Abstract
Introduction:
Smokers with posttraumatic stress disorder (PTSD) tend to lapse more quickly following a quit attempt, which might be explained by changes in PTSD symptoms during a quit attempt. The present study examines changes in PTSD symptoms, negative affect, and craving before and during a quit attempt.
Methods:
Participants in this study were 52 smokers with PTSD who completed random-alarm ecological momentary assessments of PTSD symptoms, negative affect, cigarette craving, and smoking behavior throughout a prequit phase of ad hoc smoking, a phase of abstinence from smoking, and a postlapse phase.
Results:
Relative to the prequit phase, the abstinent phase was marked by decreases in PTSD reexperiencing, avoidance, and numbing clusters (ps ≤ .01). The odds of PTSD symptom or negative affect variability from one reading in the ecological momentary assessment (EMA)to the next reading was decreased in PTSD reexperiencing, avoidance, and numbing clusters (ps ≤ .02). Smoking cravings were also mildly decreased in the abstinent and postlapse phases (ps < .01), although some cravings in both phases were rated at the maximum intensity. Increased craving was predicted by the previous EMA reading of PTSD symptoms.
Conclusions:
Results suggested that smoking abstinence is not associated with exacerbation of PTSD symptoms, but PTSD symptoms during abstinence were related to craving levels during the quit attempt.
INTRODUCTION
Based on U.S. population estimates and rates of smoking among individuals with posttraumatic stress disorder (PTSD; Fu et al., 2007; Kessler, Chiu, Demler, & Walters, 2005), nearly one in five (20%) current U.S. smokers have lifetime PTSD. Individuals with PTSD are more likely than those without PTSD to smoke (Lasser et al., 2000), especially smoke heavily (Beckham et al., 1997). Moreover, smokers with PTSD making a quit attempt tend to lapse more quickly than smokers without PTSD (Beckham et al., 2013). Ecological momentary assessment (EMA) research in smokers with PTSD has identified psychiatric symptoms as antecedents of smoking (Beckham et al., 2005, 2008) and triggers of smoking lapse and relapse (Beckham et al., 2005, 2007, 2008). Given the central role of PTSD symptoms in maintaining smoking behavior, it is worthwhile to examine how these symptoms change during the smoking cessation process.
While longitudinal data suggest that individuals who quit smoking experience improved quality of life and reduced negative affect after long-term smoking abstinence (Piper, Kenford, Fiore, & Baker, 2012), short-term exacerbation of psychiatric symptoms could reasonably be expected due to smoking withdrawal symptoms, which include negative affect. Alternatively, it is possible that PTSD symptoms are alleviated when the individual is not subjected to highly variable nicotine levels throughout each day. Despite evidence that smokers with PTSD expect that smoking relieves negative affect (Calhoun, Levin, et al., 2011), there is some evidence that nicotine may actually exacerbate PTSD symptoms (e.g., exaggerated startle response; Calhoun, Wagner, et al., 2011).
To augment research examining average symptom levels over time, it is important to examine variability in psychiatric symptoms early in the smoking cessation process (Piasecki, Jorenby, Smith, Fiore, & Baker, 2003). Research on smoking withdrawal symptoms has found that increased symptom variability, in addition to elevated mean symptoms, is associated with smoking lapse in the general population (Piasecki et al., 2003). Craving has been found to increase in the first days of a smoking cessation attempt in retrospective report studies in the general population (Hughes, Gust, Skoog, Keenan, & Fenwick, 1991), though craving trajectories are quite variable across smokers and situations. For example, observational (Dols, van den Hout, Kindt, & Willems, 2002) and experimental research (Sayette et al., 2003) indicating that smoking urges are higher when individuals expect to have the opportunity to smoke suggest that cravings would be decreased in the early stages of smoking cessation, when individuals do not plan to smoke and often do not keep cigarettes with them. In contrast with results of retrospective report studies, a study using EMA methods assessing craving several times a day before and after the quit date found that randomly sampled craving was decreased on the quit date, with continuing declines as abstinence progressed to subsequent days (Shiffman et al., 1997). Further research using EMA methods is needed on the robustness of the decline in craving and its implications for craving in the early course of abstinence in smokers with PTSD.
To date, there have been no published studies utilizing EMA methods to examine the effects of the early course of quitting smoking on PTSD symptoms, negative affect, and cigarette craving among smokers with PTSD. The present study uses random EMA methods to assess real time symptom and smoking behavior to (a) examine changes in PTSD symptoms and negative affect as smokers progress through prequit, abstinent, and postlapse phases of smoking cessation, (b) explore changes in PTSD symptoms and negative affect variability throughout smoking cessation phases, (c) examine changes in cigarette craving throughout smoking cessation phases, and (d) investigate longitudinal relationships of PTSD symptoms and negative affect with cigarette craving during a quit attempt.
H1: PTSD symptoms and negative affect will increase with the onset of abstinence and return to baseline in the postlapse phase.
H2: The variability in PTSD symptoms and negative affect will increase with the onset of abstinence and return to baseline in the postlapse phase.
H3: Craving will increase with the onset of abstinence and return to baseline in the postlapse phase.
H4a: Increases in PTSD symptoms and negative affect between EMA readings will be associated with increased cravings at the second EMA reading.
H4b: Increases in cravings between EMA readings will be associated with increased PTSD symptoms and negative affect at the second EMA reading.
METHODS
Participants
This study included 52 smokers with PTSD, with some data and more procedural details reported in an earlier publication comparing lapse in PTSD and non-PTSD smokers (Beckham et al., 2013). Eligibility criteria included smoking at least 10 cigarettes daily for the past year, willingness to make a smoking cessation attempt, and age 18–65 years. Participants were excluded for use of noncigarette forms of nicotine, major unstable medical problems (e.g., cancer, unstable angina, poorly controlled diabetes, and respiratory disorders requiring frequent hospitalization), or use of bupropion or benzodiazepines. All participants reported being in either the contemplation (n = 35) or the preparation (n = 17) stage of quitting smoking at the screening session, which means they were planning to make a quit attempt in the next 6 months.
Procedures
Participants were recruited through their providers, flyers approved by the respective institutional review boards, and/or brochures advertising a study on stress and smoking. In addition, we recruited participants by obtaining a list of potentially eligible veterans (i.e., veterans who smoke) from computerized medical records at the Durham VA Medical Center. Participants were recruited using a series of invitational letters, as described by Dillman, Smyth, and Christian (2009). Participants completed a screening session, two smoking cessation counseling sessions based on the National Cancer Institute Freshstart program (Lando, McCovern, & Barrios, 1990), and 2 weeks of electronic diary (ED) monitoring. Following the quit date, participants returned to the laboratory every other day for bioverification of smoking abstinence (expired carbon monoxide and salivary cotinine) for 1 week after the quit date. At the screening session, each participant provided sociodemographic information and smoking history and completed the Fagerström Test of Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). Baseline presence of PTSD was assessed with the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995), using the 1/2 frequency/intensity rule for symptom threshold (Blake et al., 1995). The CAPS (Weathers et al., 2004) is a semistructured interview to assess PTSD. Its strong evidence of reliability and validity has established it as the gold standard for PTSD assessment. The CAPS assesses all 17 symptoms of PTSD, rated by clinicians on a 4-point scale for frequency and a separate 4-point scale for intensity. Participants not meeting criteria for PTSD who were otherwise eligible were placed into a non-PTSD comparison group that is not included in this report.
Ecological Momentary Assessment Procedures
Participants completed EMA readings for 7 days of ad lib smoking and the first 7 days after their quit date (Beckham et al., 2013). Diary entries were time-stamped to ensure temporal accuracy (i.e., participants could not clump entries) and assess protocol adherence. Though the protocol included both random alarm and self-initiated readings, for the current report, analyses were restricted to random alarm readings to get a random sample of PTSD symptoms, negative affect, and craving. ED-initiated alarms were designed to go off randomly (a) between 2–3hr after a completed assessment during the prequit period and (b) between 1–2hr during the postquit period to provide more assessments during this critical period. Participants had a 2-min window after the alarm to begin the assessment. They were instructed to ignore or suspend (interrupt the ringing sound) during an activity in which responding would be dangerous (e.g., driving) or too costly (e.g., religious services). Additionally, participants were able to delay an assessment with a 5-min delay function. Finally, participants were able to inactivate alarms for 15–120min when they expected to be unavailable and for 4–11hr overnight for sleeping. Following any missed or skipped alarm, the next alarm was designed to go off within 30–45min. In addition to random EMA readings, participants were asked to initiate their own assessments whenever they incurred smoking lapse. As a check against potential missed reports of smoking lapse, diaries asked about smoking at the beginning of random and evening assessments.
Participants were paid $75 for screening, $25/day for ED monitoring, and up to $45 in incentive pay during the postquit week ($25 for not missing three or fewer alarms in all days between sessions; $20 for completing at least three smoking entries during the prequit phase; $20 for completing all evening postquit-week diary assessments). Participants were paid $25 for each postquit visit with no relapse (≥5 cigarettes/day for at least 3 days consecutively) by self-report and carbon monoxide reading. This totaled a maximum of $750.
ED Assessments
All full assessments included common items on smoking urge, activity, mood, and PTSD symptoms.
PTSD Symptoms:
Aside from CAPS at the screening visit, presence and severity of 15 of the 17 PTSD symptoms mentioned in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV; American Psychiatric Association, 1994) were assessed using the Davidson Trauma Scale (DTS; Davidson et al., 1997) throughout the study. The nightmare and sleep disturbance items were omitted because EMA was designed to measure symptoms during waking time. Frequency was assessed following procedures outlined in our previous work (Beckham et al., 2005). Severity was assessed on a 5-point scale, with anchors ranging from “not at all” to “extremely.” This yielded a PTSD overall summary score and symptom cluster scores at each measurement.
Negative Affect:
Participants rated the 10 negative affect severity items from the Positive And Negative Affect Schedule (Watson, Clark, & Carey, 1988; Watson, Clark, & Tellegen, 1988) on a scale of 1 (“Very slightly/not at all”) to 5 (“Extremely”), yielding a summary score.
Craving:
Participants rated smoking craving on a scale ranging from 1 to 5. Previous research has suggested that smoking urge and smoking craving are largely redundant (Shiffman et al., 1997); therefore, craving was measured with a single item asking “How strong is your urge for a cigarette right now?”
Analysis Plan
For analysis purposes, EMA data were divided into three phases: prequit, abstinent, and postlapse. The prequit phase included all EMA readings before the quit attempt, which was defined as overnight abstinence verified by carbon monoxide reading. The abstinent phase included readings between the start of the quit attempt and the first smoking lapse, which started the postlapse phase. To analyze EMA data, we used multilevel modeling (MLM). This technique is often used to model interindividual differences in intraindividual change, for example, Neupert, Mroczek, & Spiro (2008). MLM is also uniquely suited for unbalanced data (i.e., missing data and data with differing numbers of cases per individual) because of the computation of adjusted means based on the data clustered at the individual level rather than on simple arithmetic means.
Prior to conducting each model, we ran a null model (i.e., a model without predictors), which allowed us to determine the proportion of variance in the dependent variable associated with intraindividual variability (Level 1) and that associated with interindividual differences (Level 2). Slopes varying significantly across participants in the null model and yielding a −2 residual log pseudolikelihood significantly greater than the one from the null model with constrained slopes were allowed to vary. PTSD symptoms, negative affect, and craving were grand-mean standardized to facilitate both interpretation of effects and comparative analyses across measures. Due to the strong theoretical association of nicotine dependence with PTSD symptoms, negative affect, and craving, nicotine dependence was included as a covariate in all multivariate models of symptom levels and variability. To prevent overfitting, other potential covariates with conceptual relevance to PTSD symptoms and negative affect were not included, but analyses of antidepressant medication use, mean postlapse number of cigarettes per day, and veteran status found that they did not change the pattern of results.
To capture the degree to which intraindividual variability in PTSD symptoms and negative affect changed across the three phases, we calculated root mean square differences (RMSDs) for each participant at each phase (Naragon-Gainey, Simpson, Moore, Varra, & Kaysen, 2012). We then modeled RMSD as a function of phase via multivariate MLM. The RMSD can be interpreted as the average daily fluctuation. Given that high levels of PTSD symptoms and negative affect would lend themselves to greater overall variability, we controlled for centered mean symptom and affect level at each phase. Variances associated with intraindividual changes in symptom and affect variability were fixed at π2/3, or 3.29, as is custom for multilevel logistic models (Hox, 2010). Because RMSD values conformed to a zero-inflated gamma distribution, with a high incidence of zero responses and relatively few cases of positively skewed nonzero scores, modeling these variables entailed a two-part analysis. First, zero and nonzero values were modeled as a function of phase via multivariate logistic MLM. Then, nonzero values were modeled as a function of phase via multivariate gamma-corrected MLM. Analyses were conducted using PROC GLIMMIX and PROC MIXED, available via SAS (v. 9.2).
RESULTS
Demographic data are provided in Table 1. Forty-four percent of the 52 smokers with PTSD were men, and the mean age was 42.5 years. During the initial ad lib smoking phase, participants completed a mean of 3.52 alarm readings per day (SD = 1.08) and a mean total of 32.75 readings (SD = 12.35, range: 7–71). During the abstinent phase, in which participants were more frequently prompted by random alarms, the number of random alarms completed per day increased significantly to 8.93 (SD = 6.49; mean total readings = 9.24, SD = 8.42, range: 1–44), Bonferroni-corrected p < .05. After lapsing, the number of random alarms completed per day decreased significantly, Bonferroni-corrected p < .05, to prequit levels (M = 4.53, SD = 2.19; mean total readings = 22.02, SD = 10.68, range: 3–40). The mean response rate to random alarms, 69%, did not vary significantly across the study phases: F (2, 148) = 0.40, p = .67. Mean time to lapse was 1.82 days (SD = 1.82, range: 0.06–7.00 days). Twenty-one participants lapsed within 24hr. Of the remaining 28 participants, three remained abstinent up to the end of the study; hence, no postlapse data were recorded for them. Given the wide variability in time to lapse, individual differences in time to lapse (in days) were controlled for in all subsequent analyses.
Table 1.
Sociodemographic and Baseline Psychiatric Data (n = 52)
| Frequency/means (SD) | |
|---|---|
| Age, years | 42.52 (10.32) |
| Education | 12.61 (1.84) |
| SES (Hollingshead) | 57.23 (11.55) |
| Cigarettes per day | 17.56 (8.47) |
| Total years smoked | 26.40 (11.81) |
| PTSD symptoms (CAPS) | |
| Reexperiencing | 17.63 (7.91) |
| C1 Avoidance | 7.90 (4.46) |
| C2 Numbing | 18.89 (6.92) |
| Hyperarousal | 20.27 (6.73) |
| Negative affect (PANAS) | 13.83 (8.57) |
| Women (n) | 29 |
| Racial/ethnic minority (n) | 30 |
| Military veteran (n) | 13 |
| MDD, current (n) | 17 |
| MDD, lifetime (n) | 35 |
Note. CAPS = Clinician-Administered PTSD Scale; MDD = major depressive disorder; PANAS = positive and negative affect scale; PTSD = posttraumatic stress disorder; SES = socioeconomic status.
Prequit, Abstinent, and Postlapse PTSD Symptom and Negative Affect Level
The results of the null model indicated that interindividual differences accounted for 3% of the total variance in PTSD symptom and negative affect level, whereas intraindividual variability accounted for 97% (both ps < .01). PTSD symptoms and negative affect at each phase of smoking cessation are presented in Table 2. According to the full model, the multivariate effect of phase was significant [F (10, 435) = 5.71, p < .01], reflecting a decrease in symptom level and negative affect from the prequit to the abstinent phase [t (435) = -4.61, p < .01]. Although symptom levels and negative affect increased from the abstinent to the postlapse phase [t (435) = 2.57, p = .01], they remained lower in the postlapse phase than in the prequit period [t (435) = 2.24, p = .03]. The effects of nicotine dependence [F (5, 10, 318) = 0.53, p = .75] and time to lapse [F (5, 10, 318) = 0.37, p = .87] on PTSD symptoms and negative affect were not significant. As shown in the univariate models presented in Table 2, reexperiencing, numbing, and avoidance symptoms decreased significantly from the prequit to the abstinent phase. The reexperiencing symptoms remained at a lower level when participants moved from the abstinent to the postlapse phase, whereas the avoidance and numbing symptoms increased when participants moved from the abstinent to the postlapse phase. In none of the models was either nicotine dependence or time to lapse a significant predictor.
Table 2.
Summary of PTSD, Affect, and Craving Before, During, and After a Quit Attempt (n = 52)
| Prequit | Abstinent | Postlapse | F value | |
|---|---|---|---|---|
| M (SE) | M (SE) | M (SE) | ||
| Reexperiencing symptomsa, c | 2.07 (0.46) | 1.65 (0.48) | 1.62 (0.47) | 7.80** |
| Numbing symptomsa,b | 3.50 (0.65) | 2.80 (0.67) | 3.62 (0.65) | 7.25** |
| Avoidance symptomsa,b | 1.81 (0.34) | 1.42 (0.35) | 1.74 (0.34) | 5.78** |
| Hyperarousal symptomsa,c | 3.07 (0.56) | 2.46 (0.57) | 2.57 (0.56) | 9.56** |
| Negative affect | 3.72 (0.69) | 3.96 (0.71) | 3.89 (0.70) | 0.75 |
| Cravinga,c | 1.37 (0.09) | 1.09 (0.10) | 1.06 (0.09) | 21.02** |
Note. Descriptive statistics presented in this table are statistics modeled using the multilevel modeling procedures described in the Analysis Plan section.
aSignificant difference between prequit and abstinent phases, p < .05.
bSignificant difference between abstinent and postlapse phases, p < .05.
cSignificant difference between prequit and postlapse phases, p < .05.
*p < .05. **p < .01.
Symptom and Affect Variability
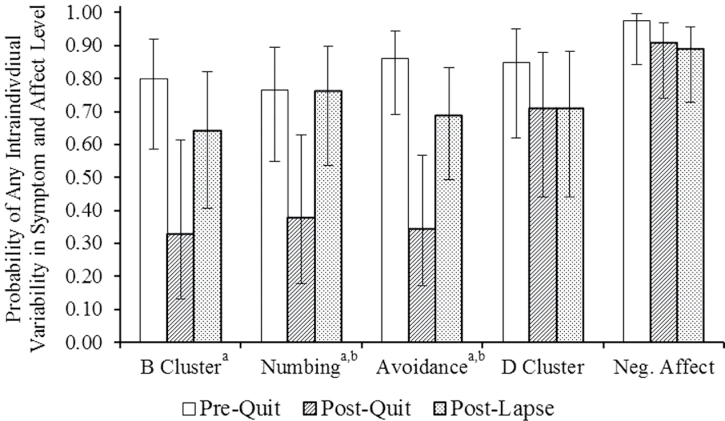
The results of the null logistic model indicated that interindividual differences in PTSD symptom and negative affect variability were significant (τ 00 = 1.42, p < .01). According to the full model, the multivariate effect of phase was significant [F (10, 315) = 2.62, p < .01] after controlling for symptom and affect means [F (5, 355) = 9.57, p < .01], nicotine dependence [F (5, 355) = 1.13, p = .34], and time to lapse [F (5, 355) = 0.31, p = .91]. The odds that a PTSD symptom or negative affect EMA reading would vary at all from the previous reading decreased from the prequit (odds ratio, OR = 1.58) to the abstinent phase (OR = 0.37, t (355) = −4.10, p < .01). These odds increased from the abstinent to the postlapse period (OR = 0.80, t (355) = 2.41, p = .02), although there was still a significant difference in the odds of variability between the postlapse and the prequit stages (t (355) = −2.14, p = .05). In univariate models, relative to the prequit phase, the odds of variability from one reading to the next in reexperiencing, numbing, and avoidance were decreased in the abstinent phase. Relative to the abstinent phase (Figure 1), odds of variability in numbing and avoidance were increased in the postlapse phase.
Figure 1.
Probability of any intraindividual variability in posttraumatic stress disorder symptoms and negative affect across the prequit, abstinent, and postlapse phases. Error bars represent 95% confidence intervals. aSignificant difference between prequit and abstinent phases, p < .05; bsignificant difference between abstinent and postlapse phases, p < .05.
The results of the null gamma model indicated that interindividual differences (σ2 = 0.24, p < .01) accounted for 47% of the variance in symptom and affect variability, and intraindividual change (τ00 = 0.27, p < .01) accounted for 53%. In the full model, the multivariate effect of phase was not significant [F (10, 171) = 0.98, p = .46] after controlling for mean levels [F (5, 171) = 6.01, p < .01], nicotine dependence [F (5, 171) = 2.18, p = .06], and time to lapse [F (5, 171) = 0.61, p = .69].
Association Between PTSD Symptoms, Negative Affect, and Nicotine Craving
To determine the degree to which craving was driven by PTSD symptoms and negative affect across each phase of the study, we modeled momentary craving as a function of PTSD symptoms and negative affect measured at the previous reading, while controlling for nicotine dependence and individual differences in both time to lapse and craving level at the previous reading. According to the null model, interindividual differences accounted for 29% of the total variance in craving level, whereas intraindividual variability accounted for 71% (both ps < .01). Previous reexperiencing symptoms (B = 0.02, p = .03), avoidance symptoms (B = 0.03, p = .01), hyperarousal symptoms (B = 0.02, p < .01), and negative affect (B = 0.03, p < .01) were all independently associated with subsequent craving. There was a trend suggesting that previous numbing symptoms were also positively associated with subsequent craving (B = 0.01, p = .05).
We next investigated the trajectory of cigarette craving across the three study phases by modeling level of craving as a function of phase and controlling for nicotine dependence and individual differences in time to lapse. The full model indicated a significant effect of phase on mean craving [F (2, 87) = 21.02, p < .01], reflecting that craving levels in the abstinent and postlapse phases were significantly lower than those in the prequit phase (ps < .01). The effect of nicotine dependence was not significant [F (1, 49) = 1.88, p = .18] nor was the effect of time to lapse [F (1, 49) = 0.03, p = .86]. Participants used the full craving scale (range: 1–5) in all three phases, with extreme cravings at the high end of the scale (i.e., a rating of “5”) being endorsed in 7.2% of prequit entries (79 of 1,094), 1.6% of abstinent-phase entries (5 of 309), and 4.9% of postlapse entries (33 of 679).
To examine associations of craving on subsequent PTSD symptoms and negative affect, we modeled symptoms and affect as a function of craving at the previous reading while controlling for symptom and affect levels at the previous reading, nicotine dependence, and time to lapse. According to the full model with covariates, the multivariate effect of prior craving was not significant [F (5, 7,228) = 1.85, p = .10].
DISCUSSION
In this study using EMA methods to follow individuals with PTSD during a quit attempt, individuals’ mean PTSD symptom levels decreased during smoking abstinence. Relative to levels observed during ad lib smoking, PTSD symptoms were significantly lower during initial abstinence and then slightly increased in the period following a smoking lapse. Negative affect did not significantly change during smoking abstinence. The frequency of changes in PTSD symptoms decreased when participants initiated smoking abstinence, suggesting that participants experienced greater short-term symptom stability when abstaining from smoking. Participants also reported decreased craving during smoking abstinence. However, increased PTSD symptoms and negative affect continued to predict elevations in craving at the next EMA reading, suggesting that PTSD and negative affect triggered cigarette cravings in individuals with PTSD, even on days with otherwise low levels of cravings.
The finding that individuals with PTSD generally reported decreased PTSD symptoms during smoking abstinence is novel. Because nicotine is a stimulant that increases heart rate, it could aggravate anxiety disorders, especially PTSD (Calhoun, Wagner, et al., 2011), which is already marked by increased physiological arousal. With the removal of nicotine from the body, individuals with PTSD could experience relief from hyperarousal symptoms as well as reduced reactivity and avoidance related to trauma reminders. Similarly, repeated changes in nicotine in the body throughout the day brought about by smoking and smoking offset could contribute to the increased symptom variability that is alleviated by smoking cessation. Our findings are consistent with some data in schizophrenia and depression (Ragg et al., 2013) and data indicating that individuals report improved PTSD symptoms months after cessation and improved quality of life as long as 3 years after cessation (Piper et al., 2012). Additionally, smoking withdrawal might be less aversive than the chronic emergence of negative affect that accompanies nicotine offset between cigarettes in regular smokers (Parrott, 1998), a potential explanation for the relative symptom stability that we observed.
The finding that craving decreased during the quit phase contrasts with the results of several studies using retrospective report of smoking craving (Hughes et al., 1991), but it is consistent with an EMA study (Shiffman et al., 1997). This finding contradicts strictly biological models of craving, which predict that withdrawal from nicotine prompts cravings. Our data are more consistent with models incorporating psychological variables such as the expectation of an opportunity to smoke. Because our participants knew they would be attending follow-up appointments with biological testing for smoking lapses, they might not have viewed themselves as having an opportunity to smoke during most of the time in the first few days of their quit attempt. Our findings generally support the view that both biological and psychological factors contribute strongly to smoking urges during a quit attempt.
This study was limited by the short follow-up period, as participants typically lapsed within a few days, and some lapsed on the first day. This resulted in relatively few assessments of the abstinent phase, with some participants quitting for less than the 24hr often used to define a quit attempt. It is possible that symptoms reported in the first few days are not representative of the symptom course over the first few weeks of abstinence. However, the duration of abstinence in smokers with PTSD from this study was similar to that in previous research (Zvolensky et al., 2008). Finally, our study provided smokers with no pharmacotherapy and provided only brief counseling to assist in their quit attempt. Although this is consistent with the majority of quit attempts, further research is needed to determine how the results of this study would generalize to individuals receiving more intensive treatment to optimize their odds of successful cessation.
Overall, results suggested that smoking cessation did not trigger symptom exacerbation in these smokers with PTSD. In fact, it is possible that these smokers experienced a small measure of symptom relief early in the course of smoking abstinence. This finding should decrease any fears that clinicians may have in targeting smoking cessation in patients with PTSD. Future research is needed to determine how pharmacotherapy and counseling influence the course of PTSD and other psychiatric symptoms during smoking cessation treatment in an effort to increase abstinence rates in this group of smokers.
FUNDING
This work was supported primarily by the National Institutes of Health grants 2R01CA081595, 2K24DA016388, R21DA019704, 1R21CA128965; by a Career Development Award from the Department of Veterans Affairs Office of Research and Development, Clinical Science Research and Development; and by the Mid-Atlantic Mental Illness Research, Education and Clinical Center, Department of Veterans Affairs (VISN 6 MIRECC) of the Department of Veterans Affairs Office of Mental Health Services.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
We would like to thank the participants who volunteered to participate in this study. The views expressed in this presentation are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health.
REFERENCES
- American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association [Google Scholar]
- Beckham J.C., Calhoun P.S., Dennis M.F., Wilson S.M., Johnson Y.C., Dedert E.A. (2013). Predictors of lapse in the first week of smoking abstinence in posttraumatic stress disorder and non-posttraumatic stress disorder smokers. Nicotine & Tobacco Research, 15, 1122–1129. 10.1093/ntr/nts252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham J.C., Dennis M.F., McClernon F.J., Mozley S.L., Collie C.F., Vrana S.R. (2007). The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addictive Behaviors, 32, 2900–2915. 10.1016/j.addbeh.2007.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham J.C., Feldman M.E., Vrana S.R., Mozley S.L., Erkanli A., Clancy C.P., Rose J.E. (2005). Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: A preliminary study. Experimental and Clinical Psychopharmacology, 13, 218–228 doi:10.1037/1064-1297.13.3.219 [DOI] [PubMed] [Google Scholar]
- Beckham J.C., Kirby A.C., Feldman M.E., Hertzberg M.A., Moore S.D., Crawford A.L., Fairbank J.A. (1997). Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addictive Behaviors, 22, 637–647 doi:0.1016/S0306- 4603(96)00071-8 [DOI] [PubMed] [Google Scholar]
- Beckham J.C., Wiley M.T., Miller S.C., Dennis M.F., Wilson S.M., McClernon F.J., Calhoun P.S. (2008). Ad lib smoking in posttraumatic stress disorder: An electronic diary study. Nicotine and Tobacco Research, 10, 1149–1157. 10.1080/14622200802123302 [DOI] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. (1995). The development of a clinician-administered posttraumatic stress disorder scale. Journal of Traumatic Stress, 8, 75–80. 0894-9867/95/0100--U075507,50/1 [DOI] [PubMed] [Google Scholar]
- Calhoun P.S., Levin H.F., Dedert E.A., Johnson Y.C, VA Mid-Atlantic Mental Illness Research, Education, and Clinical Center Registry Workgroup, & Beckham J.C. (2011). The relationship between posttraumatic stress disorder and smoking outcome expectancies among U.S. military veterans who served since September 11, 2001. Journal of Traumatic Stress, 24, 303–308. 10.1002/jts.20634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun P.S., Wagner H.R., McClernon F.J., Lee S., Dennis M.F., Vrana S.R., Beckham J.C. (2011). The effect of nicotine and trauma context on acoustic startle in smokers with and without posttraumatic stress disorder. Psychopharmacology, 215, 379–389. 10.1007/s00213-010-2144-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J.R.T., Book S.W., Colket J.T., Tupler L.A., Roth S., David D., Feldman M.E. (1997). Assessment of a new self-rating scale for posttraumatic stress disorder: The Davidson Trauma Scale. Psychological Medicine, 27, 153–160 [DOI] [PubMed] [Google Scholar]
- Dillman D.A., Smyth J.D., Christian L.M. (2009). Internet, mail, and mixed-mode surveys: The tailored design method (3rd ed.). New York: John Wiley [Google Scholar]
- Dols M., van den Hout M., Kindt M., Willems B. (2002). The urge to smoke depends on the expectation of smoking. Addiction, 97, 87–93 doi:10.1046/j.1360-0443.2002.00010.x [DOI] [PubMed] [Google Scholar]
- Fu S., McFall M., Saxon A. J., Beckham J.C., Carmody T.P., Baker D.G., Joseph A.M. (2007). Posttraumatic stress disorder and smoking: A systematic review. Nicotine & Tobacco Research, 9, 1071–1084 doi:10.1080/14622200701488418 [DOI] [PubMed] [Google Scholar]
- Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerström K.O. (1991). The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hox J.J. (2010). Multilevel analysis: Techniques and applications (2nd ed.). New York: Routledge [Google Scholar]
- Hughes J.R., Gust S.W., Skoog K., Keenan R.M., Fenwick J.W. (1991). Symptoms of tobacco withdrawal. A replication and extension. Archives of General Psychiatry, 48, 52–59 doi:10.1001/archpsyc.1991.01810250054007 [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Walters E.E. (2005). Prevalence, severity and comorbidity of 12-month DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 617–627 doi:10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando H.A., McCovern P.G., Barrios F.X. (1990). Comparative evaluation of American Cancer Society and American Lung Association smoking cessation clinics. American Journal of Public Health, 80, 554–559 doi:10.2105/AJPH.80.5.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K., Boyd J.W., Woolhander S., Himmelstein D.U., McCormick D., Bor D.H. (2000). Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association, 284, 2606–2610. 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K., Simpson T.L., Moore S.A., Varra A.A., Kaysen D.L. (2012). The correspondence of daily and retrospective PTSD reports among female victims of sexual assault. Psychological Assessment, 24, 1041–1047. 10.1037/a0028518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert S.D., Mroczek D.K., Spiro A. (2008). Neuroticism moderates the daily relation between stressors and memory failures. Psychology and Aging, 23, 287–296. 10.1037/0882-7974.23.2.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott A.C. (1998). Nesbitt’s paradox resolved? Stress and arousal modulation during cigarette smoking. Addiction, 93, 27–39 doi:10.1046/j.1360-0443.1998.931274.x [DOI] [PubMed] [Google Scholar]
- Piasecki T.M., Jorenby D.E., Smith S.S., Fiore M.C., Baker T.B. (2003). Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Experimental and Clinical Psychopharmacology, 11, 276–285 doi:10.1037/1064-1297.11.4.276 [DOI] [PubMed] [Google Scholar]
- Piper M.E., Kenford S., Fiore M.C., Baker T.B. (2012). Smoking cessation and quality of life: Changes in life satisfaction over 3 years following a quit attempt. Annals of Behavioral Medicine, 43, 262–270. 10.1007/s12160-011-9329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragg M., Gordon R., Ahmed T., Allan J. (2013). The impact of smoking cessation on schizophrenia and major depression. Austalasian Psychiatry, 21, 238–245. 10.1177/1039856213486213. [DOI] [PubMed] [Google Scholar]
- Sayette M.A., Wertz J.M., Martin C.S., Cohn J.F., Perrott M.A., Hobel J. (2003). Effects of smoking opportunity on cue-elicited urge: a facial coding analysis. Experimental Clinical Psychopharmacology, 11, 218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Engberg J., Paty J.A., Perz W.G., Gnys M., Kassel J.D., Hickcox M. (1997). A day at a time: Predicting smoking lapse from daily urge. Journal of Abnormal Psychology, 106, 104–116 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A, Carey G. (1988). Positive and negative affectivity and their relation to anxiety and depressive disorder. Journal of Abnormal Psychology, 97, 346–353 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affects: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070 [DOI] [PubMed] [Google Scholar]
- Weathers F.W., Newman E., Blake D.D., Nagy L.M., Schnurr P., Kaloupek D.G., Keane T.M. (2004). Clinician-administered PTSD scale (CAPS): Interviewer’s guide. Los Angeles, CA: Western Psychological Services [Google Scholar]
- Zvolensky M.J., Gibson L.E., Vujanovic A.A., Gregor K., Bernstein A., Kahler C., Feldner M.T. (2008). Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine & Tobacco Research, 10, 1415–1427. 10.1080/14622200802238951 [DOI] [PubMed] [Google Scholar]