Abstract
Neuropeptide W (NPW), an endogenous ligand for the G-Protein coupled receptor GPR7 (NPBWR1), is produced in neurones in the rat hypothalamus and brain stem known to be important in the control of food intake and the neuroendocrine response to stress. In previous studies, central administration of NPW during the light phase increased food and water intake and elevated prolactin and corticosterone levels in conscious, unrestrained male rats. In the present studies, central administration of siRNA reduced NPW levels in hypothalamus and resulted in a failure of angiotensin II to stimulate water drinking or increase mean arterial pressure. In addition siRNA treated animals failed to mount a significant prolactin response to immobilisation stress, while maintaining a normal corticosterone response. These results suggest that endogenous NPW may be a physiologically relevant, downstream mediator of the central actions of angiotensin II to stimulate thirst and increase arterial pressure. In addition, NPW-producing neurones appear to participate in the hypothalamic mechanisms controlling prolactin, but not corticosterone, secretion.
Keywords: Angiotensin II, Neuropeptide W, Water Drinking, Blood Pressure, Stress Hormones
Introduction
The distribution of GPR7 in hypothalamus (1) combined with the detection of GPR7 in the pituitary gland (2) strongly suggested that NPW regulated the release of pituitary hormones (3). Central NPW injection elevated plasma corticosterone and prolactin levels, and the effect on plasma corticosterone secretion could be abrogated by pre-treating the animals with a CRF receptor antagonist (4,5). Neuropeptide B (NPB), which also binds GPR7, similarly acted in brain to elevate plasma corticosterone and prolactin levels, and the effect to increase corticosterone levels was abrogated by anti-CRF anti-serum pretreatment, suggesting these GPR (NPBRW1) ligands act within the hypothalamus and control stress hormone secretion by increasing the release of CRF and prolactin releasing factors, or inhibiting dopamine release, into the median eminence and thus the hypophyseal portal vasculature (6). It has been hypothesised that the regulation of stress hormone secretion can be significantly modified by signals arising uniquely in cell populations in the brain stem that send NPW-positive projections to the hypothalamus (7) or by increased activity of NPB producing cells located within the diencephalon (6). This would suggest multiple inputs into the hypothalamus that activate several stress centers in the brain, all converging on GPR7-receptor positive cells in the PVN, ventromedial hypothalamic area, or arcuate nucleus (6).
Two models of gene compromise have added to our knowledge of the potential physiologic actions of the GPR7 ligands. NPB knockout (NPB −/−) mice exhibited hyperalgesia in response to inflammatory pain and manifested a mild, adult-onset obesity (8). GPR7 knockout male mice (GPR7 −/−) exhibited metabolic defects and adult-onset obesity, characteristic of the metabolic syndrome, while female mice (GPR7−/−, +/−) were fertile and otherwise normal, compared to wild type littermates. Males were hyperphagic and showed decreased locomotor activity and energy expenditure. The metabolic profile of the male mice suggests age-related development of insulin-resistant diabetes and a potential role for endogenous NPB and/or NPW in the regulation of glucose homeostasis and energy expenditure (9). Studies in the GPR7 knockout mice provided valuable evidence of the two peptides’ potential roles in regulating energy homeostasis.
Both NPW and NPB bind the GPR7 (NPBWR1) receptor, and thus the interpretation of the results of deleting the NPB gene is difficult because the receptor may be activated by NPW, which is not affected by the genetic manipulation (3). To investigate the physiological relevance of NPW in the stress response, we acutely compromised NPW production using small interfering RNA (siRNA) targeted against NPW. We report here that in vivo compromise of NPW production failed to significantly alter food and water intake in ad libitum fed and watered rats, and failed to alter corticosterone, but did reduce prolactin secretion during restraint stress. Compromise of the production of endogenous NPW also decreased angiotensin II (Ang II) induced water drinking and abrogated the Ang II induced increases in mean arterial pressure. These data suggest that endogenous NPW, and possibly NPB by an action on the shared receptor, may contribute to the activation of thirst, the hypothalamic regulation of prolactin secretion, and the central cardiovascular effects of angiotensin II (Ang II).
Materials and Methods
Animals
All procedures and protocols have been approved by the Saint Louis University Animal Care and Use Committee. Adult male rats (Harlan Sprague-Dawley; 250–300g) were individually housed following all surgical procedures with free access to food and water under controlled conditions (23–25°C, lights on 0600–1800h).
Reagents
NPW-23, Ang II and the NPW RIA kit (rat, mouse NPW-23) were purchased from Phoenix Pharmaceuticals, Inc. (Burlingame, CA). Small interfering RNA constructs were designed by Integrated DNA Technologies (IDT, Coralville, IA) to compromise NPW production. The TriFECTa® Kit Duplex (NM_153294, duplexes 1–3) was employed. The control construct against eGFP (AY15666.1) also was purchased from IDT.
Surgery
Animals were anaesthetised with a mixture of ketamine (60 mg/ml; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (8mg/ml; TranquiVed, VedCo, Saint Joseph, MO) at a dose of 0.1 ml per 100 g body weight, as previously described (10). A stainless-steel cannula (23 gauge, 17mm) was implanted into the right lateral cerebroventricle using a stereotaxic device. Rats were observed daily to ensure health and recovery to presurgery body weight for at least 5 days before further surgery or experimentation. Placement and patency of the cannula was confirmed by the dipsogenic effect of angiotensin II (Ang II, 50 pmol). For cardiovascular experiments, a catheter [polyethylene (PE)-50] was implanted into the left carotid artery under ketamine-xylazine anesthesia, as previously described (10). The carotid catheter was filled with heparinized saline (200 U/ml in 0.9% NaCl) and exteriorized between the shoulder blades. For restraint stress experiments, an indwelling jugular vein cannula was implanted under isoflurane-induced anaesthesia (3% in O2 for induction, 2% in O2 for maintenance; IsoSol, Vedco), and exteriorized between the shoulder blades, as previously described (11).
NPW Peptide Content
Rats were sacrificed by rapid decapitation and whole hypothalami (Boundaries: anterior, lamina terminalis with anterior commisure above and the optic chiasm below; posterior, interpeduncular fossa; dorsal, hypothalamic sulcus; ventral, tuber cinereum) and medulla [with the cerebellum removed, the medulla was dissected out from the caudal to rostral poles of the nucleus of the solitary tract (NTS)] were collected. For extraction of peptide, 1 ml of 0.2 N acetic acid was added to each tissue sample (4 C). Samples were homogenized in polypropylene tubes and homogenates centrifuged at 8,000 g for 5 min. Aliquots (20 microliter) of supernatant were taken from each sample for protein determination using the Bio-Rad Protein assay (Bio-Rad Laboratories, Inc, Hercules, CA) and a BioMate 3 spectrophotometer (Thermo Fisher Scientific, Inc, Rochester, NY). The remainder of the supernatant was dried in a rotary evaporator (Speed-vac, Savant Instruments) and later used for NPW RIA. The RIA kit was validated before use with tissue extracts. Dose-response curves for tissue extract, and increasing concentrations of the NPW added to rat tissue extract were parallel to that of synthetic NPW. The minimum detectable peptide level was 1 pg/tube, and the antibody employed displays no cross-reactivity to NPB or a variety of peptides present in the same tissue extracts including orexin, neuropeptide Y, agouti related peptide, melanocortin, alphamelanocyte stimulating hormone or ghrelin.
Metabolic Studies
Rats (250–300 g) bearing i.c.v. cannulae were habituated to metabolic cages (Nalgene; Harvard Apparatus, Holliston, MA) for at least 3 days with free access to lab chow (Cat#1811156, Test Diet, Richmond, IN) and tap water. Food and water intakes and body weight were monitored daily. Animals were injected with 2 microliters of sterile saline or 2 microliters of sterile saline (0.9% NaCl) containing 2 micrograms of siRNA targeted against NPW (Integrated DNA Technologies, Coralville, Iowa) each day for 2 days (injections at 1600 hr). Food and water intakes were recorded.
Water Intake Studies
Angiotensin II challenge
Rats bearing i.c.v. cannula were allowed to habituate to the testing room for at least three hours. Following habituation, saline (2 microliters) or saline containing 3.0 nmol NPW-23 were injected i.c.v. Fifteen minutes later, saline (2 microliters) or saline containing a threshold dose of Ang II (5 pmol) were injected i.c.v. and water intake was monitored at 5, 10, 15, 20, 30, 60, and 120 minutes, and 24 hours following the Ang II injection.
To study the physiological relevance of NPW in Ang II-induced water intake, rats bearing i.c.v. cannula were injected with 2 microliters of sterile saline containing 2 micrograms of siRNA targeted against NPW (Integrated DNA Technologies, Coralville, Iowa) or eGFP (Integrated DNA Technologies, Coralville, Iowa) each day for 2 days (injection at 1600 hr) prior to experimentation. On the experimental day, animals were allowed to habituate to the testing room for at least three hours. Following habituation, 50 pmol Ang II (our standard test dose) was centrally administered and water intake was monitored at 5, 10, 15, 30, and 60 minutes, and 24 hours following Ang II injection.
The experiment was repeated with the exception that in this group of animals the pretreatments included either 2 microliters sterile saline vehicle alone or saline containing 2 micrograms of the NPW siRNA each day for two days prior to experimentation. Angiotensin II challenge was conducted as above on the following day.
Overnight water restriction
In a third group of animals pretreated either with saline vehicle or saline containing the NPW siRNA as above, water bottles were removed at 1800 h (food present) on the day of the second siRNA injection (i.c.v.). On the following morning, animals were moved to the testing room at 0600 h and allowed to habituate for three hours. Then water bottles were returned to the cages and intakes monitored for two hours and again after twenty-four hours.
Mean Arterial Pressure in Conscious Animals
Rats bearing i.c.v. cannula were injected with 2 microliters of sterile saline or 2 microliters of saline containing 2 micrograms of siRNA targeted against NPW or eGFP each day for 2 days prior to the experiment (injection at 1600 hr), with the second day of injection corresponding to the day of carotid catheter surgery. Cardiovascular experiments in conscious animals were conducted the day after carotid implantation. On the day of the experiment, animals were habituated to a quiet room for at least 2 hours. The carotid catheter was flushed with heparinised saline (200 U/ml in 0.9% NaCl) and connected to a pressure transducer (DigiMed Blood Pressure Analyzer, Micro-Med, Louisville, KY). Baseline mean arterial pressure (MAP) and heart rate (HR) was recorded at one minute intervals. For all experiments, pre-injection baseline was calculated as the average MAP or HR for 10 minutes before i.c.v. injections of saline or saline containing 50 pmol Ang II.
Restraint Stress testing
We employed a protocol that has been previously described by our lab (12). In this model, male rats (250–300 grams) with i.c.v. cannulas (stainless steel, 23 gauge) placed in the lateral cerebral ventricle underwent a second surgery under isoflurane-induced anaesthesia (3% in O2 for induction, 2% in O2 for maintenance; IsoSol, Vedco), during which an indwelling jugular vein cannula was implanted and exteriorized on the back of the neck, as previously described (11). Animals were injected i.c.v. with 2 microliters of sterile saline or 2 microliters of saline containing 2 micrograms of siRNA targeted against NPW or eGFP each day for 2 days prior to the experiment (injection at 0900 hr), with the second day of injection corresponding to the day of jugular cannulation surgery. On the day of the experiment, rats were moved to a quiet room at 0600 and left undisturbed for at least 1 hour. Then extension tubing (PE-50) was attached to the jugular cannula, and flushed with heparinised saline (200 U heparin/ml 0.9% NaCl; MP Biochemicals, Aurora, OH). After 2 h, a baseline (time 0) blood sample (0.3 ml) was removed via the cannula, and the withdrawn volume was replaced with sterile isotonic saline (0.9% NaCl, Abbott Laboratories, North Chicago, IL). Five minutes later, a second blood sample was drawn and animals were placed in Plexiglass restrainer tubes (6.2 cm ID; Harvard Apparatus, Boston, MA), and the nose cones tightened so that the animal was held immobile for 10 min. Two more blood samples were collected while the animals were immobilised, 5 and 10 min into the period of immobilisation; then the rats were released into their home cages, and two more samples withdrawn 5 and 15 min later (12). All blood samples (0.3 ml, into heparinised syringes) were removed from conscious rats and replaced with an equal volume of 0.9% NaCl (37 C). Blood samples were kept on ice before plasma was separated (3000 × g, 3 min) and stored at −20 C until hormone assays conducted.
Hormone Radioimmunoassays
Plasma corticosterone levels were determined according to the instructions of the commercial RIA kit (rat/mouse corticosterone, ICN Biomedicals, Inc., Costa Mesa, CA). The minimum detectable hormone level was 25 ng/ml, and the intra-assay coefficient of variability was less than 10%. PRL levels in plasma were determined (4) using the kit materials provided by the National Hormone and Pituitary Program (rPRL-RP-3 standard). The minimum detectable hormone level in plasma for PRL was 0.5 ng/ml (defined as <90% B/B0) and the intra-assay coefficient of variability was less than 9%. All samples were included in a single assay.
Statistical Analyses
Data were analysed using either a Mann-Whitney U test (for the cardiovascular experiment), ANOVA with Scheffe’s multiple comparison (for the NPW-Ang II interaction experiment), or a t test (for metabolic, tissue content, siRNA water intake, and restraint stress hormone experiments). A non-parametric statistical analysis (Mann-Whitney U) was used to evaluate the blood pressure results because the data were transformed (expressed as change from pre-injection baseline) to account for the natural variation of resting blood pressure between rats (13). Significance was assigned to results that occurred with less than 5% probability.
Results
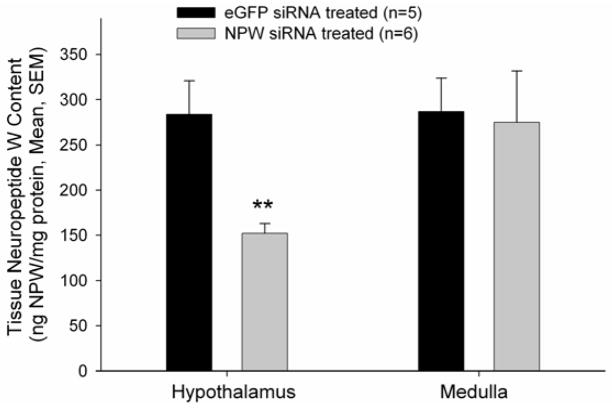
NPW has been shown to increase both food and water intake when administered i.c.v. in adult, male rats during the light phase (4). We hypothesised that compromise of the endogenous NPW production would result in decreased food and water intake during the light phase. First we demonstrated that our siRNA treatment did significantly lower NPW content in hypothalamus but not in medulla (Figure 1).
Figure 1. NPW siRNA pretreatment significantly decreased hypothalamic but not medullary NPW-immunoreactivity in male rat pretreated for two days prior to tissue harvest.
p<0.001 versus eGFP siRNA treated controls.
We then examined the effect of siRNA treatment on daily, ad libitum food and water intakes and found that rats pretreated with NPW siRNA exhibited a trend towards a decrease in both food and water intake following the first injection, but this decrease did not attain significance (data not shown). Following the final data collection time point in the metabolic studies, placement and patency of the cannula was confirmed by the dipsogenic effect of Ang II (50 pmol). We observed that the animals pretreated with NPW siRNA demonstrated a reduced dipsogenic response to Ang II compared to the saline pretreated controls. This led us to hypothesise that endogenous NPW was in some way important for the expression of the full dipsogenic response to Ang II.
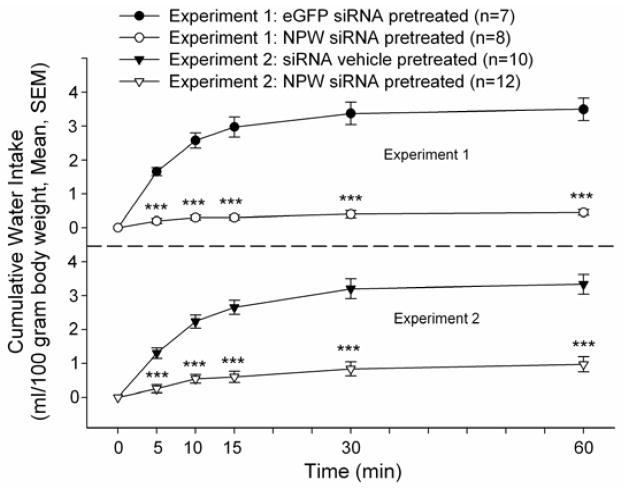
In order to test this hypothesis, an additional group of rats bearing i.c.v. cannula were similarly pretreated with siRNA targeted against NPW or eGFP for two days and injected on the following day with 2 microliters of Ang II (50 pmol). Rats that had been pretreated with NPW siRNA consumed significantly less water in response to Ang II compared to eGFP siRNA pretreated controls over the initial 60 minute observation period (Figure 2 top panel). However, there was no significant difference in cumulative water intakes between groups over the ensuing 24 hours (NPW siRNA pretreated: 12.0 ± 0.6, eGFP siRNA pretreated: 14.2 ± 1.3 ml/100 gram body weight, p=0.13).
Figure 2. NPW siRNA pretreatment significantly decreased Ang II-induced water intake compared to that observed in eGFP siRNA pretreated animals (Experiment 1, top panel) or compared to that observed in saline vehicle pretreated animals (Experiment 2, bottom panel).
Rats bearing i.c.v. cannula were injected with siRNA vehicle, or siRNA targeted against NPW or eGFP for two days prior to experimentation. Angiotensin II (50 pmol) was injected i.c.v. at time 0. ***p < 0.001 versus NPW siRNA treated animals, t test.
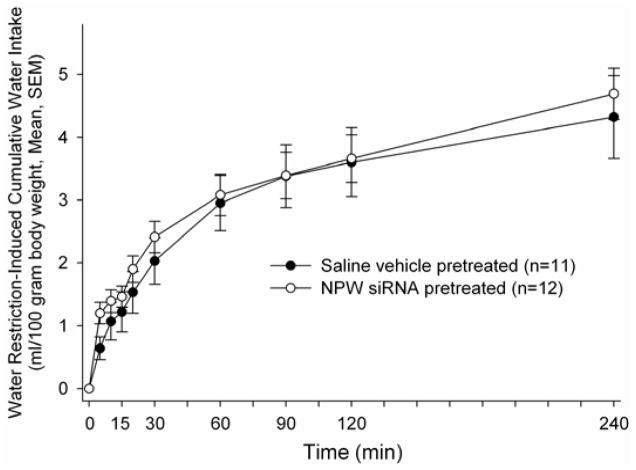
The experiment was repeated using animals pretreated either with saline vehicle or vehicle containing NPW siRNA as above. Once again, in animals pretreated with NPW siRNA, angiotensin II failed to stimulate significant water drinking compared to saline pretreated controls over the initial observation period (Figure 2 bottom panel). However, again, no significant differences in 24 hour, cumulative water drinking was observed (NPW siRNA pretreated 11.9 ± 1.4, saline pretreated 11.6 ± 0.7 ml/100 gram body weight, p = 0.85). Unlike the case when drinking was stimulated by angiotensin II administration, there were no significant differences at any time point in cumulative water intakes between the NPW siRNA pretreated and the saline pretreated animals following overnight water restriction, which is a mixed osmotic and volumetric stimulus mediated not only by circulating angiotensin II levels but also by baroreceptor activation (Figure 3).
Figure 3. NPW siRNA pretreatment failed to alter overnight water restriction-induced water drinking.
Rats bearing i.c.v. cannula were injected with siRNA targeted against NPW (open circles) or saline vehicle (closed circles) for two days prior to experimentation. Water bottles were returned to the cages at time 0.
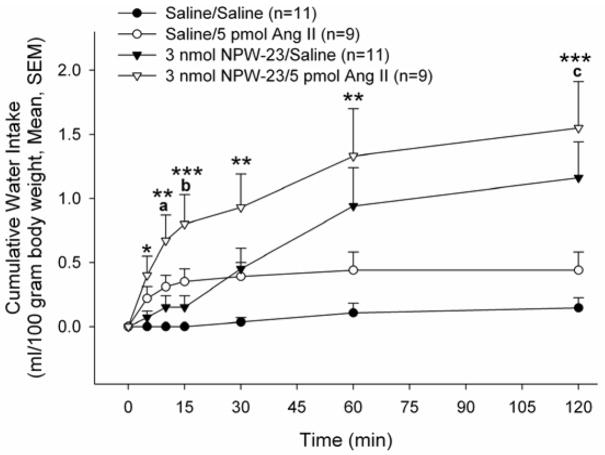
This result suggested that endogenous NPW may play a physiologically relevant role in water drinking uniquely in response to Ang II. We then verified that administration of exogenous NPW-23 could increase water consumption. Male rats bearing i.c.v. cannula were injected with saline or saline containing 3.0 nmol NPW-23. Fifteen minutes later, saline or 5 pmol Ang II (threshold dose of the peptide) was injected i.c.v. and water intake was monitored at 5, 10, 15, 30, 60, 120 minutes, and 24 hours following the Ang II injection. Only three of the eleven animals receiving two saline (vehicle) injections i.c.v. consumed water over the 120 min observation period (range: 1–2 ml over 120 min). The threshold dose of Ang II stimulated water drinking in six of the nine animals (Figure 4). Administration of NPW-23 alone stimulated water drinking in nine of eleven animals. Rats pretreated with 3.0 nmol NPW-23 prior to 5 pmol Ang II demonstrated significant increases in water intake compared to saline/saline controls (Figure 4). This increase began at 5 minutes following injection and was sustained for the entire 120-minute experiment. Animals that received the 3.0 nmol NPW-23 pretreatment prior to 5 pmol Ang II also demonstrated a significant increase in water intake compared to the 3.0 nmol NPW-23/saline treatment group at the 10 and 15 min observation periods. Similarly, water consumption in animals pretreated with NPW-23 prior to Ang II consumed more water than animals treated with the threshold dose of Ang II alone, but this difference attained significance only at the 120 min observation period. No significant differences in 24 hr cumulative water intakes were observed among groups (Saline/Saline: 7.3 ± 1.2 ml/100 g body weight, Saline/Ang II: 8.8 ± 0.8, NPW-23/Saline:: 7.6 ± 1.9, NPW-23/Ang II: 8.0 ± 0.6).
Figure 4. Additive Effects of NPW-23 and Ang II on water drinking.
Animals were pretreated with saline vehicle or NPW-23, followed 10 min later by saline or Ang II. *p<0.05, **p<0.01, ***p<0.001 versus saline/saline; a: p<0.05, b: p<0.01 versus 3.0 nmol NPW-23/saline; c: p<0.05 versus Saline/Ang II).
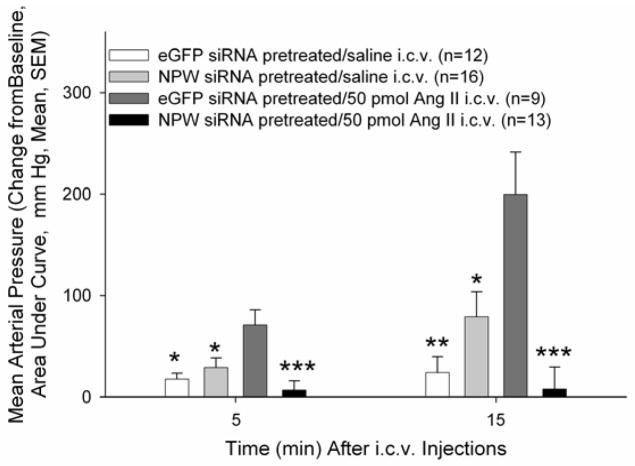
To determine if the central cardiovascular action of Ang II requires activation of NPW-producing neurons, rats were pretreated with siRNA targeting NPW production or eGFP siRNA for two days prior to experimentation. In conscious, freely moving rats, NPW siRNA pretreatment did not alter basal MAP as there were no significant differences among the four treatment groups prior to the central administration of Ang II. However, NPW siRNA treatment significantly attenuated the Ang II-induced increase in MAP observed in eGFP siRNA pretreated animals (Figure 5).
Figure 5. NPW siRNA pretreatment attenuated Ang II-induced increases in MAP.
Data represent area under curve (AUC) at 5 and 15 minutes post-injection. Data were analysed using a Mann-Whitney U test (**p<0.01, ***p<0.001, versus eGFP siRNA/saline; b: p<0.01 versus eGFP siRNA/50 pmol Ang II).
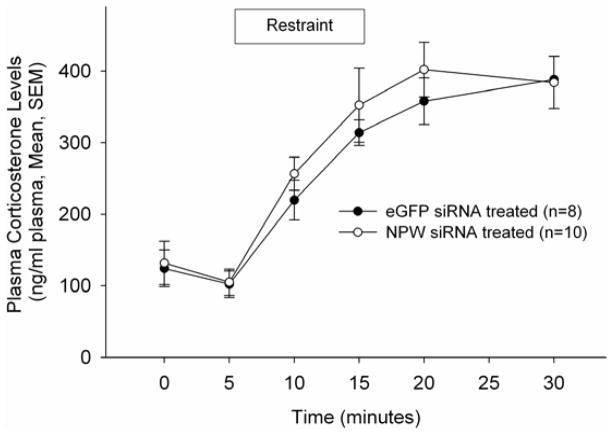
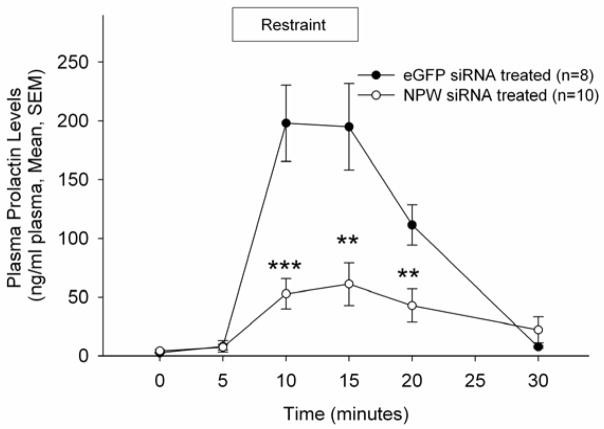
To investigate the role of NPW in the neuroendocrine response to stress, rats were pretreated as described above with siRNA targeted against NPW or eGFP and subjected to restraint stress testing (4). Prior to the initiation of restraint, in these conscious, freely moving rats, NPW siRNA pretreatment did not alter basal corticosterone or prolactin levels, nor did that pretreatment alter significantly restraint stress-induced corticosterone secretion (Figure 6). However, compromise of NPW production resulted in a significant reduction in restraint stress-induced prolactin secretion (Figure 7).
Figure 6. NPW siRNA does not alter restraint stress induced corticosterone secretion.
Animals were restrained for 10 min following collection of the second blood sample.. Data are represented as ng cortiscosterone/ml plasma.
Figure 7. NPW siRNA abrogates restraint stress induced prolactin secretion.
Animals were restrained for 10 min following collection of the second blood sample.. Data are represented as ng prolactin/ml plasma. Data were analysed using a t test; ** p<0.01, *** p<0.001, versus plasma prolactin levels in eGFP pretreated animals.
Discussion
Although central administration of exogenous NPW during the light phase stimulated food intake and water drinking (4,14), similar injections during the dark phase inhibited these behaviors (15). We have hypothesized that the diurnal variation in NPW actions is a reflection of the peptide’s action to stimulate behavioural arousal (16). Thus it is not surprising that in the 24 hour feeding paradigm, compromise of NPW production has no overall effect of cumulative food or water intakes.
On the other hand, animals pretreated with NPW siRNA failed to display a robust response to the effects of exogenously administered Ang II on water drinking, a dipsogenic signal independent of light entrainment (Figure 2). We also investigated the effect of centrally administered NPW-23 on Ang II-induced water intake and found that rats pretreated with 3.0 nmol NPW-23 prior to 5 pmol Ang II demonstrated increased water intake compared to saline treated control animals or animals treated with NPW-23 or Ang II alone (Figure 4). However, the effects of NPW-23 and Ang II treatment appeared to be only additive in nature. This result does not rule out the interaction of the two peptides. While Ang II given i.c.v. clearly reaches its site of action in the SFO, it is not clear what population of NPW receptors (i.e. GPR7) are accessed by exogenous NPW administered into the lateral cerebroventricle. Since compromise of NPW production using siRNA administered twice completely abrogated the dipsogenic action of exogenously administered Ang II, it is possible that a single injection of NPW by itself does not reach all the necessary populations of GPR7, while subsequent to Ang II administration a greater number or population of those receptor expressing neurons are activated. We can not rule out the alternative hypothesis that the NPW siRNA pretreatment itself altered the function of the AT1R expressing neurons; however, if that were the case we might have expected to observe significant effects on 24 hr food and water intakes, which we did not. Similarly, if the NPW siRNA pretreatment altered the function of the AT1R expressing neurons in the SFO, in light of the data published by Krause and colleagues (17) we might have expected to observe an effect on restraint stress-induced corticostorone levels, and we did not. These data, along with the knockdown study showing decreased water intake in response to Ang II (Figure 2), suggest that endogenous NPW may play a role in the neuronal activation of thirst in response to Ang II. Ang II has been shown to affect the activity of neurons in the PVN (18), a nucleus where NPW peptide and receptors have been localized (19). Since overnight water restriction presents both osmotic and baroreceptive stimuli to water drinking, it is not a surprise that compromise of NPW production did not alter water drinking in this paradigm (Figure 3). These data would suggest that the neuronal mechanisms originating in medullary sensory nuclei do not recruit brain-derived angiotensin II to stimulate water drinking. The failure of NPW siRNA pretreatment to alter water drinking in this model, would argue against the positive results in the angiotensin II challenge studies being a non-specific effect of the siRNA itself. An alternative explanation for the failure of NPW siRNA pretreatment to alter water drinking in response to overnight water deprivation is that the stimulus in that case generated a greater motivation to drink. This again could be explained by the stimulus of not only increased circulating Ang II levels, but also, because of the combined cellular and extracellular dehydration, baroreceptor-based mechanisms driving thirst. This volume component of thirst will be tested in future experiments employing the partial rehydration approach described by Ramsay and colleagues (20). It will also be important in future studies to test the ability of even greater dipsogenic doses of Ang II to stimulate water drinking in the siRNA pretreated rats. If higher doses overcome the inhibitory action of prior compromise of endogenous NPW production, then the Ang II – NPW circuit would have to be hypothesized to be one, but not the only, circuit through which Ang II stimulates thirst
Along with its known dipsogenic effects, Ang II also acts in brain to increase blood pressure (21). We investigated the role of NPW in the Ang II-induced pressor response and found that NPW siRNA pretreatment significantly attenuated the Ang II-induced increase in MAP (Figure 5) in conscious, freely moving rats. These results suggest that endogenous NPW-producing neurons may be downstream targets of Ang II in not only the expression of its dipsogenic but also its central cardiovascular actions as well. Although exogenous administration of NPW has been shown by us to elevate mean arterial pressure in conscious, unrestrained male rats (16) our present results do not suggest that the endogenous peptide is important in central control of resting cardiovascular function. However, a role for NPW-producing neurons in central cardiovascular control mechanisms when Ang II levels are elevated can be hypothesized. To our knowledge, these are the first experiments to identify a potential role for endogenous NPW in the mediation of these actions of Ang II.
NPW expression has been observed in several hypothalamic nuclei including the arcuate, ventromedial nucleus, paraventricular nucleus and lateral hypothalamic area (22), all sites known to be important in the control of thirst behaviors and autonomic function. Indeed there are overlaps of NPW-positive nerve fibers and GPR7 expression in arcuate nucleus, ventral and dorsal medial hypothalamic nuclei, paraventricular hypothalamic nucleus (23) and in the central amygdala, and several midbrain and brain stem structures (24,25). I125-NPW binding has been demonstrated also in the dorsal vagal complex, amygdala, bed nucleus of the stria terminalis (26), thus the ability of NPW siRNA compromise to alter these actions of Ang II is likely due to a loss of NPW’s actions in one or more of those nuclear groups. There may as well be some interaction of NPW and Ang II in SFO since I125-NPW binding has been observed in the SFO (26).
Multiple neuronal pathways regulate the secretion of ACTH and corticosterone during stress. Thus it is not surprising that the compromise of the production of endogenous NPW did not alter the corticosterone secretion in response to immobilization stress, especially since NPB utilizes the same receptors and is involved in one of these parallel pathways (4,6). If that is indeed the case, then the ability of compromise of NPW production alone to alter the central actions of Ang II that we observed here (water drinking and MAP) argues for a role for endogenous NPW but not NPB in the manifestation of those effects. Although both peptides activate the same receptor, control of their release from differing populations of neurons may be the key to understanding their unique and perhaps independent physiological relevance. Thus osmotic stimuli may uniquely activate, directly or indirectly, NPW neurons and the simple fact that pharmacologic administration of NPB results in some similar action may be merely a reflection of the exogenously applied peptide binding to the shared receptor (but not activation of endogenous NPB producing neurons in those experimental paradigms). The failure of compromise of NPW production to alter immobilization stress-induced corticosterone secretion by the same token may reflect the greater importance of endogenous NPB as a ligand for GPR7 during severe stress, and the pharmacologic action of NPW to release CRF merely a reflection of that more important role of endogenous NPB. Alternatively, unlike the results pointing to an important contribution of endogenous NPW to thirst and cardiovascular mechanisms of action of Ang II, the actions of both ligands via the shared receptor may be solely pharmacologic in nature in terms of the stress activation of the HPA axis, or due to the activation of additional neural elements by the restraint which are responsible for the activation of the HPA axis. Indeed, it has been suggested that restraint stress-induced elevations in Ang II levels, by an action in the subfornical organ control ACTH and thus corticosterone release (17). Since Ang II stimulates the central and peripheral releases of oxytocin (27,28) and oxytocin has been demonstrated under some circumstances to act directly in the anterior pituitary gland to stimulate ACTH release (29,30), Ang II generated by restraint may bypass the NPW circuitry necessary for an appropriate HPA response. Compromise of the production of endogenous NPW resulted in significantly decreased PRL secretion during restraint stress suggesting that endogenous NPW plays a physiologically relevant role in the control of PRL secretion. Since centrally administered Ang II has been shown to decrease PRL secretion in a dose-related manner (31), it would appear the increased PRL secretion following central NPW (4) occurs through a mechanism independent of Ang II. It is possible that endogenous NPW acts within the arcuate nucleus (ARC) of the hypothalamus to inhibit tuberoinfundibular neurones as both NPW peptide and GPR7 have been localized to the ARC (19, 22,32,33). Alternatively, NPW may act within the PVN to stimulate thyrotropin releasing hormone (TRH) neurones as both NPW peptide and GPR7 have been localized to this nucleus (19, 22,32,33) and bath application of NPW-23 caused depolarization of TRH expressing neurones within the PVN in brain slices (34). Thus, stressful situations may uniquely activate, directly or indirectly, NPW neurones that project to the PVN to stimulate TRH neurones and subsequently increase PRL secretion. It will be important in future studies to determine if stress activates NPW expressing neurones. Although the siRNA pretreatment was successful in decreasing whole hypothalamic NPW content, we do not know the absolute nucleus or area where a decrease in NPW caused the observed loss of Ang II action. While immunohistochemistry might be used to further elucidate the hypothalamic sites where siRNA caused a decrease in apparent peptide levels, it would not identify which of those areas was the site of the important loss of NPW action. An alternative approach, which we intend to pursue in future studies, would be to site-specifically decrease GPR7 expression in nuclei known to be important in fluid and electrolyte homeostasis, central cardiovascular control and prolactin secretion.
In summary, our studies have uncovered previously unrecognized involvement of neuropeptide W in the dipsogenic and cardiovascular actions of angiotensin II in brain. Additionally, they have provided evidence supporting the hypothesis that the pharmacologic actions of exogenous NPW-23 on prolactin secretion may be physiologically relevant. It will be important in future studies to determine if endogenous NPW production is dysregulated in pathophysiologic states of fluid and electrolyte homeostasis or cardiovascular function and if antagonism of the NPW/NPB receptor, GPR7 (NPBWR1), might be a therapeutic approach for the treatment of hypertension.
Acknowledgments
A.T. Pate was supported by a predoctoral fellowship (T32GM008306). The work was supported by National Institutes of Health Grant HL-66023 to W.K. Samson.
References
- 1.Lee DK, Nguyen T, Porter CA, Cheng R, George SR, O’Dowd BF. Two related G protein-coupled receptors: the distribution of GPR7 in rat brain and the absence of GPR8 in rodents. Brain Research Molecular Brain Research. 1999;71:96–103. doi: 10.1016/s0169-328x(99)00171-0. [DOI] [PubMed] [Google Scholar]
- 2.O’Dowd BF, Scheideler MA, Nguyen T, Cheng R, Rasmussen JS, Marchese A, Zastawny R, Heng HH, Tsui LC, Shi X. The cloning and chromosomal mapping of two novel human opioid-somatostatin-like receptor genes, GPR7 and GPR8, expressed in discrete areas of the brain. Genomics. 1995;28:84–91. doi: 10.1006/geno.1995.1109. [DOI] [PubMed] [Google Scholar]
- 3.Singh G, Davenport AP. Neuropeptide B and W: neurotransmitters in an emerging G-protein-coupled receptor system. British Journal of Pharmacology. 2006;148:1033–1041. doi: 10.1038/sj.bjp.0706825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker JR, Cardinal K, Bober C, Taylor M, Samson WK. Neuropeptide W acts in brain to control prolactin, corticosterone, and growth hormone release. Endocrinology. 2003;144:2816–2821. doi: 10.1210/en.2002-0161. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MM, Yuill EA, Baker JR, Ferri CC, Ferguson AV, Samson WK. Actions of neuropeptide W in paraventricular hypothalamus: implications for the control of stress hormone secretion. American Journal of Physiology. 2005;288:R270–R275. doi: 10.1152/ajpregu.00396.2004. [DOI] [PubMed] [Google Scholar]
- 6.Samson WK, Baker JR, Samson CK, Samson HW, Taylor MM. Central neuropeptide B administration activates stress hormone secretion and stimulates feeding in male rats. Journal of Neuroendocrinology. 2004;16:842–849. doi: 10.1111/j.1365-2826.2004.01239.x. [DOI] [PubMed] [Google Scholar]
- 7.Dun SL, Brailoiu GC, Yang J, Chang JK, Dun NJ. Neuropeptide W-immunoreactivity in the hypothalamus and pituitary of the rat. Neuroscience Letters. 2003;349:71–74. doi: 10.1016/s0304-3940(03)00804-8. [DOI] [PubMed] [Google Scholar]
- 8.Kelly MA, Beuckmann CT, Williams SC, Sinton CM, Motoike T, Richardson JA, Hammer RE, Garry MG, Yanagisawa M. Neuropeptide B-deficient mice demonstrate hyperalgesia in response to inflammatory pain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9942–9947. doi: 10.1073/pnas.0503795102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii M, Fei H, Friedman JM. Targeted disruption of GPR7, the endogenous receptor for neuropeptides B and W, leads to metabolic defects and adult-onset obesity. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10540–10545. doi: 10.1073/pnas.1334189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. American Journal of Physiology. 2009;297:R330–R336. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harms PG, Ojeda SR. A rapid and simple procedure for chronic cannulation of the rat jugular vein. Journal of Applied Physiology. 1974;36:391–392. doi: 10.1152/jappl.1974.36.3.391. [DOI] [PubMed] [Google Scholar]
- 12.Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. American Journal of Physiology. 2007;292:R382–R387. doi: 10.1152/ajpregu.00496.2006. [DOI] [PubMed] [Google Scholar]
- 13.Zar JH. Biostatistical Analysis. 2. Engelwood Cliffs, NJ: Prentice-Hall; 1984. [Google Scholar]
- 14.Levine AS, Winsky-Sommerer R, Hultron-Resendiz S, Grace MK, de Lecea L. Injection of neuropeptide W into the paraventricular nucleus of the hypothalamus increases food intake. American Journal of Physiology. 2005;288:R1727–1737. doi: 10.1152/ajpregu.00638.2003. [DOI] [PubMed] [Google Scholar]
- 15.Mondal MS, Yamaguchi H, Date Y, Shimbaa T, Toshenai K, Shimomura Y, Mori M, Nakazata M. A role for neuropeptide W in the regulation of feeding behavior. Endocrinology. 2003;144:4729–4733. doi: 10.1210/en.2003-0536. [DOI] [PubMed] [Google Scholar]
- 16.Pate AT, Yosten GLC, Samson WK. Neuropeptide W increases mean arterial pressure as a result of behavioral arousal. American Journal of Physiology. 2013 Aug 7; doi: 10.1152/ajpregu.00119.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR. Blood-borne angiotensin II acts in brain to influence behavioral and endocrine responses to restraint stress. Journal of Neuroscience. 2011;31:15009–15015. doi: 10.1523/JNEUROSCI.0892-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latchford KJ, Ferguson AV. Angiotensin depolarizes parvocellular neurons in paraventricular nucleus through modulation of putative nonselective cationic and potassium conductances. American Journal of Physiology. 2005;289:R52–R58. doi: 10.1152/ajpregu.00549.2004. [DOI] [PubMed] [Google Scholar]
- 19.Date Y, Mondal MS, Kageyama H, Ghamari-Langroudi M, Takenoya F, Yamaguchi H, Shimomura Y, Mori M, Murakami N, Shioda S, Cone RD, Nakazato M. Neuropeptide W: an anorectic peptide regulated by leptin and metabolic state. Endocrinology. 2010;151:2200–2210. doi: 10.1210/en.2009-1153. [DOI] [PubMed] [Google Scholar]
- 20.Ramsay DJ, Rolls BJ, Wood RJ. Body fluid changes which influence drinking in the water deprived rat. Journal of Physiology. 1977;266:453–469. doi: 10.1113/jphysiol.1977.sp011777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. The American Journal of Cardiology. 2002;89(2A):3A–9A. doi: 10.1016/s0002-9149(01)02321-9. discussion 10A. [DOI] [PubMed] [Google Scholar]
- 22.Takenoya F, Yagi M, Kageyama H, Shiba K, Endo K, Nonaka N, Date Y, Nakazato M, Shioda S. Distribution of neuropeptide W in the rat brain. Neuropeptides. 2010;44:99–106. doi: 10.1016/j.npep.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Ishii M, Fei H, Friedman JM. Targeted disruption of GPR7, the endogenous receptor for neuropeptides B and W, leads to metabolic defects and adult-onset obesity. Proceedings of the National Academy of Science US A. 2003;100:10540– 10545. doi: 10.1073/pnas.1334189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dun SL, Bailoiu GC, Yang K, Chang JK, Dun NJ. Neuropeptide W-immunoreactivity in the hypothalamus and pituitary of the rat. Neuroscience Letters. 2003;349:71–74. doi: 10.1016/s0304-3940(03)00804-8. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura Y, Tanaka H, Motoike T, Ishii M, Williams SC, Yanagisawa M, Sakurai T. Distribution of neuropeptide W immunoreactivity and mRNA in adult rat brain. Brain Research. 2006;1093:123–134. doi: 10.1016/j.brainres.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 26.Singh G, Maguire JJ, Kuc RE, Fidock M, Davenport AP. Identification and cellular localization of BPW1 (GPR7) receptors for the novel neuropeptide W-23 by [125I]-NPW radioligand binding and immunocytochemistry. Brain Research. 1017:222–226. doi: 10.1016/j.brainres.2004.03.079. [DOI] [PubMed] [Google Scholar]
- 27.Lang RE, Rascher W, Hell J, Unger T, Wiedemann G, Ganten D. Angiotensin stimulates oxytocin release. Life Sciences. 1981;29:1425–1428. doi: 10.1016/0024-3205(81)90005-9. [DOI] [PubMed] [Google Scholar]
- 28.Blackburn RE, Demko AD, Hoffman GE, Stricker EM, Verbalis JG. Central oxytocin inhibition of angiotensin-induced salt appetite. American Journal of Physiology. 1992:R1347–R1353. doi: 10.1152/ajpregu.1992.263.6.R1347. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs DM. Inhibition of corticotropin release during hypothermia: the role of corticotropin-releasing factor, vasopressin and oxytocin. Endocrinology. 1985;12:723–727. doi: 10.1210/endo-116-2-723. [DOI] [PubMed] [Google Scholar]
- 30.Link H, Dayanithi G, Fohr KJ, Gratzi M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH-releasing factor or arginine vasopressin-induced ACTH secretion, respectively. Endocrinology. 1992;130:2183–2191. doi: 10.1210/endo.130.4.1312449. [DOI] [PubMed] [Google Scholar]
- 31.Myers LS, Steele MK. The brain renin-angiotensin system and prolactin secretion in the male rat. Endocrinology. 1991;129:1744–1748. doi: 10.1210/endo-129-4-1744. [DOI] [PubMed] [Google Scholar]
- 32.Jackson VR, Lin SH, Wang Z, Nothacker HP, Civelli O. A study of the rat neuropeptide B/neuropeptide W system using in situ techniques. Journal of Comparative Neurology. 2006;497:367–383. doi: 10.1002/cne.20989. [DOI] [PubMed] [Google Scholar]
- 33.Kitamura Y, Tanaka H, Motoike T, Ishii M, Williams SC, Yanagisawa M, Sakurai T. Distribution of neuropeptide W immunoreactivity and mRNA in adult rat brain. Brain Research. 2006;1093:123–134. doi: 10.1016/j.brainres.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 34.Price CJ, Samson WK, Ferguson AV. Neuropeptide W has cell phenotype-specific effects on the excitability of different subpopulations of paraventricular nucleus neurones. Journal of Neuroendocrinology. 2009;21:850–857. doi: 10.1111/j.1365-2826.2009.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]