Abstract
Since the food matrix determines β-carotene availability for intestinal absorption, food matrix effects on the bioaccessibility of β-carotene from two diets were investigated in vitro and compared with in vivo data. The “mixed diet” consisted of β-carotene-rich vegetables, and the “oil diet” contained β-carotene-low vegetables with supplemental β-carotene. The application of extrinsically labeled β-carotene was also investigated. The bioaccessibility of β-carotene was 28 μg/100 μg β-carotene from the mixed diet and 53 μg/100 μg β-carotene from the oil diet. This ratio of 1.9:1 was consistent with in vivo data, where the apparent absorption was 1.9-fold higher in the oil diet than in the mixed diet. The labeled β-carotene was not equally distributed over time. In conclusion, the food matrix effects on bioaccessibility of β-carotene could be measured using an in vitro model and were consistent with in vivo data. The application of extrinsically labeled β-carotene was not confirmed.
Keywords: bioaccessibility, β-carotene, food matrix, in vitro digestion
Introduction
The absorption of β-carotene by humans comprises two steps.1,2 The first step involves the disruption of the food matrix and the solubilization of β-carotene within micelles (micellarization). This step determines the availability of β-carotene for absorption (bioaccessibility). The second step covers the entry into the intestinal cells, partial conversion into vitamin A (retinol), and entry into lymph and finally into the blood. The first step (bioaccessibility) can be studied in vitro, since micelles are readily absorbed in vivo. True absorption is determined by the first step and entry into the intestinal cells. This could be calculated from classical labor-intensive in vivo studies by using data from dietary intake and collected stools. The combined first and second steps can only be studied in vivo, by analyzing blood samples.
Thus, there is a need for a simple, quick, and realistic in vitro model to measure the food matrix effects on β-carotene absorption. The food matrix is an important determinant of the bioaccessibility of β-carotene. Factors such as food processing and the amount and type of vegetable and fruit cause food matrix effects.3,4
The computer-controlled dynamic in vitro TNO gastro-Intestinal tract Model (TIM-1)5 was selected for quantifying the bioaccessibility of β-carotene from different food matrices in this study. Compared to alternative in vitro models, TIM-1 mimics the dynamic conditions in the human gastrointestinal tract with high reproducibility, especially for fat-soluble compounds, and facilitates the study of bioaccessibility of food components in a controlled and standardized manner.6−8 TIM-1 has been validated for the digestion and bioaccessibility of various nutrients in comparison with in vivo studies. This include the bioaccessibility of, e.g., fats,8,9 water-soluble vitamins,10,11 and fat-soluble vitamins.12,13
In this study, the food matrix effects on the bioaccessibility of β-carotene from two types of diets were investigated by using TIM-1 and then compared with the results with those of an in vivo study. The diets consisted of a “mixed diet” containing β-carotene-rich vegetables and an “oil diet” containing β-carotene-low vegetables with supplemental β-carotene. In an in vivo study using these diets, the apparent absorption of β-carotene was 1.9-fold higher in the oil diet than in the mixed diet.14
This in vitro study provided an opportunity to evaluate how effectively extrinsically labeled β-carotene could be used as a tracer for measurement of absorption during in vivo studies. Ideally, the extrinsic label (administered by a capsule) should mix and equilibrate with the intrinsic β-carotene. This means that the isotopic enrichment of β-carotene should become homogeneous within the feeding sample and be equal to that observed in the bioaccessible fractions in TIM-1.
Materials and Methods
Dynamic in Vitro Gastrointestinal Model
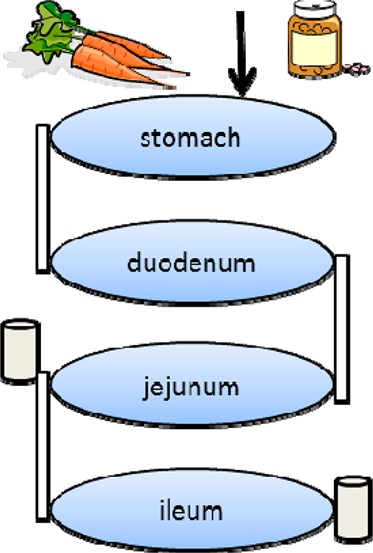
TIM-1, a dynamic in vitro gastrointestinal model, was originally described by Minekus et al.5,6 For a schematic diagram of TIM-1, see Verwei et al.15 This gastric small-intestinal model comprises four connected compartments that represent the stomach, duodenum, jejunum, and ileum, respectively. Each compartment consists of a glass outer wall with a flexible inner wall. The flexible wall is surrounded by water at 37 °C, which is used to squeeze the flexible walls and mix the chyme, simulating peristaltic movements in the gastrointestinal tract. The jejunal and ileal compartments are connected with semipermeable hollow-fiber membranes to remove digested products and especially fat-soluble compounds that were incorporated into mixed micelles.6−8 The pH values, as well as the gastric emptying and small-intestinal passage of the food, are computer-controlled according to preset curves based on information on human in vivo conditions.6
TIM-1 Experimental Design
Two diets that had already been tested in vivo14 were each evaluated using TIM-1. The difference between the two diets was the food matrix where the β-carotene was incorporated, with one diet containing vegetables high in β-carotene (mixed diet) and the second diet containing vegetables low in β-carotene but supplemented with β-carotene in salad dressing oil (oil diet). The frozen diets were defrosted at 4 °C for ∼16 h before the experiment and were homogenized to mimic the process of chewing by human teeth, to increase experimental consistency and to minimize mixing errors as only a limited amount could be introduced in the gastric compartment. A portion of the diet was put into the gastric compartment of TIM-1. During digestion, the total filtrate (bioaccessible fraction) was collected in 30-min fractions during the first 2 h and hourly fractions until the end of the experiment at 6 h. The total ileal efflux was collected hourly during the 6 h experiment. At the end of the experiment, the residues from the gastric, jejunal, and ileal compartments were collected. The ileal efflux and residues were analyzed to determine the non-bioaccessible fraction. Possibly present β-carotene content in the bile and pancreatic solutions was also analyzed. The samples were stored at −20 °C until β-carotene analysis by HPLC.
Feeding Sample
The feeding sample consisted of 120.0 g of the homogenized diet and was introduced into the gastric compartment together with 50.0 g of gastric juice, 5 g of amylase, and 37 g of water.6,8 The 120.0 g of the mixed diet contained 498 μg of β-carotene (90% as trans-β-carotene), and 120.0 g of the oil diet contained 220 μg of β-carotene (98% as trans-β-carotene) (Table 1). A capsule containing 47.0 μg of [13C10]β-carotene was introduced into TIM-1 immediately after putting the feeding sample in the gastric compartment.
Table 1. Values of Energy, Macronutrients, Fiber, Retinol, and Carotenoids of Two Diets per 100 g Wet Weighta.
| mixed diet | oil diet | |
|---|---|---|
| energy (kJ) | 584 | 570 |
| fat (g) | 4.7 | 4.3 |
| protein (g) | 4.9 | 4.8 |
| carbohydrates (g) | 17.5 | 18.2 |
| fiber (g) | 1.7 | 1.6 |
| retinol (μg) | 12 | 13 |
| total β-carotene in salad dressing oil (μg) | <1 | 156 |
| total β-carotene in vegetables and fruits (μg) | 415 | 28 |
| trans-β-carotene (μg) | 373 | 180 |
| cis-β-carotene (μg) | 42 | 4 |
| α-carotene (μg) | 75 | 3 |
| β-cryptoxanthin (μg) | 8 | 12 |
| lutein (μg) | 84 | 37 |
| zeaxanthin (μg) | 8 | 8 |
Values of energy and carbohydrates were calculated. The mixed diet contained vegetables high in β-carotene, and the oil diet contained vegetables low in β-carotene supplemented with synthetic β-carotene in salad dressing oil. For the in vitro study, the feeding sample consisted of 120.0 g of the homogenized diet.
The β-carotene in the oil diet originated from supplemental β-carotene (85%) and from vegetables and fruits (15%). The supplemental β-carotene in the oil suspension was added to salad dressing for the oil diet (all-trans β-carotene, 30% suspension in vegetable oil, Hoffmann-La Roche, Switzerland). Both diets were typical Western diets with respect to the contribution of carbohydrates, proteins, and fats to the energy intake (57%, 13%, and 30%, respectively). Both diets were prepared as duplicate diets during a previously conducted diet-controlled study in humans.14 Each of the duplicate diets was pooled, homogenized, mixed thoroughly with 2.5 mL of 20% butylhydroquinone (t-BHQ) per kilogram of food, and stored at −20 °C until analysis.
Chemical Analysis of β-Carotene in the Feeding Sample
Samples of each homogenized diet (4.0 g each) were extracted in duplicate using a mixture of methanol, tetrahydrofuran (THF), and dichloromethane (45:45:10 v/v/v). The resulting extract was saponified at room temperature overnight using a 1.5 mol/L potassium hydroxide ethanolic solution supplemented with sodium ascorbate, disodium sulfide, and glycerol. Then the mixture was extracted using diisopropyl ether, which was subsequently washed three times with water. Part of the resulting extract was evaporated to dryness and redissolved in HPLC mobile phase. The resulting solution was analyzed for β-carotene using reversed-phase HPLC with diode array detection.16,17 These analyses were performed for quality control at two laboratories (Food Analysis Laboratory at TNO, Zeist and at Human Nutrition, Wageningen University). Results from both analyses were similar.
Chemical Analysis of β-Carotene in Jejunal and Ileal Filtrates
Samples of 1000 μL each were vortex mixed with an equal volume of ethanol containing tocol as an internal standard (IS). This mixture was extracted with a 2-fold volume of hexane using vortex mixing for 2 min. Part of the resulting extract was evaporated to dryness and redissolved in eluent. The resulting solution was analyzed for β-carotene and IS using reversed-phase HPLC-diode array detection.16 The limit of precision of the known value of IS was 5%. These analyses were performed at the Food Analysis Laboratory at TNO, Zeist.
Chemical Analysis of β-Carotene in Ileal Efflux and Residues
The ileal efflux and residues had more complex and less dissoluble matrices than the filtrates and consisted of supernatants and pellets. The concentrations of carotenoids in the ileal efflux and residues were measured at the division of Human Nutrition, Wageningen University. The supernatants of both ileal efflux and residues were treated according to the procedure described by Khan et al.17 Briefly, the pellets of both ileal efflux and residues were extracted in duplicate in the presence of anhydrous sodium sulfate (4.0 g) and calcium carbonate (0.5 g), with 20 mL of THF containing 0.01% t-BHT after using a tissue grinder. The extract was filtered through a glass funnel fitted with Whatman paper no. 54. The residue was twice re-extracted with 20 mL of THF. The extract was evaporated to dryness, redissolved in methanol/THF (1:1 v/v %) containing 0.01% t-BHT, transferred into a 25.0 mL volumetric flask, and made up to volume. An amount of 25 μL was injected into the HPLC system for analysis. Within-run and between-run coefficients of variation for β-carotene were 2.6% and 7.1%, respectively.
Labeled β-Carotene in the Capsule and Measurement of Isotopic Enrichment in Filtrates
The concentration of β-carotene in the capsules was analyzed by HPLC with absorbance detection according to the method described by Khan et al.17 The capsule contained 47.0 (±0.8) μg [12,13,14,15,20,12′,13′,14′,15′,20′-13C10]-β-carotene in sunflower oil (>82% oleic acid and >9% linoleic acid; Hozol; Contined BV, Bennekom, The Netherlands). The 13C10-labeled β-carotene was synthesized at ARC Laboratories (Apeldoorn, The Netherlands) as described previously18 (isotopic incorporation >99%, isomeric purity >93% all-trans, chemical purity >98%). The capsules were designed for oil solutions and were made from bovine gelatin (Capsugel, Bornem, Belgium).
The first experiment of each diet was analyzed for isotopic enrichment in the filtrates. The degree of isotopic enrichment of β-carotene with [13C10]β-carotene in filtrates was measured by using liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry (APCI LC–MS) as described previously.19,20 β-Carotene and [13C10]β-carotene were monitored in negative ion mode at mass-to-charge ratios (m/z) 536 and 546, respectively.20 Isotopic enrichment of β-carotene in the filtrates was the proportion of labeled β-carotene to total β-carotene. These analyses were performed at the University of Illinois.
Calculations
The β-carotene bioaccessibility, the amount of bioaccessible β-carotene in μg per 100 μg of β-carotene available for digestion, was expressed as a fraction of β-carotene feeding and was calculated by the formula
where β-carotenefiltrates is the total β-carotene in the jejunal and ileal filtrate, β-carotenefeeding is the β-carotene in the feeding sample and comprises the β-carotene in 120.0 g of the diet and a capsule with 47 μg of labeled β-carotene, and β-caroteneendogenous is the β-carotene content in the bile and pancreatic solutions secreted into the duodenal compartment.
The recovery (%) (mass balance) of β-carotene was calculated by the formula
where β-caroteneileal efflux is the β-carotene content in the total material delivered from the ileal compartment, and β-caroteneresidues is the total β-carotene in the residues of stomach, duodenum, and ileum.
The proportion of isotopic enrichment (IE) of β-carotene with [13C10]β-carotene was calculated by IE in filtrates divided by IE in feeding sample and multiplied by 100.
Results
The endogenous β-carotene content of the bile and pancreatic juice used in TIM-1 was below the detection limit in all runs. There were no other sources of β-carotene than the feeding samples. The results with the mixed diet are based on triplicate experiments. The results with the oil diet are based on duplicate experiments since one experiment failed due to a defective hollow-fiber membrane unit. Because of the high recovery in both oil diet runs and similar bioaccessibilities, these duplicate runs were regarded as representative.
The bioaccessibility of β-carotene, determined by collection of all the filtrates, was 28.2 μg/100 μg β-carotene from the mixed diet and 53.4 μg/100 μg β-carotene from the oil diet (Table 2 and Figure 1). The recovery of β-carotene was 92% and 105% in the two runs with the oil diet and 88%, 94%, and 104% in the three runs with the mixed diet (Table 2).
Table 2. β-Carotene Amounts, Bioaccessibility of β-Carotene, and the Recovery of β-Carotene with the Two Diets in TIM-1 over 6 ha.
| β-carotene
in mixed diet |
β-carotene
in oil diet |
||||
|---|---|---|---|---|---|
| run 1 | run 2 | run 3 | run 1 | run 2 | |
| feeding | |||||
| diet (μg) | 498 | 498 | 498 | 220 | 220 |
| capsule (μg) | 47 | 47 | 47 | 47 | 47 |
| endogenous (μg) | nd | nd | nd | nd | nd |
| filtrates | |||||
| jejunal filtrate (μg) | 95.6 | 83.5 | 80.3 | 76.3 | 80.5 |
| ileal filtrate (μg) | 73.7 | 73.0 | 55.5 | 65.4 | 62.8 |
| ileal efflux (μg) | 264.2 | 312.0 | 233.6 | 34.8 | 44.9 |
| residues | |||||
| residue of stomach and duodenum (μg) | 31.7 | 27.4 | 67.9 | 44.0 | 62.9 |
| residue of jejunum and ileum (μg) | 48.2 | 69.7 | 40.1 | 25.8 | 29.7 |
| bioaccessibility (μg β-carotene/100 μg β-carotene)b | 31.1 | 28.7 | 24.9 | 53.1 | 53.7 |
| recovery (%)c | 94.2 | 103.8 | 87.6 | 92.3 | 105.2 |
The mixed diet contained vegetables high in β-carotene, and the oil diet contained vegetables low in β-carotene supplemented with synthetic β-carotene in salad dressing oil. nd = not detected.
The β-carotene bioaccessibility is the amount bioaccessible β-carotene in μg per 100 μg of β-carotene available for digestion and is calculated as (μg β-carotene in filtrates)/(μg β-carotene from feeding + endogenous) × 100.
Recovery (%) = (β-carotene in filtrates + ileal efflux + residues)/(β-carotene from feeding + endogenous) × 100.
Figure 1.
Cumulative β-carotene in filtrates expressed as a percentage of feeding to time after the runs (n = 3) with the mixed diet (A) and with the oil diet (B) after the runs (n = 2) in TIM-1 during 6 h.
The isotopic enrichments of β-carotene in the feeding sample compared with the isotopic enrichment of β-carotene in the filtrates varied over time from 85% to 122% in the jejunal filtrates and from 79% to 139% in the ileal filtrates (Table 3). Averaged over 6 h, the isotopic enrichment of β-carotene in the filtrate for the oil diet was closer to the theoretical value of 100% than for the mixed diet (99% v 113%). This difference in isotopic enrichment was expected because the oil diet with supplemental β-carotene was more comparable with the matrix of labeled β-carotene in the feeding sample than was the β-carotene in vegetables in the mixed diet. In the TIM-1 run with the mixed diet, the isotopic enrichment in the jejunal filtrate was lower than in the ileal filtrate, which indicates that micellarization of labeled β-carotene in the jejunal compartment lagged behind that in the ileal compartment. In contrast, the opposite was seen in the TIM-1 run with the oil diet, where micellarization of labeled β-carotene was faster in the jejunal compartment than in the ileal compartment. The isotopic enrichment increased over time in both jejunal and ileal filtrates, which means that the labeled β-carotene was not equally distributed over time in the bioaccessible fractions. Labeled β-carotene either did not fully equilibrate with the β-carotene in the food matrix or else followed a different micellarization pattern.
Table 3. Proportion of Isotopic Enrichment of β-Carotene with [13C10]β-Carotene in Filtrates Compared with That of the Feeding Sample for the Two Diets in TIM-1 over 6 h.
| mixed diet (%) | oil diet (%) | |
|---|---|---|
| jejunal filtrate 0–3 h | 85 | 106 |
| jejunal filtrate 3–6 h | 111 | 122 |
| ileal filtrate 0–3 h | 112 | 79 |
| ileal filtrate 3–6 h | 139 | 85 |
| weighted average filtrate 0–6 ha | 113 | 99 |
The measured β-carotene levels in jejunal filtrates were slightly higher than in ileal filtrates (Table 2).
Discussion
This in vitro gastrointestinal model appears useful for determination of the effects of food matrices on the bioaccessibility of β-carotene from foods, especially when fat-soluble nutrients compete for uptake in mixed micelles.
Comparing the Bioaccessibilities of β-Carotene from Both Diets
The mixed diet represented a healthy diet with high amounts of cooked vegetables and fruits. The oil diet was representative of a diet low in vegetables and fruits but high in food items fortified with β-carotene, such as those regularly consumed in industrialized societies (e.g., margarine). The oil diet and the mixed diet contained β-carotene in the physiological dose range and were designed according to the guidelines of ‘good nutrition’ from the Dutch Nutrition Board in The Hague, The Netherlands. Both diets were typical western diets with respect to the contribution of carbohydrates, proteins, and fats to the energy intake (57%, 13%, and 30%, respectively). The β-carotene amount in the oil diet was not the same as in the mixed diet; however, this was also the situation in the in vivo study.14 The absorption efficiency of β-carotene is regarded constant as long as the β-carotene consumption is within physiological ranges.1,2,21 Furthermore, the mixed diet contained a higher amount of β-carotene than the oil diet but had a lower amount bioaccessible β-carotene compared to the oil diet; in contrast, the oil diet contained a lower amount of β-carotene than the mixed diet but had a higher amount bioaccessible β-carotene compared to the mixed diet.
Bioaccessibility of β-carotene was 28 μg/100 μg β-carotene (or 28%) from the mixed diet and 53 μg/100 μg β-carotene (or 53%) from the oil diet. So, the bioaccessibility of labeled and dietary β-carotene from the oil diet was 1.9-fold higher than from the mixed diet. This 1.9-fold enhancement in apparent absorption between two diets was also found in the in vivo study.14
Although in absolute terms results of β-carotene bioaccessibility using TIM-1 are not strictly comparable with in vivo experiments, the differences in β-carotene bioaccessibilities between food matrices are still comparable with absorption estimates in humans. Thus, TIM-1 cannot be used for predicting absorption in vivo, but the system is a useful tool for measuring differences in bioaccessibility of β-carotene between various food matrices.
Comparing the Bioaccessibilities of β-Carotene Measured Using Other Models
Bioaccessibility of β-carotene from various meals, fruits, and vegetables have been reported using other in vitro digestion models. Garrett et al.22 developed an in vitro digestion model based on the method of Miller et al.,23 which simulates human gastric and pancreatic digestion as static duo-compartments. Our result of 28% bioaccessibility of β-carotene from the mixed diet is slightly higher than the reported bioaccessibility of β-carotene from meals. For example, the micellarization of β-carotene was 12–18% from a baby food meal of carrots, spinach, and chicken,22 16% from a meal of mango and chicken,24 16% from stir-fried meal of spinach, carrots, and tomato paste,25 and 18% from a salad meal of spinach, tomato, carrot, and lettuce.26
Several other studies have used the same in vitro method for single fruits or vegetables. From orange and kiwi, the micellarization of β-carotene was 34% and 47%, respectively.27 From mango, the micellarization of β-carotene was 25–39%; from mango with milk and sugar, 37–48%; from papaya, 31–34%; and from papaya with milk and sugar, 41–44%.28 The micellarization of β-carotene from carrot was 14% for carrot juice,29 17% from processed carrots,30 20% from raw carrots,31 and 33% from stir-fried carrots.31 From other vegetables, the micellarization of β-carotene was 17% from maize,32 17% from boiled spinach,29 30% from spinach puree,33 30% from spinach,27 29% from tomatoes,34 30% from boiled cassava,35,36 45% from sweet potato,27 54% from broccoli,27 and 57% from courgette.34 Our result of 28% is comparable to these as our study included a mix of different cooked vegetables and fruits.
Hedrén et al.37 developed a similar static duo-compartmental in vitro model, which estimates the maximum amount of carotenoids released, but not necessarily micellarized. Using this other in vitro model, the percentages of accessible β-carotene in homogenized, raw carrots were 21% and 30% and from cooked carrots were 27% and 39%, without and with addition of oil, respectively.37 Another study by Hedrén et al.38 showed accessible β-carotene was 39% from sweet potato, 64% from pumpkin, and 47% from cassava, which were all cooked with sunflower oil. Three other studies with this method showed a bioaccessibility of β-carotene of 19% from boiled pumpkin,39 74% from boiled carrot,39 and when cooked with vegetable oil, 11–22% from various processed sweet potato,40 21% from sweet potato, and 39% from pumpkin.41 Our results of 28% and 53% are also comparable with the results from this in vitro method.
Although these other models are temperature- and pH-controlled, they are not representative of the continuously changing variables during passage through the stomach and the small intestine. For general prediction of micellarization of β-carotene, these models are adequate. However, TIM-1 allows for the closest simulation to date of in vivo dynamic physiological processes occurring within the lumen of the stomach, duodenum, jejunum, and ileum of humans. Furthermore, TIM-1 has as major advantage that the model allows for the collection of samples during the process of digestion without disturbing or temporarily stopping the experiment and thereby allows studying the digestion process in time.
Extrinsic Labeled β-Carotene vs Time of Both Diets
When using any extrinsic labeling technique in absorption studies, the assumption must be made that the label fully mixes with the compound and equally distributes in the lumen of the small intestine. This means that in each time period of the digestion process, the isotopic enrichment of β-carotene in the filtrates should be the same as in the diet. However, during the 6 h of the TIM-1 experiment, the isotopic enrichments of β-carotene in the ileal filtrate in mixed diet runs increased and in oil diet runs decreased compared with the jejunal filtrate, which indicates discrimination for micellarization. This enrichment pattern can be explained by retarded equilibration in the mixed diet run, where the labeled β-carotene is bound and captured by the complex matrix and available later in time for digestion than in the oil diet. The isotopic enrichment of β-carotene with [13C10]β-carotene in filtrates was not constant over time. This implies that the labeled β-carotene behaved differently from the β-carotene incorporated into the matrices of vegetables and fruits high in β-carotene and behaved differently from the matrix of supplemental β-carotene in oil. The labeled β-carotene dissolved in oil was not equally distributed over time in the bioaccessible fractions, which means that during the experiment, it did not fully mix with dietary β-carotene. As suggested by the average filtrate over 6 h for the oil diet, which was 99%, the extrinsic labeling technique could be used to measure absorption in vivo if the matrix of the homogenized diet were comparable with the oily matrix of extrinsic labeled β-carotene. However, the different isotopic enrichments of β-carotene in the ileal filtrate and jejunal filtrate in the oil diet suggested different micellarization patterns for dietary β-carotene in oil and labeled β-carotene in oil.
Additional experiments are required to confirm and explain this result, which may be influenced by dissolving of the capsule, and to investigate the reproducibility of the measured isotopic enrichments in these samples with very low amounts of labeled and unlabeled β-carotene.
The advantage of this TIM-1 dynamic model is that it can address mixing effects and partitioning effects of labeled fat-soluble compounds during the digestion process. Therefore, this in vitro model could provide significant value for checking assumptions in distribution of labeled compounds in in vivo labeling studies.
In summary, the food matrix effects on bioaccessibility of β-carotene could be measured using TIM-1 and were consistent with in vivo data. The labeled β-carotene either did not fully equilibrate with the β-carotene in the food matrix or else followed a different micellarization pattern. The application of extrinsically labeled β-carotene was not confirmed.
Acknowledgments
We thank Hans Kooijman at TNO for performing the runs in TIM-1 and Tineke van Roekel-Jansen at Wageningen University for performing many analyses.
We acknowledge grant support from the Dutch Dairy Association and from the National Institutes of Health in the United States (R01 CA101052).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- U.S. Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc; National Academy Press: Washington, DC. 2001; pp 82–161. [PubMed] [Google Scholar]
- Food and Agriculture Organization /World Health Organization Joint Expert Consultation. Vitamin and mineral requirements in human nutrition, 2nd ed.; WHO Press: Geneva. 2004; pp 17–44. [Google Scholar]
- van het Hof K.; West C. E.; Weststrate J. A.; Hautvast J. G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [DOI] [PubMed] [Google Scholar]
- Veda S.; Platel K.; Srinivasan K. Enhanced bioaccessibility of β-carotene from yellow-orange vegetables and green leafy vegetables by domestic heat processing. Int. J. Food Sci. Technol. 2010, 45, 2201–2207. [Google Scholar]
- Minekus M.Development and validation of a dynamic model of the gastrointestinal tract. Doctoral thesis, University of Utrecht, Utrecht, the Netherlands. May 28, 1998; pp 1–174. [Google Scholar]
- Minekus M.; Marteau P.; Havenaar R.; Huis in ‘t Veld J. H. J. A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. ATLA, Altern. Lab. Anim. 1995, 23, 197–209. [Google Scholar]
- Minekus M.; Lelieveld J.; van den Berg H.. A dynamic model of the stomach and small intestine to study the bio-accessibility of carotenoids from vegetables and the effect of processing. In Abstracts of the Bioavailability Congress, Interlaken, Switzerland, 2001; pp 111. [Google Scholar]
- Minekus M.; Jelier M.; Xiao J.-Z.; Kondo S.; Iwatsuki K.; Kokubo S.; Bos M.; Dunnewind B.; Havenaar R. Effect of partially hydrolyzed guar gum (PHGG) on the bioaccessibility of fat and cholesterol. Biosci. Biotechnol. Biochem. 2005, 69, 932–938. [DOI] [PubMed] [Google Scholar]
- Gervais R.; Gagnon F.; Kheadr E. E.; Van Calsteren R.-M.; Farnworth E. R.; Fliss I.; Chouinard P. Y. Bioaccessibility of fatty acids from conjugated linoleic acid-enriched milk and milk emulsions studied in a dynamic in vitro gastrointestinal model. Int. Dairy J. 2009, 19, 574–581. [Google Scholar]
- Arkbåge K.; Verwei M.; Havenaar R.; Witthoft C. Bioaccessibility of folic acid and (6S)-5-methyltetrahydrofolate decreases after addition of folate-binding protein to yogurt as studied in a dynamic in vitro gastrointestinal model. J. Nutr. 2003, 133, 3678–3683. [DOI] [PubMed] [Google Scholar]
- Verwei M.; Freidig A. P.; Havenaar R.; Groten J. P. Predicted serum folate concentrations based on in vitro studies and kinetic modeling are consistent with measured folate concentrations in humans. J. Nutr. 2006, 136, 3074–3078. [DOI] [PubMed] [Google Scholar]
- Deat E.; Blanquet-Diot S.; Jarrige J.-F.; Denis S.; Beyssac E.; Alric M. Combining the dynamic TNO-gastrointestinal tract system with a Caco-2 cell culture model: Application to the assessment of lycopene and α-tocopherol bioavailability from a whole food. J. Agric. Food Chem. 2009, 57, 11314–11320. [DOI] [PubMed] [Google Scholar]
- Richelle M.; Sanchez B.; Tavazzi I.; Lambelet P.; Bortlik K.; Williamson G. Lycopene isomerisation takes place within enterocytes during absorption in human subjects. Br. J. Nutr. 2010, 103, 1800–1807. [DOI] [PubMed] [Google Scholar]
- Van Loo-Bouwman C. A.; Naber T. H. J.; van Breemen R. B.; Zhu D.; Dicke H.; Siebelink E.; Hulshof P. J. M.; Russel F. G. M.; Schaafsma G.; West C. E. Vitamin A equivalency and apparent absorption of β-carotene in ileostomy subjects using a dual-isotope dilution technique. Br. J. Nutr. 2010, 103, 1836–1843. [DOI] [PubMed] [Google Scholar]
- Verwei M.; Arkbage K.; Havenaar R.; van den Berg H.; Withoft C.; Schaafsma G. Folic acid and 5-methyltetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model. J. Nutr. 2003, 133, 2377–2383. [DOI] [PubMed] [Google Scholar]
- Broekmans W. M. R.; Berendschot T. T. J. M.; Klöpping-Ketelaars I. A. A.; de Vries A. J.; Goldbohm R. A.; Tijburg L. B. M.; Kardinaal A. F. M.; van Poppel G. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am. J. Clin. Nutr. 2002, 76, 595–603. [DOI] [PubMed] [Google Scholar]
- Khan N. C.; West C. E.; de Pee S.; Bosch D.; Phuong H. D.; Hulshof P. J. M.; Khoi H. H.; Verhoef H.; Hautvast J. G. A. J. The contribution of plant foods to the vitamin A supply of lactating women in Vietnam: a randomized controlled trial. Am. J. Clin. Nutr. 2007, 85, 1112–1120. [DOI] [PubMed] [Google Scholar]
- Lugtenburg J.; Creemers A. F. L.; Verhoeven M. A.; van Wijk A. A. C.; Verdegem P. J. E.; Monnee M. C. F.; Jansen F. J. H. M. Synthesis of 13C labeled carotenoids and retinoids. Pure Appl. Chem. 1999, 71, 2245–2251. [Google Scholar]
- van Breemen R. B.; Nikolic D.; Xu X.; Xiong Y.; van Lieshout M.; West C. E.; Schilling A. B. Development of a method for quantification of retinol and retinyl palmitate in human serum using high-performance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. J. Chromatogr. A 1998, 794, 245–251. [DOI] [PubMed] [Google Scholar]
- Zhu D.; Wang Y.; Pang Y.; Liu A.; Guo J.; Bouwman C. A.; West C. E.; van Breemen R. B. Quantitative analyses of β-carotene and retinol in serum and feces in support of clinical bioavailability studies. Rapid Commun. Mass. Spectrom. 2006, 20, 2427–2432. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization /World Health Organization. Requirements of vitamin A, iron, folate and vitamin B12. FAO/WHO Joint Expert Consultation. FAO Food and Nutrition Series, no. 23; FAO: Rome, 1988; pp 16–32. [Google Scholar]
- Garrett D. A.; Failla M. L.; Sarama R. J. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J. Agric. Food Chem. 1999, 47, 4301–4309. [DOI] [PubMed] [Google Scholar]
- Miller D. D.; Schricker B. R.; Rasmussen R. R.; van Campen D. An in vitro method for estimation of iron availability from meals. Am. J. Clin. Nutr. 1981, 34, 2248–2256. [DOI] [PubMed] [Google Scholar]
- Ornalas-Paz J.; De J.; Failla M. L.; Yahia E. M.; Gardea-Bejar A. Impact of the stage of ripening and dietary fat on in vitro bioaccessibility of β-carotene in ‘Ataulfo’ mango. J. Agric. Food Chem. 2008, 56, 1511–1516. [DOI] [PubMed] [Google Scholar]
- Garrett D. A.; Failla M. L.; Sarama R. J. Estimation of carotenoid bioavailability from fresh stir-fried vegetables using an in vitro digestion/caco-2 cell culture model. J. Nutr. 2000, 11, 574–580. [DOI] [PubMed] [Google Scholar]
- Huo T.; Ferruzzi M. G.; Schwartz S. J.; Failla M. L. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J. Agric. Food Chem. 2007, 55, 8950–8957. [DOI] [PubMed] [Google Scholar]
- O’Connell O. F.; Ryan L.; O’Brien N. M. Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr. Res. 2007, 27, 258–264. [Google Scholar]
- Veda S.; Platel K.; Srinivasan K. Varietal differences in the bioaccessibility of β-carotene from mango (Mangifera indica) and papaya (Carica papaya) fruits. J. Agric. Food Chem. 2007, 55, 7931–7935. [DOI] [PubMed] [Google Scholar]
- Reboul E.; Richelle M.; Perrot E.; Desmoulins-Malezet C.; Pirisi V.; Borel P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [DOI] [PubMed] [Google Scholar]
- Failla M. L.; Thakkar S. K.; Kim J. Y. In vitro bioaccessibility of β-carotene in orange fleshed sweet potato (Ipomoea batatas, Lam.). J. Agric. Food Chem. 2009, 57, 10922–10927. [DOI] [PubMed] [Google Scholar]
- Veda S.; Kamath A.; Platel K.; Begum K.; Srinivasan K. Determination of bioaccessibility of β-carotene in vegetables by in vitro methods. Mol. Nutr. Food Res. 2006, 50, 1047–1052. [DOI] [PubMed] [Google Scholar]
- Thakkar S. K.; Failla M. L. Bioaccessibility of pro-vitamin A carotenoids is minimally affected by non pro-vitamin A xanthophylls in maize (Zea mays sp.). J. Agric. Food Chem. 2008, 56, 11441–11446. [DOI] [PubMed] [Google Scholar]
- Ferruzzi M. G.; Failla M. L.; Schwartz S. J. Assessment of degradation and intestinal cell uptake of carotenoids and chlorophyll derivatives from spinach puree using an in vitro digestion and Caco-2 human cell model. J. Agric. Food Chem. 2001, 49, 2082–2089. [DOI] [PubMed] [Google Scholar]
- Ryan L.; O’Connell O.; O’Sullivan L.; Aherne S. A.; O’Brien N. M. Micellarisation of carotenoids from raw and cooked vegetables. Plant Foods Hum. Nutr. 2008, 63, 127–133. [DOI] [PubMed] [Google Scholar]
- Thakkar S. K.; Maziya-Dixon B.; Dixon A. G. O.; Failla M. L. β-Carotene micellarization during in vitro digestion and uptake by caco-2 cells is directly proportional to β-carotene content in different genotypes of cassava. J. Nutr. 2007, 137, 2229–2233. [DOI] [PubMed] [Google Scholar]
- Thakkar S. K.; Huo T.; Maziya-Dixon B.; Failla M. L. Impact of style of processing on retention and bioaccessibility of β-carotene in cassava (Manihot esculanta, Crantz). J. Agric. Food Chem. 2009, 57, 1344–1348. [DOI] [PubMed] [Google Scholar]
- Hedrén E.; Diaz V.; Svanberg U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. [DOI] [PubMed] [Google Scholar]
- Hedrén E.; Mulokozi G.; Scanberg U. In vitro accessibility of carotenes from green leafy vegetables cooked with sunflower oil or red palm oil. Int. J. Food Sci. Nutr. 2002, 53, 445–453. [DOI] [PubMed] [Google Scholar]
- Priyadarshani A. M. B.; Chandrika U. G. Content and in-vitro accessibility of pro-vitamin A carotenoids from Sri Lankan cooked non-leafy vegetables and their estimated contribution to vitamin A requirements. Int. J. Food Sci. Nutr. 2007, 58, 659–667. [DOI] [PubMed] [Google Scholar]
- Bengtsson A.; Alminger M. L.; Svanberg U. In vitro bioaccessibility of β-carotene from heat-processed orange-fleshed sweet potato. J. Agric. Food Chem. 2009, 57, 9693–9698. [DOI] [PubMed] [Google Scholar]
- Mulokozi G.; Hedren E.; Svanberg U. In vitro accessibility and intake of β-carotene from cooked green leafy vegetables and their estimated contribution to vitamin A requirements. Plant Foods Hum. Nutr. 2004, 59, 1–9. [DOI] [PubMed] [Google Scholar]



