Abstract
AMPK is an evolutionarily conserved energy sensor important for cell growth, proliferation, survival and metabolic regulation. Active AMPK inhibits biosynthetic enzymes like mTOR and acetyl CoA carboxylase (required for protein and lipid synthesis, respectively) to ensure that cells maintain essential nutrients and energy during metabolic crisis. Despite our knowledge about this incredibly important kinase, no specific chemical inhibitors are available to examine its function. However, one small molecule known as Compound C (also called dorsomorphin) has been widely used in cell-based, biochemical and in vivo assays as a selective AMPK inhibitor. In nearly all these reports including a recent study in glioma, the biochemical and cellular effects of Compound C has been attributed to its inhibitory action towards AMPK. While examining the status of AMPK activation in human gliomas, we observed that glioblastomas (GBMs) express copious amount of active AMPK. Compound C effectively reduced glioma viability in vitro both by inhibiting proliferation and inducing cell death. As expected, Compound C inhibited AMPK; however, all the antiproliferative effects of this compound were AMPK-independent. Instead, Compound C killed glioma cells by multiple mechanisms including activation of the Calpain/Cathepsin pathway, inhibition of AKT, mTORC1/C2, cell cycle block at G2M and induction of necroptosis and autophagy. Importantly, normal astrocytes were significantly less susceptible to Compound C. In summary, Compound C is an extremely potent anti-glioma agent but we suggest that caution should be taken in interpreting results when this compound is used as an AMPK inhibitor.
Keywords: Glioma, Compound C, AMPK
Introduction
AMP activated protein kinase (AMPK) is a serine/threonine kinase and a molecular hub for cellular metabolic control. It is a heterotrimer of catalytic α, and regulatory β and γ subunits. Mammals express two α (α1, α2), two β (β1, β2) and three γ subunits (γ1, γ2 and γ3) in a tissue specific manner (1-4). Falling energy (ATP) levels increase cellular AMP:ATP ratio resulting in increased AMP binding to AMPK with consequent phosphorylation and activation of AMPK α subunits. Full activation of AMPK requires specific phosphorylation of the α subunit at Thr172 by upstream kinases – LKB1, CAMKKβ and probably other kinases (1, 5, 6). AMPK activation is crucially important for restoring intracellular energy balance via AMPK-dependent inhibition of energy-consuming biosynthetic processes and the activation of reactions that produce ATP. Because AMPK inhibits biosynthetic pathways through its inhibition of mTOR and acetyl Co-A carboxylase (ACC), many studies correlate pharmacological AMPK activation by two indirect AMPK activators (AICAR and metformin) with reduced cancer cell proliferation. (7-11).
Compound C (6-[4-(2-Piperidin-1-ylethoxy) phenyl]-3-pyridin-4-ylpyrazolo [1,5-a]pyrimidine) is the only available agent that is used as a cell-permeable AMPK inhibitor. It has been used to rescue the antiproliferative actions of AICAR and metformin (12, 13), although the effect of Compound C alone on cell proliferation is not well documented. Surprisingly, this compound (also known as dorsomorphin) is also used as a selective inhibitor of the BMP pathway (14, 15). Indeed, in an exhaustive study of kinase specificities of inhibitors, Compound C was found to inhibit a number of kinases other than AMPK (16, 17).
Despite the existing controversy about its selectivity, Compound C is still being used as an AMPK inhibitor. In fact, in a recent study, Compound C was used as an AMPK inhibitor in vitro and in vivo to effectively reduce proliferation and growth of astrocytic tumors (18). To address the controversy and definitively determine if there is a molecular link between pharmacological AMPK inhibition by Compound C and cell proliferation, we conducted a pharmaco-genetic study. We demonstrate that Compound C is a potent cytotoxic agent that inhibits glioma proliferation in vitro through multiple mechanisms independent of AMPK. While our findings highlight the effectiveness of Compound C as an anti-glioma agent, it also warrants the development of specific pharmacological AMPK inhibitors to investigate the function of physiologically active AMPK in cancer.
Materials and Methods
Cell Culture
T98G, A172 and U87 cells were obtained from ATCC in 2012, expanded and frozen down in several aliquots. Each aliquot was thawed and used for no more than six months. ATCC uses Promega PowerPlex system to authenticate their cell lines. These cell lines were not re-authenticated by our laboratory. All glioma cells and normal astrocytes were cultured in DMEM with 10% FCS. Human primary GBM spheres were established at Ohio State University under an institutional review board-approved protocol according to NIH guidelines. Cells were maintained in DMEMF/12 supplemented with B27, EGF (10ng/ml), bFGF (10 ng/ml), Glutamax and heparin (5 mg/ml). For proliferation and viability analysis, direct counting using Trypan blue method and also a fluorescence-based method (Cell-titer-fluor; Promega) were employed. Drugs were added 24 hours post-seeding and cell viability was determined at indicated times.
Reagents
The following reagents were used at doses indicated and as described in the text and figure legends. M7GTP Sepharose (GE Healthcare), Protein A agarose (Millipore), 3MA, DMSO (Sigma), PI-RNase (BD Biosciences), ZVAD (Promega), ALLN and Compound C (EMD Chemicals).
shRNA and lentivirus
The AMPKβ1 shRNA clone (TRCN0000004770) and the nontarget hairpin were purchased from Lenti-shRNA Library Core, CCHMC. The 293T cells used to generate the shRNA lentivirus supernatant were cultured in DMEM with 10% FBS. Briefly 293T cells were co-transfected with pLKO.1 (transfer vector), Δ8.9 and VSVG vectors by Fugene HD (Roche) according to the manufacturer's instructions. The viral supernatants were collected every 24 hours for three days after the initial medium change 16 hrs of post transfection. For Lentiviral infection, cells were infected overnight with viral particles in the presence of 8ug/ml polybrene and antibiotic selection was started 48 hours post infection. Stable clones and cell populations were selected in puromycin (1 μg/ml for A172 or U87 and 2 μg/ml for T98G) and gene knockdown was assayed by immunoblotting.
Transfection
Glioma cells were transfected with pBABE puro (control) and pBABE puro Myr-Akt plasmids using jetPRIME transfection reagent (Polypus-transfection, France) following manufacturer's instructions.
Immunoblot analysis and CAP binding assay
Western blot analysis was carried out following standard methods. Glioma cells were lysed with RIPA lysis buffer (20mM Tris, 10 mM EGTA, 40mM b-glycerophosphate, 1% NP40, 2.5mM MgCl2, 2mM orthovanadate 1 mM PMSF, 1 mM DTT and protease inhibitor cocktail). For CAP-binding assay, glioma cells were washed with cold PBS and lysed in NL buffer [50mM HEPES-KOH (pH 7.5), 150 mM NaCl, 0.5% NP40, 0.1 mM GDP, 2mM Na3VO4, protease inhibitor cocktail, 1mM EDTA, 10mM beta-glycerophosphate, and 50mM NaF]. Protein lysates were incubated with 20μl of (1:1) slurry of m7GDP-agarose at 4°C for 1h, washed 4 times with the lysis buffer, resuspended in Laemmli sample buffer, boiled, and resolved by SDS-PAGE. The following antibodies (all from Cell Signaling Technology) were used – phospho AMPKThr172, AMPK, AMPK β1/β12, phospho ACCSer79, ACC, phospho S6Ser235/236, phospho 4EBP1Thr37/46, 4EBP1, mTOR, phospho Akt Ser473, phospho Akt Thr308, Akt, phospho Erk1/2Thr202/Tyr204, PARP, LC3, P62, Actin and Tubulin. Detection was performed using anti-rabbit or anti-mouse HRP-linked secondary antibodies (Cell Signaling Technology, Beverly, MA) followed by Chemiluminescence (Millipore, Billerica, MA).
Cell Cycle analysis
1.5×105 glioma cells (control) and treated with Compound C (24h) were trypsinized, washed with cold PBS, fixed with 70% cold ethanol for 1 hour and stained with propidium iodide (PI-RNAse solution, BD) for 15 minutes in the dark. Cell cycle analysis was done in a FACSCAN analyzer (BD).
Apoptosis/Necrosis Assay
Simultaneous detection of both apoptotic and necrotic cells in a single assay was done with Apoptosis and Necrosis Quantification Kit (Biotium). Quantitation Kit features Annexin V labeled with fluorescein (FITC) for staining of apoptotic cells with green fluorescence and Ethidium Homodimer III (EthD-III), a highly positively charged nucleic acid probe, which stains necrotic cells and late apoptotic cells with red fluorescence. 3×105 glioma cells (control) and treated with Compound C (72h) were harvested via trypsinization and washed with PBS. Assay protocol was as per manufacturer's instructions. Staining was followed by flow cytometry acquisition (FACSCAN analyzer-BD) of the cells to measure fluorescence in FITC and propidium iodide channels. Data was analyzed by BD FACSDiva software.
Colony Formation Assay
Minimal cell numbers required for colony formation by three types of glioma cells were optimized. Accordingly, 2×103A172 cells or 1x103 U87 cells or 0.5 ×103 T98G were seeded onto six-well culture plates in triplicates. Next day, cells were treated with Compound C with indicated doses. After a change of fresh medium 72h later, the cells were allowed to form colonies for 14 days in the absence of drug. Following removal of the medium, the wells were rinsed twice with PBS and 0.05% methylene blue solution (prepared in 50% methanol) was added to each well. Plates were incubated for 30 min at room temperature to facilitate staining of the colonies. After three rinses with distilled water, the plates were dried, photographed and colonies were counted.
Cell migration assay
Glioma cells were grown to confluence and a uniform scratch was made using a 200 ml pipet tip. Floating cells were discarded by changing the existing medium with fresh growth medium containing either DMSO (vehicle control) or Compound C. The same scratch area was photographed with a digital camera attached to a Nikon microscope. The distance between the edges of the cells migrating from two sides was measured by the Image J software.
Statistical analysis
Student's t test was used to calculate statistical significance with p < 0.05 representing a statistically significant difference.
Results
Compound C inhibits AMPK activity and proliferation of human glioma cells
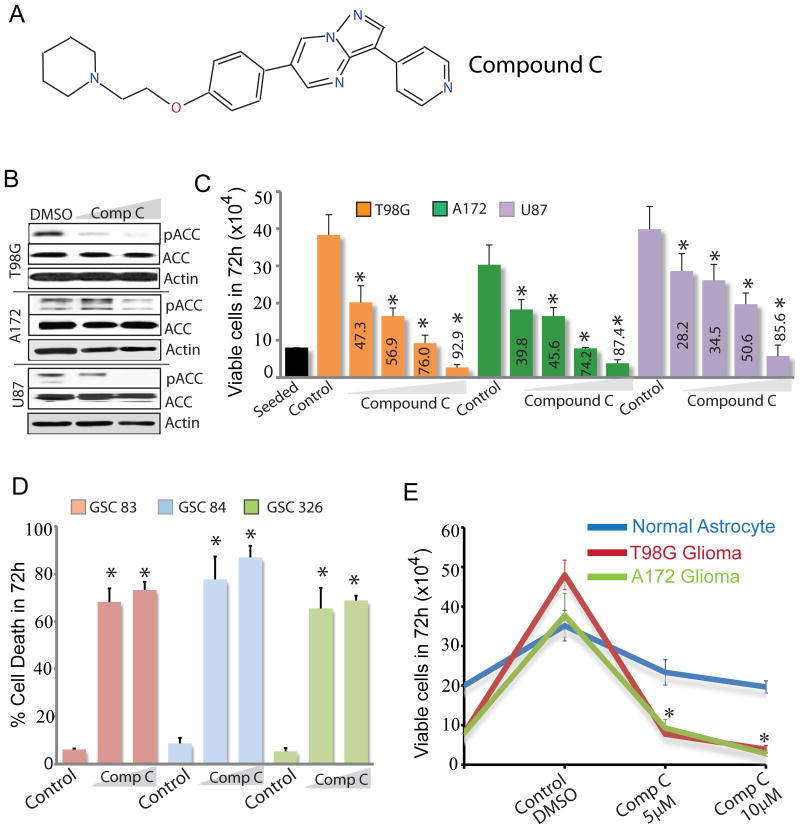
Cancer cells in solid tumors including gliomas undergo sweeping metabolic reprogramming during the process of tumorigenesis (2). Because AMPK is a key regulator of cellular energy metabolism and is activated during metabolic stress to increase cell survival, we examined if pharmacological AMPK inhibition blocks glioma cell proliferation. To test this we measured glioma cell viability in the absence or presence of the AMPK inhibitor Compound C (Fig 1A). Compound C inhibited AMPK kinase activity in a dose dependent manner in multiple glioma cells as observed by reduced phosphorylation of the canonical AMPK substrate ACC (Fig 1B). Compound C potently inhibited proliferation of established glioma cell lines T98G, A172 and U87 (Fig 1C) as well as U87 cells overexpressing the oncogene EGFR and its variant form EGFRvIII (not shown). It also significantly inhibited proliferation of a pediatric GBM cell line (not shown). In fact, the inhibitory effect of Compound C on cell proliferation was considerably more than that of the AMPK activators AICAR and metformin (not shown). Prolonged exposure to Compound C killed nearly 100% of glioma cell lines (Fig S1A). Compound C also potently killed primary GBM spheres established from freshly resected glioma tissue (Fig 1D). Importantly, the effect of Compound C on normal astrocytes was significantly less than that on glioma cells (Fig 1E). In colony formation assays, 5 μM Compound C significantly prevented formation T98G, A172 and U87 colonies (Fig S1B-E). Based on the potent cytotoxic effect of this agent on glioma cells, it is not surprising that in contrast to previous studies, Compound C did not reverse the antiproliferative effects of AICAR and metformin (Fig S1F). Collectively, our results demonstrate that Compound C is an effective anti-glioma agent with considerably less toxicity towards normal cells.
Figure 1. Compound C is a potent anti-glioma agent.
(A) Chemical structure of Compound C. (B) Immunoblots showing pACC levels in glioma cells treated with DMSO (control) or Compound C. (C) Histogram showing the dose-dependent effect of Compound C (1, 2.5, 5 and 10μM) on the viability of three glioma cell lines. Numbers inside bars represent % dead cells. (D) Histogram showing the dose-dependent effect of Compound C (5 and 10μM) on the viability of three patient-derived primary GBM sphere cultures. (E) Proliferation assay showing the anti-proliferative effect of Compound C on glioma cells relative to normal astrocytes. * P ≤ 0.001. Data shown is representative of three to five independent experiments.
Compound C inhibits glioma proliferation independent of AMPK
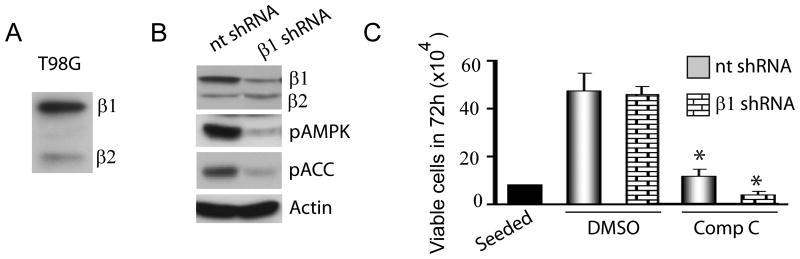
We next questioned if Compound C exerts anti-glioma action by inhibiting AMPK. To examine this we reduced AMPK activity by genetic means. The regulatory β subunits of AMPK play an obligatory role in the stability of the catalytic alpha subunits and AMPK complex formation. While examining expression of AMPK subunits in glioma cells, we observed that the regulatory β1 subunit is expressed at 80 -90% higher levels than the β2 subunit (Fig 2A). Indeed shRNA-mediated knockdown of the β1 subunit reduced 80-90% of basal AMPK activity and phosphorylated ACC levels (Fig 2B). To examine if Compound C requires AMPK to suppress proliferation, we treated control (nt; nontarget) and AMPKβ1shRNA glioma cells with 10 μM Compound C. Surprisingly, Compound C inhibited proliferation regardless of AMPK (Fig 2C) and in fact, AMPK-silenced glioma cells were more sensitive to the antiproliferative effects of Compound C. This genetic data clearly indicates that AMPK inhibition is not a mechanism by which Compound C inhibits glioma proliferation.
Figure 2. The antiproliferative effect of Compound C is AMPK-independent.
Immunoblots showing the expression of the AMPK β1 and β2 subunits in T98G glioma cells (A) and the effects of silencing the β1 subunit in reducing AMPK activity (B). Actin was used as a loading control. (C) Proliferation assay showing the effects of Compound C (5 and 10 μM) in control (nt) and β1 shRNA expressing T98G glioma cells. * P ≤ 0.001. Data shown is representative of three independent experiments.
Compound C inhibits glioma proliferation by multiple mechanisms
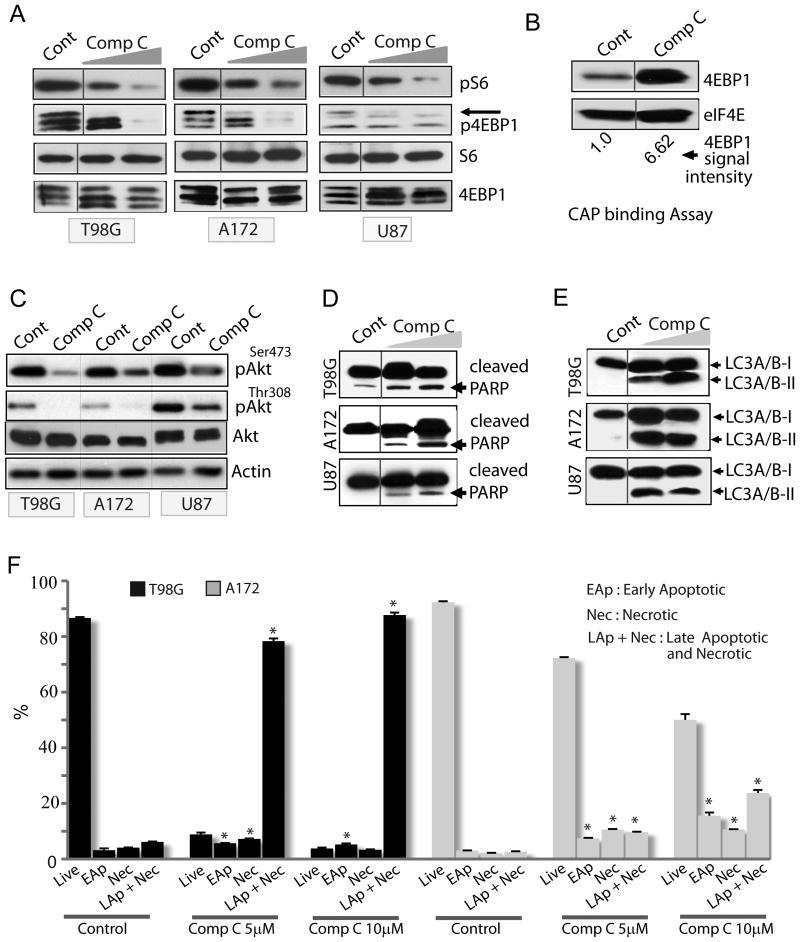
As Compound C inhibited proliferation independent of AMPK, we sought to determine its mechanism/s of inhibition. Signaling through the mTOR kinase pathway is crucial for proliferation and growth of cancer cells including gliomas (19, 20). The mTORC1 complex (containing the mTOR partner raptor) mediates its downstream effects through phosphorylation of S6 kinase1 and the protein translation initiation factor binding protein 4EBP1 (20, 21). S6K1 in turn phosphorylates the ribosomal protein S6 (22). Treatment of glioma cells with Compound C significantly inhibited S6 and 4EBP1 phosphorylation (Fig 3A) indicating that Compound C is a potent mTORC1 inhibitor. Dephosphorylated 4EBP1 sequesters the translation initiation factor eIF4E (also called CAP binding protein), thereby preventing association of eIF4E with eIF4G, thus inhibiting cap-dependent protein translation (22). We immunoprecipitated eIF4E from glioma cells treated with AMPK modulators and examined the amount of 4EBP1 bound to eIF4E. Consistent with our immunoblot results, we observed significant amount of 4EBP1 bound to eIF4E in Compound C treated cells (Fig 3B).
Figure 3. Compound C is a pleiotropic agent that affects multiple cellular pathways.
(A) Immunoblots showing the effect Compound C (5 and 10 μM) on phosphorylation of mTOR effectors (S6 and 4EBP1). In (B), eIF4E was immunoprecipitated with m7GDP-sepharose and bound 4EBP1 was detected with 4EBP1 antibody. (C-E) Immunoblots showing the effects of Compound C (5 and 10μM) in glioma cells on Akt phosphorylation (C), apoptosis (D) and autophagy (E); Note, increased processing of LC3A/BI to LC3A/BII in (E). DMSO was used as control. (F) Flow cytometry-based apoptosis/necrosis analysis of glioma cells treated with Compound C (5μM and 10μM). DMSO was used as control. * P ≤ 0.005. Data is representative of two to three independent experiments.
Akt phosphorylates and inhibits TSC2 to enhance signaling through mTORC1 (10). Thus, while the mTORC1 complex functions downstream of Akt, the mTORC2 complex (containing the mTOR partner rictor) phosphorylates and activates Akt, thus operating upstream of Akt. We examined if Compound C has any effect on mTORC2 as well. Indeed, Compound C strongly inhibited Akt phosphorylation at serine 473, the mTORC2 target site (Fig 3C). Because Akt phosphorylation by PI3K is crucial for glioma proliferation, growth and survival, we examined if the PI3K site on Akt is also affected by Compound C. Compound C robustly inhibited PI3K-mediated Akt phosphorylation at threonine 308 (Fig 3C). It however, did not inhibit MEK activity towards Erk since Erk1/2 phosphorylation was not reduced by Compound C (not shown).
To examine the mechanism of cell death, we examined if Compound C caused apoptosis. Consistent with other studies (23, 24), Compound C induced apoptosis as evident from Caspase-dependent cleavage of PARP in Compound C -treated glioma cells (Fig 3D). However, the extent of apoptosis varied among cell lines. Another protective mechanism that allows cancer cell survival is autophagy – a mechanism that can become destructive if extensive and unchecked (25). Because Akt and mTORC1 inhibits autophagy, and Compound C inhibited both kinases, we examined if autophagy is activated by Compound C in glioma cells. owever, Compound C significantly enhanced autophagy as shown by increased conversion of the microtubule-associated light chain 3 protein LC3A/B-I to LC3A/B-II (Fig 3E). To confirm this result, we transfected glioma cells with plasmid encoding LC3-EGFP before treating with Compound C. Induction of autophagy changes the diffuse cytoplasmic localization of LC3 to distinct autophagic structures (also called puncta) (25, 26). Consistent with our immunoblot results, Compound C caused redistribution of LC3 to numerous autophagic puncta (Fig S2A). Autophagy is also associated with decreased abundance of autophagic substrates including p62. As expected, Compound C reduced p62 protein levels in glioma cells (Fig S2B). To explore if necrosis is also involved in Compound C's action and to more accurately quantify cell death, we examined both apoptosis and necrosis by a flow cytometry-based assay in which apoptotic cells are detected by FITC-Annexin V reactivity while necrotic cells are detected by ethidium homodimer III. We observed that Compound C induced cell death by both necrosis and apoptosis (Fig 3F). Taken together, our results show that Compound C blocks glioma cell proliferation by multiple mechanisms.
All anti-glioma effects of Compound C are independent of AMPK
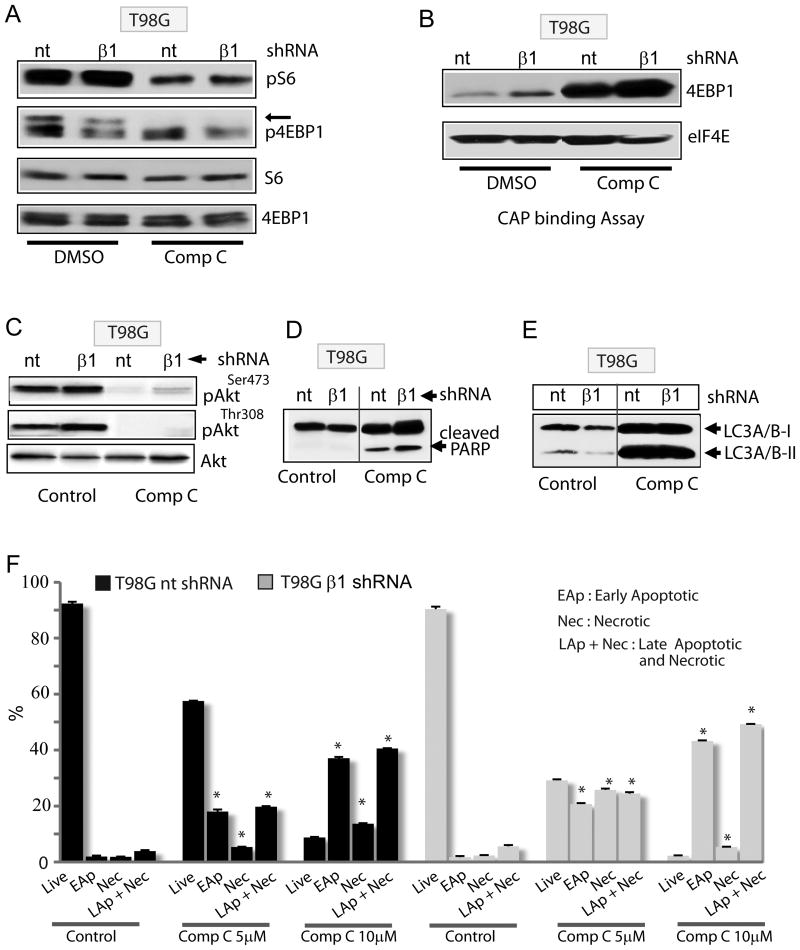
We next sought to determine whether some or all the above effects of Compound C are AMPK independent. Compound C reduced S6 and 4EBP1 phosphorylation similarly in control and AMPKβ1 knockdown cells (Fig 4A). Importantly, AMPK silencing did not block eIF4E binding to 4EBP1 (cap binding assay) in Compound C treated glioma cells (Fig 4B) suggesting that AMPK is not involved in Compound C's mTORC1 inhibition. Inhibition of Akt phosphorylation by Compound C both at serine 473 and threonine 308 was also similar in glioma cells expressing nontarget or β1 shRNA (Fig 4C) suggesting AMPK-independent inhibitory effects of this agent on mTORC2 and PI3K, respectively. In fact, Compound C-induced autophagy, apoptosis and necrosis also occurred similarly in control and AMPK-silenced glioma cells (Fig 4D-F, S2C).
Figure 4. The cellular effects of Compound C are AMPK-independent.
(A) Immunoblot showing the effect Compound C (10μM) on phosphorylation of mTOR effectors (S6 and 4EBP1) in control (nt) and AMPK β1-silenced glioma cells. In (B), eIF4E was immunoprecipitated with m7GDP-sepharose and bound 4EBP1 was detected with 4EBP1 antibody in control (nt) and AMPK β1-silenced glioma cells. (C-E) Immunoblots showing the effects of Compound C on Akt phosphorylation (C), apoptosis (D) and autophagy (E) in control (nt) and AMPK β1-silenced glioma cells. (F) Flow cytometry-based apoptosis/necrosis analysis of control (nt) and AMPK β1-silenced glioma cells treated with Compound C (5μM and 10μM). DMSO was used as control. * P ≤ 0.005. Data is representative of two to three independent experiments.
We then tested if pharmacological inhibition of autophagy or apoptosis could block Compound C's inhibitory action on cell viability. As expected, the autophagy inhibitor 3MA and the pan-Caspase inhibitor ZVAD reduced autophagy and apoptosis, respectively (Fig S2D, E). However, neither 3MA nor ZVAD was sufficient to rescue glioma cells treated with Compound C (Fig S2F). While ZVAD-treatment alone showed an expected increase in cell viability, 3MA alone caused considerable cell death suggesting that either blocking basal autophagy is detrimental to glioma cell survival or 3MA itself is toxic to the glioma cells we tested (Fig S2F).
Because Compound C inhibited Akt phosphorylation, we explored whether expression of constitutively active Akt could block the anti-viability effect of Compound C. Transfection of glioma cells with myristylated Akt increased total Akt, phosphorylated Akt and phosphorylation of the Akt substrate GSK3β (Fig S3A). However, Compound C still killed glioma cells that expressed constitutively active Akt (Fig S3B). Collectively, our results suggest that Compound C is a potent anti-glioma agent that exerts multiple pleiotropic actions to reduce glioma viability in vitro independent of AMPK.
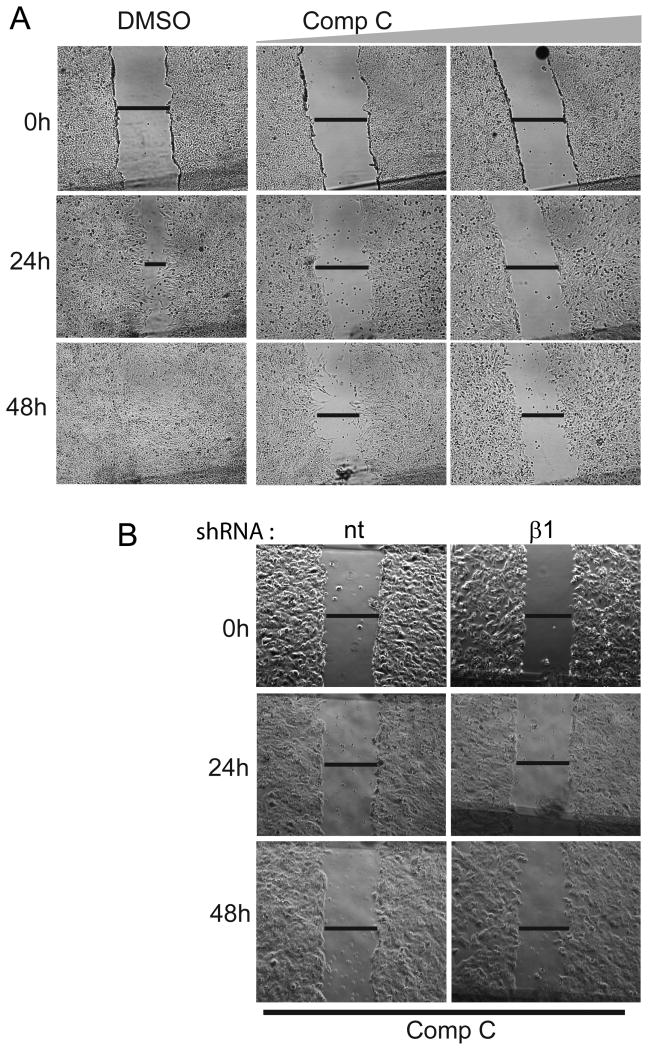
Compound C inhibits glioma cell migration independent of AMPK
AMPK has been shown to play a role in the migration of normal as well as cancer cells (27-29). In these studies AMPK function was examined by using either Compound C alone or in conjunction with AMPK silencing RNA. Because of the many AMPK-independent effects of Compound C that we observed in this study, we questioned whether inhibition of cell migration by Compound C is also AMPK-independent. Compound C strongly inhibited migration of T98G (Fig 5A), A172 and U87 glioma cells (data not shown). The effect was however not dose-dependent. The inhibition of migration was similar at 1 and 2.5 μM (not shown) as was with 5 and 10 μM (Fig 5A). While Compound C had little inhibitory effect on AMPK inhibition at 1 and 2.5 μM (not shown), it still inhibited migration suggesting that its effect is likely AMPK-independent. To definitively examine if AMPK inhibition was required for this process, we used AMPK-β1 silenced T98G cells. Compound C inhibited migration of control (nt shRNA) and AMPK-β1 silenced T98G cells similarly at all time points studied (Fig 5B), clearly indicating that the inhibitory effects of Compound C on cell migration is an AMPK-independent effect.
Figure 5. Compound C inhibits glioma cell migration independent of AMPK.
(A) Digital photographs of T98G glioma cells treated with DMSO (control) or Compound C (5 and 10μM), and (B) that of nt (control) and AMPK β1-silenced glioma T98G cells treated with Compound C (10μM) for indicated times. Note that control cells completely filled in the gap in 48 hours which was potently inhibited by Compound C in (A) and this inhibition was essentially similar in nt and β1 shRNA cells in (B). Data is representative of three independent experiments.
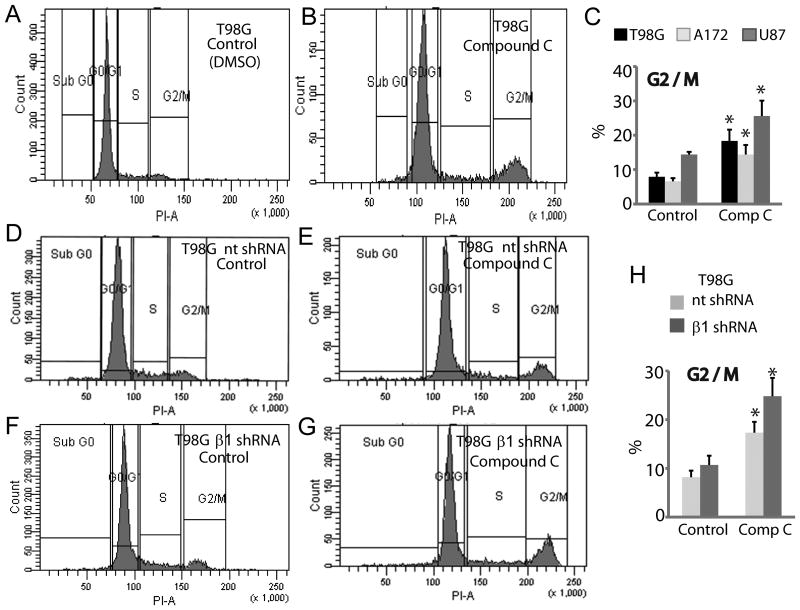
Compound C blocks glioma cell cycle at G2M independent of AMPK
We have shown that Compound C affects cell viability by inducing apoptosis and necrosis. To directly examine whether Compound C affects cell proliferation, we analyzed glioma cell cycle. Glioma cells were treated with Compound C or DMSO (control) for 24 hours and cell cycle analysis was conducted by flow cytometry. Compound C did not have any effect on the G0-G1 stage (Fig S4A) or S phase (Fig S4B). However, Compound C caused a consistent accumulation of all three glioma cells at G2M (Fig 6A-C). To examine if Compound C requires AMPK to induce cell cycle arrest, we treated control or AMPK knockdown cells with Compound C and analyzed cell cycle. AMPK silencing did not alter G0-G1 or S phase (Fig S4C, D), but consistent with AMPK's role in mitosis (30-35), AMPK knockdown cells showed a tendency towards G2-M accumulation. However, Compound C caused G2-M accumulation of glioma cells similarly in control and AMPK knockdown cells (Fig 6D-H). Collectively, our findings demonstrate that Compound C exerts cell cycle arrest in glioma cells independent of AMPK.
Figure 6. Compound C blocks glioma cell cycle at G2M independent of AMPK.
(A, B) Histograms and (C) quantitation of flow cytometry-based cell cycle analysis showing glioma cells in G2M phase that were treated for 24 hours with either DMSO (control) or Compound C (10μM). Histograms showing G2M stage occupancy of control (nt) (D, E) and AMPK β1-silenced (F, G) T98G cells treated with DMSO (control) or Compound C. (H) Quantitation of data shown in (D-G). * P < 0.001. Data shown is representative of two independent experiments.
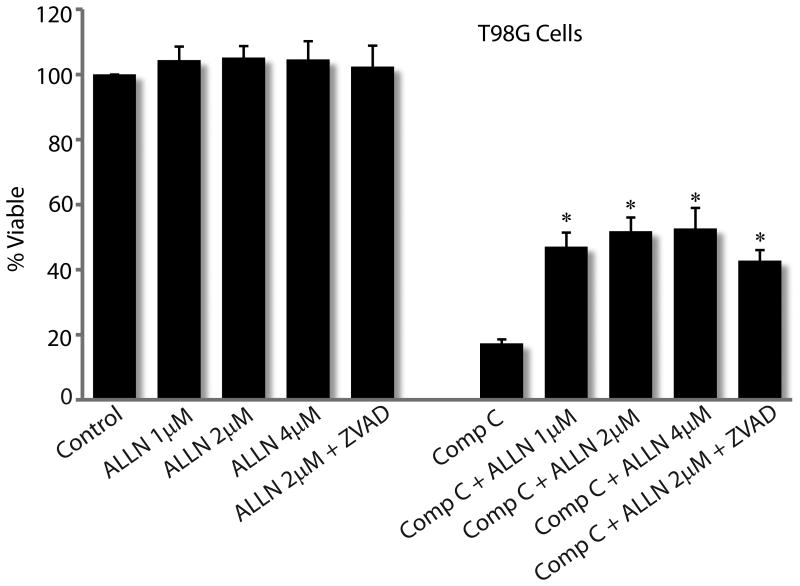
Inhibition of Calpain/Cathepsin-mediated cell death pathway partially rescues viability of Compound C treated glioma cells
Although the regulating mechanisms are still unclear, necrosis is an important mechanism of death in eukaryotic cells. Necrosis is induced by various stimuli including drugs like Smac mimetics and TNFα inhibitors. Recent studies have shown that activation of Calpain and Cathepsin proteases are involved in necrotic cell death. Since apoptosis and autophagy inhibitors failed to protect glioma cells from Compound C, and because we observed significant necrosis in Compound C treated glioma cells, we examined if Calpain and Cathepsin inhibitors rescue Compound C treated glioma cells. We used ALLN, a cell-permeable inhibitor of Calpain I, Calpain II, Cathepsin B and Cathepsin L at nanomolar concentrations. As shown in Fig 7, while Compound C alone reduced cell viability to about 17%, nearly 50% cells were alive when cells were treated with Compound C in combination with ALLN. When combined with ZVAD, ALLN did not increase viability of Compound C treated cells. This partial rescue by ALLN indicates that activation of a Calpain/Cathepsin-mediated pathway is an important mechanism by which Compound C induces glioma cell death.
Figure 7. A common Calpain/Cathepsin inhibitor partially rescues glioma cells from Compound C's inhibitory action.
Viability assay of T98G glioma cells treated with DMSO (control) or Compound C (5μM) in the presence of indicated doses of ALLN, a compound that inhibits Calpain I, Calpain II, cathepsin B and cathepsin L. * P < 0.001. Data shown is representative of two independent experiments.
Discussion
The role of physiologically active AMPK in cancer remains unknown. Because of AMPK's inhibitory effects on biosynthetic pathways and its effects on increasing insulin sensitivity (a desirable effect for the treatment of type II diabetes), efforts by both academia and industry are biased towards developing AMPK activators. As a result, unfortunately Compound C remains the only small molecule AMPK inhibitor that has been widely used to study AMPK signaling and various aspects of cell physiology including proliferation, survival and migration. The use of Compound C continues despite reports that it inhibits other kinases with a lower Km than AMPK (17). Indeed, Compound C alone is significantly cytotoxic (23) and the use of this compound to examine AMPK functions is not recommended (16).
To clearly establish that the cellular effects of Compound C are AMPK-independent, we conducted a pharmaco-genetic study in glioma cells. Our results firmly demonstrate that all cellular effects of Compound C are AMPK-independent. However, Compound C also proved to be one of the most potent anti-glioma agents among all the chemotherapy agents that we tested. These agents include mTOR Kinase inhibitors, PI3K inhibitors, DNA alkylating and microtubule disrupting agents. The primary mechanism/s by which Compound C kills cancer cells varies which likely depends on the type of cancer and the associated mutations in such cells. Others have shown that Compound C induces protective autophagy in U251 human glioma cells through AMPK-independent inhibition of Akt/mTOR pathway (36). However, in another study (37), Compound C inhibited proliferation of colorectal carcinoma cells through induction apoptosis and autophagy. Depending on the degree and the duration, autophagy can be protective or destructive to cells. Unlike the results of Vucicevic et al., (36) autophagy inhibitors (chloroquine, 3MA) failed to rescue glioma cells from the antiproliferative effects of Compound C suggesting that that autophagy induction by this agent is not protective. Our results are in contrast to the results of Vucicevic et al., and akin to the results of Yang et al (37).
Compound C also induced Caspase 3-mediated apoptosis in the glioma cells that we studied and this effect was AMPK-independent. This effect was also echoed in breast cancer cells where Compound C treatment independent of AMPK, lead to ceramide production and redistribution of Bax from the cytoplasm to the mitochondria (23). However, blocking apoptosis by the Pan-Caspase inhibitor Z-VAD failed to protect glioma cells of Compound C's effects suggesting that induction of apoptosis is only one of the many mechanisms by which Compound C kills glioma cells and that blocking apoptosis alone is not sufficient to block Compound C's antiproliferative effects.
In at least three studies, Compound C has been used to examine the role of AMPK in cell migration (27-29). In these studies although Compound C did inhibit cell migration, it was not examined if this effect was AMPK-dependent. Our studies undoubtedly demonstrate that this effect of Compound C does not require AMPK. In fact, it inhibited glioma cell migration at low doses (1 and 2.5 mM) that are insufficient to inhibit AMPK kinase activity. In most pharmacological studies, two indirect AMPK activators AICAR and metformin has been used to inhibit cancer cell proliferation and Compound C was shown to reverse the effects of AICAR and metformin (12, 13). We found that Compound C alone is an extremely potent antiproliferative agent and clearly does not reverse the effects of AICAR and metformin. Despite all the pleiotropic effects of Compound C, it is still being used as an AMPK inhibitor to examine cellular functions of AMPK in vitro and even in vivo (18). In this report (18), Compound C was used as an AMPK inhibitor, and at 10 μM it inhibited proliferation and glioma formation of human U87MG glioma cells in vivo. These Compound C studies were conducted to corroborate the genetic findings that AMPK activity is required for cancer cell proliferation. However, it was not examined if Compound C exerts the same antiproliferative effects in AMPK- silenced cells. Our pharmaco-genetic study clearly shows that although Compound C is a potent anti-glioma agent, AMPK is absolutely not required for its antiproliferative effects.
The cell cycle arrest of Compound C-treated glioma cells at G2M is in line with that observed for colorectal cancer cells (37) and is interesting in the context of glioma therapy. The DNA alkylating agent Temozolomide (TMZ) is routinely used in standard of care glioma therapy. The G2M arrest by Compound C is particularly attractive to TMZ therapy as the integrity of the G2/M checkpoint is a key determinant of TMZ cytotoxicity of glioblastoma cells (38). Interestingly, although DNA fragmentation is a hallmark of apoptotic cells that can appear in the sub-G1 fraction, we did not observe significant increase in the sub-G1 fraction of Compound C treated cells. Apoptotic cells in which DNA degradation is terminated at 50-300 kb fragments and does not proceed to internucleosomal-size fragments may not be identified as the sub-G1 cells as they are also weakly labeled in the TUNEL assay (39). Alternatively, some cells may undergo apoptosis without significant DNA digestion (40).
We finally show a novel mechanism of Compound C-mediated cell death. We observed that pharmacological inhibition of the Calpain/Cathepsin pathway partially blocked Compound C-induced death of glioma cells. This rescue although partial, was significant. Therefore, activation of this pathway is an important mechanism of cell death caused by Compound C. In summary, our results demonstrate that Compound C is a potent anti-glioma agent that kills cancer cells by multiple mechanisms, all of which are independent of AMPK.
Supplementary Material
Acknowledgments
This work was supported by the CancerFreeKids, Smith-Brinker Golf foundation, CCHMC Trustee Scholar grant and National Institute of Health (1R01NS075291-01A1) (to B.D).
Funding: This work is supported by grants awarded to B. Dasgupta by CancerFreeKids, Smith-Brinker Golf foundation, National Institute of Health (1R01NS075291-01A1).
Footnotes
Conflict of Interest: None
References
- 1.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–48. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardie DG. AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, et al. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276–95. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, et al. Ca/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Foretz M, Carling D, Guichard C, Ferré P, Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;273:14767–71. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- 8.Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci U S A. 2009;106:12932–7. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swinnen JV, Beckers A, Brusselmans K, Organe S, Segers J, Timmermans L, et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–8. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- 10.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 11.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isakovic A, Harhaji L, Stevanovic D, Markovic Z, Sumarac-Dumanovic M, Starcevic V, et al. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007;64:1290–302. doi: 10.1007/s00018-007-7080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang YC, Williams BR, Siegel JJ, Amon A. Identification of aneuploidy-selective antiproliferation compounds. Cell. 2011;144:499–512. doi: 10.1016/j.cell.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boergermann J, Kopf J, Yu P, Knaus P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int J Biochem Cell Biol. 2010;42:1802–7. doi: 10.1016/j.biocel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul BY, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bain J, Plater L, Elliott M, Shpiro N, Hastie C, Mclauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogt J, Traynor R, Sapkota GP. The specificities of small molecule inhibitors of the TGFss and BMP pathways. Cell Signal. 2011;23:1831–42. doi: 10.1016/j.cellsig.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Ríos M, Foretz M, Viollet B, Prieto A, Fraga M, Costoya JA, et al. AMPK activation by oncogenesis is required to maintain cancer cell proliferation in astrocytic tumors. Cancer Res. 2013;73:2628–38. doi: 10.1158/0008-5472.CAN-12-0861. [DOI] [PubMed] [Google Scholar]
- 19.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 20.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Livingstone M, Atas E, Meller A, Sonenberg N. Mechanisms governing the control of mRNA translation. Phys Biol. 2010;7:021001. doi: 10.1088/1478-3975/7/2/021001. [DOI] [PubMed] [Google Scholar]
- 23.Jin J, Mullen TD, Hou Q, Bielawski J, Bielawska A, Zhang X, et al. AMPK inhibitor Compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J Lipid Res. 2009;50:2389–97. doi: 10.1194/jlr.M900119-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vucicevic L, Misirkic M, Janjetovic K, Harhaji-Trajkovic L, Prica M, Stevanovic D, et al. AMP-activated protein kinase-dependent and-independent mechanisms underlying in vitro antiglioma action of compound C. Biochem Pharmacol. 2009;77:1684–93. doi: 10.1016/j.bcp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Fan QW, Cheng C, Hackett C, Feldman M, Houseman BT, Nicolaides T, et al. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal. 2010;3:ra81. doi: 10.1126/scisignal.2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–70. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano A, Kato H, Watanabe T, Min KD, Yamazaki S, Asano Y, et al. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12:583–90. doi: 10.1038/ncb2060. [DOI] [PubMed] [Google Scholar]
- 28.Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 2010;6:e1000954. doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frigo DE, Howe MK, Wittmann BM, Brunner AM, Cushman I, Wang Q, et al. CaM kinase kinase β-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 2011;71:528–37. doi: 10.1158/0008-5472.CAN-10-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, et al. Chemical genetic screen for AMPKα2 substrates uncovers a network of proteins involved in mitosis. Mol Cell. 2011;44:878–92. doi: 10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dasgupta B, Milbrandt J. AMP-activated protein kinase phosphorylates retinoblastoma protein to control mammalian brain development. Dev Cell. 2009;16:256–70. doi: 10.1016/j.devcel.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daub H, Olsen JV, Bairlein M, Gnad F, Oppermann FS, Körner R, et al. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell. 2008;31:438–48. doi: 10.1016/j.molcel.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–20. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 34.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–98. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 35.Bettencourt-Dias M, Giet R, Sinka R, Mazumdar A, Lock W, Balloux F, et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 2004;432:980–7. doi: 10.1038/nature03160. [DOI] [PubMed] [Google Scholar]
- 36.Vucicevic L, Misirkic M, Kristina J, Vilimanovich U, Sudar E, Isenovic E, et al. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy. 2011;7:40–50. doi: 10.4161/auto.7.1.13883. [DOI] [PubMed] [Google Scholar]
- 37.Yang WL, Perillo W, Liou D, Marambaud P, Wang P. AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J Surg Oncol. 2012;106:680–8. doi: 10.1002/jso.23184. [DOI] [PubMed] [Google Scholar]
- 38.Hirose Y, Berger MS, Pieper RO. Abrogation of the Chk1-mediated G2 checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res. 2001;61:5843–9. [PubMed] [Google Scholar]
- 39.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology : analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 40.Yuste VJ, Bayascas JR, Llecha N, Sanchez-Lopez I, Boix J, Comella JX. The absence of Oligonucleosomal DNA fragmentation during apoptosis of IMR-5 neuroblastoma cells. J Biol Chem. 2001;25:22323–31. doi: 10.1074/jbc.M100072200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.