Abstract
Objective
Several epidemiologic, longitudinal studies have reported that short sleep duration is a risk factor for the incidence of obesity. However, the vast majority of these studies used self-reported measures of sleep duration and did not examine the role of objective short sleep duration, subjective sleep disturbances, and emotional stress.
Design
Longitudinal, population-based study.
Subjects
We studied a random sample of 815 non-obese adults from the Penn State Cohort in the sleep laboratory for one night using polysomnography (PSG) and followed them up for a mean of 7.5 years. Subjective and objective measures of sleep as well as emotional stress were obtained at baseline. Obesity was defined as a body mass index (BMI) ≥ 30kg/m2.
Results
The incidence of obesity was 15% and it was significantly higher in women and in individuals who reported sleep disturbances, shorter sleep duration, and higher emotional stress. Significant mediating effects showed that individuals with subjective sleep disturbances who developed obesity reported the shortest sleep duration and the highest emotional stress and that subjective sleep disturbances and emotional stress were independent predictors of incident obesity. Further analyses revealed that the association between short sleep duration, subjective sleep disturbances, and emotional stress with incident obesity was stronger in young and middle-age adults. Objective short sleep duration was not associated with a significantly increased risk of incident obesity.
Conclusion
Self-reported short sleep duration in non-obese individuals at risk of developing obesity is a surrogate marker of emotional stress and subjective sleep disturbances. Objective short sleep duration is not associated with a significant increased risk of incident obesity. The detection and treatment of sleep disturbances and emotional stress should become a target of our preventive strategies against obesity.
Keywords: Incidence, Obesity, Polysomnography, Poor sleep, Sleep duration, Stress
INTRODUCTION
An overwhelming 35% of adults from the United States general population are obese.1 Obesity is associated with the development of type 2 diabetes mellitus, hypertension, heart disease, and dyslipidemia, among many other medical complications.2 Obesity’s etiology has being traditionally attributed to an imbalance between energy intake and expenditure. Therefore, both behavioral as well as endocrine-metabolic factors have been extensively studied as contributing to the development of obesity in the general population.
In the last decade, sleep-related factors, and particularly short sleep duration, have been proposed as novel factors explaining the obesity epidemic. Cross-sectional studies have shown that obese report shorter sleep duration as compared to non-obese.3 However, the longitudinal evidence has reported conflicting results regarding the association of subjective sleep duration and incident obesity in adults. Magee and Hale4 recently conducted a systematic review of longitudinal studies and concluded that there is no clear evidence supporting short sleep duration as a cause of obesity or weight gain in adults. The authors pointed to the lack of inclusion of appropriate confounding, mediating, and moderating variables in explaining the inconsistencies between studies. Furthermore, only one longitudinal study of those reviewed used objective measures of sleep (i.e., actigraphy) and did not find a significant association between short sleep duration and weigh gain.5 No study to date, however, has used polysomnography (PSG), the physiological “gold standard” method to assess sleep, to examine the relationship between sleep duration and the incidence of obesity.
We have previously suggested that the cross-sectional association between obesity and subjective short sleep duration is mediated by the presence of sleep complaints and emotional stress.6 In other words, obese individuals not only report shorter sleep duration but also a much higher prevalence of sleep difficulties and emotional stress. Given the high prevalence of obesity and of sleep difficulties (about 20%) in the general population,1,7 linking these conditions may have a greater impact in the way we understand the relationship between sleep and obesity and in the way public health policies and intervention studies targeting sleep should be designed and implemented.
The aim of the present study was to examine the longitudinal association between subjective and objective short sleep duration with incident obesity in a large, general population sample. We hypothesized that the relationship between subjective short sleep duration and incident obesity is explained by the presence of sleep difficulties and emotional stress.
MATERIALS AND METHODS
Participants
The data presented here were collected as part of a population-based study of sleep disorders, which used a two-phase protocol in order to recruit participants from various age groups.7-9 In the first phase of the study, a sample of adult men and women (age ≥20 years) was randomly selected from local telephone households in two counties of Central Pennsylvania (Dauphin and Lebanon) using the Mitofsky-Waksberg two-stage random digit dialing procedure.10 A within-household selection procedure described by Kish11 was used to select the specific man or woman to be interviewed. Telephone interviews were conducted with 4,364 age-eligible men and 12,219 age-eligible women residing in the sample households, for a total sample of 16,583 with response rates of 73.5% and 74.1%, respectively. The questionnaire employed in this interview included basic demographic and sleep information. In the second phase of this study, a subsample of 741 men and 1,000 women, selected randomly from those subjects previously interviewed by telephone, were studied in our sleep laboratory. The response rates for this phase were 67.8% and 65.8% for men and women, respectively. After giving a complete description of the study to the subjects, written informed consent was obtained.
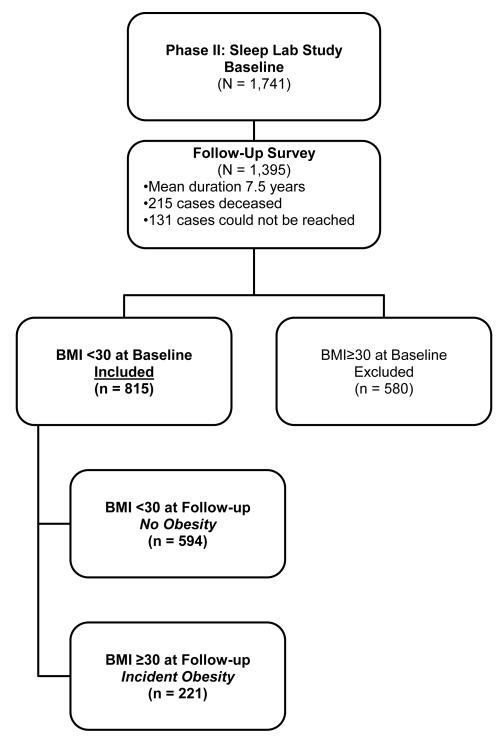
Of the 1741 subjects who completed the comprehensive sleep evaluation, 1395 subjects were followed up after an average duration of 7.5 years (mean duration of 4.5 years for women and 10.5 years for men) via telephone interview. In the Penn State Cohort Study, men were recruited first and women 5 years later. This explains the five-year difference in the follow-up period between men and women. The response rate of the follow-up study was 79.7%. However, if one considers that 215 subjects died between baseline and follow-up, then the response rate of those alive was 90.9%. After complete description of the follow-up study to the subjects, verbal informed consent was obtained. The entire study procedure was approved by the University’s Institutional Review Board. Figure 1 shows the participant flow in the study.
Figure 1.
Participants’ flow in the study.
Key measurements
Each subject selected for laboratory evaluation completed a comprehensive sleep history and physical examination. All subjects were evaluated for one night in the sleep laboratory in sound-attenuated, light- and temperature-controlled rooms. During this evaluation, each subject was continuously monitored for eight hours (fixed-time period) using 16-channel polysomnography including electroencephalogram, electrooculogram, and electromyogram. Bedtimes were adjusted to conform to subjects’ usual bedtimes, and subjects were recorded between 22:00-23:00 and 06:00-07:00. The sleep recordings were subsequently scored independently, according to Rechtschaffen and Kales criteria.12 From the objectively recorded total sleep time data, we regrouped the entire study sample into ordinal groups of ≥ 7 hours, 7-6 hours, 6-5 hours, and ≤ 5 hours of objective sleep duration.
Additional information obtained during the PSG included that assessing sleep apnea. Respiration was monitored throughout the night by use of thermocouples at the nose and mouth and thoracic strain gauges. All-night recordings of hemoglobin oxygen saturation (SpO2) were obtained with an oximeter attached to the finger. Apnea was considered present if a breath cessation exceeded 10 seconds and each apnea was categorized in terms of obstructive (chest wall movement present) or central (chest wall movement absent). In addition, hypopnea was considered present when a reduction in airflow of approximately 50% was indicated at the nose or mouth and was associated with a reduction of 4% arterial blood oxygen saturation.8,9 For the purpose of this study, the presence of SDB was defined as an obstructive apnea/hypopnea index (OHI) ≥ 5.
As part of the baseline evaluation during the telephone interview (phase 1), subjects were asked to report their height (ft) and weight (lb) and self-reported body mass index (srBMI) was calculated [srBMI = mass (lbs)/(height(ft))2 × 4.88]. As part of the physical examination during the subjects’ sleep laboratory visit at baseline (phase 2), height (cm) and weight (kg) were measured and objective body mass index (objBMI calculated [objBMI = mass (kg)/(height(m))2].
As part of this protocol we also assessed the presence of all sleep disorders, based on a standardized questionnaire completed by the subjects on the evening of their sleep laboratory visit. This questionnaire consists of 53 questions (7 demographic, 20 sleep-related, and 26 general health questions). In addition, women responded to 8 questions related to menstrual history, menopause, and hormone therapy. We subjectively assessed sleep duration with the question “How many hours do you usually sleep at night?” recorded in hours. From the self-reported sleep duration data, we regrouped the entire study sample into ordinal groups of ≥ 7 hours, 7-6 hours, 6-5 hours, and ≤ 5 hours of subjective sleep duration. Sleep related questions were qualified in terms of severity on a scale of 0-3 (0 = none, 1 = mild, 2 = moderate, 3 = severe) and duration. The presence of sleep difficulty was established on three levels of severity. First, insomnia was defined by a complaint of insomnia (i.e., a positive response to the question “Do you feel you have insomnia?”) with a duration of ≥ 1 year. Second, poor sleep was defined as a moderate-to-severe complaint (based on a mild to severe scale) of difficulty falling asleep (“Do you have difficulty falling asleep?”), difficulty staying asleep (“Do you have difficulty staying asleep?”), early final awakening (“Do you wake up in the morning earlier than desired?”), or non-restorative sleep (“Do you still feel groggy and unrefreshed after morning awakening?”). Finally, normal sleep was defined as the absence of either of these two categories. In order to create three mutually exclusive categories, none in the poor sleep group reported having insomnia and none in the normal sleeping group reported either insomnia or poor sleep. The presence of excessive daytime sleepiness (EDS) was established based on a moderate-to-severe rating on either of the following two questions: “Do you feel drowsy or sleepy most of the day but manage to stay awake?” and “Do you have any irresistible sleep attacks during the day?”.
Additional information obtained from the standardized questionnaire included assessing physical health conditions, depression, and substance use. The presence of hypertension at baseline was defined as a diastolic blood pressure ≥ 90 mm Hg and/or a systolic blood pressure ≥ 140 mm Hg or use of antihypertensive medication. The presence of diabetes at baseline was defined as a self-report of being treated for diabetes or having a fasting blood sugar ≥126 mg/dl from blood drawn the morning after the subject’s PSG. We also ascertained at baseline whether the respondent was currently treated for depression, including a history of suicidal thoughts or attempts. Participants’ daily consumption of caffeine (number of cups/day), tobacco (number of cigarettes/day), and alcohol (number of drinks/day) was also assessed.
The level of emotional stress was measured by the Minnesota Multiphasic Personality Inventory (MMPI-2).13 T scores with a mean of 50 and a standard deviation (SD) of 10 are generated for the eight major clinical scales. Scores ≥ 65 (1.5 SD above the mean) indicate a significant deviation from the original normal standardization pattern of responding and suggested an elevation at a clinically significant level.13
Follow-up measures taken through telephone interview included the standardized questionnaire that subjects completed at baseline during their sleep laboratory visit. Self-reported height (ft) and weight (lbs) were also used to calculate self-reported BMI at follow-up. Commensurate with the baseline definition, obesity at follow-up was defined by a self-reported BMI ≥ 30. The reliable use of self-reported BMI was supported by the strong positive correlation found between baseline self-reported and objectively measured BMI (r = .88; p = .00001). Of the 1395 subjects who were followed-up, 815 did not have obesity at baseline and were selected for the present study. A total of 221 subjects were incident cases of obesity and 594 did not develop obesity at follow-up.
Statistical Analyses
For inference concerning general population, such as estimations and comparisons of the prevalence rates of individual and overall subjective sleep disturbances, weighted analyses were performed to take into account the oversampling of those more-at-risk for SDB individuals in the second phase of the Penn State Cohort Study.8,9,14 The four categories of sleep duration used were (1) ≤5; (2) 5-6; (3) 6-7; (4) ≥7 hours. Multivariable logistic regression models were used to assess the independent associations of subjective or objective sleep duration, sleep difficulty, and emotional stress with incident obesity, after adjusting for significant confounding factors. We calculated the odds ratios (OR) and the 95% confidence intervals (95%CI) from these models to estimate the risk of incident obesity associated with the different predictors of interest, while simultaneously controlling for potential confounders. We report data regarding number of subjects (e.g., n) unadjusted and data referring to means, percentages, or degree of association (e.g., OR) adjusted for sampling weight. All analyses were conducted with IBM SPSS version 20.0 for Windows.
RESULTS
The overall incidence of obesity was 15.0%. Table 1 presents the demographic behavioral, and clinical characteristics of the entire sample and stratified by the presence of obesity at follow-up. After controlling for baseline BMI, women had a 2-fold risk for developing obesity as compared to men (p < .001) whereas middle-age (i.e., 41-59y) and young (i.e., ≤ 40y) adults had a 4-fold (p < .001) and almost 2-fold (p < .05) risk, respectively, of developing obesity at follow-up as compared to older adults (i.e., ≥ 60y). Univariate analyses showed that race, diabetes, and sleep apnea were associated with a significantly increased risk of incident obesity; however, these associations became non-significant after adjusting for baseline BMI. We found a significant interaction between age and BMI on the incidence of obesity so that for an increase of BMI by one unit the risk of incident obesity was greater among the younger individuals than in the older ones. We, therefore, controlled for the age by BMI interaction in our primary multivariable regression analyses and performed further secondary analyses stratified by age groups.
Table 1.
Sociodemographic, Behavioral, and Clinical Factors Associated with Incident Obesity
| Incident Obesity |
||||
|---|---|---|---|---|
| All (n = 815) |
No (n = 594) |
Yes (n = 221) |
OR (95%CI) | |
| Sex | ||||
| Male, % | 50.5 | 85.2 | 14.8 | 1.0 |
| Female, % | 49.5 | 85.1 | 14.9 | 2.15 (1.45-3.17)***,a |
| Race | ||||
| Caucasian, % | 94.7 | 85.8 | 14.2 | 1.0 |
| Non-Caucasian, % | 5.3 | 70.7 | 29.3** | 1.31 (0.67-2.55)a |
| Age, years | 48.9 (13.4) | 49.2 (13.5) | 46.8 (12.7)* | 0.95 (0.94-0.97)***,a |
| ≥ 60, % | 22.6 | 88.0 | 12.0 | 1.0 |
| 41-59, % | 48.8 | 84.7 | 15.3 | 4.15 (2.35-7.32)***,a |
| ≤ 40, % | 28.5 | 83.6 | 16.7 | 1.84 (1.12-3.01)*,a |
| Baseline BMI, kg/m2 | 24.6 (2.8) | 24.1 (2.6) | 27.3 (2.2)*** | 1.74 (1.59-1.90)*** |
| < 25, % | 51.6 | 95.3 | 4.7 | 1.0 |
| ≥ 25, % | 48.4 | 74.1 | 25.9*** | 7.04 (4.57-10.8)*** |
| Follow-up BMI, kg/m2 | 26.1 (3.7) | 25.0 (2.8) | 32.2 (2.2)** | --- |
| < 25, % | 39.3 | --- | --- | --- |
| ≥ 25, % | 45.7 | --- | --- | --- |
| ≥ 30, % | 15.0 | --- | --- | --- |
| Change in BMI, kg/m2 | 1.5 (2.6) | 0.9 (1.9) | 4.9 (2.9)*** | 2.19 (1.95-2.45)*** |
| Baseline weight, kg | 72.8 (12.6) | 71.5 (12.1) | 80.6 (12.8)*** | 1.06 (1.04-1.07)*** |
| Follow-up weight, kg | 76.5 (14.3) | 73.5 (12.1) | 94.2 (13.5)*** | --- |
| Change in weight, kg | 3.7 (7.5) | 2.0 (5.6) | 13.6 (9.2)*** | 1.29 (1.24-1.34)*** |
| Change in weight, % | 5.4 (10.4) | 3.3 (8.1) | 17.9 (13.2)*** | --- |
| ≥ 10, % | 26.0 | 18.0 | 73.0*** | 11.2 (7.71-16.3)*** |
| Caffeine, cup/day | 2.4 (2.9) | 2.3 (2.8) | 2.6 (3.3) | 1.04 (0.98-1.09) |
| None, % | 32.2 | 85.5 | 14.5 | 1.0 |
| ≥ 1 cup/day, % | 67.8 | 84.9 | 15.1 | 1.05 (0.74-1.50) |
| Cigarettes, n/day | 4.3 (11.0) | 4.6 (11.5) | 3.0 (7.2) | 0.98 (0.96-1.00) |
| None, % | 77.9 | 84.6 | 15.4 | 1.0 |
| ≥ 1/day, % | 22.1 | 86.5 | 13.5 | 0.86 (0.57-1.29) |
| Alcohol, drink/day | 1.3 (6.1) | 1.3 (6.5) | 0.9 (2.6) | 0.98 (0.93-1.03) |
| None, % | 72.1 | 84.0 | 16.0 | 1.0 |
| ≥ 1 drink/day, % | 27.9 | 88.1 | 11.9 | 0.72 (0.49-1.06) |
| Diabetes | ||||
| No, % | 90.7 | 85.7 | 14.3 | 1.0 |
| Yes, % | 9.3 | 78.8 | 21.2 | 0.66 (0.37-1.17)a |
| Hypertension | ||||
| No, % | 69.3 | 86.0 | 14.0 | 1.0 |
| Yes, % | 30.7 | 83.0 | 17.0 | 0.81 (0.55-1.21)a |
p ≤ .05;
p ≤ .01;
p ≤ .001;
adjusted for baseline BMI
Table 2 presents the univariate association between mood, emotional stress, and sleep-related factors with incident obesity. Depression was associated with a 2-fold risk of incident obesity, even after adjusting for baseline BMI (p < .05). Subjective short sleep duration (p < .05), poor sleep (p < .001), and emotional stress (p < .001) were all significantly associated with incident obesity. Among the elevated MMPI-2 scales, those related to depressed mood, anxiety, somatic complaints, emotional lability, and impulsivity were predominant in those individuals who developed obesity. Interestingly, insomnia and EDS were not significantly associated with incident obesity.
Table 2.
Mood, Stress, and Sleep Factors Associated with Incident Obesity
| Incident Obesity |
||||
|---|---|---|---|---|
| All (n = 815) |
No (n = 594) |
Yes (n = 221) |
OR (95%CI) | |
| Depression | ||||
| No, % | 86.0 | 85.8 | 14.2 | 1.0 |
| Yes, % | 14.0 | 80.8 | 19.2 | 1.94 (1.16-3.23)*,a |
| Emotional Stress | ||||
| MMPI-2 elevations, # | 0.7 (1.3) | 0.6 (1.2) | 1.2 (1.7)** | 1.48 (1.27-1.72)*** |
| 1-Hs elevated, % | 18.0 | 16.0 | 27.0* | 1.73 (1.11-2.69)* |
| 2-D elevated, % | 9.0 | 8.0 | 16.0** | 2.22 (1.31-3.77)** |
| 3-Hy elevated, % | 9.0 | 8.0 | 13.0* | 1.95 (1.13-3.38)* |
| 4-Pd elevated, % | 10.0 | 8.0 | 20.0*** | 2.75 (1.66-4.56)*** |
| 6-Pa elevated, % | 7.0 | 6.0 | 13.0** | 2.62 (1.46-4.71)** |
| 7-Pt elevated, % | 8.0 | 8.0 | 8.0 | 1.05 (0.54-2.03) |
| 8-Sc elevated, % | 6.0 | 5.0 | 10.0 | 1.88 (0.96-3.70) |
| 9-Ma elevated, % | 5.0 | 4.0 | 11.0** | 2.35 (1.17-4.74)* |
| 0-Si elevated, % | 8.0 | 8.0 | 11.0 | 1.42 (0.78-2.59) |
| Sleep Difficulty | ||||
| Normal Sleep, % | 75.0 | 86.7 | 13.3 | 1.0 |
| Poor Sleep, % | 18.9 | 77.1 | 22.9** | 1.92 (1.31-2.80)** |
| Insomnia, % | 6.1 | 89.9 | 10.1 | 0.69 (0.30-1.58) |
| Apnea/Hypopnea Index | 1.8 (5.8) | 1.4 (4.8) | 3.4 (9.4)*** | 1.01 (0.99-1.04)a |
| AHI < 5, % | 91.1 | 85.9 | 14.1 | 1.0 |
| AHI ≥ 5, % | 8.9 | 77.6 | 22.4* | 1.07 (0.61-1.87)a |
| Excessive Daytime Sleepiness | ||||
| No, % | 94.2 | 85.6 | 14.4 | 1.0 |
| Yes, % | 5.8 | 76.9 | 23.1 | 1.28 (0.64-2.59)a |
| Subjective Sleep Duration, hrs | 7.0 (1.2) | 7.0 (1.2) | 6.6 (1.2)* | 0.84 (0.73-0.97)* |
| ≥ 7 h, % | 61.9 | 86.8 | 13.2 | 1.0 |
| 6-7 h, % | 8.0 | 84.8 | 15.2 | 1.19 (0.62-2.29) |
| 5-6 h, % | 21.9 | 84.8 | 15.2 | 1.18 (0.76-1.83) |
| ≤ 5 h, % | 8.2 | 75.3 | 24.7** | 2.18 (1.25-3.78)** |
| Objective Sleep Duration, hrs | 5.9 (1.2) | 5.9 (1.2) | 6.1 (1.1)* | 1.18 (1.02-1.37)* |
| ≥ 7 h, % | 17.4 | 83.5 | 16.5 | 1.0 |
| 6-7 h, % | 37.2 | 83.8 | 16.2 | 1.00 (0.62-1.57) |
| 5-6 h, % | 22.7 | 83.7 | 16.3 | 1.00 (0.60-1.65) |
| ≤ 5 h, % | 22.7 | 89.7 | 10.3* | 0.57 (0.33-0.99)* |
p ≤ .05;
p ≤ .01;
p ≤ .001;
adjusted for baseline BMI; 1-Hs = hypochondriasis; 2-D = depression; 3-Hy = hysteria; 4-Pd = Psychopathic deviate; 6-Pa = Paranoia; 7-Pt = psychasthenia; 8-Sc = schizophrenia; 9- Ma = Hypomania; 0-Si = social introversion
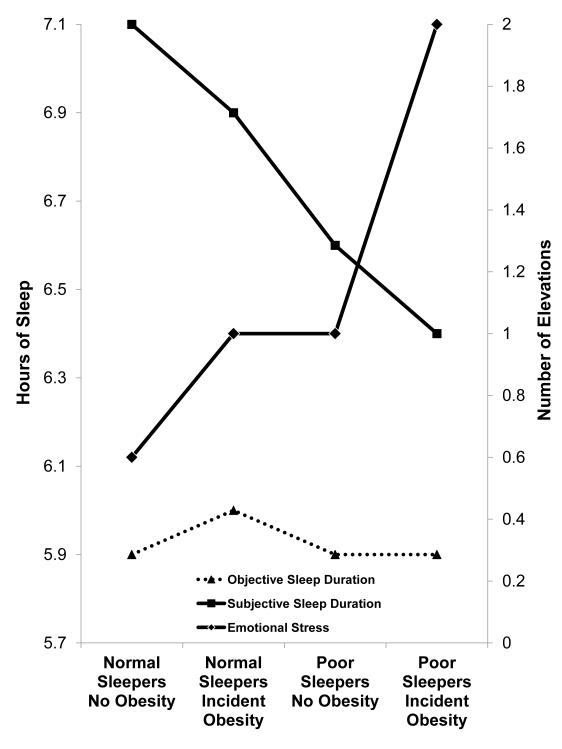
Three sets of multivariable logistic regression models examined the association of subjective or objective sleep duration, poor sleep, and emotional stress with incident obesity, after progressively adjusting for each other as well as for potential confounders. As shown in Table 3, the association between subjective short sleep duration and incident obesity became non-significant after controlling for poor sleep or emotional stress. In fact, poor sleep and emotional stress were independent predictors of incident obesity even after further controlling for gender, age, AHI, BMI, age by BMI interaction, and depression. Figure 2 depicts the mediating and additive effects of poor sleep and emotional stress in the association of subjective sleep duration with incident obesity. As shown in Figure 2, poor sleepers who developed obesity reported the shortest sleep duration and the highest levels of emotional stress, whereas normal sleepers who developed obesity reported significantly higher levels of emotional stress as compared to normal sleepers who remained non-obese. Objective short sleep duration was not associated with an increased risk of incident obesity (Table 4). In fact, the apparent negative association between objective short sleep duration and incident obesity became non-significant after controlling for potential confounders. As shown in Figure 2, there was no relationship between objective sleep duration and incident obesity, which contrasts with the linear association of subjective sleep duration with incident obesity across groups.
Table 3.
Multivariable Adjusted Odds Ratio (95% CI) of Incident Obesity and Subjective Sleep Duration, Sleep Difficulty, and Emotional Stress
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
|
Subjective Sleep Duration |
||||||
| ≥ 7 h | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 6-7 h | 1.19 (0.62-2.29) | 1.26 (0.62-2.57) | 1.25 (0.61-2.56) | 1.25 (0.60-2.60) | 1.02 (0.45-2.29) | 1.03 (0.46-2.34) |
| 5-6 h | 1.18 (0.76-1.83) | 1.15 (0.72-1.85) | 1.25 (0.77-2.03) | 1.27 (0.83-2.26) | 1.25 (0.89-2.73) | 1.27 (0.89-2.76) |
| ≤ 5 h | 2.18 (1.25-3.78)** | 1.71 (0.87-3.36) | 1.69 (0.85-3.37) | 1.68 (0.82-3.45) | 1.07 (0.48-2.39) | 1.08 (0.48-2.41) |
| Sleep Difficulty | ||||||
| Normal Sleep | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Poor Sleep | 2.48 (1.58- 3.91)*** |
2.19 (1.37- 3.48)*** |
2.07 (1.28-3.35)** | 1.80 (1.04-3.12)* | 1.78 (1.02-3.13)* | |
| Insomnia | 0.81 (0.33-1.99) | 0.81 (0.33-2.00) | 0.78 (0.30-2.00) | 0.49 (0.16-1.45) | 0.48 (0.15-1.53) | |
| Emotional Stress | 1.25 (1.11- 1.42)*** |
1.24 (1.09-1.41)*** | 1.39 (1.19-1.62)*** | 1.38 (1.17- 1.63)*** |
p < .05;
p ≤ .01;
p ≤ .001
Model 1 = subjective sleep duration
Model 2 = subjective sleep duration and sleep difficulty
Model 3 = subjective sleep duration, sleep difficulty, and emotional stress (i.e., MMPI-2 # elevations)
Model 4 = subjective sleep duration, sleep difficulty, emotional stress, gender, race, age, and AHI.
Model 5 = subjective sleep duration, sleep difficulty, emotional stress, gender, race, age, AHI, BMI, and age*BMI.
Model 6 = subjective sleep duration, sleep difficulty, emotional stress, gender, race, age, AHI, BMI, age*BMI, and depression.
Figure 2. Subjective Sleep Duration and Incident Obesity: Role of Poor Sleep and Emotional Stress.
Figure 2 depicts the additive effect of poor sleep and emotional stress on incident obesity and their role in mediating the association between shorter subjective sleep duration and incident obesity. Individuals with poor sleep who developed obesity reported the shortest sleep duration and the highest levels of emotional stress at baseline. All data adjusted for gender, race, age, diabetes, hypertension, AHI, and baseline BMI.
Table 4.
Multivariable Adjusted Odds Ratio (95% CI) of Incident Obesity and Objective Sleep Duration, Sleep Difficulty, and Emotional Stress
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
|
Objective Sleep Duration |
||||||
| ≥ 7 h | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 6-7 h | 1.00 (0.62-1.57) | 0.78 (0.45-1.35) | 0.78 (0.45-1.35) | 0.84 (0.48-1.48) | 0.86 (0.45-1.64) | 0.88 (0.46-1.67) |
| 5-6 h | 1.00 (0.60-1.65) | 0.92 (0.51-1.65) | 0.90 (0.50-1.62) | 0.97 (0.52-1.81) | 1.11 (0.55-2.25) | 1.12 (0.56-2.25) |
| ≤ 5 h | 0.57 (0.33-0.99)* | 0.50 (0.27-0.94)* | 0.46 (0.25-0.87)* | 0.44 (0.22-0.91)* | 0.51 (0.22-1.19) | 0.51 (0.22-1.18) |
| Sleep Difficulty | ||||||
| Normal Sleep | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Poor Sleep | 2.50 (1.61- 3.87)*** |
2.18 (1.39- 3.42)*** |
2.06 (1.29-3.28)** | 1.77 (1.05-3.00)* | 1.76 (1.03-3.00)* | |
| Insomnia | 0.98 (0.41-2.35) | 0.99 (0.41-2.38) | 0.97 (0.40-2.40) | 0.60 (0.21-1.74) | 0.59 (0.20-1.77) | |
| Emotional Stress | 1.25 (1.10- 1.41)*** |
1.25 (1.10-1.42)*** | 1.34 (1.15-1.56)*** | 1.34 (1.14- 1.57)*** |
p < .05;
p ≤ .01;
p ≤ .001
Model 1 = objective sleep duration
Model 2 = objective sleep duration and sleep difficulty
Model 3 = objective sleep duration, sleep difficulty, and emotional stress (i.e., MMPI-2 # elevations)
Model 4 = objective sleep duration, sleep difficulty, emotional stress, gender, race, age, and AHI.
Model 5 = objective sleep duration, sleep difficulty, emotional stress, gender, race, age, AHI, BMI, and age*BMI.
Model 6 = objective sleep duration, sleep difficulty, emotional stress, gender, race, age, AHI, BMI, age*BMI, and depression.
As shown in Table 1, individuals who developed obesity gained significantly more weight than those who did not, which indicates that individuals who developed obesity were at a true increased risk of weight gain. Nevertheless, the findings presented above remained similar and in the same direction when the association between subjective and objective sleep duration, poor sleep and emotional stress with other outcomes of weight gain (i.e., absolute change in BMI, absolute weight gain, or percent weight gain) were examined. In summary, individuals who reported short sleep duration (p< .05), poor sleep (p < .01), or higher emotional stress (p < .01) were associated with higher absolute changes in BMI and body weight and were more likely to gain 10% of body weight as compared to those who reported normal sleep duration, no sleep complaints, or lower emotional stress. In contrast, objective short sleep duration was not associated with either higher absolute changes in BMI or body weight or higher likelihood of gaining 10% of body weight.
In the analyses presented above, we systematically controlled for age, BMI, and their interaction. However, in order to better understand the role of sleep as a risk factor for incident obesity in young (i.e., ≤ 40y), middle-aged (i.e., 41-59y), and older adults (i.e., ≥ 60y), we performed secondary analyses stratified by these age groups. We found that subjective short sleep duration, poor sleep, and emotional stress were strongly associated with incident obesity in both young and middle-aged adults. Consistent with the results shown in Table 3 for the entire sample, the association between subjective short sleep duration and incident obesity in young and middle-aged adults became non-significant after adjusting for poor sleep and emotional stress, which remained as significant predictors (p < .01) after controlling for baseline BMI and other potential confounders. Interestingly, young and middle-aged adults differed in the contribution of other risk factors to incident obesity. Behavioral factors, such as smoking (p < .01), alcohol (p < .05), and caffeine (p < .05) consumption, as well as depression (p < .05) were significant predictors of incident obesity in young adults but not in middle-aged or older adults. Physical health problems such as diabetes, hypertension, and sleep apnea were significantly associated with the incidence of obesity in the middle-aged; however, the association between these physical problems and incident obesity in middle-age became non-significant after adjusting for baseline BMI. In older adults, female gender (p < .01) and poor sleep (p < .01) were significant predictors of incident obesity.
Given the significant association between female gender and incident obesity mentioned above, we also performed secondary analyses stratified by gender. We found that in men, poor sleep remained significantly associated with incident obesity (p < .05), whereas subjective short sleep duration and emotional stress were marginally associated (p = .054 and p =.064, respectively). In women, we also found that the association between subjective short sleep duration and incident obesity became non-significant after adjusting for poor sleep; however, further adjustment for emotional stress indicated that it was the best single predictor (p < .01) of incident obesity in women. Objective short sleep duration was not significantly associated with incident obesity in either men (p = .609) or women (p = .451).
DISCUSSION
This is the first study to demonstrate that the longitudinal association between subjective short sleep duration and incident obesity, particularly in the young and middle-aged, is mediated by poor sleep and emotional stress and that objective short sleep duration does not predict the incidence of obesity. Our findings provide further evidence that subjective short sleep duration is a marker of sleep complaints and emotional stress in the general population, and that these factors should become the target of intervention studies aimed at preventing the development of obesity.
As reviewed by Magee and Hale,4 about 13 studies have longitudinally examined the association between sleep duration and subsequent weight gain or incident obesity in adults. Of those, 4 studies found a significant association between subjective short sleep duration and weight gain, 4 found a significant U-shape association in which both short (i.e., ≤ 5 hours) and long (i.e., ≥ 9 hours) sleep duration were associated with weight gain, and 5 did not find a significant association between sleep duration and weight gain. Of note, only one of those studies used objective measures of sleep (i.e., actigraphy) and did not find a significant longitudinal association between short or long sleep duration and obesity or weight gain.5 The authors noted that the inconsistent results of these studies may be due in part to lack of appropriate inclusion of confounding factors or examination of mediating or moderating effects.4 The present study addressed these issues by controlling for relevant confounding factors and examining mediating and moderating effects.
Consistent with some previous studies, we found that subjective short sleep duration is associated with incident obesity. However, after controlling for complaints of poor sleep and level of emotional stress the significant association between subjective short sleep duration and incident obesity disappeared. In fact, these two factors of poor sleep and emotional stress were strong predictors of incident obesity and played an additive role between each other. As shown in Figure 2, emotional stress was significantly associated with incident obesity in normal sleepers; however, it was in those with poor sleep and incident obesity that emotional stress was greatest and subjective sleep duration shortest. The significant interaction between age and premorbid BMI and the secondary stratified analyses revealed that the effects of emotional stress are stronger in the young and the middle-aged, while complaints of poor sleep are a predictor of incident obesity across all age groups. Furthermore, the results stratified by gender showed that the effect of emotional stress is stronger in women, while complaints of poor sleep are stronger predictors of incident obesity in men. Importantly, we did not find an independent association between objective short sleep duration and incident obesity in the overall sample or after stratifying by age or gender. These results are consistent with two previous studies that used actigraphy to examine the longitudinal association of sleep duration with weigh gain and obesity.5,15 Together, these data provide further evidence that complaints of poor sleep and emotional stress, especially in the young and middle-aged, should be the focus of our research and clinical efforts in understanding the relationship between sleep and weight gain and obesity.
Interestingly, chronic insomnia was not significantly associated with an increased risk of incident obesity. There is accumulating evidence that chronic insomnia is a disorder of physiological hyperarousal, as suggested by increased activity of the hypothalamic-pituitary-adrenal (HPA) axis and increased sympathetic activation.16,17,18 Interestingly, several studies have shown increased metabolic rate in chronic insomniacs as compared to normal sleepers.19 It is, therefore, plausible that non-obese chronic insomniacs may not be at risk for developing obesity due to their increased metabolic rate and greater energy consumption. Given that chronic insomnia in the non-obese is not a risk factor for incident obesity and that in our previous cross-sectional study we showed that chronic insomnia is more prevalent in obese than non-obese individuals,6 what is the explanation of the increased prevalence of chronic insomnia in obese? In previous longitudinal studies we have shown that obesity is a risk factor for incident chronic insomnia,20,21 which indicates that the increased prevalence of chronic insomnia in the obese is secondary to obesity. In conclusion, obesity is a risk factor for chronic insomnia but chronic insomnia is not a risk factor for incident obesity.
In contrast, the exact pathophysiology associated with “poor sleep” remains unknown. It is well-established in the literature that several disorders associated with emotional stress do not necessarily exhibit the same physiological perturbations (e.g., HPA axis activity and metabolic rate). For example, major melancholic depression is associated with HPA axis hyperactivity and weight loss, whereas major atypical depression is associated with HPA axis hypoactivity and weight gain.22 Also, it has been shown that the HPA axis changes in animals prone to obesity (by consuming “comfort-food”) are different than the HPA axis alterations in stressed animals losing weight.23 In conclusion, the physiological mechanisms, including HPA axis changes, through which “poor sleep” leads to obesity are currently unknown and we hypothesize that they may be different than those associated with the pathophysiology of chronic insomnia.
Furthermore, EDS was not significantly associated with incident obesity. These data provide further evidence that EDS is a consequence of obesity rather than a premorbid risk factor for obesity.24-26 Moreover, our univariate analyses showed that non-obese sleep apneics are at significant risk of developing obesity but that this association disappeared after controlling for premorbid weight status (i.e., baseline BMI). It is plausible that there is a reciprocal interaction between obesity and sleep apnea and that metabolic abnormalities in obesity and sleep apnea are in a continuum.27,28 Our data, therefore, suggest that the presence of sleep apnea in overweight individuals leads to a natural disease progression towards obesity.
Some limitations should be taken into account when interpreting our results. First, incident obesity was defined by self-reported BMI because objectively measured BMI was not collected at follow-up. However, we found a strong positive correlation between self-reported and objectively measured BMI at baseline (r = .88) and we controlled for objectively measured baseline BMI in our multivariable logistic regression models, all of which increase our confidence about the replicability of the present findings. In addition, controlling for change in BMI over time, instead of baseline BMI, did not affect our estimation of incident obesity risk associated with each of the predictors of interest. Finally, other large epidemiological studies have also used self-reports to ascertain incident obesity.3,4 Thus, the consistency of the present findings with those of cross-sectional and longitudinal studies on the lack of association of subjective short sleep duration with obesity once factors such as sleep complaints and emotional stress are controlled for,3,4,6 increases our confidence about the replicability of the present findings. Second, the objective sleep duration in this study was based on one night of fixed-time PSG, which may not be representative of the subjects’ ad-libitum habitual sleep duration and may be affected by rebound and/or first-night effects. The lack of measurement of habitual sleep duration does not allow addressing these limitations. We used the proposed criterion of ≥ 7 hours as the cutoff point for “normal” sleep duration to commensurate with previous studies on the association of subjective sleep duration with incident obesity. However, in large epidemiologic sleep studies, i.e., Sleep Heart Health Study, Coronary Artery Risk Development in Young Adults (CARDIA) or Penn State Adult Cohort, the average objective sleep duration is about 6 hours independent of whether sleep is recorded at home (SHHS), for three consecutive nights with actigraphy (CARDIA) or in the sleep laboratory (Penn State Adult Cohort). Third, we did not use standard diagnostic criteria to define insomnia. Four lines of evidence support the validity of our definitions of insomnia and poor sleep. First, all of those that identified themselves as chronic insomniacs reported one or more of the four nighttime symptoms used in the diagnosis of the disorder (i.e., difficulty falling asleep, difficulty staying asleep, early morning awakening, or non-restorative sleep). Thus, a group of chronic insomniacs without sleep difficulties, which would indicate misclassification, does not exist. Second, the prevalence estimates in the Penn State Cohort using these definitions are similar to those of other population-based studies where insomnia syndrome and insomnia symptoms are defined as two mutually exclusive groups.7, 29 Third, in our previous studies, chronic insomnia was strongly associated with increased risk of hypertension, diabetes, and neurocognitive impairment and mortality, whereas the association of poor sleep was much smaller or non-significant.30 Fourth, in our and others’ previous studies chronic insomnia/insomnia syndrome was associated with a persistent course, 31,32 whereas poor sleep was more likely to remit and only about 1 out 5 poor sleepers developed chronic insomnia.21,32 These findings give further support to the face, construct, and predictive validity of the definitions used. Fourth, the lack of sleep duration data at follow-up precludes any analyses on the trajectory and longitudinal course of sleep duration, which is expected to decline with age. Nevertheless, the consistency of our findings with those of two previous studies using objective sleep measures in middle-aged samples, i.e., actigraphy,5,15 increases our confidence about the replicability and generalizability of the present findings. Future studies should examine the association between sleep duration, sleep complaints, emotional stress, and obesity or weight gain using multiple PSG recordings and time points.
In summary, subjective short sleep duration in young and middle-aged non-obese individuals at risk of developing obesity is a surrogate marker of emotional stress and sleep complaints. Given the high prevalence of poor sleep in the general population and the association of emotional stress with obesity, their detection and appropriate management should become the target of public health policy and clinical trials.
ACKNOWLEDGEMENTS
The work was performed at the Sleep Research & Treatment Center of the Penn State University Milton S. Hershey Medical Center and the staff (C. Criley and P. Cain) is especially commended for their efforts.
This research was in part funded by the National Institutes of Health grants R01 51931, R01 40916 and R01 64415.
Footnotes
CONFLICT OF INTEREST All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity in the United States, data from the National Health and Nutrition Examination Survey, 2009–2010. United States Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics. NCHS Data Brief. 2012;82:1–8. [Google Scholar]
- 2.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Med Clin North Am. 2011;95:919–937. doi: 10.1016/j.mcna.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen LS, Danielsen KV, Sørensen TI. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes Rev. 2011;12:78–92. doi: 10.1111/j.1467-789X.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 4.Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: a systematic review. Sleep Med Rev. 2012;16:231–241. doi: 10.1016/j.smrv.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Lin HM, Papaliaga M, Calhoun S, Vela-Bueno A, Chrousos GP, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 2008;32:801–809. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 7.Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Insomnia in Central Pennsylvania. J Psychosom Res. 2002;53:589–592. doi: 10.1016/s0022-3999(02)00450-6. [DOI] [PubMed] [Google Scholar]
- 8.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 9.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 10.Waksberg J. Sampling methods for random digit dialing. J Am Stat Assoc. 1978;73:40–46. [Google Scholar]
- 11.Kish L. Survey sampling. John Wiley & Sons, Inc; New York: 1965. [Google Scholar]
- 12.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. National Institutes of Health; Bethesda, MD: 1968. [Google Scholar]
- 13.Butcher JN, Graham JR, Ben-Porath YS, Tellegen A, Dahkstrom WG. MMPI-2: Manual for administration, scoring and interpretation. Revised edition University of Minnesota Press; Minneapolis, MN: 2001. [Google Scholar]
- 14.U.S. Department of Health and Human Services (DHHS), National Center for Health Statistics . Third National Health and Nutrition Examination Survey, 1988-1994. NHANES III laboratory data file. Centers for Disease Control and Prevention; Hyattsville, MD: 1996. [Google Scholar]
- 15.Appelhans BM, Janssen I, Cursio JF, Matthews KA, Hall M, Gold EB, Burns JW, Kravitz HM. Sleep Duration and Weight Change in Midlife Women: The SWAN Sleep Study. Obesity (Silver Spring) 2013;21:77–84. doi: 10.1002/oby.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Tsigos C, Bixler EO, Stratakis CA, Zachman K, Kales A, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet MH, Arand DL. Insomnia, metabolic rate and sleep restoration. J Intern Med. 2003;254:23–31. doi: 10.1046/j.1365-2796.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 20.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, Liao D, Calhoun S, Shaffer ML, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13:346–353. doi: 10.1016/j.sleep.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Mendoza J, Vgontzas AN, Bixler EO, Singareddy R, Shaffer ML, Calhoun SL, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35:689–697. doi: 10.5665/sleep.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 23.Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: A new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–1337. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 25.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–4515. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 26.Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea--a review. Sleep. 2012;35:605–615. doi: 10.5665/sleep.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong CW, O’Driscoll DM, Truby H, Naughton MT, Hamilton GS. The reciprocal interaction between obesity and obstructive sleep apnoea. Sleep Med Rev. 2012 Jul 17; doi: 10.1016/j.smrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5–7. doi: 10.1016/j.sleep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morin CM, Bélanger L, LeBlanc M, Ivers H, Savard J, Espie CA, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–453. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Fernandez-Mendoza J, Bixler EO, Singareddy R, Shaffer ML, Calhoun SL, et al. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep. 2012;35:61–68. doi: 10.5665/sleep.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]