Abstract
Objective
The major challenges in using amniotic fluid (AF) cultivation techniques to diagnose microbial invasion of the amniotic cavity (MIAC) are: 1) several days are typically required to obtain results, and 2) many organisms implicated in the pathogenesis of human disease are difficult to culture. Here, we compare the performance of AF culture with a novel technique for the diagnosis of MIAC that can provide results within eight hours by combining broad-range real-time polymerase chain reaction with electrospray ionization mass spectrometry (PCR/ESI-MS) to identify and quantify genomic material from bacteria and viruses in AF.
Methods
AF samples obtained by transabdominal amniocentesis from 142 women with preterm labor (PTL) and intact membranes were analyzed using cultivation techniques (aerobic, anaerobic and genital mycoplasmas) as well as PCR/ESI-MS. The prevalence and relative magnitude of intra-amniotic inflammation [AF Interleukin 6 (IL-6) concentration ≥ 2.6 ng/mL], acute histologic chorioamnionitis, spontaneous preterm delivery, and perinatal mortality were examined according to the results of these two tests.
Results
1) The prevalence of MIAC in patients with preterm labor and intact membranes was 7% using standard cultivation techniques and 12% using PCR/ESI-MS; 2) seven of ten patients with positive AF culture also had positive PCR/ESI-MS [≥17 genome equivalents per PCR reaction well (GE/well)] 3) patients with positive PCR/ESI-MS (≥17 GE/well) and negative AF cultures had significantly higher rates of intra-amniotic inflammation and histologic acute chorioamnionitis, shorter intervals to delivery [median (interquartile range-IQR)], and offspring at higher risk of perinatal mortality, than women with both tests negative [90% (9/10) vs. 32% (39/122); (p<0.001); 70% (7/10) vs. 35% (39/112); (p=0.04); 1 (IQR: <1 – 2) days vs. 25 (IQR: 5 – 51) days; (p=0.002); OR: 5.6; 95% CI: 1.4 – 22, respectively]; 5) there were no significant differences in these factors between patients with positive PCR/ESI-MS (≥17 GE/well) who had negative AF cultures compared to those with positive AF cultures; and 6) PCR/ESI-MS detected genomic material from viruses in two patients (1.4%).
Conclusion
1) Rapid diagnosis of intra-amniotic infection is possible using PCR/ESI-MS, which can provide results within 8 hours; 2) the combined use of biomarkers of inflammation and PCR/ESI-MS allows for the rapid identification of specific bacteria and viruses in women with preterm labor and intra-amniotic infection; and 3) this approach may allow for administration of timely and specific interventions to reduce morbidity attributed to infection-induced preterm birth.
Keywords: broad-range real-time polymerase chain reaction with electrospray ionization mass spectrometry, intra-amniotic inflammation, preterm delivery
Introduction
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide.1-6 Microbial invasion of the amniotic cavity (MIAC), determined with cultivation techniques, occurs in one of every four women who deliver preterm.7-43 Yet, this may be an underestimate since the diagnosis of MIAC has represented a challenge for decades.
Early investigation used transcervical catheters to evaluate the microbial state of the amniotic cavity;44-49 however, this approach was abandoned because of the problem of contamination. Thereafter, identification of bacteria by cultivation techniques in amniotic fluid (AF) retrieved by amniocentesis became the “gold standard” for the diagnosis of MIAC.10, 11, 15, 16, 19, 24, 42, 50-66 A major challenge using this approach is that culture results take several days to become available. This delay has led to the standard practice of initiating intravenous antimicrobial agents for patients with a positive Gram stain or indicators of inflammation prior to identifying the microorganism responsible for a suspected infection. 67-75 The latter assumes that inflammation is always due to infection and this is not the case.76-80
Rapid test results are needed to determine whether and which antimicrobial agents should be administered. Other decisions, such as the administration or discontinuation of tocolysis, timing of the delivery, etc., also depend on the availability of reliable information about the presence of microorganisms in the amniotic cavity. The application of polymerase chain reaction (PCR)-based techniques for the diagnosis of infectious diseases can provide results in time for rapid decision-making.81-88 Moreover, these techniques can improve the detection of microorganisms by identifying microbes which are difficult to culture, 34, 39, 42, 65, 89-102 and can provide information about anti-microbial resistance and virulence factors. 85, 103-105 It is unknown whether these techniques can be used at the point of care to inform treatment decisions in an obstetrical setting.
In this study of patients with spontaneous preterm labor (PTL) and intact membranes, AF culture was compared to a new technique using a combination of broad-range real-time PCR with electrospray ionization mass spectrometry (PCR/ESI-MS) [Ibis® technology].106-114 This technique is capable of identifying the genus and species of microorganisms in AF within 8 hours, and therefore, in time for clinical decisions.106, 107, 109-114 PCR/ESI-MS has been validated in several clinical settings106, 114-134 and has the potential to revolutionize the detection of infectious agents in modern obstetrics. The study objectives were to: 1) compare the performance of AF culture and PCR/ESI-MS in identifying MIAC in patients with PTL and intact membranes; 2) assess the magnitude of intra-amniotic inflammation in patients with MIAC identified by PCR/ESI-MS; and 3) examine the relationship between MIAC detected by PCR/ESI-MS and adverse pregnancy outcome.
Materials and Methods
Study population
A cohort study was conducted by searching the clinical database and bank of biological samples of Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD) (Detroit, MI) to identify patients with a diagnosis of spontaneous PTL with intact membranes. Patients were included if they met the following criteria: 1) had a singleton gestation; 2) presented with PTL and intact membranes; and 3) had an amniocentesis (trans-abdominal amniocentesis) performed between 20 and 35 weeks of gestation with microbiological studies. Patients were excluded from the study if: 1) rupture of the chorioamniotic membranes occurred before AF collection; or 2) chromosomal or structural fetal anomaly was present. Perinatal mortality was defined as the occurrence of fetal or neonatal death.
All patients provided written informed consent and the use of biological specimens and clinical data for research purposes was approved by the Institutional Review Boards of NICHD and Wayne State University.
Sampling procedures
Patients with preterm labor and intact membranes who underwent transabdominal ultrasound-guided amniocentesis for evaluating possible MIAC (within the standard of care at Hutzel Women's Hospital) were eligible for the study. AF was immediately transported in a capped sterile syringe to the clinical laboratory where it was cultured for aerobic and anaerobic bacteria, including genital mycoplasmas. Evaluation of white blood cell (WBC) count, glucose concentration and Gram stains of AF were also performed shortly after collection. AF not required for clinical assessment was centrifuged for 10 minutes at 4°C shortly after the amniocentesis, and the supernatant was aliquoted and stored at -70°C until analysis. The presence of intra-amniotic inflammation was assessed by determination of AF interleukin-6 (IL-6) concentration by ELISA. AF IL-6 concentrations were determined for research purposes, and such results were not used in patient management.
Detection of microorganisms with cultivation and molecular methods
AF was analyzed using cultivation techniques (aerobic, anaerobic and genital mycoplasmas) as well as with PCR/ESI-MS (Ibis® Technology - Athogen, Carlsbad, CA). Briefly, DNA was extracted from 300 uL of AF using a method that combines bead-beating cell lysis with a magnetic-bead based extraction method.135, 136 The extracted DNA was amplified on the bacterial artificial chromosome (BAC) spectrum assay according to the manufacturer's instructions. PCR/ESI-MS can identify 3400 bacteria and 40 Candida spp, which are represented in the platform's signature database.112, 114, 137 A total of 200 μL of extract was used per sample. For viral detection, the nucleic acids were extracted from 300 uL of AF using a method that combines chemical lysis with a magnetic-bead based extraction method. The extracted RNA/DNA was amplified on the broad viral assay according to the manufacturer's instructions. In the 8 wells, there were fourteen primer pairs used to detect the following viruses: Herpes simplex virus 1 (HHV-1), Herpes simplex virus 2 (HHV-2), Varicella-zoster virus (HHV-3), Epstein-Barr virus (HHV-4), Cytomegalovirus (HHV-5), Kaposi's sarcoma-associated herpes virus (HHV-8), Human adenoviruses, Human enteroviruses, BK polyomavirus, JC polyomavirus and Parvovirus B19.137
After PCR amplification, 30-μL aliquots of each PCR product were desalted and analyzed via Electrospray-Ionization Time-of-Flight Mass Spectrometer (ESI-MS) as previously described.112, 116 The presence of microorganisms was determined by signal processing and triangulation analysis of all base composition signatures obtained from each sample and compared to a database. Along with organism identification, the ESI-MS analysis includes a Q-score and level of detection (LOD). The Q-score, a rating between 0 (low) and 1 (high), represents a relative measure of the strength of the data supporting identification; only Q-scores ≥ 0.90 were reported for the BAC Spectrum assay.132 The LOD describes the amount of amplified DNA present in the sample: this is calculated with reference to an internal calibrant, as previously described,108 and is reported herein as genome equivalents per PCR reaction well (GE/well). The bacterial/viral genome load per mL of AF (GE/mL) is equal to the GE/well multiplied by 133.33. The sensitivity (LOD) of the Ibis assay for the detection of bacteria in blood is in average 100 CFU/mL (95% CI, 6 – 600 CFU/mL).114 A comparison of detection limits between blood and amniotic fluid showed that the assays have comparable detection limits (100 CFU/mL). The sensitivity (LOD) for the broad viral in plasma ranges from 400 copies/mL to 6600 copies/Ml.138 A comparison of detection limits between amniotic fluid and plasma was performed by spiking known amounts of a DNA virus (HHV-5) and an RNA virus (Human enterovirus) into amniotic fluid and plasma. Detection limits in amniotic fluid were similar to plasma, ranging from ∼800 to 1600 copies/mL (depending upon the specific microorganism).
Determination of IL-6 in amniotic fluid
AF concentrations of IL-6 were determined to assess the magnitude of the intra-amniotic inflammatory response. We used a sensitive and specific enzyme immunoassay obtained from R&D Systems (Minneapolis, MN). Briefly, the immunoassay utilized the quantitative sandwich enzyme immunoassay technique, and the concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for IL-6 were 8.7% and 4.6%, respectively. The detection limit of the IL-6 assay was 0.09 pg/mL.
Clinical definitions
Preterm labor was diagnosed by the presence of at least two regular uterine contractions every 10 minutes associated with cervical changes in patients with a gestational age between 20 and 36 6/7 weeks. Preterm delivery was defined as having occurred prior to the 37th week of gestation. Acute histological chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes.139-141 Intra-amniotic inflammation was diagnosed when IL-6 AF concentration was ≥ 2.6 ng/mL.41, 142 MIAC was defined according to the results of AF culture and PCR/ESI-MS analysis.99, 101, 143, 144 Intra-amniotic infection was defined as a combination of MIAC with intra-amniotic inflammation.
Statistical analysis
The Kolmogorov-Smirnov test and visual plot inspection were used to assess the normality of continuous data distributions. Spearman's non-parametric correlation coefficients were calculated. The Kruskal-Wallis and Mann Whitney U tests were used to test for differences in arithmetic variable distributions. The X2 or Fischer's exact test were used to test for differences in proportions, as appropriate. Quantile regression models were fit to test for median differences adjusting for potential confounders, including gestational age and cervical dilatation at amniocentesis. Kaplan-Meier survival curves were plotted and the Mantel-Haenszel log-rank test was used to test for differences in time-to-spontaneous preterm delivery. Logistic and Cox proportional hazards regression models were fit to examine magnitudes of association. A cutoff for classifying PCR/ESI-MS results according to the microbial inoculum size (genome copies/well) in each sample was selected upon inspection of a receiver operating characteristic (ROC) curve for the identification of intra-amniotic inflammation among patients with detectable gene copies by PCR/ESI-MS. For all analyses, a two-tailed p value <0.05 was considered significant. SPSS v.15.0 (SPSS, Chicago, IL) was used to analyze the data.
Results
Characteristics of the study population
Baseline characteristics of the study population are displayed in Table I. The median [interquartile range (IQR)] gestational age at amniocentesis was 30 (IQR: 25 - 32) weeks. The median gestational age at delivery was 34 (IQR: 27 - 37) weeks. Extraplacental membranes were examined for 92% (131/142) of the study population. The prevalence of histological acute chorioamnionitis was 41% (53/131).
Table I. Maternal characteristics and demographic data of the study population.
| Median (IQR) or % (n/N) | |
|---|---|
| Maternal age (years) | 23 (20 - 26.3) |
| Body mass index (kg/m2) | 24 (21 - 30) |
| Cervical dilatation at admission (cm) | 3 (2.0 - 3.5) |
| AF glucose (mg/dL) | 25 (19 - 31) |
| AF white blood cell count (cells/mm3) | 2 (0 - 12) |
| GA at amniocentesis (weeks) | 30 (25 - 32) |
| GA at delivery (weeks) | 34 (27 - 37) |
| Birthweight (grams) | 2190 (930 - 2700) |
| AF culture positive | 7 (10/142) |
| Detection of bacteria and/or virus genome by PCR/ESI-MS | 21 (30/142) |
| Positive PCR-ESI-MS (GE/well ≥ 17) | 12 (17/142) |
AF: amniotic fluid; GA: gestational age; GE/well: genome equivalent per PCR reaction; IQR: interquartile range; PCR: polymerase chain reaction; ESI-MS: electrospray ionization mass spectrometry.
The prevalence of MIAC and microbial diversity
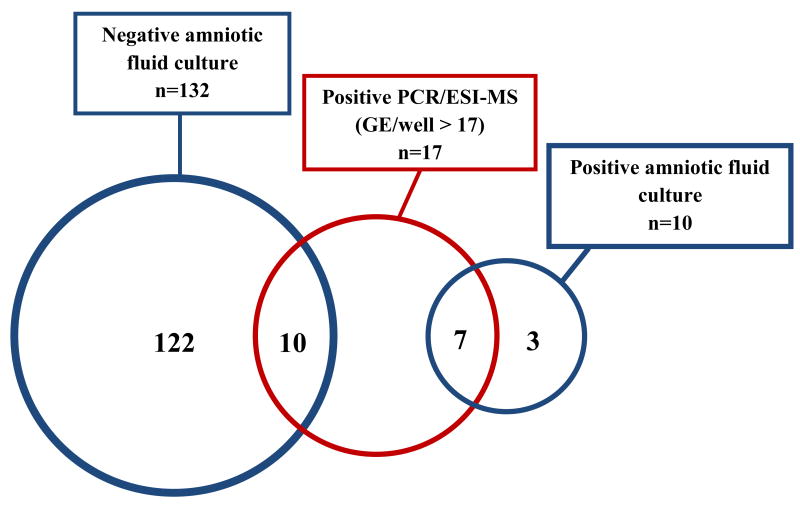
The prevalence of MIAC based on a positive AF culture was 7% (10/142). PCR/ESI-MS detected amplified DNA in 21% (30/142) of the AF samples (Table I). Upon inspection of a ROC curve for the identification of intra-amniotic inflammation (IL-6 ≥ 2.6 ng/mL) among patients with detectable DNA from bacteria and/or virus using PCR/ESI-MS (Figure 1), results were classified according to microbial burden as positive or negative. Positive tests had 17 or more GE/well, whereas negative tests had fewer than 17 GE/well, or genomic material was undetected. Figure 2 shows a Venn diagram describing the combined results of AF culture and PCR/ESI-MS. Seven of the 17 patients with positive PCR/ESI-MS (>17 GE/well) had positive AF cultures.
Figure 1.
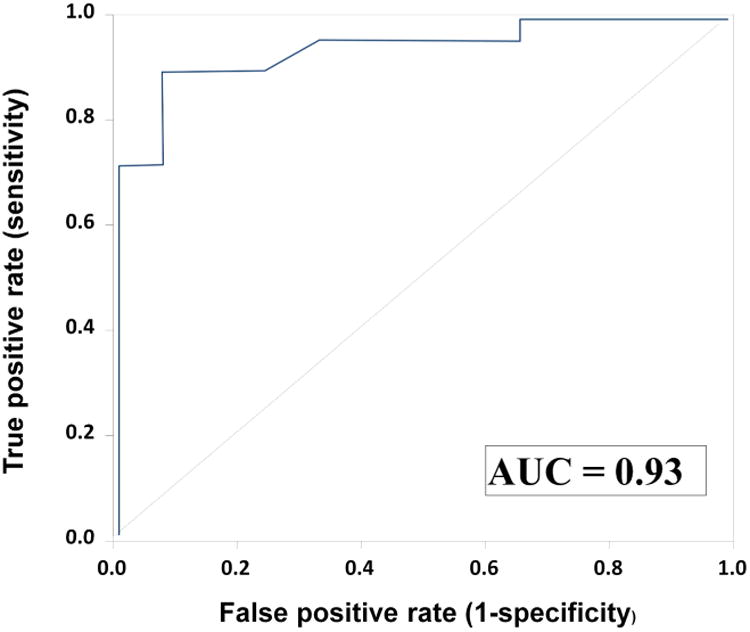
Receiver operator characteristic curve analysis of PCR/ESI-MS genome equivalents per PCR reaction well (GE/well) results for the identification of intra-amniotic inflammation (amniotic fluid IL-6 ≥ 2.6 ng/mL) among patients with detectable DNA of bacteria and/or viruses. Among patients with detectable DNA of bacteria or viruses using PCR/ESI-MS, a GE/well ≥17 had an area under the ROC curve of 0.93 (95% CI 0.83 – 1.0; p<0.001), a positive predictive value of 94.1% (16/17), and a negative predictive value of 84.6% (11/13) for the identification of intra-amniotic inflammation.
Figure 2.
Bacteria and viruses detected in amniotic fluid of patients with preterm labor using standard cultivation techniques vs. PCR/ESI-MS. Amniotic fluid culture includes routine cultivation techniques for bacteria (aerobes, anaerobes, and genital mycoplasmas). PCR/ESI-MS refers to broad range polymerase chain reaction (PCR) and Electrospray-Ionization Mass Spectrometry (ESI-MS).
Microorganisms detected in the amniotic fluid of patients with preterm labor and intact membranes using standard cultivation techniques versus PCR/ESI-MS.
PCR: polymerase chain reaction; ESI-MS: electrospray ionization mass spectrometry; GE/well: genome equivalent per PCR reaction.
For each patient with detectable genomic material by PCR/ESI-MS or positive AF cultures, the microorganisms identified, concentrations of inflammatory markers in amniotic fluid, gestational age at delivery, as well as the presence or absence of histologic acute chorioamnionitis are shown in Table II. The most frequent microorganism identified by PCR/ESI-MS was Ureaplasma parvum, (instead of Ureaplasma urealyticum, which was the most common microorganism identified by standard AF culture). Fusobacterium nucleatum, Gardnella vaginalis, Mycoplasma hominis and Acinetobacter junii were only detected by PCR/ESI-MS. Two patients (1.4%) had a positive viral assay for Human Enterovirus using PCR/ESI-MS. One of these two patients also had genomic material consistent with Acinetobacter Junii.
Table II.
Amniotic fluid inflammatory profile, delivery information, placenta pathology and perinatal mortality in patients with microbial invasion of the amniotic cavity according to amniotic fluid cultures vs. GE/well results using PCR/ESI-MS.
| Amniotic Fluid Culture and PCR/ESI-MS Results | Amniotic fluid (AF) test results | Delivery information | Histologic Placenta Lesions | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Patient number | Group | AF culture | PCR/ESI-MS bacteria |
GE/well bacteria |
PCR/ESI-MS viruses |
GE/well viruses |
AF-IL6 (ng/mL) |
Intra-amniotic inflammation |
AF Gram stain |
AF Glucose | AF WBC | GA at amniocentesis (weeks) |
GA at delivery (weeks) | Interval- amniocentesis-to delivery (days) |
Acute histological chorioamnionitis |
Severity of histological chorioamnionitis |
Perinatal mortality |
| 1 | Positive AF Culture and positive PCR/ESI-MS (n=7) | Ureaplasma spp | Ureaplasma parvum | 575 | No detected | 0 | 29.8 | Yes | Neg | 10 | 490 | 32.4 | 32.6 | 1 | Yes | 2 | No |
| 2 | Candida albicans | Candida albicans | 215 | No detected | 0 | 201.3 | Yes | Neg | 10 | 2160 | 26.3 | 26.4 | 1 | Yes | 3 | No | |
| 3 | Ureaplasma spp | Ureaplasma parvum Fusobacterium nucleatum | 1296 | No detected | 0 | 275.5 | Yes | Pos | ND | 50 | 24.9 | 26.0 | 8 | NI | NI | Yes | |
| 4 | Ureaplasma spp | Ureaplasma parvum | 1795 | No detected | 0 | 86 | Yes | Neg | 10 | 500 | 32.9 | 33.0 | 1 | Yes | 2 | No | |
| 5 | Streptococcus | Gardnerella vaginalis Mycoplasma hominis | 6983 | No detected | 0 | 185.5 | Yes | Neg | 10 | 1920 | 31.7 | 31.9 | 1 | Yes | 3 | No | |
| 6 | Ureaplasma urealyticum | Ureaplasma urealyticum | 1484 | No detected | 0 | 9.9 | Yes | Pos | 20 | 10 | 32.1 | 32.7 | 4 | Yes | 2 | No | |
| 7 | Bacteroides | Fusobacterium nucleatum | 20 | No detected | 0 | 517.8 | Yes | Neg | 10 | 295 | 22.6 | 22.6 | 0 | Yes | 3 | Yes | |
|
| |||||||||||||||||
| 8 | Negative AF culture and GE/Well ≥ 17 (n=10) | Negative | No detected | 0 | Human Enterovirus | >1000 | 6.0 | Yes | Neg | 21 | 0 | 21.6 | 21.7 | 1 | No | No | Yes |
| 9 | Negative | Fusobacterium nucleatum | 27 | No detected | 0 | 46.8 | Yes | Neg | 24 | 1 | 25 | 25.7 | 5 | Yes | 4 | Yes | |
| 10 | Negative | Ureaplasma urealyticum | 768 | No detected | 0 | 265.5 | Yes | Neg | 11 | 357 | 20.4 | 20.4 | 0 | Yes | 3 | Yes | |
| 11 | Negative | Acinetobacter junii | 68 | Human Enterovirus | 24 | 77.7 | Yes | Neg | 20 | 0 | 24.6 | 24.7 | 1 | Yes | 1 | No | |
| 12 | Negative | Acinetobacter junii | 41 | No detected | 0 | 0.28 | No | Neg | 30 | 2 | 29.1 | 39.9 | 75 | No | No | No | |
| 13 | Negative | Sneathia species | 280 | No detected | 0 | 290.7 | Yes | Pos | 10 | 440 | 26 | 26.0 | 0 | Yes | 3 | No | |
| 14 | Negative | Pseudomonas entomophila/putida | 23 | No detected | 0 | 13.1 | Yes | Neg | 17 | 13 | 33.9 | 33.9 | 0 | No | No | No | |
| 15 | Negative | Ureaplasma parvum | 571 | No detected | 0 | 118.5 | Yes | Neg | 10 | 2750 | 26.7 | 27.0 | 2 | Yes | 3 | No | |
| 16 | Negative | Candida albicans | 57 | No detected | 0 | 96.3 | Yes | Pos | 10 | 1292 | 32.4 | 32.6 | 1 | Yes | 2 | No | |
| 17 | Negative | Sneathia species | 152 | No detected | 0 | 263.3 | Yes | Pos | 10 | 960 | 23.6 | 23.6 | 0 | Yes | 4 | Yes | |
|
| |||||||||||||||||
| 18 | Negative AF culture and GE/Well < 17 (n=13) | Negative | Aeromonas caviae | 3 | No detected | 0 | 0.6 | No | Neg | ND | 10 | 30.1 | 30.1 | 0 | No | No | No |
| 19 | Negative | Acinetobacter junii | 14 | No detected | 0 | 1.6 | No | Neg | 30 | 3 | 32.1 | 33.6 | 10 | No | No | No | |
| 20 | Negative | Moraxella osloensis | 3 | No detected | 0 | 1.0 | No | Neg | 29 | 8 | 32.4 | 39.9 | 52 | No | No | No | |
| 21 | Negative | Staphylococcus aureus | 5 | No detected | 0 | 0.6 | No | Neg | 30 | 0 | 33.3 | 37.6 | 30 | No | No | No | |
| 22 | Negative | Staphylococcus aureus | 7 | No detected | 0 | 1.6 | No | Neg | 10 | 0 | 34.1 | 34.9 | 5 | No | No | No | |
| 23 | Negative | Acidovorax sp. | 11 | No detected | 0 | 0.4 | No | Neg | 29 | 20 | 27 | 27.3 | 2 | No | No | No | |
| 24 | Negative | Streptococcus species | 3 | No detected | 0 | 1.0 | No | Neg | 10 | 0 | 34.1 | 36.9 | 19 | Yes | 2 | No | |
| 25 | Negative | Lactobacillus acidophilus/crispatus | 5 | No detected | 0 | 2.3 | No | Neg | 25 | 1 | 28.1 | 39.7 | 81 | Yes | 2 | No | |
| 26 | Negative | Ureaplasma parvum | 8 | No detected | 0 | 0.2 | No | Neg | 31 | 0 | 22.3 | 25.3 | 21 | Yes | 3 | No | |
| 27 | Negative | Gardnerella vaginalis | 5 | No detected | 0 | 16.5 | Yes | Neg | 52 | 0 | 28.4 | 35.4 | 49 | No | No | No | |
| 28 | Negative | Ureaplasma parvum | 4 | No detected | 0 | 0.2 | No | Neg | 22 | 2 | 26.4 | 27.0 | 4 | No | No | No | |
| 29 | Negative | Staphylococcus aureus | 8 | No detected | 0 | 51.8 | Yes | Neg | 25 | 5 | 29.7 | 33.9 | 29 | No | No | No | |
| 30 | Negative | Pantoea dispersa | 3 | No detected | 0 | 0.9 | No | Neg | 54 | 2 | 27.4 | 28.3 | 6 | No | No | No | |
|
| |||||||||||||||||
| 31 | Positive AF culture and Negative PCR/ESI-MS (n=3) | Ureaplasma urealyticum | No detected | 0 | No detected | 0 | 0.2 | No | Neg | 24 | 0 | 34.6 | 35.1 | 4 | Yes | 1 | No |
| 32 | Ureaplasma urealyticum | No detected | 0 | No detected | 0 | 0.4 | No | Neg | 19 | 2 | 29.1 | 39.3 | 71 | No | No | No | |
| 33 | Staphylococcus aureus | No detected | 0 | No detected | 0 | 0.2 | No | Neg | 20 | 225 | 31 | 36.9 | 41 | No | No | No | |
GA= gestational age; AF = amniotic fluid; WBC = white blood cells; IL-6 = Interleukin-6; PCR = polymerase chain reaction; ESI-MS = electrospray ionization mass spectrometry; GE/well: genome equivalent per PCR reaction; NI = not information
Severity of Acute histological chorioamnionitis:
Stage 1 - Early: Acute Subchorionitis/Chorionitis
Stage 2 - Intermediate: Acute Choriamnionitis
Stage 3 - Necrotizing Chorioamnionitis
Stage 4 - Subacute Chorioamnionitis
The relationship between the presence and burden of microorganisms in the amniotic fluid and intra-amniotic inflammation
The microbial inoculum size, expressed as GE/well, was significantly correlated with AF concentration of IL-6 and the AF WBC count [Spearman's rho (ρ) =0.63, p <0.001 and ρ=0.67, p <0.0001]. Exclusion of patients with positive AF cultures did not alter these findings [AF IL-6: ρ =0.61 p =0.002 and AF WBC: ρ =0.51, p =0.01].
Figures 3A and 3B display AF concentrations of IL-6 and AF WBC count, respectively, among four study groups according to results of PCR/ESI-MS and AF cultures. The first study group had positive AF culture and positive PCR/ESI-MS (≥17 GE/well) (n=7). The second study group included patients with positive PCR/ESI-MS (≥17 GE/well) who had negative AF cultures (n=10). The third and fourth study groups included patients with negative PCR/ESI-MS (<17 GE/well) who had negative (n=122) or positive (n=3) AF cultures, respectively.
Figure 3.
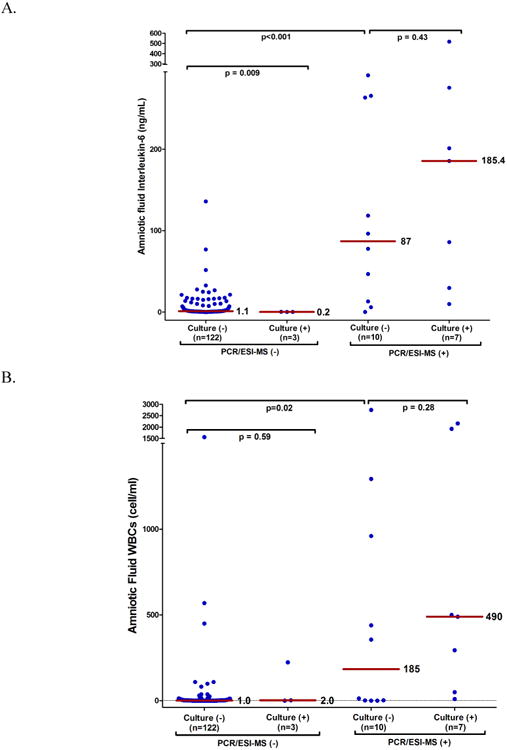
Markers of infection in amniotic fluid according to the results of standard AF culture and PCR/ESI-MS. Amniotic fluid concentrations of Interleukin-6 (Panel A) and white blood cells (WBCs) count (Panel B) as a function of PCR/ESI-MS and AF culture results. There was no significant difference in the median AF IL-6 concentration and WBC count between patients with negative AF culture and a positive PCR/ESI-MS (GE/well ≥17) and those patients with positive AF culture and positive PCR/ESI-MS (p= 0.43 and 0.28, respectively). Among patients with negative AF culture, those with a positive PCR/ESI-MS had a significantly higher median AF IL-6 concentration and WBC count than those patients with both tests negative (p<0.001 and p=0.02, respectively). The median AF IL-6 concentration in patients with positive AF culture and negative PCR/ESI-MS (GE/well < 17) was always < 2.6 ng/mL.
Amniotic fluid IL-6 concentration and white blood cell count according to the results of standard AF cultures and PCR/ESI-MS.
Among patients with positive PCR/ESI-MS (≥17 GE/well), there was no significant difference in the median AF IL-6 concentration between those with (n=7) and without (n=10) positive AF cultures [185.4 (IQR: 30 – 275.4) vs. 87 (IQR: 11.3 - 264) ng/mL, p=0.43] (Figure 3A). The median AF IL-6 concentration was significantly higher in patients with positive PCR/ESI-MS results (≥ 17 GE/well) and negative AF cultures than among: 1) women with positive AF cultures and negative PCR/ESI-MS results (n=3); and 2) patients (n=122) with negative AF cultures and negative PCR/ESI-MS [87 (IQR: 11.3 - 264) ng/mL vs. 0.2 (IQR: 0.2 -0.3), and 1.1 (IQR: 0.7- 5) ng/mL, respectively, p<0.001]. However, the median AF IL-6 concentration was significantly higher in patients without MIAC (by AF culture and PCR/ESI-MS) than in patients with positive AF culture and negative PCR/ESI-MS (p=0.009) (Figure 3A).
Differences in the median AF WBC count among the four study groups were consistent with those of AF IL-6. There were no differences in the median WBC count among patients with positive PCR/ESI-MS (≥ 17 GE/well) who had positive (n=7) or negative (n=10) AF cultures [490 (IQR: 50-1920) vs. 185 (IQR: 0.7-1043) cell/mm3, p=0.28]. The median AF WBC count among these two study groups was significantly greater than the median AF WBC count of the two study groups with negative PCR/ESI-MS (<17 GE/well), irrespective of the AF culture results (p<0.0001) (Figure 3B). Differences in the median AF IL-6 and WBC concentrations among the four study groups were consistent both in direction and statistical significance when fitting a quantile regression model to adjust for potential confounders, including gestational age and cervical dilatation at amniocentesis [data not shown].
Figure 4A shows the prevalence of intra-amniotic inflammation (IL-6 ≥ 2.6 ng/mL) by the combined results of AF culture and PCR/ESI-MS. Intra-amniotic inflammation was diagnosed in 70% (n=7/10) and 94% (n=16/17) of patients with positive AF culture and a positive PCR/ESI-MS (≥17 GE/well) results, respectively. These patients accordingly had intra-amniotic infection (i.e., MIAC and intra-amniotic inflammation).
Figure 4.
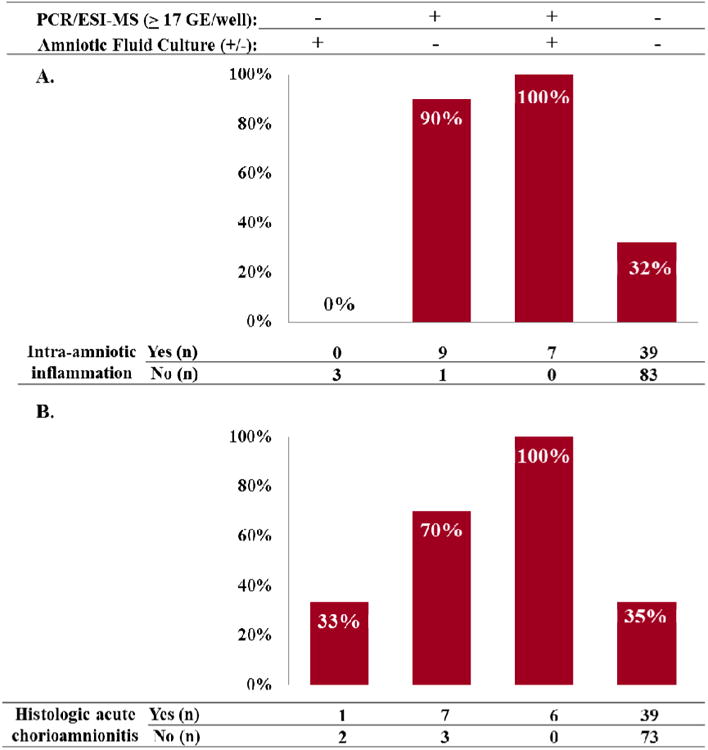
Prevalence of intra-amniotic inflammation (Panel A) and histologic acute chorioamnionitis (Panel B) according to the results of PCR/ESI-MS and amniotic fluid cultures. Intra-amniotic inflammation was diagnosed in 70% (n=7/10) and 94% (n=16/17) of patients with positive AF culture and positive PCR/ESI-MS (≥17 GE/well) results, respectively. Patients with positive PCR/ESI-MS (GE/well ≥ 17) and negative AF culture have a prevalence of histological acute chorioamnionitis of 70% (7/10). In contrast, the prevalence of histological acute chorioamnionitis in patients with negative AF culture and negative PCR/ESI-MS (GE/well < 17) was 35% (39/112).
Prevalence of intra-amniotic inflammation and histologic acute chorioamnionitis by PCR/ESI-MS and amniotic fluid culture results
PCR: polymerase chain reaction; ESI-MS: electrospray ionization mass spectrometry.
All but one participant with a positive PCR/ESI-MS (≥ 17 GE/well) and a negative AF cultures had intra-amniotic inflammation [90%, (9/10)]. These patients were significantly more likely to have intra-amniotic inflammation than women with negative tests, adjusting for gestational age and cervical dilatation at amniocentesis [odds ratio (OR) 12.2, 95% confidence interval (CI) 1.5 – 97] (Table III). None of the three women with positive AF cultures and negative PCR/ESI-MS had intra-amniotic inflammation. Importantly, 32% (39/122) of the patients with negative results for both tests (AF culture and PCR/ESI-MS), which are considered “sterile” samples, had intra-amniotic inflammation (Figure 4A).
Table III. Magnitudes of association among combined test results and intra-amniotic inflammation and histologic acute chorioamnionitis.
| Intra-amniotic inflammation | Histologic acute chorioamnionitis | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | |||
| Unadjusted | ||||||
|
| ||||||
| PCR/ESI-MS (+) – AF (+) | 31.7 | 1.5 | 691 | 25.8 | 1.1 | 591 |
| PCR/ESI-MS (+) – AF (-) | 13.4 | 2.1 | 85 | 4.3 | 1.1 | 17 |
| PCR/ESI-MS (-) – AF (+) | 0.3 | 0.01 | 9 | 1.2 | 0.1 | 13 |
| PCR/ESI-MS (-) – AF (-) | 1 | reference | 1 | reference | ||
|
| ||||||
| Adjusted* | ||||||
|
| ||||||
| PCR/ESI-MS (+) – AF (+) | 47.8 | 1.8 | >999 | 32.6 | 1.3 | 788 |
| PCR/ESI-MS (+) – AF (-) | 12.2 | 1.5 | 97 | 5.1 | 1.01 | 26 |
| PCR/ESI-MS (-) – AF (+) | 0.9 | 0.02 | 35 | 1.7 | 0.2 | 20 |
| PCR/ESI-MS (-) – AF (-) | 1 | reference | 1 | reference | ||
AF: Amniotic fluid; GE/well: genome equivalent per well; PCR: polymerase chain reaction; ESI-MS: Electrospray ionization mass spectrometry.
Note: adjusted for cervical dilation and gestational age at amniocentesis; Firth's penalized maximum likelihood estimation was performed where necessary to resolve separation issues.
The relationship between detectable microorganisms in the amniotic fluid and acute histological chorioamnionitis
Figure 4B shows the prevalence of histologic acute chorioamnionitis among the four study groups, according to the results of PCR/ESI-MS and AF culture. Each of the seven patients with positive AF cultures and positive PCR/ESI-MS results (GE/well ≥ 17) had histologic acute chorioamnionitis. Histologic acute chorioamnionitis was diagnosed in 70% (7/10) of patients with a positive PCR/ESI-MS (≥ 17 GE/well) and negative AF cultures. These patients were significantly more likely to have acute histological chorioamnionitis than women with negative tests, adjusting for gestational age and cervical dilatation at amniocentesis [OR 5.1, 95%CI 1.01-26, respectively] (Table III). One of the three women with positive AF culture and negative PCR/ESI-MS had mild histologic acute chorioamnionitis (stage 1 subchorionitis/chorionitis) (Figure 4B).
The observations described in the previous paragraph, combined with the parameters assessing intra-amniotic inflammation, reveals that a positive AF culture among patients with negative PCR/ESI-MS results likely reflect either early-phase microbial invasion, in which an inflammatory response is not detectable, or contamination of the specimen (false-positive culture).
The presence of microorganisms in the amniotic fluid by molecular techniques and the risk of spontaneous preterm delivery
Table IV presents the positive predictive values (PPV) of AF culture and positive PCR/ESI-MS (≥ 17 GE/well) for the identification of patients who delivered spontaneously prior to 37 and 32 weeks, respectively. The PPV of a positive AF culture for spontaneous delivery before 37 and 32 weeks was 90% and 66.7%, respectively. In contrast, the PPV of a positive PCR/ESI-MS (≥ 17 GE/well) was 94.1% and 91.7% for the same outcomes. Similarly, the PPV of a positive PCR/ESI-MS (≥ 17 GE/well) and a positive AF culture for the identification of patients who delivered within 2 days of amniocentesis were 76.5% (13/17) and 50% (5/10), respectively (Table IV).
Table IV. Positive predictive value (PPV) of GE/well reported by PCR/ESI-MS and AF cultures for the identification of adverse pregnancy outcomes.
| sPTD < 37 weeks | sPTD < 32 weeks | Delivery within 7 days of amniocentesis | Delivery within 2 days of amniocentesis | |
|---|---|---|---|---|
| Prevalence | 79.6% (113/142) | 52.6% (50/95) | 37.3% (53/142) | 21.1% (30/142) |
|
| ||||
| PPV of positive culture | 90% (9/10) | 66.7% (4/6) | 70% (7/10) | 50% (5/10) |
| PPV of PCR/ESI/MS (GE/well ≥17) | 94.1% (16/17) | 91.7% (11/12) | 88.2% (15/17) | 76.5% (13/17) |
AF: amniotic fluid; PCR: polymerase chain reaction; ESI-MS: electrospray ionization mass spectrometry; GE/well: genome equivalent per PCR reaction.
Presence and burden of microorganisms in amniotic fluid and the interval to spontaneous preterm delivery
Kaplan-Meier survival estimates, censoring subjects in whom labor was induced for maternal or fetal indications, show that the amniocentesis-to-delivery interval was significantly shorter in patients with positive PCR/ESI-MS results (≥ 17 GE/well) and negative AF culture than in patients with negative results for both tests [1 (IQR: <1 – 2) days vs. 25 (IQR: 5 – 51) days; p=0.002]. There were no significant differences in the median amniocentesis-to-delivery interval between patients who had positive PCR/ESI-MS (≥ 17 GE/well) with negative AF cultures [1 (IQR: <1 – 2) days] and those patients with positive findings for both tests [1 (IQR: 1 – 8) days] (p=0.6) (Figure 5).
Figure 5.
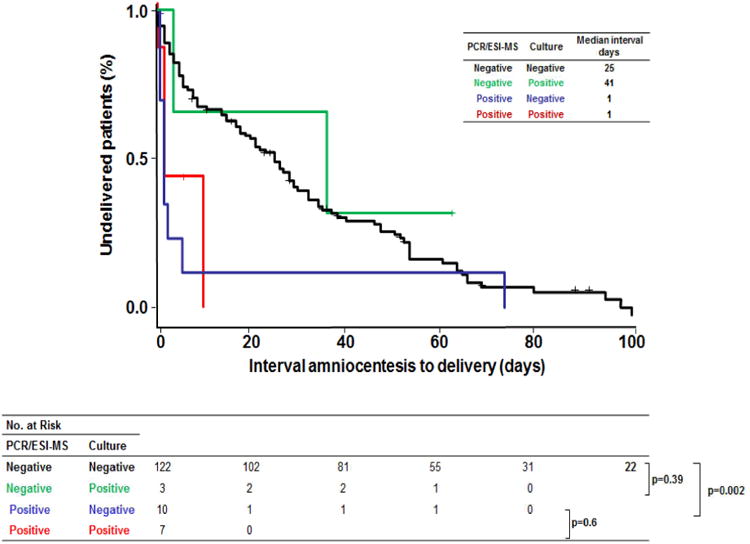
Kaplan-Meier survival analysis of amniocentesis-to-delivery interval (days) according to amniotic fluid cultures and PCR/ESI-MS results. Patients in whom labor was induced were censored and are represented by crosses. Among patients with negative AF cultures, the amniocentesis-to-delivery interval was significantly shorter in patients with a positive PCR/ESI-MS than in those with a negative PCR/ESI-MS results [1 (IQR: < 1 – 2) days vs. 25 (IQR: 5 – 51) days; p=0.002]. There were no significant differences in the median amniocentesis-to-delivery interval between patients who had a positive PCR/ESI-MS with a negative AF culture [1 (IQR: < 1 – 2) days] and those patients with both tests positive [1 (IQR: 1 – 8) days] (p=0.6).
Kaplan-Meier survival analysis of amniocentesis-to-delivery interval (days) according to amniotic fluid cultures and PCR/ESI-MS results.
Figure 6 shows a forest plot of the hazard ratios (HR) describing the relative risks of spontaneous preterm delivery (sPTD) for patients with positive PCR/ESI-MS (≥ 17 GE/well) and/or AF cultures compared to those with negative tests, adjusted for gestational age at amniocentesis and cervical dilatation. The risks (i.e., hazard) of sPTD at <37 weeks and, separately, <32 weeks, were significantly greater among patients with positive PCR/ESI-MS results (≥ 17 GE/well), both with and without positive AF cultures, when compared to women with negative tests [sPTD <37: AF+, HR 3.9 (95%CI 1.7-9.1) and AF-, HR 3.8 (95%CI 1.8-8.1); sPTD <32: AF+, HR 3.3 (95%CI 1.1-10.1) and AF-, HR 5.2 (95%CI 2-13.4)].
Figure 6.
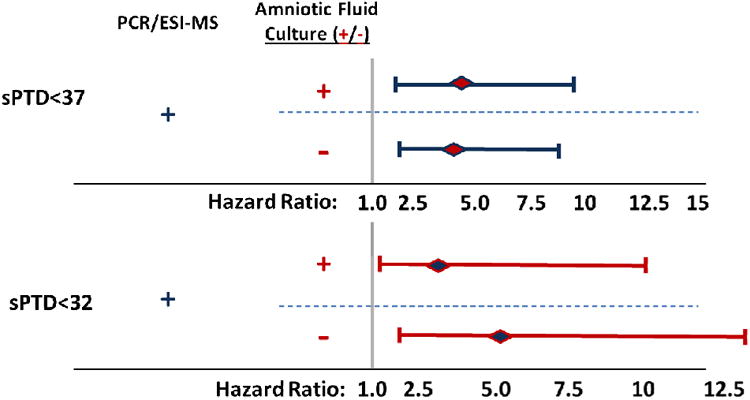
Forest plot of hazard ratios for spontaneous preterm delivery (<37, <32 weeks) by PCR/ESI-MS and amniotic fluid culture results compared to patients with negative tests. The risks of spontaneous preterm delivery (sPTD) <37 and, separately, <32 weeks, were significantly greater among patients with positive PCR/ESI-MS (GE/well ≥17), both with and without positive AF cultures, when compared to women with negative tests.
Forest plot of hazard ratios for spontaneous preterm delivery (<37, <32 weeks) by PCR/ESI-MS and amniotic fluid culture findings compared to patients with negative tests.
Detection of microbial invasion of the amniotic cavity using cultivation and molecular techniques and the risk of perinatal death
The prevalence of perinatal death (fetal and neonatal death) differed significantly among the four study groups defined by the results of AF culture and PCR/ESI-MS (p=0.03) (Figure 7). Twenty percent of perinatal death (4/19) occurred in the 7% (10/142) of women with positive PCR/ESI-MS and negative AF culture results. Offspring of these women were at fivefold greater risk of perinatal mortality than those of women with negative AF culture and negative PCR/ESI-MS results (OR 5.6; 95% CI 1.4-22). There was no difference in the rate perinatal death for offspring of women with positive PCR/ESI-MS according to the results of AF culture [positive culture 29% (2/7) vs. negative culture 40% (4/10); p=1.0].
Figure 7.
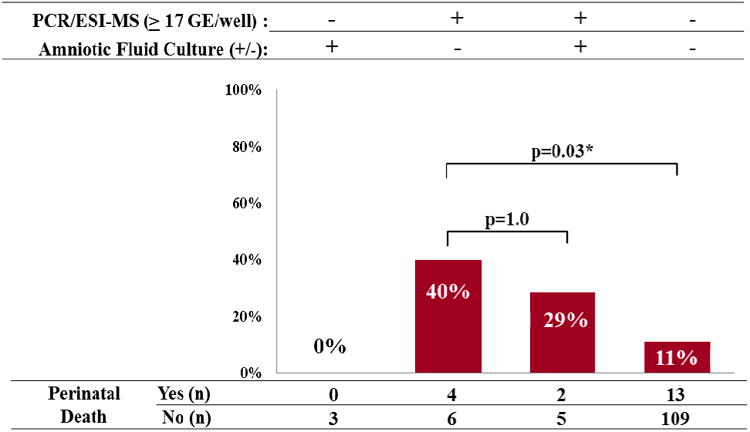
Prevalence of perinatal death (fetal and neonatal deaths) by the combined results of amniotic fluid culture and PCR/ESI-MS. Patients with positive PCR/ESI-MS (≥17 GE/well) and negative AF cultures had significantly higher rates of perinatal mortality, than women with both tests negative [40% (4/10) vs. 11% (13/122); (p=0.03)]. There was no difference in the rate of perinatal death for offspring of women with positive PCR/ESI-MS according to the results of AF culture [positive culture 29% (2/7) vs. negative culture 40% (4/10); p=1.0].
Prevalence of perinatal death (fetal and neonatal deaths) by the combined results of amniotic fluid culture and PCR/ESI-MSI.
Comment
Principal findings of the study
1) The prevalence of MIAC in patients with preterm labor and intact membranes was 7% using standard cultivation techniques and 12% using PCR/ESI-MS; 2) patients with positive PCR/ESI-MS (≥17 GE/well) and negative AF cultures had higher rates of intra-amniotic inflammation and histologic acute chorioamnionitis, shorter intervals to delivery, and their offspring were at greater risk of perinatal mortality than patients with negative results for both tests; 3) there were no differences in these factors when comparing patients with positive PCR/ESI-MS (≥17 GE/well) who had positive to those with negative AF cultures; and 4) genomic material from viruses in AF were detected in two patients [1.4% (2/142)]. Taken together, these findings strongly suggest that PCR/ESI-MS can be used as a rapid test to diagnose MIAC and guide patient management.
Microbial invasion of the amniotic cavity detected by cultivation and molecular microbiologic techniques
A key transition in human life is the emergence from a sterile to a non-sterile environment at the time of birth.145 The amniotic fluid is sterile under normal circumstances.43, 146-148 Upon conducting systematic investigations using cultivation and molecular techniques, we found that the overwhelming majority of women with a normal pregnancy outcome do not have evidence of bacteria 62, 147, 149, 150 or viruses in the amniotic cavity.151-154 In contrast, 25 – 40% of patients with preterm labor and intact membranes who deliver preterm have evidence of MIAC, 7-43, 155, 156 whereas 50-75% of women with pre-labor rupture of membranes (PROM) have MIAC.25,53,101,156
The prevalence of MIAC in patients with PROM is a function of the obstetrical circumstances that prompted investigation of the microbial state in the amniotic cavity. For example, the earlier the gestational age at presentation with preterm labor or preterm PROM, the higher the frequency of MIAC.20, 26, 41, 99, 101, 157-159 More importantly, in patients admitted with PROM, the frequency of MIAC increases from 39% (24/61) without labor, to 75% (36/48) with the onset of spontaneous preterm labor (p=0.0004).53 This unique data was obtained before routine administration of antibiotics in patients with preterm PROM, and is the basis for the clinical dogma that the onset of labor in these women is frequently due to subclinical intra-amniotic infection.
Using cultivation techniques, MIAC has been identified in several obstetrical disorders with the prevalence ranging from 10–12% in patients with preterm labor and intact membranes,9-16, 20-27, 30-37, 61 to as high as 51% in pregnant women diagnosed with acute cervical insufficiency (Table V).160-163 These cultivation-based studies provide strong evidence for the involvement of microorganisms in multiple pregnancy complications. Yet, cultivation techniques represent a minimum estimate of the prevalence of MIAC.39, 99, 156, 164, 165
Table V. Microbial invasion of the amniotic cavity (MIAC) in obstetric disorders as determined by amniotic fluid studies obtained by transabdominal amniocentesis using cultivation techniques.
| Obstetric disorders | Authors | Prevalence of MIAC | ||
|---|---|---|---|---|
| % | (n/N) | |||
| Spontaneous labor at term with intact membranes | Romero et al. (1993) | 18.8 | (17/90) | 62 |
| Gomez et al. (1994) | 15.6 | (14/90) | 63 | |
| Yoon et al. (2003) | 6.3 | (12/190) | 149 | |
|
| ||||
| Preterm labor with intact membranes | Miller et al. (1980) | 20 | (15) | 9 |
| Bobbit et al. (1981) | 25 | (8/31) | 11 | |
| Wallace and Herrick (1981) | 12 | (3/25) | 10 | |
| Wahbeh et al. (1984) | 21.2 | (7/33) | 12 | |
| Hameed et al. (1984) | 10.8 | (4/37) | 13 | |
| Weible and Randall (1985) | 3 | (1/35) | 353 | |
| Leigh et al. (1986) | 12 | (7/59) | 15 | |
| Gravett et al. (1986) | 24 | (13/54) | 14 | |
| Duff and Kopelman (1987) | 4 | (1/24) | 354 | |
| Romero et al (1988) | 9.8 | (4/41) | 16 | |
| Romero et al. (1989) | 9.1 | (24/264) | 20 | |
| Skoll et al. (1989) | 5.5 | (7/127) | 21 | |
| Romero et al. (1989) | 9.1 | (24/64) | 20 | |
| Romero et al. (1990) | 13.8 | (15/109) | 23 | |
| Romero et al. (1990) | 13.7 | (23/168) | 22 | |
| Romero et al. (1991) | 12.8 | (25/195) | 24 | |
| Harger et al. (1991) | 0 | (0/38) | 355 | |
| Gauthier et al. (1991) | 15.9 | (18/113) | 25 | |
| Coultrip et al. (1992) | 11.2 | (12/107) | 27 | |
| Watts et al. (1992) | 19 | (20/105) | 26 | |
| Romero et al. (1993) | 9.2 | (11/120) | 61 | |
| Coultrip et al. (1994) | 13 | (12/89) | 356 | |
| Yoon et al. (1996) | 10.8 | (11/102) | 357 | |
| Markenson et al (1997) | 9.3 | (5/54) | 197 | |
| Gomez et al. (1998) | 10.7 | (11/103) | 32 | |
| Hussey et al. (1998) | 12.6 | (16/127) | 31 | |
| Kara et al. (1998) | 33.8 | (25/74) | 358 | |
| Oyarzun et al. (1998) | 12 | (6/50) | 359 | |
| Rizzo et al. (1998) | 12.5 | (18/144) | 33 | |
| Greci et al. (1998) | 8.7 | (9/103) | 360 | |
| Yoon et al. (1998) | 11.6 | (21/181) | 30 | |
| Elimian et al. (1998) | 11.5 | (12/104) | 35 | |
| Gonzalez/Bosquet et al (1999) | 11.5 | (13/113) | 36 | |
| Locksmith et al. (1999) | 13.6 | (6/44) | 37 | |
| Ovalle et al. (2000) | 23.8 | (15/63) | 38 | |
| Yoon et al. (2001) | 10 | (21/209) | 41 | |
|
| ||||
| Prelabor premature rupture of membranes without labor | Garite et al. (1979) | 30 | (9/30) | 50 |
| Garite and Freeman (1982) | 23.3 | (20/86) | 361 | |
| Cotton et al. (1984) | 17 | (7/41) | 52 | |
| Zlatnik et al. (1984) | 31 | (9/29) | 362 | |
| Broekhuizen et al. (1985) | 28.3 | (15/53) | 66 | |
| Vintzileos et al. (1986) | 22.2 | (12/54) | 363 | |
| Felnstein et al. (1986) | 20 | (12/50) | 364 | |
| Romero et al. (1988) | 25.6 | (41/160) | 53 | |
| Gauthier et al. (1991) | 53.8 | (49/91) | 25 | |
| Coultrip et al. (1992) | 41.4 | (12/29) | 27 | |
| Gauthier and Meyer (1992) | 47.9 | (56/117) | 365 | |
| Romero et al. (1993) | 38.2 | (42/110) | 60 | |
| Font et al. (1995) | 56.8 | (21/37) | 366 | |
| Averbuch et al. (1995) | 35.6 | (32/90) | 367 | |
| Carroll et al. (1996) | 30.9 | (30/97) | 368 | |
| Gomez et al. (1998) | 57.7 | (30/52) | 32 | |
| Hussey et al. (1998) | 15.4 | (4/26) | 31 | |
|
| ||||
| Prelabor premature rupture of membranes in labor | Romero et al. (1988) | 75 | (36/48) | 53 |
|
| ||||
| Spontaneous rupture of membranes at term | Romero et al. (1992) | 34.3 | (11/32) | 59 |
|
| ||||
| Sonographic short cervix | Gomez et al. (2005) | 7 | (28/401) | 369 |
| Hassan et al. (2006) | 9 | (5/57) | 350 | |
| Vaisbuch et al. (2010) | 4.3 | (2/47) | 370 | |
|
| ||||
| Cervical insufficiency | Romero et al. (1992) | 51.5 | (17/33) | 160 |
| Mays et al. (2000) | 39 | (7/18) | 161 | |
| Lee et al. (2008) | 8 | (4/52) | 162 | |
| Bujold et al. (2008) | 47 | (7/15) | 163 | |
|
| ||||
| Twin gestations with preterm labor and intact membranes | Romero et al. (1990) | 11.9 | (5/42) | 371 |
| Mazor et al. (1996) | 12 | (9/74) | 372 | |
| Yoon et al. (1997) | 35 | (7/20) | 373 | |
|
| ||||
| Meconium stained amniotic fluid in preterm gestations | Romero et al. (1991) | 33 | (10/30) | 216 |
|
| ||||
| Meconium stained amniotic fluid in term gestations | Romero et al. (2013) | 19.6 | (16/66) | 374 |
|
| ||||
| Placenta previa | Madan et al. (2010) | 5.7 | (2/35) | 375 |
|
| ||||
| Idiopathic vaginal bleeding | Gomez et al. (2005) | 14 | (16/114) | 376 |
|
| ||||
| Pregnancy with intra-uterine device | Kim et al (2010) | 45.9 | (45/98) | 377 |
The advantages of molecular techniques for the identification of microorganisms
Molecular testing for the detection of microorganisms is considered to be the “diagnostic tool for the new millennium”.85, 166, 167 In the U.S. alone, 5 million cases of infectious disease-related illness are reported annually, and many are considered to be undiagnosed.168 Accordingly, the application of these tools to acute care settings is a major emphasis.
In the context of pregnancy, identification and culture of bacteria from AF has been considered the “gold standard” for the diagnosis of MIAC.10, 11, 15, 16, 19, 24, 42, 50-66 However, successful cultivation of an organism requires knowledge of the conditions necessary to support growth of specific microorganisms.169 Often, these conditions are unknown, rendering standard techniques inadequate in detecting many organisms implicated in human diseases.170-174 For example, Treponema pallidum, the organism responsible for syphilis, has remained difficult to culture for decades.175, 176 In addition, cultivation methods are frequently unreliable (and prone to false-negative results) when antibiotics are administered before the biological fluid is obtained for culture.67-75 Moreover, the results generally take days, and are often not available in time to inform clinical decisions (e.g. antibiotic administration). This is why physicians have adopted the standard approach of beginning intravenous antibiotic therapy without knowing the etiologic agent responsible for a suspected infection.67-75
The realization that only a small fraction of the microbial world is readily culturable in clinical laboratories 177-181 prompted the development of PCR-based diagnostic techniques for infectious diseases.83-88 The advantages of molecular techniques are that they allow broad-spectrum microbial detection, evaluation of emerging novel infections, assessment of antimicrobial resistance profiling,85, 103, 105 virulence factors,85, 104 and relatively low cost.85 These methods were used to identify agents responsible for multiple conditions previously considered as being of unknown etiology (e.g. Tropheryma whipplei, the agent of Whipple's disease,182, 183 Mycobacterium genavense, a cause of disseminated infections in AIDS patients,184 Ehrlichia chaffeensis, an agent of human tick-borne monocytic ehrlichiosis,185 and Bartonella henselae, the agent of Trench fever).186-188 However, implementation of PCR techniques in clinical practice has not been easy. Bacteria, viruses, and fungi must be investigated using separate assays, and the time required to obtain results is longer than when using emerging techniques, such as PCR/ESI-MS.
Broad-range PCR with ESI-MS for the rapid detection of microorganisms
Broad-range PCR methods for the detection of bacteria are based on the fundamental premise that 16S rRNA gene is evolutionarily conserved exclusively in bacterial species.189, 190 The detection of this gene in a sterile biological fluid (such as amniotic fluid) accordingly indicates the presence of bacteria. This approach has been used by many investigators to identify bacteria in amniotic fluid using species-specific 34, 39, 42, 65, 89, 92, 93, 95-97, 100, 191-196 or broad-range PCR.90-92, 99, 101, 143, 144, 197-200 Most of the studies have shown that broad-range PCR assays and specific assays are superior to culture in the detection of microorganisms in the amniotic fluid.90-92, 99, 101, 143, 144, 197-200 Yet, until recently, these techniques have remained research procedures because the time required to obtain results has precluded their use as a point of care test.
A fundamental advantage of the PCR/ESI-MS technology over conventional PCR methods using broad primers is that mass spectrometry allows the rapid identification of the organism.109, 111 PCR/ESI-MS results can be obtained within 8 hours.106, 112, 113 This is a key feature since failure to identify pathogens in time to inform clinical decisions is a major impediment to the treatment of infectious diseases. The PCR/ESI-MS system is also able to quantify the amount of genomic material present in a biological sample.108, 109 Genome quantification is useful in clinical circumstances including the selection of antimicrobial agents and the monitoring of therapeutic benefit. This can also be used in selecting the route of delivery (vaginal vs. cesarean delivery) in cases of HIV infection.201-207 Other examples in which this approach has been valuable include visceral leishmaniasis, 208-210 infectious mononucleosis,211-213 and mortality risk assessment in patients with pneumococcal pneumonia.214, 215
PCR/ESI-MS technology also uses the concept of “triangulation” to distinguish among microorganisms. This term refers to taking measurements from multiple loci distributed across the microbial genome.108, 109 Subsequently, ESI-MS is used to rapidly determine the precise mass-to-charge ratio of the fragments amplified based upon their nucleotide composition to create a signature that allows for the identification of a large number of microorganisms 109, 112 at the genus and species levels.112, 116, 120-122, 131
Broad-range PCR with ESI-MS for the rapid detection of intra-amniotic infection
The identification of bacteria in amniotic fluid is a pathological finding, which we have defined as “microbial invasion of the amniotic cavity”.18, 24, 40, 57, 59, 60, 62, 143, 144, 160, 165, 216-220 The distinction between such invasion and infection is predicated on the host response to the invading microorganism(s).23, 42, 43, 60, 149, 156, 157, 165, 217, 218, 220-236 Thus, intra-amniotic infection refers to the combination of MIAC with intra-amniotic inflammation.40, 41, 43, 162, 165, 219, 235, 237-239 In this study, MIAC was diagnosed by PCR/ESI-MS in 12% (n=17/142) of the study population, and 94% (n=16/17) of these patients exhibited a host inflammatory response consistent with intra-amniotic infection (AF IL-6 ≥ 2.6 ng/mL). In contrast, 70% (n=7/10) of patients with positive amniotic fluid cultures had such a host response, although each of the seven cases with both tests positive met the criteria for intra-amniotic infection. Accordingly, use of PCR/ESI-MS resulted in a twofold greater prevalence of intra-amniotic infection (5% vs. 11%).
Viral infection in patients with preterm labor
The role of viral infection in spontaneous preterm labor has not been examined rigorously. Previous studies using PCR-based methods have shown conflicting results describing the prevalence of viral invasion into the amniotic cavity (VIAC), ranging from non-detectable, to as high as 6.4%.151-153, 240-242 In this study, we used PCR/ESI-MS to detect multiple organisms in the same sample, (viruses and bacteria), as co-infection may occur.109 However, only two patients (1.4%) had positive results for Enterovirus. Recently, Gervasi et al., using specific PCR assays for a panel of viruses reported that the prevalence of VIAC during the second trimester was 2.2%.154 In that study, Gervasi et al. collected amniotic fluid samples from asymptomatic patients during the second trimester.154 The presence of viral nucleic acid in AF was not associated with detectable inflammation as reflected by AF WBC count, glucose and IL-6 concentrations between patients with and without VIAC.154 Similarly, Baschat et al., suggested that the isolation of viruses in the amniotic cavity was not associated with adverse maternal or perinatal outcome.153
Recent studies suggest that systemic viral infections may predispose pregnant mice to the effect of microbial products such as endotoxin.243 Specifically, pregnant mice infected with a herpesvirus were more likely to have spontaneous preterm labor and deliver following endotoxin administration than those who were not exposed to the virus.243, 244 Further studies are required to determine if this is a mechanism of disease operative in humans. It is well known that viral infections of the respiratory tract predispose to bacterial infections and pneumonia.245-248 The same may be the case in the lower genital tract.249 However, if the viral infections are not systemic (and associated with a symptomatic state), but are localized to the uterine cervix, or other parts of the genital tract, the identification of subclinical infection would pose an important challenge. Yet, given the high prevalence and diversity of viruses, as well as the severity of the consequences of viral predisposition to bacterial infections, this possibility cannot be ignored.
True-positive, false-negative and false-positive PCR/ESI-MS results
A challenge when interpreting the results of molecular microbiologic techniques is differentiating true- from false-positive results. PCR methods can amplify extremely small quantities of microbial DNA such that contamination of a specimen may appear as a positive result.
In this study, we used the host response to microbial invasion (e.g. intra-amniotic inflammation, histologic acute chorioamnionitis, and the onset of spontaneous preterm delivery) to assess the clinical significance of microbial detection by PCR/ESI-MS. We found that the microbial burden (expressed as gene copies/well) was significantly correlated with each marker of intra-amniotic inflammation, even when excluding patients with positive amniotic fluid cultures. The prevalence of intra-amniotic inflammation and histologic acute chorioamnionitis in patients with negative cultures and positive PCR/ESI-MS was 90% and 70%, respectively. Patients with positive PCR/ESI-MS results and negative amniotic fluid cultures also had a significantly shorter interval to spontaneous preterm delivery than women with negative tests. Moreover, neonates born to mothers with positive PCR/ESI-MS and negative amniotic fluid cultures were at five-fold greater risk of perinatal mortality than those born to mothers with negative tests. Together, these findings strongly support the conclusion that a positive PCR/ESI- MS (≥17 GE/well) result represents a true positive finding, even when amniotic fluid cultures are negative.
Bacterial cultures are considered a sensitive test for the identification of microorganisms when the conditions for supporting growth of that particular microorganism are optimal. Even one organism can form a colony in culture under ideal circumstances; yet, one organism may be insufficient for detection using PCR methods.250 In previous studies, we identified patients with positive amniotic fluid cultures who had negative PCR using broad primers and specific assays.94, 99, 101, 194 These results were attributed to possible DNA degradation,251 low inoculum size,252 etc. The observations herein suggest that false negative PCR/ESI-MS results are an extremely rare phenomenon. None of the three patients with positive amniotic fluid cultures and negative PCR/ESI-MS results in this study had intra-amniotic inflammation, only one had mild histologic acute chorionitis/subchorionitis, and all three delivered after 35 weeks of gestation without major complications. It is accordingly doubtful that positive amniotic fluid culture with negative PCR/ESI-MS results constitutes a true-positive culture.
Acidovorax sp. was identified by PCR/ESI-MS in one patient who had a negative amniotic fluid culture. The microbial burden in this case was low, (11 GE/well), the amniotic fluid Gram stain was negative, there was no evidence of intra-amniotic infection (by amniotic fluid IL-6, glucose, WBC count, or histologic acute chorioamnionitis); yet, the patient delivered before the 28th week of gestation. Acidovorax sp. has been identified as a contaminant in other studies 253-255 and thus, it is unlikely that PCR/ESI-MS identified true infection, responsible for the process that led to preterm delivery. Preterm labor, in this case, is most likely attributable to a mechanism other than intra-amniotic infection. Further studies are necessary to investigate the frequency of false positive and negative PCR/ESI-MS results using fresh samples collected in a clinical setting.
Clinical value of the rapid detection of MIAC in preterm labor
Therapy to reduce the rate of preterm birth in patients with symptoms of preterm labor has focused almost exclusively on tocolytic agents.256-266 However, these efforts have had limited success.263, 265, 267-269 This is partly attributable to the syndromic nature of preterm labor,164, 165, 219, 270-272 which may require an etiology-based approach to diagnosis, treatment, and prevention.
MIAC and subclinical intra-amniotic inflammation are implicated in at least one-third of the cases of spontaneous preterm labor and delivery.7-43, 155, 156 Furthermore, intra-amniotic infection has been recognized as predisposing to cerebral palsy 273-285 and chronic lung disease.41, 286-301 However, with the exception of asymptomatic bacteriuria,302-306 prophylactic antibiotic treatment has not been successful in the prevention of preterm birth,307-331 and some have claimed that it may increase the rate of preterm delivery.332
The ineffectiveness of antibiotics has been attributed to the inclusion in randomized clinical trials of patients who do not evidence of infection, and therefore cannot benefit from antimicrobial therapy,309, 314-320, 322-324, 326-331, 333 the choice and timing of antimicrobial agents,334 the presence of biofilm 335-340 in which bacteria are refractory to antimicrobial treatment, etc. Benefits of antimicrobial treatment could include eradication of intra-amniotic infection in early phases, down regulation of the inflammatory cascade that leads to preterm labor and delivery, prevention of an exaggerated inflammatory response syndrome that may cause fetal injury, and even congenital neonatal sepsis.
Animal models of intra-amniotic infection have shown that early administration of antibiotics at, or within 12 hours after inoculation of bacteria in AF reduces the bacterial burden in blood, the peritoneum, the uterus, and amniotic fluid.334, 341-343 Moreover, Grigsby et al. have shown that in an animal model of intra-amniotic infection, generated by the inoculation of Ureaplasma parvum, the administration of specific maternal antibiotic therapy (Azithromycin) can eradicate Ureaplasma parvum from the amniotic fluid and fetal lungs, prolonging pregnancy.344 Eradication of MIAC with parenteral antibiotic has also been documented in patients with a short cervix as well as in patients with preterm labor.345-349 For example, Hassan et al. reported that in patients with short cervix and positive AF culture for Ureaplasma urealyticum, AF cultures were sterile following 7 days of intravenous Azithromycin and three of the four cases delivered at term.350
The rapid, high-throughput technique used in this study is a promising option that facilitates broader and faster identification of MIAC in patients with preterm labor and intact membranes, and is applicable to other complications of pregnancy. Accordingly, this technique may render standard cultivation methods of limited value as it would allow targeting of specific interventions for at risk patients, including hospitalization, delivery, or transfer to high level facilities with specialized neonatal care. Future studies are required to clarify whether timely administration of specific antibiotic therapy for intra-amniotic infection could improve perinatal outcomes. Yet, as noted previously by our group,309, 345, 346, 351, 352 such studies may be difficult to conduct under the current framework of regulations protecting human subjects from research risks, since withholding antimicrobial therapy from immune-compromised patients (the human fetus) would most likely be ethically unacceptable.
Strengths and limitations
This is the first report of the use of PCR/ESI-MS in the amniotic fluid of patients with PTL and intact membranes, as well as the first direct comparison of this technology with standard cultivation techniques. These results provide evidence of the importance of the number of genome copies of bacteria during intra-amniotic infection. Future studies are required to assess the performance of PCR/ESI-MS in freshly collected samples.
Conclusion
1) Rapid diagnosis of intra-amniotic infection is possible using PCR/ESI-MS, which can provide results within 8 hours; 2) the combined use of biomarkers of inflammation and PCR/ESI-MS allows for the rapid identification of specific bacteria in cases of preterm labor and intra-amniotic inflammation; and 3) this approach may allow for administration of timely and specific interventions to reduce morbidity attributed to infection-induced preterm birth.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C.
We would like to acknowledge for the several contributions of Jack Sobel, MD, Chief of Infectious Diseases at Wayne State University, Ronald Lamont, BSc, MB, ChB, MD, FRCOG, Jacques Ravel, PhD, and David Relman, MD, and Sharon Hillier PhD, who have worked in collaboration with the Perinatology Research Branch in previous projects related to microbial invasion of the amniotic cavity and the vaginal microbiome.
Footnotes
This work was presented, in part, as an abstract at the 14th International Symposium for Immunology of Reproduction (2013). Boston, Massachusetts, USA.
References
- 1.World Health Organization. WHO Library Cataloguing-in-Publication. World Health Organization; 2012. Born too soon: the global action report in preterm birth. [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010-2011. Pediatrics. 2013;131:548–558. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimes Mo., editor. March of dimes: Premature Birth. 2009. [Google Scholar]
- 5.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 6.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 7.Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med. 1977;19:8–12. [PubMed] [Google Scholar]
- 8.Bobitt JR, Ledger WJ. Amniotic fluid analysis. Its role in maternal neonatal infection. Obstet Gynecol. 1978;51:56–62. [PubMed] [Google Scholar]
- 9.Miller JM, Jr, Pupkin MJ, Hill GB. Bacterial colonization of amniotic fluid from intact fetal membranes. Am J Obstet Gynecol. 1980;136:796–804. doi: 10.1016/0002-9378(80)90458-5. [DOI] [PubMed] [Google Scholar]
- 10.Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol. 1981;57:483–486. [PubMed] [Google Scholar]
- 11.Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981;140:947–952. doi: 10.1016/0002-9378(81)90090-9. [DOI] [PubMed] [Google Scholar]
- 12.Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol. 1984;148:739–743. doi: 10.1016/0002-9378(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 13.Hameed C, Tejani N, Verma UL, Archbald F. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am J Obstet Gynecol. 1984;149:726–730. doi: 10.1016/0002-9378(84)90111-x. [DOI] [PubMed] [Google Scholar]
- 14.Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986;67:229–237. doi: 10.1097/00006250-198602000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Leigh J, Garite TJ. Amniocentesis and the management of premature labor. Obstet Gynecol. 1986;67:500–506. [PubMed] [Google Scholar]
- 16.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Emamian M, Wan M, Yarkoni S, McCormack W, Mazor M, Hobbins JC. The value of the leukocyte esterase test in diagnosing intra-amniotic infection. Am J Perinatol. 1988;5:64–69. doi: 10.1055/s-2007-999657. [DOI] [PubMed] [Google Scholar]
- 18.Romero R, Scharf K, Mazor M, Emamian M, Hobbins JC, Ryan JL. The clinical value of gas-liquid chromatography in the detection of intra-amniotic microbial invasion. Obstet Gynecol. 1988;72:44–50. [PubMed] [Google Scholar]
- 19.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 20.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 21.Skoll MA, Moretti ML, Sibai BM. The incidence of positive amniotic fluid cultures in patients preterm labor with intact membranes. Am J Obstet Gynecol. 1989;161:813–816. doi: 10.1016/0002-9378(89)90407-9. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 25.Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol. 1991;165:1105–1110. doi: 10.1016/0002-9378(91)90480-f. [DOI] [PubMed] [Google Scholar]
- 26.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Coultrip LL, Grossman JH. Evaluation of rapid diagnostic tests in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1992;167:1231–1242. doi: 10.1016/s0002-9378(11)91694-9. [DOI] [PubMed] [Google Scholar]
- 28.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–612. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 29.Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol. 1996;88:1034–1040. doi: 10.1016/s0029-7844(96)00339-0. [DOI] [PubMed] [Google Scholar]
- 30.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92:77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 31.Hussey MJ, Levy ES, Pombar X, Meyer P, Strassner HT. Evaluating rapid diagnostic tests of intra-amniotic infection: Gram stain, amniotic fluid glucose level, and amniotic fluid to serum glucose level ratio. Am J Obstet Gynecol. 1998;179:650–656. doi: 10.1016/s0002-9378(98)70059-6. [DOI] [PubMed] [Google Scholar]
- 32.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 33.Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. Ultrasonographic assessment of the uterine cervix and interleukin-8 concentrations in cervical secretions predict intrauterine infection in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 1998;12:86–92. doi: 10.1046/j.1469-0705.1998.12020086.x. [DOI] [PubMed] [Google Scholar]
- 34.Oyarzun E, Yamamoto M, Kato S, Gomez R, Lizama L, Moenne A. Specific detection of 16 micro-organisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol. 1998;179:1115–1119. doi: 10.1016/s0002-9378(98)70115-2. [DOI] [PubMed] [Google Scholar]
- 35.Elimian A, Figueroa R, Canterino J, Verma U, Aguero-Rosenfeld M, Tejani N. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet Gynecol. 1998;92:72–76. doi: 10.1016/s0029-7844(98)00123-9. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, Gasser I, Bermejo B, Cabero L. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med. 1999;8:155–158. doi: 10.1002/(SICI)1520-6661(199907/08)8:4<155::AID-MFM3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Locksmith GJ, Clark P, Duff P, Schultz GS. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet Gynecol. 1999;94:1–6. doi: 10.1016/s0029-7844(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 38.Ovalle A, Martinez MA, Gomez R, Saez J, Menares I, Aspillaga C, Schwarze JE. Premature labor with intact membranes: microbiology of the amniotic fluid and lower genital tract and its relation with maternal and neonatal outcome. Rev Med Chil. 2000;128:985–995. [PubMed] [Google Scholar]
- 39.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, Jun JK. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–1137. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 41.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Wennerholm UB, Hagberg H. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120–128. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Lockwood CJ. Pathogenesis of Spontaneous Preterm Labor. In: Creasy RK, Resnik R, Iams J, editors. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. Sixth. Philadelphia: Elsevier; 2009. pp. 521–543. [Google Scholar]
- 44.Blanco JD, Gibbs RS, Krebs LF, Castaneda YS. The association between the absence of amniotic fluid bacterial inhibitory activity and intra-amniotic infection. Am J Obstet Gynecol. 1982;143:749–755. doi: 10.1016/0002-9378(82)90004-7. [DOI] [PubMed] [Google Scholar]
- 45.Gibbs RS, Blanco JD, Hnilica VS. Inorganic phosphorus and zinc concentrations in amniotic fluid: correlation with intra-amniotic infection and bacterial inhibitory activity. Am J Obstet Gynecol. 1982;143:163–166. doi: 10.1016/0002-9378(82)90647-0. [DOI] [PubMed] [Google Scholar]
- 46.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Blanco JD, Gibbs RS, Krebs LF. Inhibition of group B streptococci by amniotic fluid from patients with intra-amniotic infection and from control subjects. Am J Obstet Gynecol. 1983;147:247–250. doi: 10.1016/0002-9378(83)91105-5. [DOI] [PubMed] [Google Scholar]
- 48.Blanco JD, Gibbs RS, Krebs LF. A controlled study of amniotic fluid immunoglobulin levels in intraamniotic infection. Obstet Gynecol. 1983;61:450–453. [PubMed] [Google Scholar]
- 49.Blanco JD, Gibbs RS, Malherbe H, Strickland-Cholmley M, St Clair PJ, Castaneda YS. A controlled study of genital mycoplasmas in amniotic fluid from patients with intra-amniotic infection. J Infect Dis. 1983;147:650–653. doi: 10.1093/infdis/147.4.650. [DOI] [PubMed] [Google Scholar]
- 50.Garite TJ, Freeman RK, Linzey EM, Braly P. The use of amniocentesis in patients with premature rupture of membranes. Obstet Gynecol. 1979;54:226–230. [PubMed] [Google Scholar]
- 51.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 52.Cotton DB, Hill LM, Strassner HT, Platt LD, Ledger WJ. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol. 1984;63:38–43. [PubMed] [Google Scholar]
- 53.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, Hobbins JC. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–666. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Asrat T, Nageotte MP, Garite TJ, Gocke SE, Dorchester W. Gram stain results from amniocentesis in patients with preterm premature rupture of membranes--comparison of maternal and fetal characteristics. Am J Obstet Gynecol. 1990;163:887–889. doi: 10.1016/0002-9378(90)91089-u. [DOI] [PubMed] [Google Scholar]
- 56.Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol. 1991;31:331–336. doi: 10.1111/j.1479-828x.1991.tb02814.x. [DOI] [PubMed] [Google Scholar]
- 57.Romero R, Ghidini A, Mazor M, Behnke E. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34:769–778. doi: 10.1097/00003081-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 58.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 59.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, Parra M, Behnke E, Montiel F, Cassell GH. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129–133. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, Baumann P, Araneda H, Kenney JS, Cotton DB, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 61.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 62.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, Insunza A, Montiel F, Behnke E, Cassell GH. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38:543–548. [PubMed] [Google Scholar]
- 63.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–210. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 64.Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol. 1999;11:541–547. doi: 10.1097/00001703-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Nikolaitchouk N, Wennerholm UB, Hagberg H. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–431. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 66.Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66:316–321. [PubMed] [Google Scholar]
- 67.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244:379–386. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 68.Wing DA, Park AS, Debuque L, Millar LK. Limited clinical utility of blood and urine cultures in the treatment of acute pyelonephritis during pregnancy. Am J Obstet Gynecol. 2000;182:1437–1440. doi: 10.1067/mob.2000.106135. [DOI] [PubMed] [Google Scholar]
- 69.Gilstrap LC, 3rd, Ramin SM. Urinary tract infections during pregnancy. Obstet Gynecol Clin North Am. 2001;28:581–591. doi: 10.1016/s0889-8545(05)70219-9. [DOI] [PubMed] [Google Scholar]
- 70.Wing DA. Pyelonephritis in pregnancy: treatment options for optimal outcomes. Drugs. 2001;61:2087–2096. doi: 10.2165/00003495-200161140-00006. [DOI] [PubMed] [Google Scholar]
- 71.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 72.Kumar A. Optimizing antimicrobial therapy in sepsis and septic shock. Crit Care Clin. 2009;25:733–751. viii. doi: 10.1016/j.ccc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Jolley JA, Wing DA. Pyelonephritis in pregnancy: an update on treatment options for optimal outcomes. Drugs. 2010;70:1643–1655. doi: 10.2165/11538050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 74.Tam CS, O'Reilly M, Andresen D, Lingaratnam S, Kelly A, Burbury K, Turnidge J, Slavin MA, Worth LJ, Dawson L, Thursky KA. Use of empiric antimicrobial therapy in neutropenic fever. Australian Consensus Guidelines 2011 Steering Committee. Intern Med J. 2011;41:90–101. doi: 10.1111/j.1445-5994.2010.02340.x. [DOI] [PubMed] [Google Scholar]
- 75.Polin RA. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129:1006–1015. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 76.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 77.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 78.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 79.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 80.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Relman DA, Falkow S. Identification of uncultured microorganisms: expanding the spectrum of characterized microbial pathogens. Infect Agents Dis. 1992;1:245–253. [PubMed] [Google Scholar]
- 82.Relman DA. Emerging infections and newly-recognised pathogens. Neth J Med. 1997;50:216–220. doi: 10.1016/s0300-2977(97)00016-8. [DOI] [PubMed] [Google Scholar]
- 83.Harder TC, Hufnagel M, Zahn K, Beutel K, Schmitt HJ, Ullmann U, Rautenberg P. New LightCycler PCR for rapid and sensitive quantification of parvovirus B19 DNA guides therapeutic decision-making in relapsing infections. J Clin Microbiol. 2001;39:4413–4419. doi: 10.1128/JCM.39.12.4413-4419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng VC, Yam WC, Hung IF, Woo PC, Lau SK, Tang BS, Yuen KY. Clinical evaluation of the polymerase chain reaction for the rapid diagnosis of tuberculosis. J Clin Pathol. 2004;57:281–285. doi: 10.1136/jcp.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang S, Rothman RE. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. 2004;4:337–348. doi: 10.1016/S1473-3099(04)01044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alfa MJ, Sepehri S, De Gagne P, Helawa M, Sandhu G, Harding GK. Real-time PCR assay provides reliable assessment of intrapartum carriage of group B Streptococcus. J Clin Microbiol. 2010;48:3095–3099. doi: 10.1128/JCM.00594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bauer M, Reinhart K. Molecular diagnostics of sepsis--where are we today? Int J Med Microbiol. 2010;300:411–413. doi: 10.1016/j.ijmm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 88.Gardella C, Huang ML, Wald A, Magaret A, Selke S, Morrow R, Corey L. Rapid polymerase chain reaction assay to detect herpes simplex virus in the genital tract of women in labor. Obstet Gynecol. 2010;115:1209–1216. doi: 10.1097/AOG.0b013e3181e01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17(Suppl 1):S148–153. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 90.Jalava J, Mantymaa ML, Ekblad U, Toivanen P, Skurnik M, Lassila O, Alanen A. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103:664–669. doi: 10.1111/j.1471-0528.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 91.Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN, Eschenbach DA. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–1232. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 92.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG. 2002;109:527–533. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 93.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–521. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 94.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, Jun JK. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 95.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, Kalish RB, Witkin SS. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 96.Yi J, Yoon BH, Kim EC. Detection and biovar discrimination of Ureaplasma urealyticum by realtime PCR. Mol Cell Probes. 2005;19:255–260. doi: 10.1016/j.mcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 97.Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, Martinez M, Gamonal J. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007;78:1249–1255. doi: 10.1902/jop.2007.060368. [DOI] [PubMed] [Google Scholar]
- 98.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, Yoon BH. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203:211, e211–218. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzun E, Relman DA. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez N, Fernandez C, Zamora Y, Berdasquera D, Rivera JA. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: association with pregnancy outcomes. J Matern Fetal Neonatal Med. 2011;24:47–50. doi: 10.3109/14767058.2010.482609. [DOI] [PubMed] [Google Scholar]
- 103.Jungkind D. Tech.Sight. Molecular testing for infectious disease. Science. 2001;294:1553–1555. doi: 10.1126/science.294.5546.1553. [DOI] [PubMed] [Google Scholar]
- 104.Singh DV. Hexaplex PCR for rapid detection of virulence factors. Expert Rev Mol Diagn. 2003;3:781–784. doi: 10.1586/14737159.3.6.781. [DOI] [PubMed] [Google Scholar]
- 105.Bhattacharya S. Early diagnosis of resistant pathogens: how can it improve antimicrobial treatment? Virulence. 2013;4:172–184. doi: 10.4161/viru.23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ecker DJ, Sampath R, Blyn LB, Eshoo MW, Ivy C, Ecker JA, Libby B, Samant V, Sannes-Lowery KA, Melton RE, Russell K, Freed N, Barrozo C, Wu J, Rudnick K, Desai A, Moradi E, Knize DJ, Robbins DW, Hannis JC, Harrell PM, Massire C, Hall TA, Jiang Y, Ranken R, Drader JJ, White N, McNeil JA, Crooke ST, Hofstadler SA. Rapid identification and strain-typing of respiratory pathogens for epidemic surveillance. Proc Natl Acad Sci U S A. 2005;102:8012–8017. doi: 10.1073/pnas.0409920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ecker DJ, Sampath R, Willett P, Wyatt JR, Samant V, Massire C, Hall TA, Hari K, McNeil JA, Buchen-Osmond C, Budowle B. The Microbial Rosetta Stone Database: a compilation of global and emerging infectious microorganisms and bioterrorist threat agents. BMC Microbiol. 2005;5:19. doi: 10.1186/1471-2180-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hofstadler SA, Sampath R, Blyn LB, Eshoo MW, Hall TA, Jiang Y, Drader JJ, Hannis JC, Sannes-Lowery KA, Cummins LL, Libby B, Walcott DJ, Schink A, Massire C, Ranken R, Gutierrez J, Manalili S, Ivy C, Melton R, Levene H, Barret-Wilt G, Li F, Zapp V, White N, Samant V, McNeil JA, Knize D, Robbins D, Rudnick K, Desai A, Moradi E, Ecker DJ. TIGER: the universal biosensor. International Journal of Mass Spectrometry. 2005;242:23–41. [Google Scholar]
- 109.Sampath R, Hall TA, Massire C, Li F, Blyn LB, Eshoo MW, Hofstadler SA, Ecker DJ. Rapid identification of emerging infectious agents using PCR and electrospray ionization mass spectrometry. Ann N Y Acad Sci. 2007;1102:109–120. doi: 10.1196/annals.1408.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ecker DJ, Sampath R, Massire C, Blyn LB, Hall TA, Eshoo MW, Hofstadler SA. Ibis T5000: a universal biosensor approach for microbiology. Nat Rev Microbiol. 2008;6:553–558. doi: 10.1038/nrmicro1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ecker DJ, Massire C, Blyn LB, Hofstadler SA, Hannis JC, Eshoo MW, Hall TA, Sampath R. Molecular Genotyping of Microbes by Multilocus PCR and Mass Spectrometry: A new Tool for Hospital Infection Control and Public Health Surveillance. In: Caugant DA, editor. Molecular epidemiology of Microorganisms, Methods in Molecular Biology. Gumana Press, a part of Springer Science + Business Media; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ecker DJ, Sampath R, Li H, Massire C, Matthews HE, Toleno D, Hall TA, Blyn LB, Eshoo MW, Ranken R, Hofstadler SA, Tang YW. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn. 2010;10:399–415. doi: 10.1586/erm.10.24. [DOI] [PubMed] [Google Scholar]
- 113.Wolk DM, Kaleta EJ, Wysocki VH. PCR-electrospray ionization mass spectrometry: the potential to change infectious disease diagnostics in clinical and public health laboratories. J Mol Diagn. 2012;14:295–304. doi: 10.1016/j.jmoldx.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Metzgar D, Frinder M, Lovari R, Toleno D, Massire C, Blyn LB, Ranken R, Carolan HE, Hall TA, Moore D, Hansen CJ, Sampath R, Ecker DJ. Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J Clin Microbiol. 2013;51:2670–2678. doi: 10.1128/JCM.00966-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sampath R, Hofstadler SA, Blyn LB, Eshoo MW, Hall TA, Massire C, Levene HM, Hannis JC, Harrell PM, Neuman B, Buchmeier MJ, Jiang Y, Ranken R, Drader JJ, Samant V, Griffey RH, McNeil JA, Crooke ST, Ecker DJ. Rapid identification of emerging pathogens: coronavirus. Emerg Infect Dis. 2005;11:373–379. doi: 10.3201/eid1103.040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ecker JA, Massire C, Hall TA, Ranken R, Pennella TT, Agasino Ivy C, Blyn LB, Hofstadler SA, Endy TP, Scott PT, Lindler L, Hamilton T, Gaddy C, Snow K, Pe M, Fishbain J, Craft D, Deye G, Riddell S, Milstrey E, Petruccelli B, Brisse S, Harpin V, Schink A, Ecker DJ, Sampath R, Eshoo MW. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J Clin Microbiol. 2006;44:2921–2932. doi: 10.1128/JCM.00619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sampath R, Russell KL, Massire C, Eshoo MW, Harpin V, Blyn LB, Melton R, Ivy C, Pennella T, Li F, Levene H, Hall TA, Libby B, Fan N, Walcott DJ, Ranken R, Pear M, Schink A, Gutierrez J, Drader J, Moore D, Metzgar D, Addington L, Rothman R, Gaydos CA, Yang S, St George K, Fuschino ME, Dean AB, Stallknecht DE, Goekjian G, Yingst S, Monteville M, Saad MD, Whitehouse CA, Baldwin C, Rudnick KH, Hofstadler SA, Lemon SM, Ecker DJ. Global surveillance of emerging Influenza virus genotypes by mass spectrometry. PLoS One. 2007;2:e489. doi: 10.1371/journal.pone.0000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eshoo MW, Whitehouse CA, Zoll ST, Massire C, Pennella TT, Blyn LB, Sampath R, Hall TA, Ecker JA, Desai A, Wasieloski LP, Li F, Turell MJ, Schink A, Rudnick K, Otero G, Weaver SC, Ludwig GV, Hofstadler SA, Ecker DJ. Direct broad-range detection of alphaviruses in mosquito extracts. Virology. 2007;368:286–295. doi: 10.1016/j.virol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 119.Blyn LB, Hall TA, Libby B, Ranken R, Sampath R, Rudnick K, Moradi E, Desai A, Metzgar D, Russell KL, Freed NE, Balansay M, Broderick MP, Osuna MA, Hofstadler SA, Ecker DJ. Rapid detection and molecular serotyping of adenovirus by use of PCR followed by electrospray ionization mass spectrometry. J Clin Microbiol. 2008;46:644–651. doi: 10.1128/JCM.00801-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hannis JC, Manalili SM, Hall TA, Ranken R, White N, Sampath R, Blyn LB, Ecker DJ, Mandrell RE, Fagerquist CK, Bates AH, Miller WG, Hofstadler SA. High-resolution genotyping of Campylobacter species by use of PCR and high-throughput mass spectrometry. J Clin Microbiol. 2008;46:1220–1225. doi: 10.1128/JCM.02158-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wolk DM, Blyn LB, Hall TA, Sampath R, Ranken R, Ivy C, Melton R, Matthews H, White N, Li F, Harpin V, Ecker DJ, Limbago B, McDougal LK, Wysocki VH, Cai M, Carroll KC. Pathogen profiling: rapid molecular characterization of Staphylococcus aureus by PCR/electrospray ionization-mass spectrometry and correlation with phenotype. J Clin Microbiol. 2009;47:3129–3137. doi: 10.1128/JCM.00709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hujer KM, Hujer AM, Endimiani A, Thomson JM, Adams MD, Goglin K, Rather PN, Pennella TT, Massire C, Eshoo MW, Sampath R, Blyn LB, Ecker DJ, Bonomo RA. Rapid determination of quinolone resistance in Acinetobacter spp. J Clin Microbiol. 2009;47:1436–1442. doi: 10.1128/JCM.02380-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Metzgar D, Baynes D, Myers CA, Kammerer P, Unabia M, Faix DJ, Blair PJ. Initial identification and characterization of an emerging zoonotic influenza virus prior to pandemic spread. J Clin Microbiol. 2010;48:4228–4234. doi: 10.1128/JCM.01336-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deyde VM, Sampath R, Garten RJ, Blair PJ, Myers CA, Massire C, Matthews H, Svoboda P, Reed MS, Pohl J, Klimov AI, Gubareva LV. Genomic signature-based identification of influenza A viruses using RT-PCR/electro-spray ionization mass spectrometry (ESI-MS) technology. PLoS One. 2010;5:e13293. doi: 10.1371/journal.pone.0013293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eshoo MW, Crowder CD, Li H, Matthews HE, Meng S, Sefers SE, Sampath R, Stratton CW, Blyn LB, Ecker DJ, Tang YW. Detection and identification of Ehrlichia species in blood by use of PCR and electrospray ionization mass spectrometry. J Clin Microbiol. 2010;48:472–478. doi: 10.1128/JCM.01669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen KF, Blyn L, Rothman RE, Ramachandran P, Valsamakis A, Ecker D, Sampath R, Gaydos CA. Reverse transcription polymerase chain reaction and electrospray ionization mass spectrometry for identifying acute viral upper respiratory tract infections. Diagn Microbiol Infect Dis. 2011;69:179–186. doi: 10.1016/j.diagmicrobio.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaleta EJ, Clark AE, Cherkaoui A, Wysocki VH, Ingram EL, Schrenzel J, Wolk DM. Comparative analysis of PCR-electrospray ionization/mass spectrometry (MS) and MALDI-TOF/MS for the identification of bacteria and yeast from positive blood culture bottles. Clin Chem. 2011;57:1057–1067. doi: 10.1373/clinchem.2011.161968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Massire C, Ivy CA, Lovari R, Kurepina N, Li H, Blyn LB, Hofstadler SA, Khechinashvili G, Stratton CW, Sampath R, Tang YW, Ecker DJ, Kreiswirth BN. Simultaneous identification of mycobacterial isolates to the species level and determination of tuberculosis drug resistance by PCR followed by electrospray ionization mass spectrometry. J Clin Microbiol. 2011;49:908–917. doi: 10.1128/JCM.01578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gu Z, Hall TA, Frinder M, Walsh TJ, Hayden RT. Evaluation of repetitive sequence PCR and PCR-mass spectrometry for the identification of clinically relevant Candida species. Med Mycol. 2012;50:259–265. doi: 10.3109/13693786.2011.600341. [DOI] [PubMed] [Google Scholar]
- 130.Bhatia NS, Farrell JJ, Sampath R, Ranken R, Rounds MA, Ecker DJ, Bonomo RA. Identification of Streptococcus intermedius central nervous system infection by use of PCR and electrospray ionization mass spectrometry. J Clin Microbiol. 2012;50:4160–4162. doi: 10.1128/JCM.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schuetz AN, Huard RC, Eshoo MW, Massire C, Della-Latta P, Wu F, Jenkins SG. Identification of a novel Acinetobacter baumannii clone in a US hospital outbreak by multilocus polymerase chain reaction/electrospray-ionization mass spectrometry. Diagn Microbiol Infect Dis. 2012;72:14–19. doi: 10.1016/j.diagmicrobio.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 132.Brinkman CL, Vergidis P, Uhl JR, Pritt BS, Cockerill FR, Steckelberg JM, Baddour LM, Maleszewski JJ, Edwards WD, Sampath R, Patel R. PCR-Electrospray Ionization Mass Spectrometry for Direct Detection of Pathogens and Antimicrobial Resistance from Heart Valves in Patients with Infective Endocarditis. J Clin Microbiol. 2013;51:2040–2046. doi: 10.1128/JCM.00304-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Farrell JJ, Sampath R, Ecker DJ, Bonomo RA. “Salvage Microbiology”: Detection of Bacteria Directly from Clinical Specimens following Initiation of Antimicrobial Treatment. PLoS One. 2013;8:e66349. doi: 10.1371/journal.pone.0066349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jordana-Lluch E, Carolan HE, Gimenez M, Sampath R, Ecker DJ, Quesada MD, Modol JM, Armestar F, Blyn LB, Cummins LL, Ausina V, Martro E. Rapid diagnosis of bloodstream infections with PCR followed by mass spectrometry. PLoS One. 2013;8:e62108. doi: 10.1371/journal.pone.0062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, Soloski MJ, Ecker DJ, Schutzer SE, Aucott JN. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One. 2012;7:e36825. doi: 10.1371/journal.pone.0036825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shin JH, Ranken R, Sefers SE, Lovari R, Quinn CD, Meng S, Carolan HE, Toleno D, Li H, Lee JN, Stratton CW, Massire C, Tang YW. Detection, identification, and distribution of fungi in bronchoalveolar lavage specimens by use of multilocus PCR coupled with electrospray ionization/mass spectrometry. J Clin Microbiol. 2013;51:136–141. doi: 10.1128/JCM.01907-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.A Broad Range of Tests to Meet Your Needs. www.athogen.com/consulting-services/microbial-tests.html.In.
- 138.Legoff J, Feghoul L, Mercier-Delarue S, Dalle JH, Scieux C, Cherot J, Sicre de Fontbrune F, Baruchel A, Socie G, Simon F. Broad-Range PCR/Electrospray Ionization Mass Spectrometry for Detection and Typing of Adenovirus and Other Opportunistic Viruses in Stem Cell Transplant Patients. J Clin Microbiol. 2013 doi: 10.1128/JCM.01978-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, Kim JC, Kadar N, Romero R. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 140.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26(Suppl A):S114–117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 141.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 142.Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, Yoon BH. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292, e291–295. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 143.DiGiulio DB, Gervasi M, Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Seok KS, Gomez R, Mittal P, Gotsch F, Chaiworapongsa T, Oyarzun E, Kim CJ, Relman DA. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med. 2010;38:503–513. doi: 10.1515/JPM.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.DiGiulio DB, Gervasi MT, Romero R, Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Seok KS, Gomez R, Mittal P, Gotsch F, Chaiworapongsa T, Oyarzun E, Kim CJ, Relman DA. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med. 2010;38:495–502. doi: 10.1515/JPM.2010.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Romero R, Korzeniewski SJ. Are infants born by elective cesarean delivery without labor at risk for developing immune disorders later in life? Am J Obstet Gynecol. 2013;208:243–246. doi: 10.1016/j.ajog.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12:111–117. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 147.Seong HS, Lee SE, Kang JH, Romero R, Yoon BH. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol. 2008;199:375, e371–375. doi: 10.1016/j.ajog.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gervasi MT, Romero R, Bracalente G, Erez O, Dong Z, Hassan SS, Yeo L, Yoon BH, Chaiworapongsa T. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40:329–343. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yoon BH, Romero R, Moon J, Chaiworapongsa T, Espinoza J, Kim YM, Edwin S, Kim JC, Camacho N, Bujold E, Gomez R. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J Matern Fetal Neonatal Med. 2003;13:32–38. doi: 10.1080/jmf.13.1.32.38. [DOI] [PubMed] [Google Scholar]
- 150.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med. 2006;19:693–697. doi: 10.1080/14767050600927353. [DOI] [PubMed] [Google Scholar]
- 151.McLean LK, Chehab FF, Goldberg JD. Detection of viral deoxyribonucleic acid in the amniotic fluid of low-risk pregnancies by polymerase chain reaction. Am J Obstet Gynecol. 1995;173:1282–1286. doi: 10.1016/0002-9378(95)91371-8. [DOI] [PubMed] [Google Scholar]
- 152.Van den Veyver IB, Ni J, Bowles N, Carpenter RJ, Jr, Weiner CP, Yankowitz J, Moise KJ, Jr, Henderson J, Towbin JA. Detection of intrauterine viral infection using the polymerase chain reaction. Mol Genet Metab. 1998;63:85–95. doi: 10.1006/mgme.1997.2651. [DOI] [PubMed] [Google Scholar]
- 153.Baschat AA, Towbin J, Bowles NE, Harman CR, Weiner CP. Prevalence of viral DNA in amniotic fluid of low-risk pregnancies in the second trimester. J Matern Fetal Neonatal Med. 2003;13:381–384. doi: 10.1080/jmf.13.6.381.384. [DOI] [PubMed] [Google Scholar]
- 154.Gervasi MT, Romero R, Bracalente G, Chaiworapongsa T, Erez O, Dong Z, Hassan SS, Yeo L, Yoon BH, Mor G, Barzon L, Franchin E, Militello V, Palu G. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neonatal Med. 2012;25:2002–2013. doi: 10.3109/14767058.2012.683899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Berg TG, Philpot KL, Welsh MS, Sanger WG, Smith CV. Ureaplasma/Mycoplasma-infected amniotic fluid: pregnancy outcome in treated and nontreated patients. J Perinatol. 1999;19:275–277. doi: 10.1038/sj.jp.7200185. [DOI] [PubMed] [Google Scholar]
- 156.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 157.Hitti J, Tarczy-Hornoch P, Murphy J, Hillier SL, Aura J, Eschenbach DA. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks' gestation or less. Obstet Gynecol. 2001;98:1080–1088. doi: 10.1016/s0029-7844(01)01567-8. [DOI] [PubMed] [Google Scholar]
- 158.Soto E, Romero R, Richani K, Yoon BH, Chaiworapongsa T, Vaisbuch E, Mittal P, Erez O, Gotsch F, Mazor M, Kusanovic JP. Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J Matern Fetal Neonatal Med. 2009;22:983–992. doi: 10.3109/14767050902994747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kacerovsky M, Pliskova L, Bolehovska R, Skogstrand K, Hougaard DM, Tsiartas P, Jacobsson B. The impact of the microbial load of genital mycoplasmas and gestational age on the intensity of intraamniotic inflammation. Am J Obstet Gynecol. 2012;206:342, e341–348. doi: 10.1016/j.ajog.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 160.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, Mazor M, Treadwell MC, Cotton DB. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–1091. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 161.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652–655. doi: 10.1016/s0029-7844(99)00633-x. [DOI] [PubMed] [Google Scholar]
- 162.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633, e631–638. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 163.Bujold E, Morency AM, Rallu F, Ferland S, Tetu A, Duperron L, Audibert F, Laferriere C. Bacteriology of amniotic fluid in women with suspected cervical insufficiency. J Obstet Gynaecol Can. 2008;30:882–887. doi: 10.1016/S1701-2163(16)32967-X. [DOI] [PubMed] [Google Scholar]
- 164.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 165.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Relman DA. The biological century: coming to terms with risk in the life sciences. Nat Immunol. 2010;11:275–278. doi: 10.1038/ni0410-275. [DOI] [PubMed] [Google Scholar]
- 167.Relman DA. Microbial genomics and infectious diseases. N Engl J Med. 2011;365:347–357. doi: 10.1056/NEJMra1003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Increase in National Hospital Discharge Survey rates for septicemia--United States, 1979-1987. MMWR Morb Mortal Wkly Rep. 1990;39:31–34. [PubMed] [Google Scholar]
- 169.Houpikian P, Raoult D. Traditional and molecular techniques for the study of emerging bacterial diseases: one laboratory's perspective. Emerg Infect Dis. 2002;8:122–131. doi: 10.3201/eid0802.010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fournier PE, Raoult D. Nonculture Laboratory Methods for the Diagnosis of Infectious Endocarditis. Curr Infect Dis Rep. 1999;1:136–141. doi: 10.1007/s11908-996-0020-x. [DOI] [PubMed] [Google Scholar]
- 171.Yeo SF, Wong B. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin Microbiol Rev. 2002;15:465–484. doi: 10.1128/CMR.15.3.465-484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.White PL, Archer AE, Barnes RA. Comparison of non-culture-based methods for detection of systemic fungal infections, with an emphasis on invasive Candida infections. J Clin Microbiol. 2005;43:2181–2187. doi: 10.1128/JCM.43.5.2181-2187.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jones TF, Gerner-Smidt P. Nonculture diagnostic tests for enteric diseases. Emerg Infect Dis. 2012;18:513–514. doi: 10.3201/eid1803.111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Didelot X, Bowden R, Wilson DJ, Peto TE, Crook DW. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet. 2012;13:601–612. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fitzgerald T. In vitro cultivation of Treponema pallidum: a review. Bull World Health Organ. 1981;59:787–101. [PMC free article] [PubMed] [Google Scholar]
- 176.Ho EL, Lukehart SA. Syphilis: using modern approaches to understand an old disease. J Clin Invest. 2011;121:4584–4592. doi: 10.1172/JCI57173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pace NR. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 178.Relman DA. The search for unrecognized pathogens. Science. 1999;284:1308–1310. doi: 10.1126/science.284.5418.1308. [DOI] [PubMed] [Google Scholar]
- 179.Amann R. Who is out there? Microbial aspects of biodiversity. Syst Appl Microbiol. 2000;23:1–8. doi: 10.1016/S0723-2020(00)80039-9. [DOI] [PubMed] [Google Scholar]
- 180.Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J. Metagenomic pyrosequencing and microbial identification. Clin Chem. 2009;55:856–866. doi: 10.1373/clinchem.2008.107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Flores GE, Henley JB, Fierer N. A direct PCR approach to accelerate analyses of human-associated microbial communities. PLoS One. 2012;7:e44563. doi: 10.1371/journal.pone.0044563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wilson KH, Blitchington R, Frothingham R, Wilson JA. Phylogeny of the Whipple's-disease-associated bacterium. Lancet. 1991;338:474–475. doi: 10.1016/0140-6736(91)90545-z. [DOI] [PubMed] [Google Scholar]
- 183.Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple's disease. N Engl J Med. 1992;327:293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 184.Bottger EC, Teske A, Kirschner P, Bost S, Chang HR, Beer V, Hirschel B. Disseminated “Mycobacterium genavense” infection in patients with AIDS. Lancet. 1992;340:76–80. doi: 10.1016/0140-6736(92)90397-l. [DOI] [PubMed] [Google Scholar]
- 185.Anderson BE, Dawson JE, Jones DC, Wilson KH. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Welch DF, Pickett DA, Slater LN, Steigerwalt AG, Brenner DJ. Rochalimaea henselae sp nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol. 1992;30:275–280. doi: 10.1128/jcm.30.2.275-280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brenner DJ, O'Connor SP, Winkler HH, Steigerwalt AG. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol. 1993;43:777–786. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 188.Bergmans AM, Groothedde JW, Schellekens JF, van Embden JD, Ossewaarde JM, Schouls LM. Etiology of cat scratch disease: comparison of polymerase chain reaction detection of Bartonella (formerly Rochalimaea) and Afipia felis DNA with serology and skin tests. J Infect Dis. 1995;171:916–923. doi: 10.1093/infdis/171.4.916. [DOI] [PubMed] [Google Scholar]
- 189.Chen K, Neimark H, Rumore P, Steinman CR. Broad range DNA probes for detecting and amplifying eubacterial nucleic acids. FEMS Microbiol Lett. 1989;48:19–24. doi: 10.1016/0378-1097(89)90139-0. [DOI] [PubMed] [Google Scholar]
- 190.Greisen K, Loeffelholz M, Purohit A, Leong D. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol. 1994;32:335–351. doi: 10.1128/jcm.32.2.335-351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, Jun JK. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. American journal of obstetrics and gynecology. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 192.Holst RM, Mattsby-Baltzer I, Wennerholm UB, Hagberg H, Jacobsson B. Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta Obstet Gynecol Scand. 2005;84:551–557. doi: 10.1111/j.0001-6349.2005.00708.x. [DOI] [PubMed] [Google Scholar]
- 193.Holst RM, Jacobsson B, Hagberg H, Wennerholm UB. Cervical length in women in preterm labor with intact membranes: relationship to intra-amniotic inflammation/microbial invasion, cervical inflammation and preterm delivery. Ultrasound Obstet Gynecol. 2006;28:768–774. doi: 10.1002/uog.3837. [DOI] [PubMed] [Google Scholar]
- 194.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–268. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kacerovsky M, Pliskova L, Bolehovska R, Musilova I, Hornychova H, Tambor V, Jacobsson B. The microbial load with genital mycoplasmas correlates with the degree of histologic chorioamnionitis in preterm PROM. Am J Obstet Gynecol. 2011;205:213, e211–217. doi: 10.1016/j.ajog.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 196.Holst RM, Hagberg H, Wennerholm UB, Skogstrand K, Thorsen P, Jacobsson B. Prediction of microbial invasion of the amniotic cavity in women with preterm labour: analysis of multiple proteins in amniotic and cervical fluids. BJOG. 2011;118:240–249. doi: 10.1111/j.1471-0528.2010.02765.x. [DOI] [PubMed] [Google Scholar]
- 197.Markenson GR, Martin RK, Tillotson-Criss M, Foley KS, Stewart RS, Jr, Yancey M. The use of the polymerase chain reaction to detect bacteria in amniotic fluid in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1997;177:1471–1477. doi: 10.1016/s0002-9378(97)70093-0. [DOI] [PubMed] [Google Scholar]
- 198.Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol. 2004;21:319–323. doi: 10.1055/s-2004-831884. [DOI] [PubMed] [Google Scholar]
- 199.Miralles R, Hodge R, McParland PC, Field DJ, Bell SC, Taylor DJ, Grant WD, Kotecha S. Relationship between antenatal inflammation and antenatal infection identified by detection of microbial genes by polymerase chain reaction. Pediatr Res. 2005;57:570–577. doi: 10.1203/01.PDR.0000155944.48195.97. [DOI] [PubMed] [Google Scholar]
- 200.Marconi C, de Andrade Ramos BR, Peracoli JC, Donders GG, da Silva MG. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am J Reprod Immunol. 2011;65:549–556. doi: 10.1111/j.1600-0897.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 201.Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, Kornegay J, Jackson B, Moye J, Hanson C, Zorrilla C, Lew JF. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 202.Read JS. Cesarean section delivery to prevent vertical transmission of human immunodeficiency virus type 1. Associated risks and other considerations. Ann N Y Acad Sci. 2000;918:115–121. doi: 10.1111/j.1749-6632.2000.tb05479.x. [DOI] [PubMed] [Google Scholar]
- 203.Marcollet A, Goffinet F, Firtion G, Pannier E, Le Bret T, Brival ML, Mandelbrot L. Differences in postpartum morbidity in women who are infected with the human immunodeficiency virus after elective cesarean delivery, emergency cesarean delivery, or vaginal delivery. Am J Obstet Gynecol. 2002;186:784–789. doi: 10.1067/mob.2002.122251. [DOI] [PubMed] [Google Scholar]
- 204.Gross E, Burr CK. HIV counseling and testing in pregnancy. N J Med. 2003;100:21–26. quiz 67-28. [PubMed] [Google Scholar]
- 205.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, Clark JE. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE11-14. [PubMed] [Google Scholar]
- 206.Senise J, Bonafe S, Castelo A. The management of HIV-infected pregnant women. Curr Opin Obstet Gynecol. 2012;24:395–401. doi: 10.1097/GCO.0b013e328359f11e. [DOI] [PubMed] [Google Scholar]
- 207.Cotter AM, Brookfield KF, Duthely LM, Gonzalez Quintero VH, Potter JE, O'Sullivan MJ. Duration of membrane rupture and risk of perinatal transmission of HIV-1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482, e481–485. doi: 10.1016/j.ajog.2012.10.862. [DOI] [PubMed] [Google Scholar]
- 208.Mary C, Faraut F, Lascombe L, Dumon H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol. 2004;42:5249–5255. doi: 10.1128/JCM.42.11.5249-5255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pourabbas B, Ghadimi Moghadam A, Pouladfar G, Rezaee Z, Alborzi A. Quantification of Leishmania infantum kinetoplast DNA for monitoring the response to meglumine antimoniate therapy in visceral leishmaniasis. Am J Trop Med Hyg. 2013;88:868–871. doi: 10.4269/ajtmh.12-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Molina I, Fisa R, Riera C, Falco V, Elizalde A, Salvador F, Crespo M, Curran A, Lopez-Chejade P, Tebar S, Perez-Hoyos S, Ribera E, Pahissa A. Ultrasensitive real-time PCR for the clinical management of visceral leishmaniasis in HIV-Infected patients. Am J Trop Med Hyg. 2013;89:105–110. doi: 10.4269/ajtmh.12-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lay ML, Lucas RM, Ratnamohan M, Taylor J, Ponsonby AL, Dwyer DE. Measurement of Epstein-Barr virus DNA load using a novel quantification standard containing two EBV DNA targets and SYBR Green I dye. Virol J. 2010;7:252. doi: 10.1186/1743-422X-7-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sakamoto Y, Mariya Y, Kubo K. Quantification of Epstein-Barr virus DNA is helpful for evaluation of chronic active Epstein-Barr virus infection. Tohoku J Exp Med. 2012;227:307–311. doi: 10.1620/tjem.227.307. [DOI] [PubMed] [Google Scholar]
- 213.Ruf S, Wagner HJ. Determining EBV load: current best practice and future requirements. Expert Rev Clin Immunol. 2013;9:139–151. doi: 10.1586/eci.12.111. [DOI] [PubMed] [Google Scholar]
- 214.Rello J, Lisboa T, Lujan M, Gallego M, Kee C, Kay I, Lopez D, Waterer GW. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest. 2009;136:832–840. doi: 10.1378/chest.09-0258. [DOI] [PubMed] [Google Scholar]
- 215.Werno AM, Anderson TP, Murdoch DR. Association between pneumococcal load and disease severity in adults with pneumonia. J Med Microbiol. 2012;61:1129–1135. doi: 10.1099/jmm.0.044107-0. [DOI] [PubMed] [Google Scholar]
- 216.Romero R, Hanaoka S, Mazor M, Athanassiadis AP, Callahan R, Hsu YC, Avila C, Nores J, Jimenez C. Meconium-stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1991;164:859–862. doi: 10.1016/0002-9378(91)90529-z. [DOI] [PubMed] [Google Scholar]
- 217.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–220. doi: 10.1002/9780470514269.ch13. discussion 220-203. [DOI] [PubMed] [Google Scholar]
- 218.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, Svinarich D, Cotton DB. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–113. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 219.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. In: Elder MG, Romero R, Lamont RF, editors. Preterm labor. New York: Churchill Livingstone; 1997. pp. 29–49. [Google Scholar]
- 220.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, Kim KS. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–1260. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 221.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–341. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 222.Romero R, Avila C, Sepulveda W, et al. The role of systemic and intrauterine infection in preterm labor. In: Fuchs A, Fuchs F, Stubblefield P, editors. Preterm Birth: Causes, Prevention, and Management. New York: McGraw-Hill Inc.; 1993. p. 97. [Google Scholar]
- 223.Baumann P, Romero R, Berry S, Gomez R, McFarlin B, Araneda H, Cotton DB, Fidel P. Evidence of participation of the soluble tumor necrosis factor receptor I in the host response to intrauterine infection in preterm labor. Am J Reprod Immunol. 1993;30:184–193. doi: 10.1111/j.1600-0897.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 224.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–948. [PubMed] [Google Scholar]
- 225.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 226.Gomez R, Ghezzi F, Romero R, Yoon BH, Mazor M, Berry SM. Two thirds of human fetuses with microbial invasion of the amniotic cavity have a detectable systemic cyto-kine response before birth. Am J Obstet Gynecol. 1997;176:S14. [Google Scholar]
- 227.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 228.Hsu CD, Meaddough E, Hong SF, Aversa K, Lu LC, Copel JA. Elevated amniotic fluid nitric oxide metabolites and interleukin-6 in intra-amniotic infection. J Soc Gynecol Investig. 1998;5:21–24. doi: 10.1016/s1071-5576(97)00099-3. [DOI] [PubMed] [Google Scholar]
- 229.Hsu CD, Meaddough E, Aversa K, Hong SF, Lu LC, Jones DC, Copel JA. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol. 1998;179:1267–1270. doi: 10.1016/s0002-9378(98)70144-9. [DOI] [PubMed] [Google Scholar]
- 230.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–1143. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 231.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, Edwin SS. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000;182:135–141. doi: 10.1016/s0002-9378(00)70502-3. [DOI] [PubMed] [Google Scholar]
- 232.Keelan JA, Yang J, Romero RJ, Chaiworapongsa T, Marvin KW, Sato TA, Mitchell MD. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol Reprod. 2004;70:253–259. doi: 10.1095/biolreprod.103.016204. [DOI] [PubMed] [Google Scholar]
- 233.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, Adashi EY. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 234.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–416. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kacerovsky M, Musilova I, Khatibi A, Skogstrand K, Hougaard DM, Tambor V, Tosner J, Jacobsson B. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2012;25:2014–2019. doi: 10.3109/14767058.2012.671873. [DOI] [PubMed] [Google Scholar]
- 237.Shim SH, Yoon BH, Romero R, Hong JS, Kim G, Sohn YK, et al. The frequency and the clinical significant of intraamniotic inflammation in patients with preterm premature rupture of membrane. Am J Obstet Gynecol. 2003;189:S83. [Google Scholar]
- 238.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 239.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294, e291–296. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 240.Wenstrom KD, Andrews WW, Bowles NE, Towbin JA, Hauth JC, Goldenberg RL. Intrauterine viral infection at the time of second trimester genetic amniocentesis. Obstet Gynecol. 1998;92:420–424. doi: 10.1016/s0029-7844(98)00210-5. [DOI] [PubMed] [Google Scholar]
- 241.Naresh A, Simhan H. Absence of viruses in amniotic fluid of women with PPROM: a case series. J Reprod Immunol. 2012;96:79–83. doi: 10.1016/j.jri.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 242.Bopegamage S, Kacerovsky M, Tambor V, Musilova I, Sarmirova S, Snelders E, de Jong AS, Vari SG, Melchers WJ, Galama JM. Preterm prelabor rupture of membranes (PPROM) is not associated with presence of viral genomes in the amniotic fluid. J Clin Virol. 2013 doi: 10.1016/j.jcv.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 243.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, Means RE. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. American journal of reproductive immunology. 2011;65:110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hament JM, Kimpen JL, Fleer A, Wolfs TF. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol. 1999;26:189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 246.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918-19 influenza pandemic. Emerg Infect Dis. 2008;14:1193–1199. doi: 10.3201/eid1408.071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Racicot K, Cardenas I, Wunsche V, Aldo P, Guller S, Means RE, Romero R, Mor G. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013;191:934–941. doi: 10.4049/jimmunol.1300661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Smith CJ, Osborn AM. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol Ecol. 2009;67:6–20. doi: 10.1111/j.1574-6941.2008.00629.x. [DOI] [PubMed] [Google Scholar]
- 251.Katsoulis J, Heitz-Mayfield LJ, Weibel M, Hirschi R, Lang NP, Persson GR. Impact of sample storage on detection of periodontal bacteria. Oral Microbiol Immunol. 2005;20:128–130. doi: 10.1111/j.1399-302X.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- 252.von Wintzingerode F, Gobel UB, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev. 1997;21:213–229. doi: 10.1111/j.1574-6976.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 253.Sun W, Dong L, Kaneyama K, Takegami T, Segami N. Bacterial diversity in synovial fluids of patients with TMD determined by cloning and sequencing analysis of the 16S ribosomal RNA gene. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:566–571. doi: 10.1016/j.tripleo.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 254.Hong PY, Croix JA, Greenberg E, Gaskins HR, Mackie RI. Pyrosequencing-based analysis of the mucosal microbiota in healthy individuals reveals ubiquitous bacterial groups and micro-heterogeneity. PLoS One. 2011;6:e25042. doi: 10.1371/journal.pone.0025042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Vaneechoutte M, Janssens M, Avesani V, Delmee M, Deschaght P. Description of Acidovorax wautersii sp nov to accommodate clinical isolates and an environmental isolate, most closely related to Acidovorax avenae. Int J Syst Evol Microbiol. 2013;63:2203–2206. doi: 10.1099/ijs.0.046102-0. [DOI] [PubMed] [Google Scholar]
- 256.Cox SM, Sherman ML, Leveno KJ. Randomized investigation of magnesium sulfate for prevention of preterm birth. Am J Obstet Gynecol. 1990;163:767–772. doi: 10.1016/0002-9378(90)91065-k. [DOI] [PubMed] [Google Scholar]
- 257.Treatment of preterm labor with the beta-adrenergic agonist ritodrine. The Canadian Preterm Labor Investigators Group. N Engl J Med. 1992;327:308–312. doi: 10.1056/NEJM199207303270503. [DOI] [PubMed] [Google Scholar]
- 258.Papatsonis DN, Van Geijn HP, Ader HJ, Lange FM, Bleker OP, Dekker GA. Nifedipine and ritodrine in the management of preterm labor: a randomized multicenter trial. Obstet Gynecol. 1997;90:230–234. doi: 10.1016/S0029-7844(97)00182-8. [DOI] [PubMed] [Google Scholar]
- 259.Romero R, Sibai BM, Sanchez-Ramos L, Valenzuela GJ, Veille JC, Tabor B, Perry KG, Varner M, Goodwin TM, Lane R, Smith J, Shangold G, Creasy GW. An oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double-blind, placebo-controlled trial with tocolytic rescue. Am J Obstet Gynecol. 2000;182:1173–1183. doi: 10.1067/mob.2000.95834. [DOI] [PubMed] [Google Scholar]
- 260.Valenzuela GJ, Sanchez-Ramos L, Romero R, Silver HM, Koltun WD, Millar L, Hobbins J, Rayburn W, Shangold G, Wang J, Smith J, Creasy GW. Maintenance treatment of preterm labor with the oxytocin antagonist atosiban. The Atosiban PTL-098 Study Group. Am J Obstet Gynecol. 2000;182:1184–1190. doi: 10.1067/mob.2000.105816. [DOI] [PubMed] [Google Scholar]
- 261.Smith GN, Walker MC, Ohlsson A, O'Brien K, Windrim R. Randomized double-blind placebo-controlled trial of transdermal nitroglycerin for preterm labor. Am J Obstet Gynecol. 2007;196:37, e31–38. doi: 10.1016/j.ajog.2006.10.868. [DOI] [PubMed] [Google Scholar]
- 262.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 263.Conde-Agudelo A, Romero R, Kusanovic JP. Nifedipine in the management of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204:134, e131–120. doi: 10.1016/j.ajog.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ. 2012;345:e6226. doi: 10.1136/bmj.e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Conde-Agudelo A, Romero R. Transdermal nitroglycerin for the treatment of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2013 doi: 10.1016/j.ajog.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hadar E, Melamed N, Aviram A, Raban O, Saltzer L, Hiersch L, Yogev Y. Effect of an oxytocin receptor antagonist (atosiban) on uterine electrical activity. Am J Obstet Gynecol. 2013;209:384, e381–387. doi: 10.1016/j.ajog.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 267.Sanchez-Ramos L, Kaunitz AM, Gaudier FL, Delke I. Efficacy of maintenance therapy after acute tocolysis: a meta-analysis. Am J Obstet Gynecol. 1999;181:484–490. doi: 10.1016/s0002-9378(99)70582-x. [DOI] [PubMed] [Google Scholar]
- 268.Berkman ND, Thorp JM, Jr, Lohr KN, Carey TS, Hartmann KE, Gavin NI, Hasselblad V, Idicula AE. Tocolytic treatment for the management of preterm labor: a review of the evidence. Am J Obstet Gynecol. 2003;188:1648–1659. doi: 10.1067/mob.2003.356. [DOI] [PubMed] [Google Scholar]
- 269.Dodd JM, Crowther CA, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database Syst Rev. 2012;12:CD003927. doi: 10.1002/14651858.CD003927.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Romero R. The child is the father of the man. Prenat Neonat Med. 1996;1:8–11. [Google Scholar]
- 271.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 272.Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–211. [PubMed] [Google Scholar]
- 274.Dammann O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol. 1998;5:190–201. doi: 10.1016/s1071-9091(98)80034-x. [DOI] [PubMed] [Google Scholar]
- 275.Paneth N. Classifying brain damage in preterm infants. J Pediatr. 1999;134:527–529. doi: 10.1016/s0022-3476(99)70231-3. [DOI] [PubMed] [Google Scholar]
- 276.Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol. 2000;182:1404–1413. doi: 10.1067/mob.2000.106180. [DOI] [PubMed] [Google Scholar]
- 277.Grether JK, Nelson KB, Walsh E, Willoughby RE, Redline RW. Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Arch Pediatr Adolesc Med. 2003;157:26–32. doi: 10.1001/archpedi.157.1.26. [DOI] [PubMed] [Google Scholar]
- 278.Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res. 2006;59:50–55. doi: 10.1203/01.pdr.0000191141.21932.b6. [DOI] [PubMed] [Google Scholar]
- 279.Dammann O, Leviton A. Inflammation, brain damage and visual dysfunction in preterm infants. Semin Fetal Neonatal Med. 2006;11:363–368. doi: 10.1016/j.siny.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 280.McElrath TF, Allred EN, Boggess KA, Kuban K, O'Shea TM, Paneth N, Leviton A. Maternal antenatal complications and the risk of neonatal cerebral white matter damage and later cerebral palsy in children born at an extremely low gestational age. Am J Epidemiol. 2009;170:819–828. doi: 10.1093/aje/kwp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.O'Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, Leviton A. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Leviton A, Allred EN, Kuban KC, Hecht JL, Onderdonk AB, O'Shea TM, Paneth N. Microbiologic and histologic characteristics of the extremely preterm infant's placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr Res. 2010;67:95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, Cohen AS, Yudkoff M, Elovitz MA. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. Journal of neuroscience research. 2010;88:1872–1881. doi: 10.1002/jnr.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29:663–671. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Breen K, Brown A, Burd I, Chai J, Friedman A, Elovitz MA. TLR-4-dependent and -independent mechanisms of fetal brain injury in the setting of preterm birth. Reproductive sciences. 2012;19:839–850. doi: 10.1177/1933719112438439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 287.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 288.Jobe AH, Newnham JP, Willet KE, Moss TJ, Gore Ervin M, Padbury JF, Sly P, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med. 2000;162:1656–1661. doi: 10.1164/ajrccm.162.5.2003044. [DOI] [PubMed] [Google Scholar]
- 289.Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C, Possmayer F, Hallman M, Ikegami M. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol. 2000;182:401–408. doi: 10.1016/s0002-9378(00)70231-6. [DOI] [PubMed] [Google Scholar]
- 290.Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate. 2001;79:205–209. doi: 10.1159/000047092. [DOI] [PubMed] [Google Scholar]
- 291.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2:27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Willet KE, Kramer BW, Kallapur SG, Ikegami M, Newnham JP, Moss TJ, Sly PD, Jobe AH. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2002;282:L411–420. doi: 10.1152/ajplung.00097.2001. [DOI] [PubMed] [Google Scholar]
- 293.Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol. 2003;8:29–38. doi: 10.1016/s1084-2756(02)00190-2. [DOI] [PubMed] [Google Scholar]
- 294.Dammann O, Leviton A, Gappa M, Dammann CE. Lung and brain damage in preterm newborns, and their association with gestational age, prematurity subgroup, infection/inflammation and long term outcome. BJOG. 2005;112(Suppl 1):4–9. doi: 10.1111/j.1471-0528.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- 295.Maxwell NC, Davies PL, Kotecha S. Antenatal infection and inflammation: what's new? Curr Opin Infect Dis. 2006;19:253–258. doi: 10.1097/01.qco.0000224819.42729.2e. [DOI] [PubMed] [Google Scholar]
- 296.Moss TJ, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, Ikegami M, Jobe AH. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol. 2008;198:122, e121–128. doi: 10.1016/j.ajog.2007.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123:1314–1319. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 298.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Polglase GR, Hillman NH, Ball MK, Kramer BW, Kallapur SG, Jobe AH, Pillow JJ. Lung and systemic inflammation in preterm lambs on continuous positive airway pressure or conventional ventilation. Pediatr Res. 2009;65:67–71. doi: 10.1203/PDR.0b013e318189487e. [DOI] [PubMed] [Google Scholar]
- 300.Polglase GR, Dalton RG, Nitsos I, Knox CL, Pillow JJ, Jobe AH, Moss TJ, Newnham JP, Kallapur SG. Pulmonary vascular and alveolar development in preterm lambs chronically colonized with Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol. 2010;299:L232–241. doi: 10.1152/ajplung.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kallapur SG, Kramer BW, Knox CL, Berry CA, Collins JJ, Kemp MW, Nitsos I, Polglase GR, Robinson J, Hillman NH, Newnham JP, Chougnet C, Jobe AH. Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep. J Immunol. 2011;187:2688–2695. doi: 10.4049/jimmunol.1100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73:576–582. [PubMed] [Google Scholar]
- 303.Smaill F. Antibiotic versus no treatment for asymptomatic bacteriuria in pregnancy. In: Chalmers I, editor. Oxford Database of Perinatal Trials. Oxford University Press; 1991. [Google Scholar]
- 304.Hall DR, Theron GB, van der Horst W. Significance and treatment of asymptomatic bacteriuria during pregnancy. Int J Gynaecol Obstet. 1997;57:179–180. doi: 10.1016/s0020-7292(97)02819-1. [DOI] [PubMed] [Google Scholar]
- 305.Villar J, Gulmezoglu AM, de Onis M. Nutritional and antimicrobial interventions to prevent preterm birth: an overview of randomized controlled trials. Obstet Gynecol Surv. 1998;53:575–585. doi: 10.1097/00006254-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 306.Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2007:CD000490. doi: 10.1002/14651858.CD000490.pub2. [DOI] [PubMed] [Google Scholar]
- 307.Duff P, Lee ML, Hillier SL, Herd LM, Krohn MA, Eschenbach DA. Amoxicillin treatment of bacterial vaginosis during pregnancy. Obstet Gynecol. 1991;77:431–435. [PubMed] [Google Scholar]
- 308.Lugo-Miro VI, Green M, Mazur L. Comparison of different metronidazole therapeutic regimens for bacterial vaginosis. A meta-analysis. JAMA. 1992;268:92–95. [PubMed] [Google Scholar]
- 309.Romero R, Sibai B, Caritis S, Paul R, Depp R, Rosen M, Klebanoff M, Sabo V, Evans J, Thom E, et al. Antibiotic treatment of preterm labor with intact membranes: a multicenter, randomized, double-blinded, placebo-controlled trial. Am J Obstet Gynecol. 1993;169:764–774. doi: 10.1016/0002-9378(93)90003-2. [DOI] [PubMed] [Google Scholar]
- 310.Hillier SL, Lipinski C, Briselden AM, Eschenbach DA. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol. 1993;81:963–967. [PubMed] [Google Scholar]
- 311.Livengood CH, 3rd, McGregor JA, Soper DE, Newton E, Thomason JL. Bacterial vaginosis: efficacy and safety of intravaginal metronidazole treatment. Am J Obstet Gynecol. 1994;170:759–764. doi: 10.1016/s0002-9378(94)70278-0. [DOI] [PubMed] [Google Scholar]
- 312.Joesoef MR, Hillier SL, Wiknjosastro G, Sumampouw H, Linnan M, Norojono W, Idajadi A, Utomo B. Intravaginal clindamycin treatment for bacterial vaginosis: effects on preterm delivery and low birth weight. Am J Obstet Gynecol. 1995;173:1527–1531. doi: 10.1016/0002-9378(95)90644-4. [DOI] [PubMed] [Google Scholar]
- 313.Cox SM, Bohman VR, Sherman ML, Leveno KJ. Randomized investigation of antimicrobials for the prevention of preterm birth. Am J Obstet Gynecol. 1996;174:206–210. doi: 10.1016/s0002-9378(96)70395-2. [DOI] [PubMed] [Google Scholar]
- 314.McDonald HM, O'Loughlin JA, Vigneswaran R, Jolley PT, Harvey JA, Bof A, McDonald PJ. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis flora (Gardnerella vaginalis): a randomised, placebo controlled trial. Br J Obstet Gynaecol. 1997;104:1391–1397. doi: 10.1111/j.1471-0528.1997.tb11009.x. [DOI] [PubMed] [Google Scholar]
- 315.Vermeulen GM, Bruinse HW. Prophylactic administration of clindamycin 2% vaginal cream to reduce the incidence of spontaneous preterm birth in women with an increased recurrence risk: a randomised placebo-controlled double-blind trial. Br J Obstet Gynaecol. 1999;106:652–657. doi: 10.1111/j.1471-0528.1999.tb08363.x. [DOI] [PubMed] [Google Scholar]
- 316.Stetzer BP, Mercer BM. Antibiotics and preterm labor. Clin Obstet Gynecol. 2000;43:809–817. doi: 10.1097/00003081-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 317.Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, Heine RP, Nugent RP, Fischer ML, Leveno KJ, Wapner R, Varner M. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 318.Kurkinen-Raty M, Vuopala S, Koskela M, Kekki M, Kurki T, Paavonen J, Jouppila P. A randomised controlled trial of vaginal clindamycin for early pregnancy bacterial vaginosis. BJOG. 2000;107:1427–1432. doi: 10.1111/j.1471-0528.2000.tb11660.x. [DOI] [PubMed] [Google Scholar]
- 319.Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:989–994. doi: 10.1016/s0140-6736(00)04234-3. [DOI] [PubMed] [Google Scholar]
- 320.Kekki M, Kurki T, Pelkonen J, Kurkinen-Raty M, Cacciatore B, Paavonen J. Vaginal clindamycin in preventing preterm birth and peripartal infections in asymptomatic women with bacterial vaginosis: a randomized, controlled trial. Obstet Gynecol. 2001;97:643–648. doi: 10.1016/s0029-7844(01)01321-7. [DOI] [PubMed] [Google Scholar]
- 321.Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, Ernest JM, Heine RP, Wapner RJ, Trout W, Moawad A, Leveno KJ, Miodovnik M, Sibai BM, Van Dorsten JP, Dombrowski MP, O'Sullivan MJ, Varner M, Langer O, McNellis D, Roberts JM. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487–493. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 322.King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev. 2002:CD000246. doi: 10.1002/14651858.CD000246. [DOI] [PubMed] [Google Scholar]
- 323.Andrews WW, Sibai BM, Thom EA, Dudley D, Ernest JM, McNellis D, Leveno KJ, Wapner R, Moawad A, O'Sullivan MJ, Caritis SN, Iams JD, Langer O, Miodovnik M, Dombrowski M. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin-positive women. Obstet Gynecol. 2003;101:847–855. doi: 10.1016/s0029-7844(03)00172-8. [DOI] [PubMed] [Google Scholar]
- 324.Guaschino S, Ricci E, Franchi M, Frate GD, Tibaldi C, Santo DD, Ghezzi F, Benedetto C, Seta FD, Parazzini F. Treatment of asymptomatic bacterial vaginosis to prevent pre-term delivery: a randomised trial. Eur J Obstet Gynecol Reprod Biol. 2003;110:149–152. doi: 10.1016/s0301-2115(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 325.Klein LL, Gibbs RS. Use of microbial cultures and antibiotics in the prevention of infection-associated preterm birth. Am J Obstet Gynecol. 2004;190:1493–1502. doi: 10.1016/j.ajog.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 326.Goldenberg RL, Mwatha A, Read JS, Adeniyi-Jones S, Sinkala M, Msmanga G, Martinson F, Hoffman I, Fawzi W, Valentine M, Emel L, Brown E, Mudenda V, Taha TE. The HPTN 024 Study: the efficacy of antibiotics to prevent chorioamnionitis and preterm birth. Am J Obstet Gynecol. 2006;194:650–661. doi: 10.1016/j.ajog.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 327.Eschenbach DA. Bacterial vaginosis: resistance, recurrence, and/or reinfection? Clin Infect Dis. 2007;44:220–221. doi: 10.1086/509584. [DOI] [PubMed] [Google Scholar]
- 328.Nygren P, Fu R, Freeman M, Bougatsos C, Klebanoff M, Guise JM. Evidence on the benefits and harms of screening and treating pregnant women who are asymptomatic for bacterial vaginosis: an update review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;148:220–233. doi: 10.7326/0003-4819-148-3-200802050-00008. [DOI] [PubMed] [Google Scholar]
- 329.Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, Stamilio DM, Appleby D, Clothier B, Sammel MD, Jeffcoat M. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS) Am J Obstet Gynecol. 2010;202:147, e141–148. doi: 10.1016/j.ajog.2009.10.892. [DOI] [PubMed] [Google Scholar]
- 330.Lamont RF, Nhan-Chang CL, Sobel JD, Workowski K, Conde-Agudelo A, Romero R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;205:177–190. doi: 10.1016/j.ajog.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2013;1:CD000262. doi: 10.1002/14651858.CD000262.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Gulmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev. 2011:CD000220. doi: 10.1002/14651858.CD000220.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Romero R, Chaiworapongsa T, Kuivaniemi H, Tromp G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: a role for genetic epidemiology in the prevention of preterm birth. Am J Obstet Gynecol. 2004;190:1509–1519. doi: 10.1016/j.ajog.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 334.Fidel P, Ghezzi F, Romero R, Chaiworapongsa T, Espinoza J, Cutright J, Wolf N, Gomez R. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern Fetal Neonatal Med. 2003;14:57–64. doi: 10.1080/jmf.14.1.57.64. [DOI] [PubMed] [Google Scholar]
- 335.Romero R, Kusanovic JP, Espinoza J, Gotsch F, Nhan-Chang CL, Erez O, Kim CJ, Khalek N, Mittal P, Goncalves LF, Schaudinn C, Hassan SS, Costerton JW. What is amniotic fluid ‘sludge’? Ultrasound Obstet Gynecol. 2007;30:793–798. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, Nhan-Chang CL, Erez O, Kim CJ, Espinoza J, Goncalves LF, Vaisbuch E, Mazaki-Tovi S, Hassan SS, Costerton JW. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135, e131–135. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J. 2000;46:S47–52. doi: 10.1097/00002480-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 338.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 339.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 340.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 341.McDuffie RS, Jr, Blanton SJ, Shikes RH, Gibbs RS. A rabbit model for bacterially induced preterm pregnancy loss: intervention studies with ampicillin-sulbactam. Am J Obstet Gynecol. 1991;165:1568–1574. doi: 10.1016/0002-9378(91)90406-h. [DOI] [PubMed] [Google Scholar]
- 342.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 343.Gravett MG, Sadowsky DW, Witkin SS, Novy MJ. Annual Scientific Meeting. Infectious Diseases Society for Obsetrics and Gynecology; 2003. Early but not delayed antibiotic treatment prevents preterm delivery following experimental intraamniotic infection. [Google Scholar]
- 344.Grigsby PL, Novy MJ, Sadowsky DW, Morgan TK, Long M, Acosta E, Duffy LB, Waites KB. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol. 2012;207:475, e471–475, e414. doi: 10.1016/j.ajog.2012.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Romero R, Scioscia AL, Edberg SC, Hobbins JC. Use of parenteral antibiotic therapy to eradicate bacterial colonization of amniotic fluid in premature rupture of membranes. Obstet Gynecol. 1986;67:15S–17S. doi: 10.1097/00006250-198603001-00005. [DOI] [PubMed] [Google Scholar]
- 346.Romero R, Hagay Z, Nores J, Sepulveda W, Mazor M. Eradication of Ureaplasma urealyticum from the amniotic fluid with transplacental antibiotic treatment. Am J Obstet Gynecol. 1992;166:618–620. doi: 10.1016/0002-9378(92)91686-5. [DOI] [PubMed] [Google Scholar]
- 347.Mazor M, Chaim W, Horowitz S, Leiberman JR, Glezerman M. Successful treatment of preterm labour by eradication of Ureaplasma urealyticum with erythromycin. Arch Gynecol Obstet. 1993;253:215–218. doi: 10.1007/BF02766648. [DOI] [PubMed] [Google Scholar]
- 348.Mazor M, Chaim W, Meirovitz M, Yohay D, Leiberman JR, Glezerman M. Eradication of viridans streptococci from the amniotic cavity by parenteral antibiotic administration. A case report. J Reprod Med. 1995;40:820–822. [PubMed] [Google Scholar]
- 349.Morency AM, Rallu F, Laferriere C, Bujoldg E. Eradication of intra-amniotic Streptococcus mutans in a woman with a short cervix. J Obstet Gynaecol Can. 2006;28:898–902. doi: 10.1016/S1701-2163(16)32269-1. [DOI] [PubMed] [Google Scholar]
- 350.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, Sorokin Y. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Mazor M, Chaim W, Maymon E, Hershkowitz R, Romero R. The role of antibiotic therapy in the prevention of prematurity. Clin Perinatol. 1998;25:659–685. x. [PubMed] [Google Scholar]
- 352.Ovalle A, Romero R, Gomez R, Martinez MA, Nien JK, Ferrand P, Aspillaga C, Figueroa J. Antibiotic administration to patients with preterm labor and intact membranes: is there a beneficial effect in patients with endocervical inflammation? J Matern Fetal Neonatal Med. 2006;19:453–464. doi: 10.1080/14767050600852668. [DOI] [PubMed] [Google Scholar]
- 353.Weible DR, Randall HW., Jr Evaluation of amniotic fluid in preterm labor with intact membranes. J Reprod Med. 1985;30:777–780. [PubMed] [Google Scholar]
- 354.Duff P, Kopelman JN. Subclinical intra-amniotic infection in asymptomatic patients with refractory preterm labor. Obstet Gynecol. 1987;69:756–759. [PubMed] [Google Scholar]
- 355.Harger JH, Meyer MP, Amortegui A, Macpherson TA, Kaplan L, Mueller-Heubach E. Low incidence of positive amnionic fluid cultures in preterm labor at 27-32 weeks in the absence of clinical evidence of chorioamnionitis. Obstet Gynecol. 1991;77:228–234. doi: 10.1097/00006250-199102000-00013. [DOI] [PubMed] [Google Scholar]
- 356.Coultrip LL, Lien JM, Gomez R, Kapernick P, Khoury A, Grossman JH. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1994;171:901–911. doi: 10.1016/s0002-9378(94)70057-5. [DOI] [PubMed] [Google Scholar]
- 357.Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol. 1996;87:231–237. doi: 10.1016/0029-7844(95)00380-0. [DOI] [PubMed] [Google Scholar]
- 358.Kara M, Ozden S, Arioglu P, Cetin A. The significance of amniotic fluid interleukin-6 levels in preterm labour. Aust N Z J Obstet Gynaecol. 1998;38:403–406. doi: 10.1111/j.1479-828x.1998.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 359.Oyarzun E, Gomez R, Rioseco A, Gonzalez P, Gutierrez P, Donoso E, Montiel F. Antibiotic treatment in preterm labor and intact membranes: a randomized, double-blinded, placebo-controlled trial. J Matern Fetal Med. 1998;7:105–110. doi: 10.1002/(SICI)1520-6661(199805/06)7:3<105::AID-MFM1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 360.Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, Curet LB. Is amniotic fluid analysis the key to preterm labor?. A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol. 1998;179:172–178. doi: 10.1016/s0002-9378(98)70269-8. [DOI] [PubMed] [Google Scholar]
- 361.Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol. 1982;59:539–545. [PubMed] [Google Scholar]
- 362.Zlatnik FJ, Cruikshank DP, Petzold CR, Galask RP. Amniocentesis in the identification of inapparent infection in preterm patients with premature rupture of the membranes. J Reprod Med. 1984;29:656–660. [PubMed] [Google Scholar]
- 363.Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ, Escoto DT, Mirochnick MH. Qualitative amniotic fluid volume versus amniocentesis in predicting infection in preterm premature rupture of the membranes. Obstet Gynecol. 1986;67:579–583. [PubMed] [Google Scholar]
- 364.Feinstein SJ, Vintzileos AM, Lodeiro JG, Campbell WA, Weinbaum PJ, Nochimson DJ. Amniocentesis with premature rupture of membranes. Obstet Gynecol. 1986;68:147–152. [PubMed] [Google Scholar]
- 365.Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol. 1992;167:1092–1095. doi: 10.1016/s0002-9378(12)80044-5. [DOI] [PubMed] [Google Scholar]
- 366.Font GE, Gauthier DW, Meyer WJ, Myles TD, Janda W, Bieniarz A. Catalase activity as a predictor of amniotic fluid culture results in preterm labor or premature rupture of membranes. Obstet Gynecol. 1995;85:656–658. doi: 10.1016/0029-7844(95)00026-n. [DOI] [PubMed] [Google Scholar]
- 367.Averbuch B, Mazor M, Shoham-Vardi I, Chaim W, Vardi H, Horowitz S, Shuster M. Intra-uterine infection in women with preterm premature rupture of membranes: maternal and neonatal characteristics. Eur J Obstet Gynecol Reprod Biol. 1995;62:25–29. doi: 10.1016/0301-2115(95)02176-8. [DOI] [PubMed] [Google Scholar]
- 368.Carroll SG, Papaioannou S, Ntumazah IL, Philpott-Howard J, Nicolaides KH. Lower genital tract swabs in the prediction of intrauterine infection in preterm prelabour rupture of the membranes. Br J Obstet Gynaecol. 1996;103:54–59. doi: 10.1111/j.1471-0528.1996.tb09515.x. [DOI] [PubMed] [Google Scholar]
- 369.Gomez R, Romero R, Nien JK, Chaiworapongsa T, Medina L, Kim YM, Yoon BH, Carstens M, Espinoza J, Iams JD, Gonzalez R. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2005;192:678–689. doi: 10.1016/j.ajog.2004.10.624. [DOI] [PubMed] [Google Scholar]
- 370.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, Dong Z, Yeo L, Mittal P, Yoon BH, Romero R. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433, e431–438. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J, Mazor M, Brekus CA, Hobbins JC. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol. 1990;163:757–761. doi: 10.1016/0002-9378(90)91063-i. [DOI] [PubMed] [Google Scholar]
- 372.Mazor M, Hershkovitz R, Ghezzi F, Maymon E, Horowitz S, Leiberman JR. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand. 1996;75:624–627. doi: 10.3109/00016349609054686. [DOI] [PubMed] [Google Scholar]
- 373.Yoon BH, Park KH, Koo JN, Kwon JH, Jun JK, Syn HC, Romero R. Intraamniotic infection of twin pregnancies with preterm labor. Am J Obstet Gynecol. 1997;176:S35. [Google Scholar]
- 374.Romero R, Hyun Yoon B, Chaemsaithong P, Cortez J, Park CW, Gonzalez R, Behnke E, Hassan S, Gotsch F, Chaiworapongsa T, Yeo L. Bacteria and Endotoxin in Meconium-Stained Amniotic Fluid at Term: Could Intra-amniotic Infection Cause Meconium Passage? J Matern Fetal Neonatal Med. 2013 doi: 10.3109/14767058.2013.844124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Madan I, Romero R, Kusanovic JP, Mittal P, Chaiworapongsa T, Dong Z, Mazaki-Tovi S, Vaisbuch E, Alpay Savasan Z, Yeo L, Kim CJ, Hassan SS. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38:275–279. doi: 10.1515/JPM.2010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, Chaiworapongsa T, Espinoza J, Gonzalez R. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–37. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 377.Kim SK, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Mittal P, Chaiworapongsa T, Pacora P, Ogge G, Gomez R, Yoon BH, Yeo L, Lamont RF, Hassan SS. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD) J Perinat Med. 2010;38:45–53. doi: 10.1515/JPM.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]