studies that have specifically focused on testing ME smoking cessation interventions for low-SES smokers (Okuyemi et al., 2007, 2013), and none that we were able to identify, which delivered ME treatment to low-income populations in primary health care centers.
The current study tested the relative efficacy of two adjunctive interventions among low-SES smokers (uninsured or Medicaid) seen in a primary care setting. The first intervention consisted of an 8-week prescription for the nicotine patch combined with brief physician advice, education in the use of the patch, and a brief follow-up telephone call with a health educator (standard treatment). The second intervention consisted of an 8-week prescription for the nicotine patch, brief physician advice, and an ME intervention delivered through counseling sessions that included behavioral skills training. We hypothesized that the ME intervention would increase cessation rates compared with the standard treatment condition.
METHODS
Adult smokers were recruited during routine health care visits at three hospital-based primary care clinics located in separate inner-city hospitals in southern New England. To be eligible for the study, patients had to be more than 18 years of age; a current, regular smoker (at least 10 cigarettes/day for the past 3 months); speak English or Spanish; be uninsured or a Medicaid recipient; and be available for follow-up. Participants did not have to agree to quit smoking to enroll. Individuals were excluded if they had medical conditions that precluded the use of the nicotine patch (e.g., unstable angina, uncontrolled hypertension, psoriasis); were currently using smokeless tobacco, NRT, or other smoking cessation treatment; or were pregnant or nursing.
To maximize enrollment of smokers with diverse levels of readiness to quit smoking, proactive recruitment strategies were employed: all smokers were identified by clinic personnel during registration when they checked in for their physician visit and were invited to participate in a study of smoking patterns and smoking cessation. Recruitment procedures emphasized that patients did not have to quit smoking, or even be interested in quitting smoking to enroll. The study Research Assistant (RA) screened interested individuals for eligibility and administered informed consent following procedures approved by the Miriam Hospital Institutional Review Board.
Immediately after providing consent, enrolled participants (n = 846) completed baseline surveys using laptop computers. Upon completion of the last question item, the computer used a random number program to assign participants at random to one of two treatment conditions: standard of care (SC) or ME. The SC intervention was designed to provide smoking cessation assistance following guidelines for the current best practices standard of care (Fiore, Hatsukami, & Baker, 2002). One-hour training sessions were held prior to the beginning of recruitment for all attending and resident physicians, as well as fellows and interns. These training sessions provided an introduction to the study, its purpose and procedures, educational materials concerning smoking cessation treatment guidelines from the Agency for Health Care Policy and Research (AHCPR; “Clinician’s Guide”), and training in applying the “five As” (Ask, Assess, Advise, Assist, Arrange follow-up). Thus, study protocol required physicians to implement the first four points, following a “four As” model for all patients enrolled in this study. The fifth “A,” Arrange follow-up, was handled by the study Health Educator as part of the intervention protocol. Thus, SC subjects were asked about their smoking status, assessed for nicotine dependence, and advised to quit smoking by their physician who also offered assistance with quitting (nicotine patches, self-help pamphlets and/or referral to the RI state quitline) to smokers who were interested in quitting.
Participants in the ME condition received all components of the SC intervention, plus a 45-min individual counseling session with study Health Educators, who were smoking cessation specialists fluent in both English and Spanish. This counseling session used motivational interviewing (MI) techniques (Miller & Rollnick, 1991). For participants who were ready to quit, the session also included behavioral skills training for smoking cessation. Participants in the ME intervention arm who decided to quit during this baseline visit were given two follow-up telephone counseling calls: one on their quit day and one call 2 weeks later. Those choosing not to quit were called 2 and 4 weeks later for follow-up counseling. The content of these calls focused on reviewing topics discussed during the in-person counseling session, the participant’s thoughts regarding quitting smoking, and a reevaluation of their readiness to quit. Participants who elected to quit smoking and set a quit date either at the in-person counseling session or during the follow-up telephone calls were provided with the nicotine patch, behavioral skills training, and two additional follow-up phone calls (on the quit date and 2 weeks later), following the same protocol as those participants who had chosen to quit during their baseline visit. The content of the follow-up calls for those electing to quit was to review progress of the current quit attempt, provide positive reinforcement of efforts to sustain quitting, and problem solve around barriers and issues arising during quitting.
ME interventionists were trained and supervised by licensed clinical psychologists. Ongoing fidelity was monitored through selected session observation and weekly clinical supervision. All counseling sessions were tape recorded, and 20% of tapes were selected at random for review by the study intervention coordinator, a PhD psychologist who was certified in MI. The number and duration of all counseling calls were logged by study counselors so that they could be included in analyses. Regular, weekly meetings were conducted to review the intervention procedures and results of counseling tape audits to enhance treatment fidelity. All participants were assessed at baseline and four follow-up assessments (1, 2, 6, and 12 months after baseline). Study RAs made a minimum of three attempts to reach participants by telephone to conduct follow-up assessments. Those not responding to phone calls were mailed reminder letters, and an additional three attempts were made the following week.
Measures
Participants completed questionnaires assessing sociodemographic information, smoking history, and psychological variables (described below). All assessment instruments, consent forms, self-help manuals, and other study materials were available in English and Spanish. To achieve linguistic and conceptual equivalence between Spanish and English versions of the assessment instruments, back-translation methods were used (Becerra & Shaw, 1988). English language instruments were translated into Spanish by a bilingual staff member and then translated back to English by another bilingual staff member who had not seen the original English instrument. Discrepancies in meaning between the two English versions were clarified or removed, with modifications made as necessary in either the English and/or the Spanish versions to arrive at equivalence.
Ethnicity and Acculturation
In addition to standard demographic questions assessing age, gender, marital status, and education level, participants were asked to identify their ethnic group, whether or not they were born in the United States (yes/no), and their degree of language fluency. Language fluency was assessed using a single question asking participants to describe how well they spoke English and Spanish (5-point scale: Only English, English better than Spanish, Both languages equally well, Spanish better than English, Only Spanish). Prior research in Hispanic and White populations has shown that language fluency is highly correlated with more extensive measures of acculturation (Kaplan, Nápoles-Springer, Stewart & Pérez-Stable, 2001) and has been used as an indicator of acculturation in large-scale epidemiological studies (e.g., Kerner, Breen, Tefft & Silsby, 1998). Therefore, language fluency was used to separate Hispanic subjects into a less-acculturated group (n = 138) that was exclusively or primarily Spanish speaking, and a highly acculturated group (n = 60) that was at least as fluent in English (highly acculturated Hispanics).
Smoking Variables
Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Self-reported tobacco use for the previous 7 days was assessed using the timeline followback (TLFB; Sobell & Sobell, 1992). The TLFB is a calendar-based interview that asks participants to recall the frequency of substance use. The TLFB has been used extensively in assessing the use of a variety of substances, as well as health behaviors (Sobell & Sobell, 1992). TLFB reports of tobacco abstinence were confirmed using expired carbon monoxide testing with a Bedfont MicroSmokerlyzer™ machine with ≥5 ppm as the cutoff indicating a positive smoking result (Benowitz, Bernert, Caraballo, Holiday, & Wang, 2009). Nicotine patch use was measured at each follow-up point through interview with the participant asking whether and how many of the study provided patches had been used since the previous contact, and whether the participant had purchased and used any other quit-smoking medications (including NRT) not provided by the study.
Motivation to quit smoking was assessed using the Contemplation Ladder (Biener & Abrams, 1991). Perceived benefits and barriers to smoking cessation were assessed using the Smoking Decisional Balance Scale (short form), a six-item measure of the perceived benefits (positive decisional balance) and perceived barriers (negative decisional balance) of smoking. The pros and cons subscales have high internal consistency with α = 0.88 and 0.89, respectively (Velicer, Diclemente, Rossi, & Prochaska, 1990). Self-efficacy for smoking cessation was assessed using the nine-item short form of the Smoking Temptations questionnaire (Velicer, DiClemente, Prochaska, & Brandenburg, 1985), which has demonstrated good validity and internal consistency (α: 0.80–0.90). It includes subscales assessing temptation to smoke when prompted by social, mood, or habit cues. Based on a factor analysis of the data at hand, we employed a three-item subscale measuring temptation to smoke in negative affect situations (α = 0.80) and a six-item subscale measuring temptation to smoke in social/habit situations (α = 0.84). Perceived vulnerability to smoking-related illness was assessed by summing four items asking participants (a) if they thought they had symptoms of an illness that is caused or was made worse by smoking (yes/no); (b) to rate their health relative to a nonsmoker their own age (1 = much worse to 5 = much better); (c) the extent to which their overall health has been affected by smoking (1 = not at all to 5 = very much); and (d) how much they believed that quitting smoking could improve their health (1 = not at all to 5 = very much). Depression symptoms were assessed using the 20-item Center for Epidemiologic Studies Depression Scale, which has demonstrated good reliability and validity (Radloff, 1977) and is available in both English and Spanish. The four-item Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983) was used to assess the degree to which participants perceived their environment and experiences as stressful. The scale correlates well with scores on life-event measures and has demonstrated adequate reliability. It was used because stress has been implicated in problems quitting smoking (Cohen & Lichtenstein, 1990).
Analytic Plan
Our primary outcome was biochemically verified 7-day point prevalence abstinence (p.p.a.) at each of the four follow-up points (1, 2, 6, and 12 months). We were primarily interested in differences in 7-day PPA rates by study condition (SC, ME), after adjusting for study dropout. Using a two-tailed α = 0.05, we had 80% power for estimating a 6% versus 12% abstinence rate among those given the minimal intervention and motivational intervention, respectively, based on n = 360 subjects per cell at 6-month follow-up. With a 15% projected attrition rate, this is translated into n = 423 subjects per arm at baseline.
Generalized estimating equation (GEE) models (Liang et al., 2002) were used to account for the repeated-measures aspect of our study. In particular, robust SEs based on a working independence correlation matrix were calculated in PROC GENMOD of SAS/STAT 9.2 (SAS Institute, 2010) to correct the naive SEs produced by logistic regression. GEE models tend to be sensitive to attrition bias, as they require that missingness at follow-up be completely at random (MCAR) in the nomenclature of Little and Rubin (1987). To minimize this bias, study participants were grouped into four strata according to their last follow-up visit, with intermittent missingness assumed to be MCAR. Separate models were fit to each group, and results subsequently combined using pattern-mixture methodology (Park & Lee, 1999).
A further complication arose from the presence of perirandomization attrition (refusal to follow through with follow-up assessment or intervention at any point in the project, after having successfully completed the baseline assessment and been randomized to a particular study arm). In contrast to postrandomization attrition (the more traditional notion of study dropout after some follow-up assessment and/or treatment participation), such study participants contributed no follow-up observations at all and could not be accommodated as a distinct group within a pattern mixture model. Rather, they induced selection bias that was dealt with using two-stage instrumental variable (IV) techniques popular in the econometrics literature (Heckman, 1979). These require that the hazard of perirandomization attrition be first estimated using logistic regression techniques applied to baseline data alone, and then used a covariate to de-bias the regression coefficients of the outcome model (Leigh, Ward, & Fries, 1993). The selection hazard (inverse Mills ratio) can be derived as the ratio of a probability density to its survivor function. Under a logit link, it equals the selection probability, simplifying interpretation of the results.
Regarding model choice, it should be noted that providing a parsimonious description of perirandomization attrition was not a primary study goal. Therefore, we chose to retain selection model predictors at a less stringent significance level than the customary 5% (D’Agostino, 1998). On the other hand, our impetus to fit as comprehensive a model as possible was tempered by the fact that covariate missingness limited the number of subjects for which the selection hazard could be estimated, thus reducing the effective sample size for analyzing the primary outcome.
When using a two-stage IV approach, one needs to adjust the SEs in the outcome model for the fact that the selection hazard is itself estimated with error. For cross-sectional outcomes, a procedure for inflating the SEs is available in Stata 13.0 (StataCorp, 2013). However, no extensions to longitudinal data are currently available, and we had to implement the IV approach using simulation. For all subjects with available baseline data, the subject-specific selection hazards were simulated 10,000 times from their joint sampling distribution and entered as covariates in the outcome model. The resulting CIs for the GEE regression coefficients were centered at their simulation average and have lengths that reflect both within- and between-simulation variability; convergence monitoring indicated that they are correct to two decimal places in the log–odds ratio scale.
RESULTS
Participant characteristics are summarized in Table 1. The sample was 68.7% (n = 581) female with a mean age of 39.6 (SD = 11.4). Racial/ethnic diversity was substantial, with 52.8% (n = 447) of participants identifying as White/European American, 23.4% (n = 198) identified as Latino/Hispanic, 13.1% (n = 111) as African American, and 10.6% (n = 90) identified as being of “Other” racial/ethnic backgrounds. The majority of participants were unemployed (75.9%; n = 204), 41.4% (n = 350) were single/never married, and 27.7% (n = 234) were divorced or separated.
Table 1.
Demographic Characteristics (N = 846)
| Variable | N | % |
|---|---|---|
| Gender | ||
| Male | 264 | 31.2 |
| Female | 581 | 68.7 |
| Ethnicity | ||
| European American | 447 | 52.8 |
| Hispanic/Latino | 198 | 23.4 |
| African American | 111 | 13.1 |
| Other | 90 | 10.6 |
| Age (M/SD) | 39.6 | 11.4 |
| Employment status | ||
| Unemployed | 642 | 75.9 |
| Employed | 204 | 24.1 |
| Marital status | ||
| Single | 350 | 41.4 |
| Divorced/separated | 234 | 27.7 |
| Married | 160 | 18.9 |
| Living with partner | 59 | 7 |
| Widowed | 42 | 5 |
| Condition | ||
| Motivational | 440 | 52 |
| Standard care | 406 | 48 |
Dropout and Missing Data
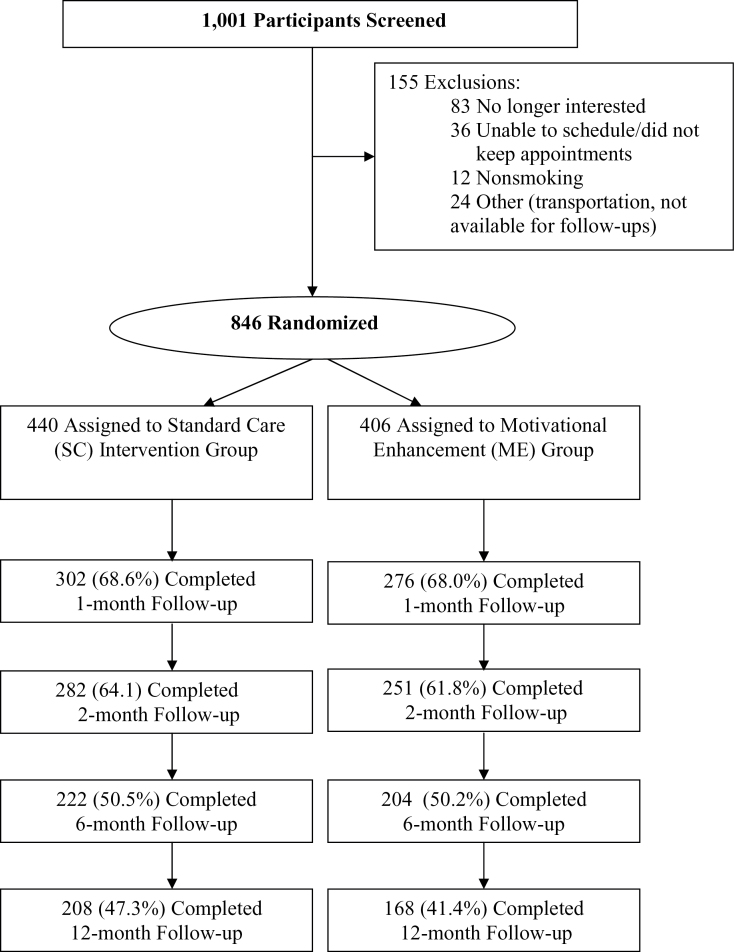
Participants were randomized to the ME (n = 406) or SC (n = 440) arms of the study. Dropout rates by last study visit attended are shown in Table 2 and in the Consort Diagram (Figure 1). It appears that 16% of subjects in each study arm (66 from SC and 71 from ME) dropped out immediately after their baseline visit. Cumulative dropout by month 12 reached 58.6% in SC (n = 238) and 52.7% in ME (n = 232). Results highlight the need to take both perirandomization and postrandomization attrition into account when modeling abstinence rates.
Table 2.
Abstinence Rates Over Time by Dropout Pattern Within Study Arm
| Study arm | Last visit attended | Abstinence rate (%) | |||||
|---|---|---|---|---|---|---|---|
| N | % | 1 month | 2 months | 6 months | 12 months | ||
| SC (N = 440) | Baseline | 71 | 16.1 | ||||
| 1 month | 30 | 6.8 | 40 | ||||
| 2 months | 68 | 15.5 | 38 | 33 | |||
| 6 months | 63 | 14.3 | 37 | 37 | 20 | ||
| 12 month | 208 | 47.3 | 39 | 40 | 30 | 28 | |
| ME (N = 406) | Baseline | 66 | 16.3 | ||||
| 1 month | 38 | 9.4 | 37 | ||||
| 2 months | 60 | 14.8 | 35 | 32 | |||
| 6 months | 74 | 18.2 | 42 | 37 | 24 | ||
| 12 months | 168 | 41.4 | 38 | 45 | 33 | 29 | |
Note. Values in italics show study completers. ME = motivational enhancement; SC = standard care.
Figure 1.
Consort diagram: participant flow.
Logistic regression analyses indicated that racial/ethnic background, treatment site, motivational readiness to quit smoking, size and composition of the household, marital and employment status, temptation to smoke in negative affect situations, and previous use of NRT were potentially significant predictors of perirandomization attrition. Study arm (ME vs. SC) was included by default but failed to attain statistical significance. The final selection model is shown in Table 3, with covariates standardized so that the baseline odds of perirandomization attrition (odds ratio [OR] = 1:10, 95% CI = 1:20–1:5) apply to a reference group of White participants in SC recruited from the Miriam Hospital, who were in either full-time or part-time employment, had not been widowed, and came from a household of median size comprising two adults and a single child. They had no plans of quitting within the next 30 days, had a negative affect score equal to the sample median, and had not previously used a nicotine patch. Negative affect was scaled so that its regression coefficient captures the effect of an increase from the sample median to the 3rd quartile.
Table 3.
Logistic Regression Model for Perirandomization Attrition
| Model terms | OR | |||
|---|---|---|---|---|
| PE | LCL | UCL | p value | |
| Odds for referent group | 0.10 | 0.05 | 0.20 | <.001 |
| Rhode Island Hospital | 1.99 | 1.15 | 3.45 | .014 |
| St. Joseph’s Hospital | 1.72 | 0.93 | 3.15 | .077 |
| Less-acculturated Hispanic/Latino | 1.45 | 0.84 | 2.51 | .194 |
| Highly acculturated Hispanic/Latino | 2.41 | 1.24 | 4.69 | .009 |
| African American | 1.14 | 0.61 | 2.13 | .675 |
| Other | 1.06 | 0.58 | 1.95 | .834 |
| Plans to quit within 30 days | 1.57 | 1.06 | 2.32 | .027 |
| Number of minors in household | 1.13 | 0.98 | 1.29 | .085 |
| Number of other adults in household | 1.09 | 0.97 | 1.23 | .162 |
| Motivational enhancement group | 0.97 | 0.66 | 1.44 | .889 |
| Temptation to smoke (negative affect) | 0.84 | 0.69 | 1.03 | .080 |
| Unemployed | 0.82 | 0.52 | 1.29 | .390 |
| Previous use of patch | 0.47 | 0.23 | 0.95 | .033 |
| Widowed | 0.25 | 0.06 | 1.04 | .054 |
Note. The reference group used for the regression model for Table 3: Non-Hispanic White participants in SC who were in either full or part-time employment, had not been widowed, and came from a household of median size comprising two adults and a single child. They had no plans of quitting within the next 30 days, had a negative affect score equal to the sample median, and had not previously used a nicotine patch. OR = odds ratio; PE = point estimate; LCL = 95% lower confidence limit; UCL = 95% upper confidence limit. Values in italics are the odds of experiencing the outcome of the referent group.
Smoking Outcomes
Due to missing covariates at baseline, our selection model allowed us to calculate perirandomization attrition hazards for only 810 of our 846 subjects. A GEE pattern mixture model for 7-day p.p.a. at follow-up that controlled for selection bias found no statistically significant differences in the longitudinal trajectories of abstinence rates, when stratifying these subjects by last follow-up visit attended. Therefore, we decided to treat all postrandomization missingness as completely at random and estimated a single GEE model for smoking abstinence across all 810 subjects, adjusted for perirandomization attrition alone.
Since the overall aim of the study was to evaluate the effect of ME on 7-day p.p.a. over and above physician advice combined with NRT, we entered self-reported patch use in the 8 weeks following the setting of a target quit date as a covariate, centered at the SC end-of-treatment median of 4 weeks. In addition to patch use, we adjusted for differences in the number of telephone contacts received, centered at the SC end-of-treatment median of two calls. Therefore, our reference group is composed of SC subjects who reported using a 4-week supply of patches (at 1-month follow-up) and received two telephone calls, but no ME therapy; the latter was designated as the “Treatment” effect and became the narrowly circumscribed target of our estimation procedure. Further, the selection hazard rate was standardized so that its coefficient estimates the effect of increasing the propensity for early dropout from zero (subjects certain to attend at least the first follow-up visit postrandomization) to its sample median of 0.15, that is, the characteristic of study participants which at baseline were predicted to have 15% probability of dropping out immediately and contributing no follow-up measurements whatsoever. Finally, race/ethnicity was also entered into the model, with highly and less acculturated Hispanics retained as potentially distinct groups.
With the effect of baseline variables mostly subsumed by the selection hazard term, the remaining covariates were predominantly time varying in nature: cumulative self-reported patch use, cumulative number of phone contacts, positive decisional balance, perceived stress, and temptation to smoke in both social/habit and negative affect situations. To aid interpretation, we chose to standardize these variables so that their regression coefficients capture the effect of an increase from their baseline median to their baseline 3rd quartile: positive decisional balance (median = 10, 3rd quartile = 12), social/habit (median = 13, 3rd quartile = 15), and negative affect (median = 23, 3rd quartile = 26). Perceived stress (median = 8, 3rd quartile = 10) showed a threshold effect, that is, a linear effect above the median and no effect at low levels; therefore, only its positive part was entered in the regression, with low values replaced by zero.
Results are shown in Table 4, with the GEE CIs and p values corrected for estimation uncertainty via simulation. As seen in Table 4, the odds of abstinence in our reference group were approximately reduced by half at each of the first three follow-up visits but stabilized at 6 months. Converting odds to abstinence rates, we find that the latter declined from 22% at the 1-month follow-up (95% CI = 0.12–0.65) to 12% at the 2-month follow-up (95% CI = 0.05–0.24). After dropping further to 7% at the 6-month follow-up (95% CI = 0.03–0.17), they stabilized at that level through the 12-month follow-up (95% CI = 0.03–0.15).
Table 4.
Logistic Regression Model for 7-Day Point Prevalence Abstinence: Adjusted for Covariates and Corrected for Selection Bias Due to Perirandomization Attrition
| Model terms | OR | |||
|---|---|---|---|---|
| PE | LCL | UCL | p value | |
| Odds for referent group | ||||
| 1 month | 0.28 | 0.12 | 0.65 | .003 |
| 2 months | 0.13 | 0.05 | 0.31 | <.001 |
| 6 months | 0.07 | 0.03 | 0.15 | <.001 |
| 12 months | 0.07 | 0.03 | 0.17 | <.001 |
| Less-acculturated Latino/Hispanic | 1.99 | 0.95 | 4.20 | .070 |
| Highly acculturated Latino/Hispanic | 2.01 | 0.80 | 5.06 | .139 |
| Patch use (weeks): 1 m | 1.70 | 1.29 | 2.24 | <.001 |
| Patch use (weeks): 2 m | 1.58 | 1.33 | 1.89 | <.001 |
| Patch use (weeks): 6 m | 1.19 | 1.01 | 1.39 | .042 |
| Patch use (weeks):12 m | 1.14 | 0.95 | 1.36 | .180 |
| Number of telephone contacts | 1.26 | 0.96 | 1.66 | .087 |
| Positive decisional balance | 1.19 | 1.01 | 1.39 | .036 |
| Perceived Stress Scale > 8 | 0.72 | 0.52 | 0.99 | .045 |
| Temptation to smoke (positive affect/habit) | 0.83 | 0.71 | 0.97 | .021 |
| Temptation to smoke (negative affect) | 0.47 | 0.40 | 0.55 | <.001 |
| Motivational enhancement group | 0.97 | 0.36 | 2.64 | .952 |
| Early dropout hazard | 0.72 | 0.37 | 1.40 | .322 |
| Early dropout hazard: motivational enhancement group | 0.68 | 0.31 | 1.48 | .327 |
Note. The reference group used for the regression model for Table 4: Non-Hispanic White or African American subjects in SC who reported using a 4-week supply of patches and received two telephone calls by 1-month follow-up. They reported median values at baseline in all psychological measures included in the model and had a baseline covariate profile associated with a 15% chance of dropping out immediately after their baseline visit. OR = odds ratio; PE = point estimate; LCL = 95% lower confidence limit; UCL = 95% upper confidence limit. Values in italics are the odds of experiencing the outcome of the referent group.
Increased patch use during the 8 weeks following setting a target quit date raised the odds of abstinence at both the 1-month (OR = 1.70, 95% CI = 1.29–2.24) and 2-month follow-ups (OR = 1.58, 95% CI = 1.33–1.89), but its effect dissipated over time, weakening during the maintenance period from a borderline significant result at 6 months (OR = 1.19, 95% CI = 1.01–1.39) to a still positive but nonsignificant result at 12 months (OR = 1.14, 95% CI = 0.95–1.36). In contrast, the effect of an additional telephone contact remained constant throughout the study period, increasing the odds of abstinence by a quarter (OR = 1.26, 95% CI = 0.96–1.66), a clinically significant effect size that attained only borderline statistical significance (p = .09). One-quartile increases in positive decisional balance raised the odds of abstinence by a fifth (OR = 1.19, 95% CI = 1.01–1.39) at all follow-up points. Increases in smoking temptation were even stronger predictors of continued smoking, with one-quartile increases in negative affect situations more than halving the odds of abstinence (OR = 0.47, 95% CI = 0.40–0.55), and one-quartile increases in social/habit situations reducing them by a sixth (OR = 0.83, 95% CI = 0.71–0.97). Finally, high perceived stress levels at follow-up (10 units on the Cohen scale) reduced the odds of abstinence by about a quarter (OR = 0.72, 95% CI = 0.52–0.99).
Increases in the early dropout hazard from 0 (typical of subjects deemed certain to attend the first follow-up) to the sample median of 0.15 (typical of subjects estimated to have been 15% likely to drop out immediately randomization, who nevertheless did end up attending the first follow-up) adversely affected smoking outcomes, reducing the odds of abstinence by 28% (OR = 0.72, 95% CI = 0.37–1.40) among SC subjects. Although not statistically significant (p = .32), inclusion of the hazard rate as a covariate allows one to calculate the marginal benefit from adding a ME component to a standard nicotine replacement treatment regimen for individuals judged certain to attend at least one follow-up visit and hence to receive a positive dose of ME if randomized to the ME group: subjects with estimated zero hazard of early dropout apparently derived no direct benefit from the intervention, after adjusting for all other covariates in the model (OR = 0.97, 95% CI = 0.36–2.64).
However, ME subjects appeared to benefit from the intervention indirectly via its effects on psychological constructs predictive of abstinence, as well as on treatment dose received. In particular, they experienced larger decreases than SC subjects in smoking temptations in both social/habit (p = .029) and negative affect (p = .021) situations, although these differences dissipated past the 1-month follow-up. They also completed more counseling calls during the intervention phase (median: ME = 3 vs. SC = 2, p < .0001) and reported more weeks of patch use (median: ME = 4 vs. SC = 3) at both the 6-month (p = .032) and 12-month (p = .083) follow-ups.
DISCUSSION
This study sought to test the efficacy of two smoking cessation interventions (ME vs. SC) among low-SES smokers (uninsured or Medicaid) seen in a primary care setting. Study findings indicated no effects of the intervention on psychological predictors of abstinence, other than transient effects on smoking self-efficacy at the 1-month follow-up that dissipated over time. This finding of limited effectiveness of ME as a component of smoking interventions is consistent with a meta-analysis of clinical trials that used adaptations of MI and found low efficacy of the MI approach for smoking in the general population (Burke, Arkowitz, & Menchola, 2003). Additionally, our findings are consistent with the work of Okuyemi et al. (2007), who also tested a motivational focused smoking cessation trial conducted in low-income housing developments (83% African American) and failed to find the addition of a MI component to NRT effective for smoking cessation (Okuyemi et al., 2007, 2013).
Findings from the current study highlight the importance of several factors relating to tobacco cessation in a sample of low-SES smokers. In the current study, ethnic and racial minority participants had a significantly higher level of attrition compared with nonminority individuals. Moreover, highly acculturated Hispanics, in particular, had the highest levels of study dropout. These findings suggest a need for developing innovative retention strategies and treatment interventions that specifically target and address the needs of ethnically diverse individuals involved in smoking cessation treatment. Such strategies and interventions may be enhanced by accounting for the level of acculturation, as well as for stress associated with acculturation. Smoking in response to negative affect was associated with treatment retention; however, it was also predictive of continued smoking at follow-up. Previous studies have also noted the relationship between negative affective states, such as depression, and successful tobacco cessation (Benjet, Wagner, Borges, & Medina-Mora, 2004; Berlin & Covey, 2006; Blalock, Robinson, Wetter, & Cinciripini, 2006; Catley et al., 2005). Integrating psychotherapeutic approaches that are designed to manage and decrease negative affect may enhance smoking cessation interventions and improve treatment retention and outcomes. In the current study, providing techniques for managing stress was included in the behavioral skills component of the ME intervention; however, a more pronounced and targeted emphasis may be required to fully address the role of negative affective states in smoking cessation. Patients planning to quit smoking should be screened for depressive symptoms and if indicated, referred to specialists for additional therapy. The use of quit-smoking medications, particularly those that have an antidepressant effect (i.e., Zyban) should also be considered.
In this study, greater use of the nicotine patch significantly increased the odds of abstinence at both the 1-month and 2-month follow-up assessments. However, the protective effect of extended patch use in the 8-week period following the choice of a target quit date was attenuated at the 6-month follow-up and did not significantly raise the odds of abstinence at the 12-month follow-up. This finding indicates a weakening of the relationship between increased patch use during treatment and abstinence during the maintenance period. It may be that patch use is a more critical predictor of abstinence earlier in the quitting process when withdrawal and craving-related symptoms are more prominent. During the maintenance period, behavioral relapse prevention skills may become a more critical factor in maintaining abstinence. Alternatively, this may provide a strong rationale for the continued use of nicotine replacement for prolonged periods following initial cessation, in addition to the use of behavioral methods, to help prevent relapse. Future studies can examine the impact of including behavioral skill training components that specifically target the maintenance period and take into account the extended trajectory of the quit process.
Low-income smokers present numerous challenges for offering and implementing smoking cessation interventions. Practical barriers include cost and access to health care services in settings that are also able to provide smoking cessation aids, counseling, and continuity of care. These smokers are often challenged on a daily basis to find and sustain employment and stable housing arrangements, which compete with other activities and priorities. It is not clear, however, that low-income smokers are less motivated to quit smoking compared with those who are less socioeconomically disadvantaged (Okuyeme et al., 2013). Many low-income smokers, though, suffer from additional burdens related to depression, alcohol, and substance abuse, which may complicate seeking help for smoking cessation and adhering to treatment. Access to comprehensive health care settings, which address multiple health care needs and which integrate smoking cessation services, is required to more completely address this problem. There are several limitations in the current study. Women were over-represented in our sample; therefore, it is unclear whether findings would have been different if a more balanced distribution of genders had been recruited. This study also experienced significant attrition after baseline, despite multiple attempts to reach participants by phone and mail. Although our analytic strategy accounted for missing data, high levels of study attrition reduced the precision of our estimates at the 6-month and 12-month follow-ups. Dropouts were not selective, insofar as there was no differential dropout according to treatment condition. We are not aware of other studies that encountered similar issues with perirandomization attrition (16.3% for SC; 16.1% for ME) as we found in the present study. Thus, we believe our analytic strategy is among the first to have been utilized to address this issue. Our 12-month postrandomization attrition rates (an additional 42.4% for SC; 36.6% for ME) were also higher than is typically encountered in clinical smoking cessation studies; however, we attribute this in large part to high residential mobility in this low-income primary care population over the course of an entire year. The study by Okuyemi et al. (2013) in a homeless population achieved follow-up rates between 72% and 78% at 6 months, which is higher than we observed (60%–62%). However, they did not follow participants out to 12 months. Despite limitations, this study provides findings that continue to be relevant for interventions among low-SES smokers, given recent public policy initiatives aimed at increasing Medicaid coverage for tobacco cessation.
CONCLUSIONS
This study highlights the importance of a number of pretreatment variables affecting study retention, including acculturation status. Accounting for its transient effects on smoking self-efficacy, the addition of an ME component to standard care for smoking cessation did not substantially improve other potential psychological predictors of abstinence at follow-up. It may be that brief motivational interventions need additional telephone or in-person contacts to be effective. Alternatively, individuals interested in joining this study may have already been motivated to quit, and additional motivational intervention was not poised to be effective above and beyond the impact of nicotine replacement medications. Although studies have shown motivational interventions are effective for helping those in earlier stages of readiness (e.g., contemplation) move toward readiness to quit, over 90% of the participants in this study were already in the preparation stage (ready to quit in next 30 days).
The two-stage IV approach used in this study helped to overcome problems associated with perirandomization attrition, a common problem in interventions targeting low-income and hospital-recruited populations (e.g., Neuner et al., 2009; Okuyemi et al., 2013). Finally, the methods used in this study may hold promise for research applications that utilize low-contact methods.
FUNDING
This work was supported by the National Institutes of Health, National Institute on Drug Abuse (R01DA010860) to Dr. Niaura.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
BCB, RN, and DBA developed the study design. BCB, RN, DBA, PJS, MF, MDS, and MMA implemented the study, recruited participants, delivered the intervention, and assisted with data organization. GDP conducted the statistical analyses. MAD, GDP, and RN were the primary writers of the manuscript. All authors were involved in editing the manuscript.
REFERENCES
- Aveyard P., Begh R., Parsons A., West R. (2012). Brief opportunistic smoking cessation interventions: A systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction (Abingdon, England), 107, 1066–1073. 10.111/j/1360-0443.2011.03770.x [DOI] [PubMed] [Google Scholar]
- Baker A., Richmond R., Haile M., Lewin T. J., Carr V. J., Taylor R. L. … Wilhelm K. (2006). A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. American Journal of Psychiatry, 163, 1934–1942. 10.117/appi.ajp.163.11.1934 [DOI] [PubMed] [Google Scholar]
- Becerra R., Shaw D. (1988). The Hispanic elderly: A research reference guide. New York: University Press [Google Scholar]
- Benjet C., Wagner F. A., Borges G. G., Medina-Mora M. E. (2004). The relationship of tobacco smoking with depressive symptomatology in the Third Mexican National Addictions Survey. Psychological Medicine, 34, 881–888. 10.1017/S0033291703001600 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Bernert J. T., Caraballo R. S., Holiday D. B., Wang J. (2009). Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. American Journal of Epidemiology, 169, 236–248. 10.1093/aje/kwp215 [DOI] [PubMed] [Google Scholar]
- Berlin I., Covey L. S. (2006). Pre-cessation depressive mood predicts failure to quit smoking: The role of coping and personality traits. Addiction, 101, 1814–1821. 10.111/j.1360-0443.2006.01616.x [DOI] [PubMed] [Google Scholar]
- Biener L., Abrams D. B. (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10, 360–365. 10.1037/0278-6133.10.5.360 [DOI] [PubMed] [Google Scholar]
- Blalock J. A., Robinson J. D., Wetter D. W., Cinciripini P. M. (2006). Relationship of DSM-IV-based depressive disorders to smoking cessation and smoking reduction in pregnant smokers. American Journal on Addictions, 15, 268–277. 10.1080/10550490600754309 [DOI] [PubMed] [Google Scholar]
- Burke B. L., Arkowitz H., Menchola M. (2003). The efficacy of motivational interviewing: A meta-analysis of controlled clinical trials. Journal of Consulting and Clinical Psychology, 71, 843–861. 10.1037/0022-006X.71.5.843 [DOI] [PubMed] [Google Scholar]
- Catley D., Harris K. J., Okuyemi K. S., Mayo M. S., Pankey E., Ahluwalia J. S. (2005). The influence of depressive symptoms on smoking cessation among African Americans in a randomized trial of bupropion. Nicotine & Tobacco Research, 7, 859–870. 10.1080/14622200500330118 [DOI] [PubMed] [Google Scholar]
- CDC (2008). State medicaid coverage for tobacco-dependence treatments: United States, 2006. Morbidity and Mortality Weekly Report, 57, 177–122 [PubMed] [Google Scholar]
- CDC (2010). State medicaid coverage for tobacco-dependence treatments—United States, 2009. Morbidity and Mortality Weekly Report, 59, 1340–1343 [PubMed] [Google Scholar]
- CDC (2011a). Current cigarette smoking prevalence among working adults—United States, 2004–2010. Morbidity and Mortality Weekly Report, 60, 1305–1309 [PubMed] [Google Scholar]
- CDC (2011b). Vital signs: Current cigarette smoking among adults aged ≥18 years—United States, 2005–2010. Morbidity and Mortality Weekly Report, 60, 1207–1212 [PubMed] [Google Scholar]
- CDC (2012). Current cigarette smoking among adults—United States 2011. Morbidity and Mortality Weekly Report, 61, 889–894 [PubMed] [Google Scholar]
- Coates R. J., Ogden L., Monroe J. A., Buehler J., Yoon P. W., Collins J. L. Centers for Disease Control and Prevention (CDC) (2012). Conclusions and future directions for periodic reporting on the use of adult clinical preventive services of public health priority–United States. Morbidity and Mortality Weekly Report, 61, 73–78 [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. 10.2307/2136404 [PubMed] [Google Scholar]
- Cohen S., Lichtenstein E. (1990). Partner behaviors that support quitting smoking. Journal of Consulting and Clinical Psychology, 58, 304–309. 10.1037/0022- 006X.58.3.304 [DOI] [PubMed] [Google Scholar]
- D’Agostino R. B. (1998). Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in Medicine, 17, 2265–2281 [DOI] [PubMed] [Google Scholar]
- Ferketich A. K., Khan Y., Wewers M. E. (2006). Are physicians asking about tobacco use and assisting with cessation? Results from the 2001-2004 national ambulatory medical care survey (NAMCS). Preventive Medicine, 43, 472–476. 10.1016/j.ypmed.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Fiore M. C., Hatsukami D. K., Baker T. B. (2002). Effective tobacco dependence treatment. Journal of the American Medical Association, 288, 1768–1771. 10.1001/jama.288.14.1768 [DOI] [PubMed] [Google Scholar]
- Goldstein M. G., DePue J. D., Monroe A. D., Lessne C. W., Rakowski W., Prokhorov A. … Dube C. E. (1998). A population-based survey of physician smoking cessation counseling practices. Preventive Medicine, 27(Pt 1), 720–729. 10.1006/pmed.1998.0350 [DOI] [PubMed] [Google Scholar]
- Haug N. A., Svikis D. S., Diclemente C. (2004). Motivational enhancement therapy for nicotine dependence in methadone-maintained pregnant women. Psychology of Addictive Behaviors, 18, 289–292 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. 10.111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Heckman C. J., Egleston B. L., Hofmann M. T. (2010). Efficacy of motivational interviewing for smoking cessation: A systematic review and meta-analysis. Tobacco Control, 19, 410–416. 10.1136/tc.2009.033175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. J. (1979). Sample selection bias as specification error. Econometrica, 47, 153–161 [Google Scholar]
- Hettema J. E., Hendricks P. S. (2010). Motivational interviewing for smoking cessation: A meta-analytic review. Journal of Consulting and Clinical Psychology, 78, 868–884. 10.1037/a0021498 [DOI] [PubMed] [Google Scholar]
- Kaplan C. P., Nápoles-Springer A., Stewart S. L., Pérez-Stable E. J. (2001). Smoking acquisition among adolescents and young Latinas: The role of socioenvironmental and personal factors. Addictive Behaviors, 26, 531–550. 10.1016/S0306-4603(00)00143-X [DOI] [PubMed] [Google Scholar]
- Kerner J. F., Breen N., Tefft M. C., Silsby J. (1998). Tobacco use among multi-ethnic Latino populations. Ethnicity & Disease, 8, 167–183 [PubMed] [Google Scholar]
- Leigh J. P., Ward M. M., Fries J. F. (1993). Reducing attrition bias with an instrumental variable in a regression model: Results from a panel of rheumatoid arthritis patients. Statistics in Medicine, 12, 1005–1018 doi:10.1002/sim.4780121102 [DOI] [PubMed] [Google Scholar]
- Liang K. Y., Heagerty P., Zeger S. L., Diggle P. J. (2002). Analysis of longitudinal data. Oxford, UK: Clarendon Press [Google Scholar]
- Little R. A., Rubin D. B. (1987). Statistical analysis with missing data. New York, NY: Wiley [Google Scholar]
- Lumley J., Chamberlain C., Dowswell T., Oliver S., Oakley L., Watson L. (2009). Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews, 3. 10.1002/14651858.CD001055.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. R., Rollnick S. (1991). Motivational interviewing: Preparing people to change addictive behavior. New York: Guilford Press [Google Scholar]
- Neuner B., Weiss-Gerlach E., Miller P., Martus P., Hesse D., Spies C. (2009). Emergency department-initiated tobacco control: A randomised controlled trial in an inner city university hospital. Tobacco Control, 18, 283–293 doi:10.1136/tc.2008.028753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi K. S., Goldade K., Whembolua G. L., Thomas J. L., Eischen S., Sewali B. … Des Jarlais D. (2013). Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: A randomized controlled trial. Addiction (Abingdon, England), 108, 1136–1144. 10.1111/add.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi K. S., James A. S., Mayo M. S., Nollen N., Catley D., Choi W. S., Ahluwalia J. S. (2007). Pathways to health: A cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low-income housing. Health Education & Behavior, 34, 43–54 doi:10.1177/1090198106288046 [DOI] [PubMed] [Google Scholar]
- Park T., Lee S. Y. (1999). Simple pattern-mixture models for longitudinal data with missing observations: Analysis of urinary incontinence data. Statistics in Medicine, 18, 2933–2941. 10.1002/(SICI)1097-0258(19991115)18:21<2933::AID-SIM233>3.0.CO;2-X [DOI] [PubMed] [Google Scholar]
- Persson L. G., Hjalmarson A. (2006). Smoking cessation in patients with diabetes mellitus: Results from a controlled study of an intervention programme in primary healthcare in Sweden. Scandinavian Journal of Primary Health Care, 24, 75–80 doi:10.1080/02813430500439395 [DOI] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychosocial Measurement, 1, 385–401. 10.1177/014662167700100306 [Google Scholar]
- Reid J. L., Hammond D., Boudreau C., Fong G. T., Siahpush M. ITC Collaboration (2010). Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four western countries: Findings from the International Tobacco Control Four Country Survey. Nicotine & Tobacco Research, 12, S20–S33. 10.1093/ntr/ntq051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute (2010). SAS/STAT 9.2 User’s Guide. Cary, NC: SAS Institute Inc [Google Scholar]
- Smedslund G., Berg R. C., Hammerstrøm K. T., Steiro A., Leiknes K. A., Dahl H. M., Karlsen K. (2011). Motivational interviewing for substance abuse. Cochrane Database of Systematic Reviews, 5. 10.1002/14651858.CD008063.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit E. S., Fidler J. A., West R. (2011). The role of desire, duty and intention in predicting attempts to quit smoking. Addiction (Abingdon, England), 106, 844–851. 10.111/j.1360-0443.2010.03317.x [DOI] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B. (1992). Timeline follow-back: A technique for assessing self reported alcohol consumption. In Litten R. Z., Allen J. (Eds.), Measuring alcohol consumption: Psychosocial and biological methods (pp. 41–72). Princeton, New Jersey: Humana Press [Google Scholar]
- Soria R., Legido A., Escolano C., López Yeste A., Montoya J. (2006). A randomised controlled trial of motivational interviewing for smoking cessation. The British Journal of General Practice, 56, 768–774 [PMC free article] [PubMed] [Google Scholar]
- StataCorp (2013). Statistical Software: Release 13.0. College Station, TX: Stata Corporation [Google Scholar]
- Stein M. D., Weinstock M. C., Herman D. S., Anderson B. J., Anthony J. L., Niaura R. (2006). A smoking cessation intervention for the methadone-maintained. Addiction (Abingdon, England), 101, 599–607. 10.111/j.1360-0443.2006.01406.x [DOI] [PubMed] [Google Scholar]
- Velicer W. F., DiClemente C. C., Prochaska J. O., Brandenburg N. (1985). Decisional balance measure for assessing and predicting smoking status. Journal of Personality and Social Psychology, 48, 1279–1289. 10.1037/0022-3514.48.5.1279 [DOI] [PubMed] [Google Scholar]
- Velicer W. F., Diclemente C. C., Rossi J. S., Prochaska J. O. (1990). Relapse situations and self-efficacy: An integrative model. Addictive behaviors, 15, 271–283. 10.1016/0306-4603(90)90070-E [DOI] [PubMed] [Google Scholar]
- Wadland W. C., Stoffelmayr B., Ives K. (2001). Enhancing smoking cessation of low-income smokers in managed care. Journal of Family Practice, 50, 138–144 [PubMed] [Google Scholar]