Abstract
Problem
Pigtail macaques, Macaca nemestrina (PT) are more susceptible to vaginal transmission of simian immunodeficiency virus (SIV) and other sexually transmitted diseases (STD) than rhesus macaques (RM). However, comparative studies to explore the reasons for these differences are lacking.
Method of Study
Here we compared differences in hormone levels and vaginal mucosal anatomy and thickness of RM and PT through different stages of the menstrual cycle. Concentrations of plasma estradiol (E2) and progesterone (P4) were determined weekly, and vaginal biopsies examined at day 0 and 14 of the menstrual cycle.
Results
Consistent changes in vaginal epithelial thickness occurred at different stages of the menstrual cycle. In both species, the vaginal epithelium was significantly thicker in the follicular than in luteal phase. Keratinized epithelium was strikingly much more prominent in RM, especially during the luteal phase. Further, the vaginal epithelium was significantly thinner and the P4:E2 ratio was higher in PT during luteal phase than RM.
Conclusions
Striking anatomical differences in the vaginal epithelium between rhesus and pigtail macaques combined with differences in P4:E2 ratio support the hypothesis that thinning and less keratinization of the vaginal epithelium may be involved in the greater susceptibility of pigtail macaques to vaginal transmission of SIV or other STD.
Keywords: Menstrual cycle, vagina, epithelium, mucosa, estradiol, progesterone, Macaca nemestrina, Rhesus Macaque, SIV/HIV transmission
Introduction
The vast majority of HIV infections occur through heterosexual transmission (1). However, the mechanisms of vaginal transmission are not well understood. Further, recent studies suggest HIV transmission rates are significantly higher in women taking hormonal contraceptives (2), suggesting hormonal fluctuations affect vaginal susceptibility to HIV transmission, and potentially other sexually transmitted diseases (STDs). Nonhuman primates, particularly pigtail (Macaca nemestrina, PT) and rhesus macaques (Macaca mulatta, RM) are commonly used as models for studying the transmission, pathogenesis, and prevention of HIV using simian immunodeficiency virus (SIV), or simian-human immunodeficiency chimeric virus (SHIV)(3). However, there are important differences between these species that should be considered when selecting either model for vaginal transmission or pathogenesis studies. Although both species usually have menstrual cycles throughout the year, cycles in RM are more sporadic, and they are “seasonal” breeders that only produce offspring in the spring and summer months (4). However (and more like humans) PT have regular menstrual cycles, and give birth to offspring throughout the year (3,5). In addition, due to the similarities in their menstrual cycles, reproductive anatomy, physiology, and vaginal flora, PT are used as a model for various STDs common in humans (5,6). Moreover, PT have recently been shown to be more susceptible to vaginal chlamydial and trichomonal infections than RM (6). Further, pigtails support higher levels of SIV replication and progress to AIDS faster than other non-human primate (NHP) species (7). Finally, PT have higher states of immune activation and different frequencies of memory cells than RM, which may contribute to an accelerated progression to AIDS (8).
Although the mechanisms are not understood, it has been suggested that PT are also more susceptible to vaginal SIV/SHIV transmission than RM (3). Pigtails are readily infected with SHIV using relatively low doses administered vaginally (9), whereas rhesus are more resistant to the same dose and stock of virus (reviewed in (3)). Moreover, pigtails have been shown to be more susceptible during the luteal phase of the menstrual cycle, when progesterone levels are highest (10)Thus, we hypothesized that the thickness and/or integrity of the vaginal epithelium of PT may differ from RM, which may explain their increased susceptibility to vaginal SIV transmission as well as their higher susceptibility to other STD (6).
Previous studies in RM and women have shown that vaginal HIV/SIV transmission occurs even in the absence of a cervix, indicating that the vaginal mucosa alone is sufficient for transmission (11,12). Further, studies have suggested that both rhesus (13) and pigtail macaques (10) are more susceptible to SIV/SHIV infection in the luteal phase of the menstrual cycle. Like humans, the thickness of the vaginal epithelium changes throughout the menstrual cycle in rhesus macaques (14,15). However, comparative studies of the vaginal anatomy and changes in vaginal thickness throughout the menstrual cycle have not been performed in PT and RM.
Here we compared histologic differences between the vagina of PT and RM at day 0 (luteal phase) and day 14 (follicular phase) of the menstrual cycle. We found that PT macaques have a significantly thinner vaginal epithelium, and less keratinization than RM, particularly in the luteal stage of the menstrual cycle. This suggests that a thinner and less keratinized vaginal epithelium may predispose PT to more vaginal infections, including STD and SIV/SHIV.
Materials and Methods
Animals and menstrual observations
Twelve normal cycling female rhesus and 12 pigtail macaques ranging from 4 to 21 years of age were used. All animals were housed and maintained at the Tulane National Primate Research Center in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care. To determine stages of the menstrual cycle, all animals were placed on daily menses watch, and the first day of menses was considered day zero of the menstrual cycle. However, and in contrast to RM, PT macaques did not always have heavy menstrual flow that could be detected by observation, so in addition to daily observations, weekly vaginal swabs were atraumatically applied to the vagina of PT to detect menstrual bleeding using Weck-Cel® ophthalmic sponges (Medtronic Xomed Inc. Jacksonville, FL, USA).
Estrogen and Progesterone Levels
Plasma was collected weekly from EDTA-anti-coagulated blood and stored at -80C until assayed. Levels of estradiol-17b (E2) and progesterone (P4) were measured at the Endocrine Technology and Support Core Lab at the Oregon National Primate Research Center, Oregon Health and Science University. Sensitivity to the E2-extraction radioimmunoassay (RIA) ranged from <2.5 -5 pg/tube to 750 pg/tube. The overall inter-assay variation for steroid extraction RIA was less than 15% while the intra-assay variations did not exceed 10%.
Vaginal biopsy and vaginal thickness measurement
Two vaginal 5mm biopsies were performed by placing the animals in ventral recumbency and taking biopsies at the 12 and either the 3 or 9 o'clock position at both day 0 (peak luteal) and day 14 (peak follicular) phase of the cycle.
The vaginal epithelial thickness was measured using ImageProPlus software, version 4.5 (Media Cybernetics, Silver Springs, MD, USA) on appropriately oriented H&E stained sections using a Leica microscope (Leica Microsystems Inc., Bannockbum, IL, USA) interfaced to a digital camera (Spot Insight color camera; Diagnostic Instruments Inc., Sterling Heights, MI, USA). The software was calibrated for each objective using a stage micrometer. An average of 16 images were collected from each section taken at 100x magnification. For each image, ten measurements were taken by applying an electronic grid to eliminate observer bias, and measurements were taken from the point where each gridline intersected the basal epithelium using ImageProPlus software (Fig. 1A).
Figure 1.
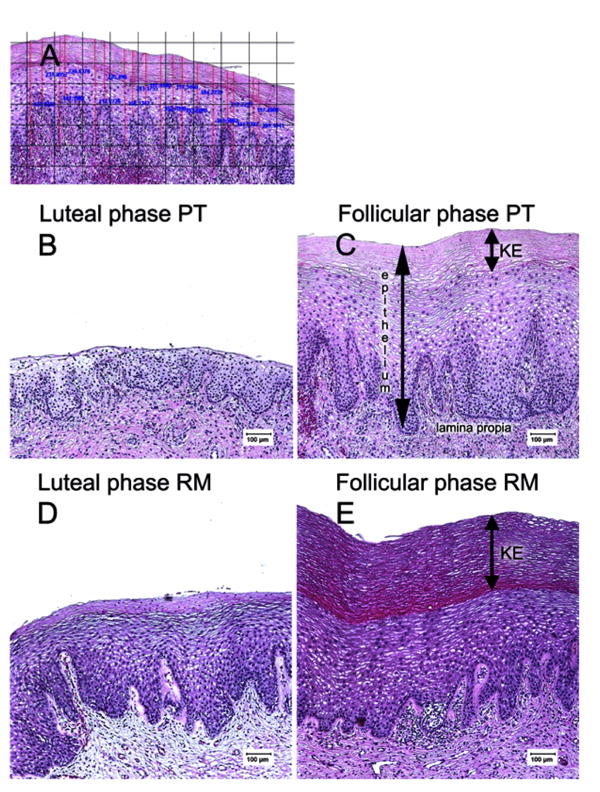
Comparative histology of the vaginal epithelium of rhesus and pigtail macaques. A): Technique for measuring epithelial thickness. To eliminate observer bias, an electronic grid was placed on images of vaginal epithelium and 16 measurements were taken at the intersection of each vertical gridline and the basal lamina, taking two measurements (thinnest and thickest) between each set of gridlines. All measurements were taken at 100x magnification. (B-E): Vaginal biopsies from the same normal pigtail (B, C) or rhesus (D, E) macaque at day 0 (luteal phase, B, D) and peak follicular (C, E) stage of the menstrual cycle. Note that the keratinized layer (KE) is essentially absent in the luteal phase of pigtail macaques (B). Also note the epithelium is markedly thinner in the luteal phase of both macaque species.
Statistical Analyses
The Wilcoxon rank-sum test was used to perform intra-group comparisons, and the Mann-Whitney U test was used for inter-group comparisons. Where appropriate, values are indicated as mean ± SD. Statistical analyses were performed using the GraphPad Instat 5 (GraphPad Software, Inc., San Diego, CA) and the SAS System (SAS Institute, Cary, NC). P values less than 0.05 were considered significant.
Results
Vaginal epithelial thickness
As expected, changes in vaginal thickness during the menstrual cycle were consistent in both species, as both had significantly thinner epithelium during the luteal phase of the cycle, and thicker epithelium at day 14 (when estradiol was dominant)(Fig. 1B-E). Individual animals of both species also showed consistently thicker vaginal epithelium at day 14 of the cycle compared to day 0 (Fig. 2). However, the most striking difference between the two species was the absence of keratinization of the vagina of most PT macaques on day 0 (Fig. 1B). Of the 12 PT examined, only two had measurable keratin on day 0, making the differences between PT and RM highly significant (P=0.0033)(Fig. 3A). On day 14, five of 12 PT and all RM had visible keratin, but RM still had significantly thicker layers of keratinized epithelium than PT (P=0.019)(Fig. 3B).
Figure 2.
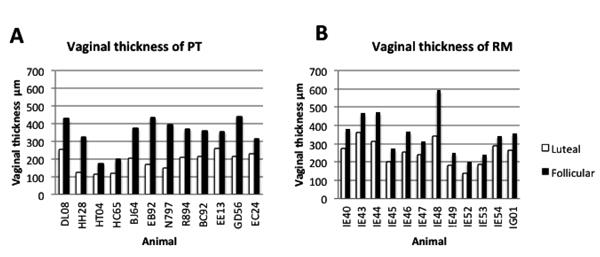
Comparison of vaginal epithelial thicknesses of each animal examined in this study at day 0 (luteal; open bars) and day 14 (follicular; filled bars) between PT (A) and RM (B). The vaginal mucosa was consistently thinner under influence of progesterone and thicker during estradiol dominance in each animal.
Figure 3.
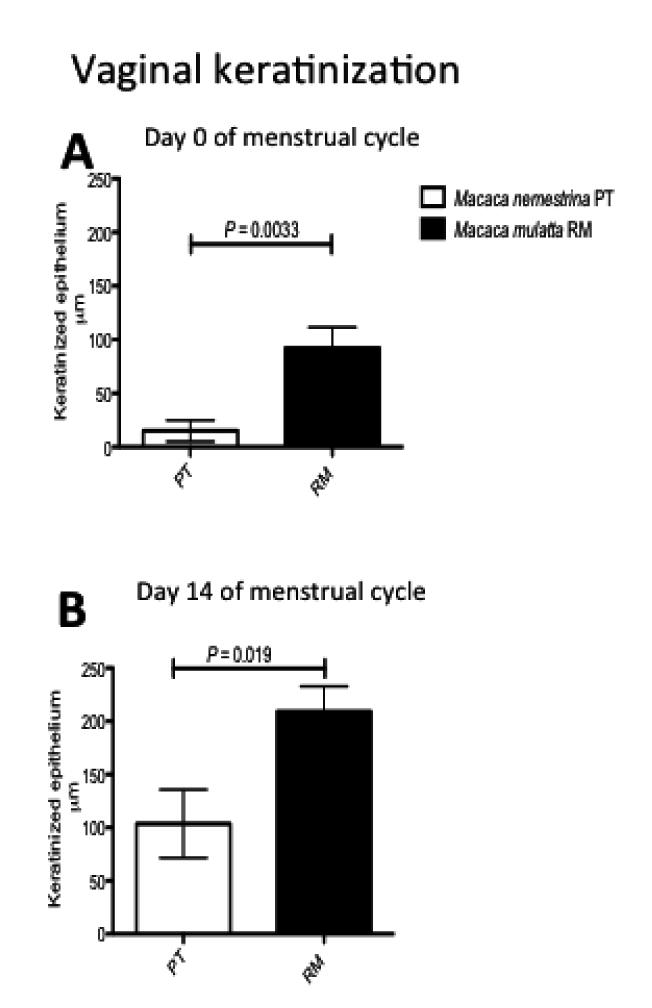
Comparison of thickness of the keratinized layer in the vagina of rhesus (filled bars) or pigtail (open bars) at day 0 (top) or day 14 (bottom) of the menstrual cycle. Note keratinization is essentially absent in PT macaques during the luteal phase (day 0).
Rhesus also had significantly thicker overall vaginal mucosal epithelium than PT when measuring the mean total thickness (RM = 0.263 mm; PT = 0.188 mm; P= 0.043) thinnest points (RM 0.199 mm; PT 0.08 mm; P=0.003) or thickest points (P<0.035) at peak luteal stage (Fig. 4A). There was also a trend for thicker vaginal epithelia in RM compared to PT at day 14 (follicular stage) but significant differences were limited to measurements of the thinnest point at this stage of the cycle (P=0.03)(Fig.4B). There were also significant interspecies differences in overall vaginal epithelial thickness between day 0 and 14 (Fig. 4C).
Figure 4.
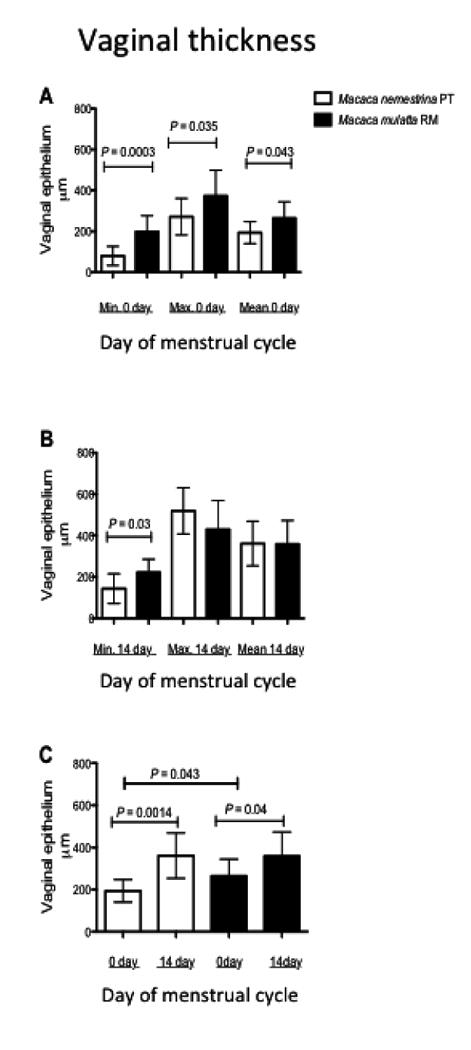
Comparison of total vaginal mucosal thickness between PT and RM in day 0 (progesterone dominance) and day 14 (estradiol dominance) of the menstrual cycle. Note (A) significant differences in mean vaginal epithelial thickness between the two species on day zero (RM 0.263 mm; PT 0.188 mm; P= 0.043). Minimum thicknesses (thinnest points) in the epithelium of the macaques were also significantly different on day zero (RM 0.199 mm; PT 0.08 mm; P=0.003.). Significant differences in the overall vaginal epithelial thickness in the follicular phase were limited to differences in the thinnest point (RM 0.222 mm, PT 0.143mm; P=0.03) (B). There were also significant inter-species differences between mean vaginal thickness on day 0 and 14 (C). P values less than 0.05 were considered significant.
Hormone fluctuations throughout the menstrual cycle
Peripheral blood was collected weekly to determine progesterone and estradiol levels. Progesterone fluctuations throughout the menstrual cycle of both subspecies were similar, with consistently rising levels through the cycle peaking at day 21 (Fig. 5A). Significant differences were detected between day 0 and 21 (P<0.02 PT; P<0.001 RM). In RM, progesterone was significantly higher on day 14 (P= 0.048) than on day 0 (Fig. 5A). The estradiol peak in both macaques was on day 14 of the cycle as expected (Fig. 5B). However, estradiol levels were higher in PT throughout the cycle (Mean 92.7± 7.8 n=75) than RM (Mean 79.14 ± 4.1 n=116) consistent with previous findings (16) although these differences were not significant. Further, progesterone and estradiol ratios were higher in PT than RM on day zero (PT ratio P4:E2= 14.7; RM ratio P4:E2=11.53), which correlated with the significantly thinner epithelium of PT on day 0 of the cycle (Fig.5C-D). In contrast to RM where the P4:E2 ratio was slightly increased on day 14 (P4:E2= 12.59), the ratio in PT was lower than on day 0 which correlated with significant thinning of the vaginal wall between day 0 and 14. In general, PT had a 1.9 fold increase in vaginal thickness between day 0 and 14 (Fig. 2A) whereas the difference was only 1.3 fold greater in RM (Fig. 2B).
Figure 5.
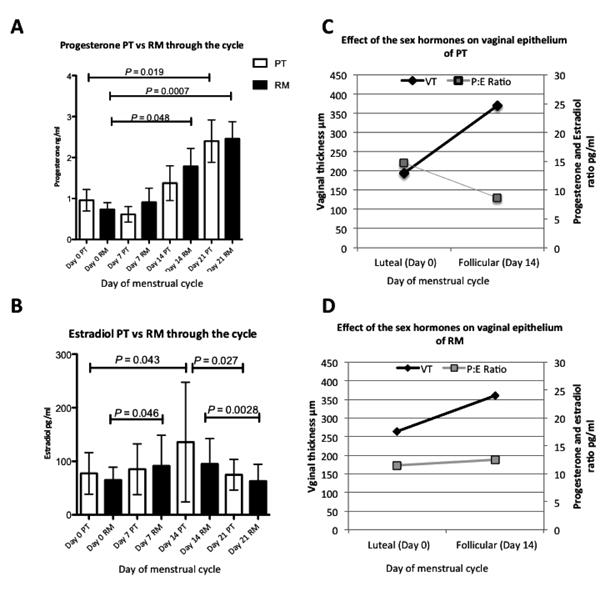
Comparison of progesterone (A) and estradiol (B) fluctuations through the menstrual cycle of rhesus and pigtail macaques. The first day of the menses was considered day 0. Open bars represent progesterone means of 12 PT and filled bars 12 RM +/- standard deviation of the means measured throughout the cycle. Also note progesterone levels (A), consistently rose through the cycle peaking at day 21 in both macaques. Significant differences were detected between day 0 and 21 for both species (P<0.02 PT; P<0.01 RM). Also RM have significantly higher progesterone levels on day 14 than on day 0 (P= 0.048). (B) Peak estradiol levels in both macaques were on day 14 with significant differences between day 0 and day 14 for PT (P=0.043), and day 14 and 21 for RM (P= 0.027). Significant differences in progesterone levels of RM were limited to between day 0 and day 7(P=0.046) and day14 and 21(P= 0.0028). (C-D) Correlation of progesterone and estradiol ratios (P:E) with vaginal epithelial thickness (VT) in PT (C) and RM (D).
Discussion
The present study demonstrates that both PT and RM have similar changes occurring throughout the menstrual cycle as women, including thinning of the vaginal epithelium during menses (luteal phase) and thickening during the period of estrogen dominance, or the follicular stage. However, and unlike women, rhesus macaques have significantly greater levels of superficial vaginal keratinization throughout the menstrual cycle. Pigtails also have a keratinizing vaginal epithelium, but this is thinner than in RM, and essentially disappears during the luteal phase, making the vagina more similar to that of women, at least during the luteal phase. Pigtails also have a longer follicular phase (17-19 days) than rhesus, and therefore an extended period of estradiol production (16). However, typically, high hormone levels in blood are characteristic of primates with low numbers of hormonal receptors in vaginal tissues (17), which may explain why the pigtails had higher levels of estradiol, yet less keratinization. Alternatively (or in addition) the ratio of P4:E2 may be more important in epithelial thickness, as the P4:E2 ratio was higher in the PT on day 14, suggesting the lack of a keratinized epithelium at this stage is due to progesterone dominance.
Thickening of the vaginal epithelium is the result of estrogenic influences on epithelial cell maturation (18). With insufficient estrogen, the vaginal walls become thin and atrophic, which is a common condition in postmenopausal women (19). Accumulating evidence suggests that vaginal mucosal thinning increases HIV transmission rates in women (3). For example, macaque studies have shown progestin compounds (Depo-provera, progesterone implants) markedly thin the vaginal epithelium and increase vaginal SIV transmission rates (11). Recent studies in women have shown that Depo-provera use also increases HIV transmission rates (20). Finally, topical estrogen, when applied to the vagina of ovariectomized macaques results in thickening of the vaginal epithelium and increased protection from vaginal SIV transmission (18), indirectly indicating that the increased transmission rates in progesterone treated macaques are due to changing thickness of the vaginal epithelium and not immunosuppressive effects of exogenous progesterone use. Finally, both rhesus (13), and pigtail (10) macaques in the luteal phase (no hormone treatment) have been shown to be more susceptible to vaginal SIV/SHIV transmission, and pigtails are even more susceptible to other sexually transmitted diseases including Trichomas and Chlamydia sp (5,6). Since Depo-provera use in women produces a very similar thinning of the vaginal epithelium to that of the natural luteal phase (15), we hypothesize that this vaginal thinning increases a woman's susceptibility to HIV-1 transmission irrespective of whether it occurs naturally during menses, or after progestin treatment. In summary, converging evidence indicates that estrogen thickens the vaginal wall and decreases susceptibility to infection, and conversely, progesterone dominance and a thinner vaginal epithelium predisposes females to increased rates of SIV/HIV transmission and possibly other sexually transmitted diseases.
To our knowledge, this is the first direct comparison of the vaginal histology of PT and RM macaques throughout the menstrual cycle. Importantly, we found that RM have marked keratinization of the vaginal epithelium throughout the cycle, whereas PT lose this keratinized layer during menses, making the vaginal epithelium more similar to that of women. The loss of this keratinized layer may make the vagina more susceptible to viral penetration or infection with other pathogens. In addition, the minimum vaginal thickness of the PT in both stages of the cycle was significantly thinner than in RM (Fig. 3C P<0.043). In conclusion, the histology of the pigtail vagina during the luteal phase is slightly more similar to humans than rhesus macaques, which may also explain why PT are more susceptible to the STD of women than rhesus macaques.
The loss of keratinization of the vaginal epithelium in the luteal phase of the PT was the most striking difference between the two species. Keratinization of the vaginal epithelium is a normal feature of most mammals including rats (21) dogs (22) and mice (23). In fact, murine models of vaginal transmission usually require administration of Depo-provera or other progestin hormones to facilitate transmission. Cyclic changes occur throughout the estrus cycles of most mammals including increased keratinization during estrus, presumably as a protective mechanism against the anticipated trauma of intercourse. Thus, keratinization may be a protective mechanism to prevent STD, or exposure of sensitive layers of the vagina to foreign antigens introduced during intercourse. Although primates do not have estrus cycles, the layers of squamous epithelium markedly increase during the follicular stage that accompanies ovulation. However, the vaginal epithelium of humans is considered a “non-keratinizing” epithelium, and has apparently lost the capacity to form this protective barrier. Instead, it appears humans have become dependent on a symbiotic “infection” with acid-producing lactobacilli, which are fed through the glycogen produced by vaginal epithelial cells, resulting in an acidic pH that affords protection of the vagina from most STD. However, numbers and levels of lactobacilli and glycogen appear to be lower in nonhuman primates, including rhesus and PT (24,25). Since PT have neither lactobacilli, acidic vaginal pH, or thick keratinization of the vaginal epithelium in the luteal phase, they may be even more susceptible to vaginal infections than either humans or RM.
The effects of sex hormones on vaginal epithelium and susceptibility to HIV infection in humans and non-human primates have been the subject of study and debate (18,26,27). The current study shows that PT macaques, which are more susceptible to SIV and STD transmission than RM, have a thinner and less keratinized vaginal epithelium. This is consistent with the barrier hypothesis, in that vaginal epithelial thinning is most likely responsible for the increased transmission rates shown in macaques and women taking progesterone-based contraceptives.
Although other factors may also be involved in the greater susceptibility of PT to SIV infection and disease progression, these data argue that thinning of the vaginal epithelium allows greater contact of virus or pathogens with the underlying target cells, and greater susceptibility of PT to vaginal transmission of SIV/HIV or other STD.
Acknowledgments
The authors gratefully acknowledge Dr. K.-Y. Francis Pau (Oregon National Primate Research Center) for hormone analyses and endocrinology advice. We also thank Terri and Kelsi Rasmussen, Megan Gardner, Meagan Watkins, and the Division of Veterinary Medicine and the Anatomic Pathology Core lab at the Tulane National Primate Research Center. This work was supported by NIH grants U19 AI076981, R01 AI084793, a Faculty Enhancement Grant from Tulane University, the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through grant no. OD011104-51.
References
- 1.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nature reviews Microbiology. 2003;1(1):25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 2.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, Coombs RW, Baeten JM. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey RS, Shattock RJ, Klasse PJ, Moore JP. Animal models for microbicide studies. Curr HIV Res. 2012 doi: 10.2174/157016212799304715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker ML, Gordon TP, Wilson ME. Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biol Reprod. 1983;29(4):841–848. doi: 10.1095/biolreprod29.4.841. [DOI] [PubMed] [Google Scholar]
- 5.Patton DL, Sweeney YT, Agnew KJ, Balkus JE, Rabe LK, Hillier SL. Development of a nonhuman primate model for Trichomonas vaginalis infection. Sex Transm Dis. 2006;33(12):743–746. doi: 10.1097/01.olq.0000218871.89901.61. [DOI] [PubMed] [Google Scholar]
- 6.Henning T, Fakile Y, Phillips C, Sweeney E, Mitchell J, Patton D, Sturdevant G, Caldwell HD, Secor WE, Papp J, Hendry RM, McNicholl J, Kersh E. Development of a pigtail macaque model of sexually transmitted infection/HIV coinfection using Chlamydia trachomatis, Trichomonas vaginalis, and SHIV(SF162P3) J Med Primatol. 2011;40(4):214–223. doi: 10.1111/j.1600-0684.2011.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klatt NR, Canary LA, Vanderford TH, Vinton CL, Engram JC, Dunham RM, Cronise HE, Swerczek JM, Lafont BA, Picker LJ, Silvestri G, Brenchley JM. Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques. J Virol. 2012;86(2):1203–1213. doi: 10.1128/JVI.06033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, Martin MA, Levine AD, Estes JD, Brenchley JM. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010;3(4):387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, Grohskopf LA, Monsour M, Butera S, Folks TM. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005;191(2):164–173. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 10.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, Kersh EN. High Susceptibility to Repeated, Low-Dose, Vaginal SHIV Exposure Late in the Luteal Phase of the Menstrual Cycle of Pigtail Macaques. J Acquir Immune Defic Syndr. 2011;57(4):261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 11.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2(10):1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 12.Kell PD, Barton SE, Edmonds DK, Boag FC. HIV infection in a patient with Meyer-Rokitansky-Kuster-Hauser syndrome. J Royal Soc Med. 1992;85(11):706–707. doi: 10.1177/014107689208501119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Research & Human Retroviruses. 1998;14(Suppl 1):S119–123. [PubMed] [Google Scholar]
- 14.Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS. Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol. 2006;190(3):829–835. doi: 10.1677/joe.1.06873. [DOI] [PubMed] [Google Scholar]
- 15.Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, Pan Z, Morrison CS, Chen-Mok M, Archer DF, Gabelnick HL. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60(1):15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 16.Blakley GB, Beamer TW, Dukelow WR. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Laboratory animals. 1981;15(4):351–353. doi: 10.1258/002367781780953059. [DOI] [PubMed] [Google Scholar]
- 17.Hendricks AG, Dukelow WR. Nonhuman Primates in Biomedical Research. London: Academic Press; 1995. Biology and management. In: Bennett BT, Abee CR, eds. [Google Scholar]
- 18.Smith SM, Mefford M, Sodora D, Klase Z, Singh M, Alexander N, Hess D, Marx PA. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS. 2004;18(12):1637–1643. doi: 10.1097/01.aids.0000131393.76221.cc. [DOI] [PubMed] [Google Scholar]
- 19.Panjari M, Davis SR. Vaginal DHEA to treat menopause related atrophy: a review of the evidence. Maturitas. 2011;70(1):22–25. doi: 10.1016/j.maturitas.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, Coombs RW, Baeten JM. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chateau D, Geiger JM, Samama B, Boehm N. Vaginal keratinization during the estrous cycle in rats: a model for evaluating retinoid activity. Skin pharmacology : the official journal of the Skin Pharmacology Society. 1996;9(1):9–16. doi: 10.1159/000211385. [DOI] [PubMed] [Google Scholar]
- 22.Rehm S, Stanislaus DJ, Williams AM. Estrous cycle-dependent histology and review of sex steroid receptor expression in dog reproductive tissues and mammary gland and associated hormone levels. Birth Defects Res B Dev Reprod Toxicol. 2007;80(3):233–245. doi: 10.1002/bdrb.20121. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi G. Antagonistic effects of estradiol dipropionate and progesterone on the histology of the vagina and uterus of the mouse. The Journal of experimental zoology. 1984;232(1):151–155. doi: 10.1002/jez.1402320118. [DOI] [PubMed] [Google Scholar]
- 24.Spear GT, Gilbert D, Sikaroodi M, Doyle L, Green L, Gillevet PM, Landay AL, Veazey RS. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res Hum Retroviruses. 2010;26(2):193–200. doi: 10.1089/aid.2009.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirmonsef P, Gilbert D, Veazey RS, Wang J, Kendrick SR, Spear GT. A Comparison of Lower Genital Tract Glycogen and Lactic Acid Levels in Women and Macaques: Implications for HIV and SIV Susceptibility. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2005;308(5728):1582–1583. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 27.Baeten JM, Lavreys L, Overbaugh J. The influence of hormonal contraceptive use on HIV-1 transmission and disease progression. Clin Infect Dis. 2007;45(3):360–369. doi: 10.1086/519432. [DOI] [PubMed] [Google Scholar]


