Abstract
Background and PurposeCannabinoid CB2 receptors mediate immunomodulation. Here, we investigated the effects of CB2 receptor ligands on leukocyte-endothelial adhesion and inflammatory mediator release in experimental endotoxin-induced uveitis (EIU).
Experimental ApproachEIU was induced by intraocular injection of lipopolysaccharide (LPS, 20 ng·μL−1). Effects of the CB2 receptor agonist, HU308 (1.5% topical), the CB2 receptor antagonist, AM630 (2.5 mg·kg−1 i.v.), or a combination of both compounds on leukocyte-endothelial interactions were measured hourly for 6 h in rat iridial vasculature using intravital microscopy. Anti-inflammatory actions of HU308 were compared with those of clinical treatments for uveitis - dexamethasone, prednisolone and nepafenac. Transcription factors (NF-κB, AP-1) and inflammatory mediators (cytokines, chemokines and adhesion molecules) were measured in iris and ciliary body tissue.
Key ResultsLeukocyte-endothelium adherence was increased in iridial microvasculature between 4–6 h after LPS. HU308 reduced this effect after LPS injection and decreased pro-inflammatory mediators: TNF-α, IL-1β, IL-6, CCL5 and CXCL2. AM630 blocked the actions of HU-308, and increased leukocyte-endothelium adhesion. HU-308 decreased levels of the transcription factors NF-κB and AP-1, while AM630 increased levels of NF-κB. Topical treatments with dexamethasone, prednisolone or nepafenac, failed to alter leukocyte adhesion or mitigate LPS-induced increases in inflammatory mediators during the 6 h of EIU.
Conclusion and ImplicationsActivation of CB2 receptors was anti-inflammatory in a model of acute EIU and involved a reduction in NF-κB, AP-1 and inflammatory mediators. CB2 receptors may be promising drug targets for the development of novel ocular anti-inflammatory agents.
Linked ArticlesThis article is part of a themed section on Cannabinoids 2013. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-6
Keywords: cannabinoid, cannabinoid 2 receptor, uveitis, endotoxin-induced uveitis
Introduction
The endocannabinoid system is an endogenous lipid ligand signalling system consisting of two GPCRs, the cannabinoid CB1 and CB2 receptors, the endogenous ligands including N-arachidonoylethanolamine (AEA) and 2-arachidonoyl glycerol, and their respective cognate biosynthetic and degradative enzymes (Pertwee, 2005; Pacher et al., 2006; Di Marzo, 2009; receptor nomenclature follows Alexander et al., 2013). CB1 receptors are found throughout the nervous system and in the periphery and their activation modulates presynaptic release of neurotransmitters (Scotter et al., 2010). The CB2 receptors are highly localized on immune cells and their stimulation with either endogenous or exogenous cannabinoids is associated with immunomodulatory effects (see De Petrocellis and Di Marzo, 2009; Tanasescu and Constantinescu, 2010).
Recently, several studies have reported that modulating CB2 receptors in experimental models of sepsis decreased inflammation (Lehmann et al., 2011; 2012), and deleting these receptors reduced survival (Tschop et al., 2009). Attenuation of the inflammatory response by activation of CB2 receptors has also been reported in the brain. In a study of pial vessels forming the blood–brain barrier, using a model of LPS-induced encephalitis (Ramirez et al., 2012), activation of CB2 receptors decreased adhesion molecules in the brain tissue and leukocyte-endothelial adhesion in the pial vessels.
To date, the involvement of CB2 receptors in immune responses in the eye has not been extensively examined. However, activation of CB2 receptors had anti-inflammatory effects in the retina in a chronic experimental model of autoimmune uveoretinitis, and was associated with inhibition of leukocyte trafficking in vivo and reduction of inflammatory mediators in vitro (Xu et al., 2007). The immunomodulatory actions of CB2 receptors have not been investigated in the anterior chamber of the eye and, while these receptors have been found in porcine trabecular meshwork tissue (Zhong et al., 2005), other studies have not detected their expression in anterior ocular tissues (Straiker et al., 1999). Furthermore, in contrast to the CB1 receptors, there are conflicting reports on the role of drugs activating CB2 receptors in the eye, including their effects on intraocular pressure and aqueous humour outflow (Zhong et al., 2005; Szczesniak et al., 2011; see Tomida et al., 2004).
Here, we have examined the effects of modulating the CB2 receptors in the anterior iridial microvasculature in an experimental model of endotoxin-induced uveitis (EIU). This model is a widely used animal model of human bacterially derived uveitis, involving inflammation of the uveal tract. The uveal tract comprises the middle layer of the eye, including the iris, ciliary body and uvea. Uveitis causes upwards of 10% of vision loss globally accounting for a prevalence of 38 to 370 per 100 000 individuals depending on genetic, geographic and environmental variables (Nussenblatt, 1990; Chang and Wakefield, 2002). Current treatments for uveitis include topical, periocular or systemic administration of corticosteroids, or non-steroidal anti-inflammatory drugs (NSAIDs). Despite these available treatments, some patients become refractory to chronic use of these drugs, and several of the drugs can cause significant side effects, including decreases in wound healing, corneal epithelium toxicity, increased intraocular pressure, cataracts and glaucoma (Rosenbaum et al., 1980; Cheng et al., 1995; Raizman, 1996; Giuliano, 2004; Comstock and Decory, 2012). Therefore, identification of novel anti-inflammatory therapeutic agents with fewer side effects for the treatment of uveitis is needed.
The model of EIU can be induced via systemic or intraocular injection of LPS (Rosenbaum et al., 1980; Caspi, 2006; Xie et al., 2010). The inflammatory response induced by LPS occurs through its interaction with Toll-like receptor 4 (TLR4) on leukocytes and endothelium. Stimulation of TLR4 activates cellular inflammatory pathways, including those including the transcription factors NF-κB and AP-1, with resultant release of cytokines, chemokines and increased expression of adhesion molecules (Johnson and Lapadat, 2002; Akira and Takeda, 2004; Cho and Kim, 2009; Aomatsu et al., 2008; Andonegui et al., 2009). The key inflammatory mediators released during uveitis include cytokines (TNF-α, IL-1β, IL-6, IFN-γ and IL-10), chemokines (CCL5 and CXCL2) and adhesion molecules, including soluble vascular cell adhesion molecule (sVCAM), and intracellular adhesion molecule (ICAM; Murray et al., 1990; Smith et al., 1998; Becker et al., 2001, 2000; Koizumi, 2003; Lehmann et al., 2012). The inflammatory cytokines and chemokines released during inflammation are critical in the activation and recruitment of leukocytes. Adhesion molecules are an essential component for leukocyte-endothelial interactions at the inflamed site for leukocyte tethering, rolling and adhesion to the endothelium (Becker et al., 2001). Pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, are released from macrophages, monocytes, fibroblasts and endothelial cells, and are increased during uveitis (Murray et al., 1990; De Vos et al., 1994; Olszewski et al., 2007). The anti-inflammatory cytokine, IL-10, is produced by monocytes during acute inflammation and probably contributes to the resolution of ocular inflammation (Caspi, 1999). The chemokines CCL5 and CXCL2 are secreted by endothelial cells (Läubli et al., 2009) and macrophages (Kawamura et al., 2012) respectively. Once released, these chemokines attract immune cells to the site of inflammation. CCL5 attracts mononuclear cells (Läubli et al., 2009), while CXCL2 is a chemoattractant for neutrophils and macrophages (Kawamura et al., 2012). Additionally, the adhesion molecules, ICAM-1 and VCAM-1, are up-regulated on endothelial cells during ocular inflammation playing a vital role for the adhesion of leukocytes to the endothelium (Silverman et al., 2001). Although leukocyte activation and recruitment are important components of the immune response, allowing for wound healing and pathogen clearance, an escalation of the initial inflammatory response in the vasculature of the eye leads to pathology and vision loss (Bellingan, 2000).
Our study utilized in vivo real-time non-invasive imaging and ex vivo analysis of inflammatory mediators in an acute model of EIU generated by intraocular LPS injection. We examined the immunomodulatory effects of both an agonist and an antagonist of the CB2 receptor and compared these with clinically effective immunosuppressive agents (Becker et al., 2000; Gaynes and Onyekwuluje, 2008; Siddique and Shah, 2011; LeHoang, 2012). Our results provide novel data demonstrating that activation of CB2 receptors is anti-inflammatory in the eye. The immunosuppressive actions of topically applied agonists of CB2 receptors in this acute EIU model had a rapid onset, were more efficacious than clinically relevant topical steroid and NSAID agents, and were mediated by a decrease in the transcription factors NF-κB and AP-1 with resultant reduction in several key pro-inflammatory mediators.
Methods
Animals
All animal care and experimental procedures complied with the Canadian Council for Animal Care guidelines (http://www.ccac.ca/) and were approved by the Dalhousie University Committee on Laboratory Animals. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010; http://www.nc3rs.org.uk/). A total of 100 animals were used in the experiments described here.
Male Lewis rats (250–400 g; Charles River Laboratories International Inc., Wilmington, MA, USA) were kept on a light/dark cycle (07:00–19:00), and fed ad libitum. Animals were anaesthetized by i.p. injection of sodium pentobarbital (65 mg·kg−1; Ceva Sante Animale, Montreal, QC, Canada). Depth of anaesthesia was assessed by toe-pinch every 15 min. The femoral vein and artery were catheterized using Intramedic non-radiopaque polyethylene tubing (PE 50; Clay Adams, Sparks, MD, USA). Both catheters were attached to intravenous (i.v.) infusion pumps (Lifecare 5000 Plum Infusion Pump; Abbott, IL, USA) in conjunction with the Marquette Eagle 4000 Patient Monitor (General Electric, North Bergen, NJ, USA). Haemodynamic variables, including heart rate and mean arterial pressure were measured from the arterial catheter every 15 min. The venous catheter was used for the administration of drug, anaesthetic agents and fluorochromes. Animals were killed, under anaesthesia, by i.v. injection of KCl (0.142 mL·kg−1).
Endotoxin-induced uveitis (EIU)
Intravitreal injection of either LPS or saline was given into the left eye, through the pars plana, under a WILD M37 dissecting microscope (Leitz Canada, Kitchener, ON, Canada) using a Hamilton syringe (Hamilton Company, Reno, NV, USA) fitted to a 30G needle. Control animals received 5 μL of sterile saline, while experimental animals were injected with 100 ng LPS (Escherichia coli, 026:B6, Sigma-Aldrich, Oakville, ON, Canada) in 5 μL of saline. Once the injection was completed, the puncture wound was glued shut with 3M Vetbond Tissue Adhesive (3M Animal Products, St. Paul, MN, USA). This technique was modified from Becker et al., 2000. Animals were studied for the first 6 h post-injection, a time where leukocyte adhesion and extravasation was occurring with no overt changes in gross tissue morphology (Supporting Information Fig. S1).
Intravital microscopy
The iridial microcirculation was observed with the Olympus OV 100 Small Animal Imaging System (ON, Canada). The OV100 contains an MT-20 light source and a DP70 CCD camera (Suetsugu et al., 2011). Fluorescence excitation was generated by a xenon lamp (150W), and eight position excitation filters to block for rhodamine-6G (excitation 515–560 nm, emission 590 nm) and FITC (450–490 nm, emission 520 nm). Images were captured by a black and white DP70 CCD C-mount camera in real time and recorded in a digital format by the software Wassabi (Hamatsu, Herrsching, Germany) on a computer connected to the OV100. Rats were placed in a stereotactic frame (Kopf, CA, USA), and Tear-Gel (Novartis Pharmaceuticals Canada Inc., Dorval, QC, Canada) was placed on the cornea to prevent dehydration of the tissue throughout the experiment. Rhodamine-6G (1.5 mL·kg−1; Sigma-Aldrich) and FITC-albumin (1 mL·kg−1; Sigma-Aldrich) were injected i.v. 15 min before initiating intravital microscopy (IVM). The fluorescent dye rhodamine-6G labelled mitochondria in leukocytes within the vessel and FITC-albumin allowed for the visualization of blood flow through the vasculature. Animals were placed on a heating pad to maintain body temperature at 37°C. IVM was carried out in four regions of interest. In each region of interest, four randomly selected vessels were observed, and 30 s recordings were made per hour of each vessel for the duration of the experiment. For IVM studies, data were analysed off-line without knowledge of the treatment groups. Recordings of the iridial microcirculation were analysed using the imaging software ImageJ (National Institute of Health, USA). The length, measured between points of branching and diameter of the vessels were used to calculate the volume within the vessel. Adhering leukocytes were defined as white blood cells that were attached to the vessel wall and were immobile for a 30 s observation period; adhering cells were reported as the number of cells per square millimetre of endothelial surface.
Eyes were enucleated, bi-dissected at the corneal-scleral junction to obtain anterior uveal tissue and flash frozen in liquid nitrogen and stored at –80°C for subsequent analyses of inflammatory mediators (cytokines, chemokines and adhesion molecules) or fixed in 4% paraformaldehyde for haematoxylin and eosin (H&E) staining and light microscopy.
Measurement of inflammatory markers
Frozen tissue was homogenized in 100 μL of PBS and 1% BSA, supplemented with a protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Homogenates were centrifuged at 13 000× g for 15 min at 4°C, and supernatant was collected. The Bio-Rad Protein Assay (Mississauga, ON) was used to determine protein concentration according to the manufacturer's instructions. Protein was diluted to a concentration of 3620 μg·mL−1 in PBS then diluted 1:1 in rat diluent provided within the Procarta Multiplex Cytokine Assay Kit (Freemont, CA, USA). Samples were analysed using a Procarta Multiplex Cytokine Assay kit from Affymetrix and the Bio-Rad 200 instrument with Bio-Plex software according to the manufacturer's instructions. Samples were run in duplicate and tested for levels of TNF-α, IL-1β, IL-6, IL-10, INF-γ, CCL5, CXCL2, sVCAM and ICAM. Standard curves for each cytokine were generated using the reference cytokine concentrations supplied with the kit.
Histology
Following fixation, eyes were immersed in 30% sucrose for 24–72 h at 4°C and the lens removed. Whole eyes were then frozen in OCT compound and sectioned (10 μm) using a Leica CM1950 cryostat (Leica Microsystems Inc., Concord, ON, Canada). Tissue sections were cut at 10 μm, mounted onto slides and stained with H&E. Stained sections were visualized using light microscopy (Supporting Information Fig. S1).
qRT-PCR
RNA was harvested from supernatant from homogenized tissue in PBS and protease inhibitor cocktail (Sigma) using the Trizol® (Invitrogen; Life Technologies Inc., Burlington, ON, Canada) extraction method according to the manufacturer's instructions. Reverse transcription reactions were carried out with SuperScript III® reverse transcriptase (+RT; Invitrogen), or without (−RT) as a negative control for use in subsequent PCR experiments according to the manufacturer's instructions. Two micrograms of RNA were used per RT reaction. qRT-PCR was conducted using the LightCycler® system and software (version 3.0; Roche, Laval, QC, Canada). Reactions were composed of a primer-specific concentration of MgCL2 (Supporting Information Table S2), 0.5 μM each of forward and reverse primers (Supporting Information Table S2), 2 μL of LightCycler FastStart Reaction Mix SYBR Green I, and 1 μL cDNA to a final volume of 20 μL with distilled water (Roche). The PCR program was 95°C for 10 min, 50 cycles of 95°C 10 s, a primer-specific annealing temperature for 5 s, and 72°C for 10 s (Supporting Information Table S2). Experiments included sample-matched RT controls, a no-sample distilled water control and a standard control containing product-specific cDNA of a known concentration. cDNA abundance was calculated by comparing the cycle number at which a sample entered the logarithmic phase of amplification to a standard curve generated by amplification of cDNA samples of known concentration (LightCycler Software version 4.1; Roche). Here qRT-PCR data were normalized to the expression of hypoxanthine-guanine phosphoribosyltransferase (HPRT).
Data analysis
Individual animals in each of the treatment groups were coded and derived data analysed without knowledge of the treatment groups. All data are presented as means ± SEM and were analysed with the statistical software GraphPad Prism v.5 (GraphPad Software Inc., San Diego, CA, USA). Haemodynamic variables were analysed by two-way anova and Bonferroni post hoc testing after confirmation of normal distribution according to Kolmogorov–Smirnov. IVM data and inflammatory mediators were analysed by one-way anova with Dunnett's post hoc test, comparing all experimental groups to the LPS-treated group. qRT-PCR data were analysed by one-way anova with Tukey's test. P < 0.05 was considered statistically significant.
Materials
Briefly, rats were divided into 11 groups (n = 7–15). Saline solution (0.9% sodium chloride) was obtained from Hospira (Montreal, QC, Canada). The CB2 receptor agonist, HU308 (4-[4-(1,1-dimethylheptyl)-2,6-dimethoxyphenyl]-6,6-dimethylbicyclo[3.1.1]hept-2-ene-2-methanol), was obtained from Cayman Chemical (Ann Arbor, MI, USA) and dissolved in Tocrisolve™ 100 (Tocris Bioscience, Ellisville, MO, USA) for topical application at 1.5% (w v(1). The CB2 receptor antagonist, AM630 (6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone) was obtained from Tocris Bioscience and stock solutions prepared in DMSO (Sigma-Aldrich Canada Ltd) and administered i.v. at 2.5 mg·kg(1 in a 1:1 dilution with saline. In addition, immunosuppressive and anti-inflammatory pharmacological agents at doses currently used in the treatment of ocular inflammation, including Maxidex™ (0.1% dexamethasone, Alcon, Fort Worth, TX, USA), Nevanac™ (0.1% nepafenac, Alcon) and Pred Forte™ (1% prednisolone, Allergan, Irvine, CA, USA), were also tested, and their effects were compared with CB receptor ligands. All drugs were given as 5 μL of topical ophthalmic drops, except AM630, which was given i.v over a 15 min period. All topical drugs were administered 15 min after the intravitreal injection. BP and heart rate were monitored throughout the experimental time course using a Marquette Eagle 4000 Patient Monitor (General Electric) and did not significantly differ in any of the treatment groups (data shown in Supporting Information Table S1A and S1B).
Results
Leukocyte-endothelial interactions in the iridial microcirculation
Figure 1A–B shows representative IVM images of the iridial microvasculature at 6 h from control eyes (Figure 1A), and eyes from animals with EIU following an intravitreal injection of 100 ng of LPS in 5 μL of saline (Figure 1B). The histogram in Figure 1C shows that the number of firmly adhering leukocytes in the microvasculature was significantly increased at 4, 5 and 6 h, in comparison to the control group at the same time-points. There was no significant difference between leukocyte adhesion in animals challenged with LPS alone or LPS plus the topical vehicle, Tocrisolve®, or LPS plus i.v. DMSO, or LPS plus both i.v. DMSO and topical Tocrisolve at 6 h.
Figure 1.
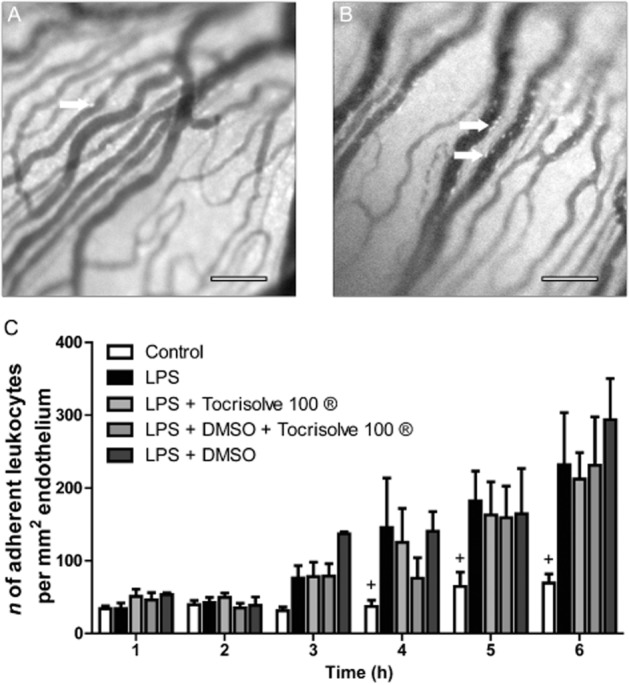
(A and B) Representative intravital microscopy images of iridial microcirculation in rat eye showing adherent leukocytes at 6 h after intravitreal injection of (A) saline and (B) LPS. Arrows indicate adherent leukocytes. Scale bar = 100 μm. (C) Bar graph represents the time course for the mean number of adherent leukocytes in the following groups: control (n = 11); LPS (n = 15); LPS + the following vehicles (n = 6 for each vehicle group) including Tocrisolve100® (topical); DMSO (i.v.); Tocrisolve100 + DMSO. Values represent mean ± SEM. + P < 0.05 compared with the LPS group.
Figure 2A–D shows representative images of the iridial microcirculation obtained in LPS-treated (100 ng) eyes and eyes treated with LPS and the following treatments: the CB2 receptor agonist, HU308 (1.5%), the CB2 receptor antagonist, AM630 (2.5 mg·kg−1) and AM630 + HU308. The doses of cannabinoid drugs were based on route of delivery, and the dose-response for inhibition of leukocytes adhering to the endothelium (Supporting Information Fig. S2). For topical HU308, a dose of 1.5% was chosen based on the IC50 (0.8%) and Emax (1.5%) for inhibition of leukocytes adhering to the endothelium in EIU. This agonist, is highly selective for CB2 receptors with a Ki of >10 μM for CB1 receptors and a Ki of 22.7 ± 3.9 nM for CB2 receptors (Hanus et al., 1999; Rajesh et al., 2007). Antagonist dose was based on previously published studies (Szczesniak et al., 2011; Lehmann et al., 2012). Topical application of the CB2 receptor agonist HU308 (1.5%) significantly attenuated the number of leukocytes adhering to the endothelium at 4, 5 and 6 h, compared with LPS alone (Figure 2E). Treatment with the CB2 receptor antagonist AM630 (2.5 mg·kg−1, i.v.) significantly exacerbated leukocyte adhesion to the endothelium at 4, 5 and 6 h, compared with LPS alone. Figure 2 also shows that no significant difference was found in leukocyte-endothelial adhesion at 6 h between LPS-injected eyes and in LPS-injected eyes of animals treated with AM630 + HU308.
Figure 2.
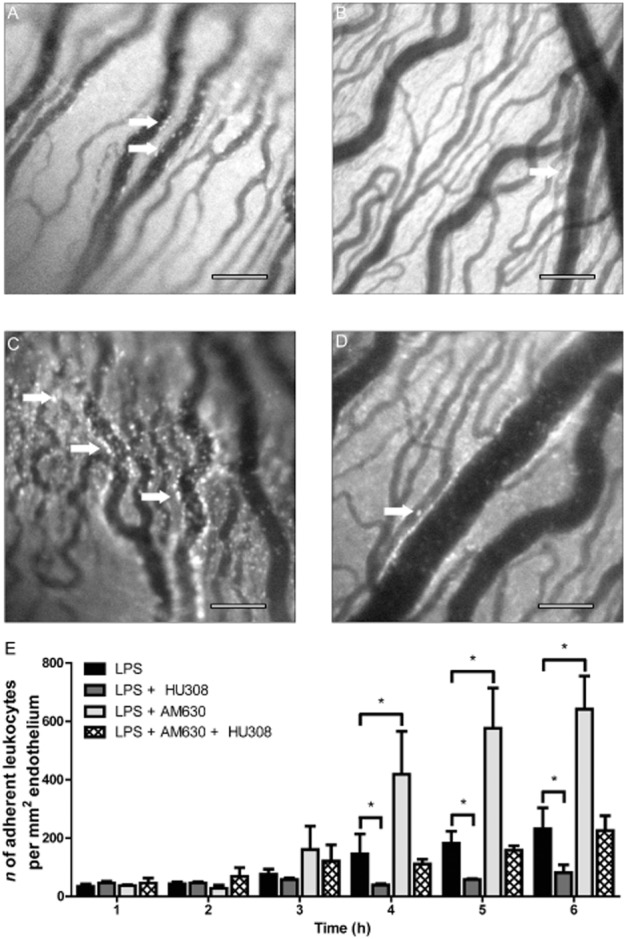
(A–D) Representative intravital microscopy images of iridial microcirculation in rat eyes at 6 h after intravitreal LPS injection in the following groups: (A) LPS injection only, (B) LPS + CB2 receptor agonist, HU308 (1.5%, topical), (C) LPS + CB2 receptor antagonist, AM630 (2.5 mg·kg−1, i.v.) and (D) LPS + AM630+ HU308. Arrows indicate adherent leukocytes. Scale bar = 100 μm. (E) Bar graph represents the time course for the mean number of adherent leukocytes in for the above groups including: LPS (n = 15), LPS + HU308 (n = 12), AM630 (n = 8) and AM630 + HU308 (n = 7). Values represent mean ± SEM. *P < 0.05 compared with the LPS group.
Effects of cannabinoids on ocular inflammatory mediators
In order to determine the potential mechanisms contributing to the anti-inflammatory effects following activation of CB2 receptors, measurements of pro-inflammatory and anti-inflammatory cytokines released following immune cell recruitment and activation were obtained from anterior ocular tissue (iris and ciliary body), in the absence and presence of cannabinoid treatments (Table 1). The most applicable inflammatory molecules were chosen for measurement based on animal and clinical studies of uveitis and LPS-induced inflammation.
Table 1.
Levels of inflammatory mediators (pg·mL−1) in extracts of the iris and ciliary body
| Control | LPS | LPS + HU308 | LPS + AM630 | LPS + AM630 + HU308 | |
|---|---|---|---|---|---|
| TNF-α | 5.9 ± 4.1* | 52.6 ± 18.12+ | 0.82 ± 0.6* | 16.8 ± 7.1* | 5.9 ± 4.2* |
| IL-1β | 264.2 ± 71.4* | 5572 ± 1076+ | 603 ± 214* | 3564 ± 1453+ | 550 ± 137* |
| IL-6 | 8.9 ± 2.2* | 32.9 ± 5.1+ | 7.5 ± 2.3* | 16.5 ± 3.7* | 7.2 ± 2.3* |
| IL-10 | 11.1 ± 1.8 | 18 ± 1.5 | 6.9 ± 1.3* | 14.2 ± 3.0 | 12.9 ± 2.7 |
| IFN-γ | 1.3 ± 0.4 | 2.5 ± 3.8 | 0.8 ± 0.4* | 1.4 ± 0.4 | 1.2 ± 0.6 |
| CCL5 | 53.2 ± 17.9* | 289 ± 49+ | 66.2 ± 17.1* | 192 ± 57.3+ | 51.1 ± 13.2* |
| CXCL2 | 101 ± 34.6* | 2023 ± 418+ | 213 ± 58.7* | 852 ± 362* | 288 ± 102* |
| sVCAM | 214 ± 34.3 | 363 ± 50.5 | 191 ± 27.1 | 360 ± 82 | 244 ± 43.4 |
| ICAM | 1304 ± 234 | 2118 ± 368 | 1208 ± 116 | 2333 ± 557 | 1589 ± 271 |
Data shown (mean ± SEM) are from control, LPS and LPS with treatments with CB receptor ligands: CB2 receptor agonist, HU308, CB2 receptor antagonist AM630 and combination of AM630 + HU308, n = 8–11,P < 0.05 to control, *P < 0.05 compared with LPS.
Induction of EIU by intravitreal LPS injection significantly increased inflammatory mediators, TNF-α, IL-1β, IL-6, CCL5 and CXCL2 in the anterior uveal tissues of the rat eye. Topical application of HU308, resulted in a significant decrease in cytokines, TNF-α, IL-1β, IL-6, IL-10, IFN-γ, as well as chemokines, CCL5 and CXCL2, but had no effect on adhesion molecule levels. Treatment with AM630 alone reduced LPS-induced inflammatory mediators, decreasing levels of TNF-α, IL-10 and CXCL2, compared with untreated EIU animals. A combination of both AM630 and HU308, decreased the release of pro-inflammatory cytokines, TNF-α, IL-1β, IL-6 and the chemokines CXCL2 and CXC5 but failed to attenuate the levels of adhesion molecules measured.
Effects of CB receptor ligands on mRNA for NF-κβ and AP-1 during ocular inflammation
The LPS pro-inflammatory response is mediated by TLR4, which in turn can either directly (Akira and Takeda, 2004) or indirectly (Johnson and Lapadat, 2002) activate the transcription factors NF-κB and activator protein-1 (AP-1). This results in increased inflammatory mediators, cytokines, chemokines and adhesion molecules (Cho and Kim, 2009).
Figure 3A shows that an intravitreal injection of LPS significantly increased mRNA levels of NF-κB, compared with those in the saline-injected control group. Topical application of HU308 decreased mRNA levels of NF-κB, compared with those in the group treated with LPS. In contrast, treatment with AM630 significantly increased mRNA levels of NF-κB, compared to LPS-treated animals. Combination treatment with AM630 and HU308, however, significantly decreased mRNA levels of NF-κB, compared with LPS-treated animals.
Figure 3.
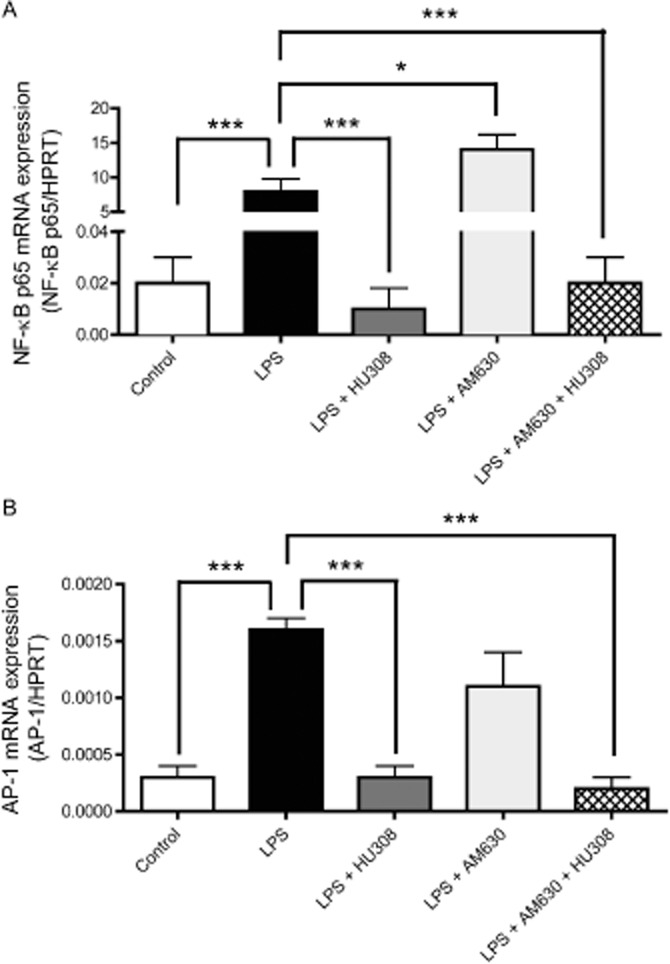
(A and B) mRNA expression levels of inflammatory transcription factors: (A) NF-κB and (B) AP-1 in the uveal tissues, including the iris and ciliary body, at 6 h in the following treatment groups: control (n = 9), LPS (n = 6), LPS + HU308 (n = 13), LPS + AM630 (n = 10) and LPS + AM630 + HU308 (n = 10). Values (mean ± SEM) are reported compared with the housekeeping gene HPRT. *P < 0.05, ***P < 0.001; compared with the EIU group.
Figure 3B shows that ocular inflammation induced by LPS increased mRNA levels of the transcription factor AP-1 in comparison to the control group. The levels of AP-1mRNA were significantly decreased in HU-308-treated animals in comparison to LPS-only. No significant difference was found in mRNA levels of AP-1 in the LPS-only group and the group treated with AM630. Combination treatment with both AM630 and HU308 decreased AP-1 mRNA levels in LPS-treated animals eight-fold, compared with the LPS-only treated group.
Effects of clinically used immunosuppressive drugs in EIU
Leukocyte-endothelial interactions in the iridial microcirculation were also examined in EIU following treatment with topical dexamethasone, prednisolone and nepafenac. These drugs are used clinically at 0.1–1%, respectively, to treat uveitis (Cheng et al., 1995; Gaynes and Onyekwuluje, 2008). Figure 4A–D shows representative images of the iridial microcirculation and leukocytes within the vasculature following LPS injection in untreated eyes (Figure 4A), following treatment with 0.1% dexamethasone (Figure 4B), 1% prednisolone (Figure 4C) or 0.1% nepafenac (Figure 4D). Figure 4E shows the mean number of adherent leukocytes per mm2 of endothelium over the 6 h time course in EIU in untreated eyes, and following either corticosteroid or NSAID treatments. In this acute model of EIU, all three of these drugs at doses used clinically, or previously reported to reduce inflammation in experimental models (Medeiros et al., 2008; Hariprasad et al., 2009; Kanai et al., 2012), failed to reduce leukocyte adhesion in the iridial vasculature within the 6 h observation period following LPS, compared with that in the LPS-only group.
Figure 4.
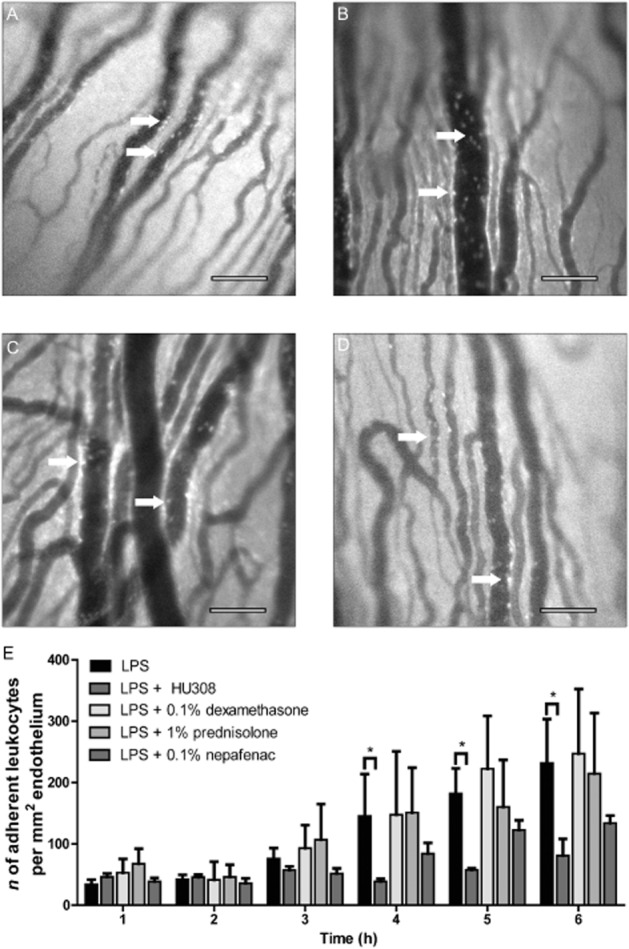
(A–D) Representative intravital microscopy images of iridial microcirculation in rat eyes at 6 h after intravitreal LPS injection in the following groups: (A) LPS injection only (n = 15), (B) LPS + dexamethasone (0.1% topical), (C) prednisolone (1% topical) and (D) nepafenac (0.1% topical). Arrows indicate adherent leukocytes. Scale bar = 100 μm. (E) Bar graph represents the time course for the mean number of adherent leukocytes in the above treatment groups (n = 9 for all groups). Values represent mean ± SEM. *P < 0.05; compared with the LPS group.
Examination of levels of inflammatory mediators after topical application of 0.1% dexamethasone, 1% prednisolone and 0.1% nepafenac in the EIU model (Table 2) was consistent with intravital measurements of leukocyte-endothelial adhesion, where there was no significant change in the number leukocyte adhesion to the endothelium as compared with LPS. With the exception of prednisolone, which significantly decreased the levels of IL-1β at 6 h, the steroids, dexamethasone or prednisolone and the NSAID, nepafenac, did not significantly reduce the concentrations of inflammatory mediators measured in the iris and ciliary body tissue, compared with those in untreated LPS-only animals (Table 2).
Table 2.
Levels of inflammatory mediators (pg·mL−1) in extracts of the iris and ciliary body
| LPS | LPS + dexamethasone | LPS + prednisolone | LPS + nepafenac | |
|---|---|---|---|---|
| TNF-α | 52.6 ± 18.1+ | 31.6 ± 21.3 | 24.7 ± 13.1 | 15.4 ± 6.7 |
| IL-1β | 5572 ± 1076+ | 2639 ± 798 | 2381 ± 1203* | 2888 ± 947 |
| IL-6 | 32.9 ± 5.1+ | 29.7 ± 9.9+ | 24.8 ± 5.6 | 14.5 ± 5 |
| IL-10 | 18 ± 1.5 | 16.53 ± 2.7 | 16.5 ± 6.2 | 13.5 ± 2.0 |
| IFN-γ | 2.5 ± 3.8 | 1.3 ± 0.5 | 2.1 ± 0.9 | 2.2 ± 0.8 |
| CCL5 | 289 ± 49+ | 196 ± 57 | 143 ± 49 | 277 ± 69+ |
| CXCL2 | 2023 ± 418+ | 1994 ± 852+ | 1199 ± 365 | 1340 ± 531 |
| sVCAM | 363 ± 50.5 | 410 ± 81.6 | 390 ± 142 | 381 ± 41.2 |
| ICAM | 2118 ± 368 | 2174 ± 352 | 2676 ± 926 | 2616 ± 393 |
Data shown (mean ± SEM) are from control, LPS and LPS with dexamethasone, prednisolone or nepafenac treatments for uveitis, n = 7–11,P < 0.05 to control, *P < 0.05 compared with LPS.
Discussion
Our results provide novel findings demonstrating that activation of ocular CB2 receptors in a model of EIU, by the synthetic cannabinoid agonist, HU-308, attenuated leukocyte-endothelial adhesion in the iridial microvasculature and reduced the release of pro-inflammatory mediators (TNF-α, IL-1β, IL-6, INF-γ, CCL5 and CXCL2) and the transcription factors (NF-κβ and AP-1), which mediate the transcription of pro-inflammatory genes (Johnson and Lapadat, 2002; Akira and Takeda, 2004). Furthermore, this is the first time that a topical cannabinoid treatment has been shown to be anti-inflammatory in an experimental model of acute ocular inflammation.
Immunomodulatory effects of CB2 receptor activation
CB2 receptors are up-regulated following inflammation in several different tissues (including ileum, pancreas and throughout the CNS) and the consequent increase in CB2 receptor signalling may play a role in the physiological and homeostatic mechanisms evoked to resolve injury (D'Argenio et al., 2006; Cabral et al., 2008; Michler et al., 2013). To date, several studies have suggested that activation of CB2 receptors is anti-inflammatory in the vasculature of different tissues, such as intestinal, pial and retinal vessels (Xu et al., 2007; Lehmann et al., 2012; Ramirez et al., 2012). Our results are supportive of these findings, and further show that the decrease in leukocyte-endothelial adhesion seen in the iridial microvasculature following topical application of the CB2 receptor agonist, HU308, is mediated via CB2 receptors. Blockade of this receptor by its antagonist, AM630, prevented the anti-inflammatory effects of HU308. Our data are in agreement with earlier findings that systemic treatment with a CB2 receptor antagonist, SR144528, in combination with a CB2 receptor agonist, JWH133 prevented the anti-inflammatory actions of CB2 receptor activation in pial vessels during LPS-induced inflammation in an encephalitis model (Ramirez et al., 2012). However, we found, in EIU, that application of AM630 actually increased the number of adherent leukocytes compared to the LPS-only group. The increase in the number of adhering leukocytes observed when AM630 was given during EIU suggests endogenous activation of CB2 receptors. This increase in adherent leukocytes may result from several factors, including up-regulation of CB2 receptors and increased production of endogenous endocannabinoids and metabolites during inflammation (D'Argenio et al., 2006; Cabral et al., 2008; Michler et al., 2013). The increased production of AEA during inflammation could be anti-inflammatory, given that AEA can attenuate inflammatory mediators released by macrophages, including IL-6, NOS and COX-2 (Chang et al., 2001). Therefore, the potentiation of leukocyte-endothelium adhesion by AM630 in EIU could have been due to the blockade of the anti-inflammatory effects of AEA on CB2 receptors. Additionally, increased production of AEA metabolites during inflammation, including N-arachidonoyl glycine can activate the pro-migratory lipid receptor, GPR18 (McHugh et al., 2010). N-arachidonoyl glycine can also induce the expression of COX-2, which then metabolizes N-arachidonoyl glycine to pro-inflammatory PGs (Prusakiewicz et al., 2002; Mestre et al., 2006; Lowin et al., 2012), which could result in enhanced leukocyte-endothelial interactions in the presence of CB2 receptor block by AM630.
Cannabinoid modulation of inflammatory mediators
Leukocyte-endothelial interactions depend on inflammatory mediators that are released during inflammation. As previously reported for other studies of ocular inflammation, we measured an increase in multiple inflammatory mediators during the induction of EIU (Murray et al., 1990; De Vos et al., 1994; Olszewski et al., 2007). These included TNF-α, IL-1β, IL-6, CCL5 and CXCL2. Activation of CB2 receptors by HU308 in EIU produced a decrease in several key pro-inflammatory cytokines including, TNF-α, IL-1β and IL-6. This finding is in line with previous in vivo and in vitro studies carried out in different tissues and cell types, including the intestine (Lehmann et al., 2012), pial vasculature (Ramirez et al., 2012) and isolated macrophages and dendritic cells (Selvi et al., 2008; Greineisen and Turner, 2010). A decrease in the pro-inflammatory cytokine TNF-α would limit leukocyte infiltration by halting the up-regulation of adhesion molecules and macrophage activation. A reduction in IL-1β would reduce infiltration of polymorphonuclear cells and consequent breakdown of the blood-retinal barrier and decreased release of IL-6 would impede macrophage activation (Ooi et al., 2006).
We also found that activation of CB2 receptors reduced levels of the chemokines CCL5 and CXCL2 in the anterior chamber of the LPS-treated eye. Such reduced chemokine levels could decrease mononuclear and neutrophil chemoattraction into the ocular tissue (Läubli et al., 2009; Kawamura et al., 2012). Our results are consistent with, earlier data from a intravital study of systemic endotoxemia in which application of HU308 suppressed elevated levels of CCL5 and CXCL2 in intestinal tissues of LPS-treated animals (Lehmann et al., 2012). Other studies have also indicated that activation of CB2 receptors decreases the levels of these chemokines thereby attenuating macrophage and neutrophil migration respectively (Raborn et al., 2008; De Filippo et al., 2013). However, in our study, activation of ocular CB2 receptors by HU308 failed to decrease the adhesion molecules ICAM and sVCAM, a finding comparable to those from other studies using CB2 receptor agonists in models of inflammation and sepsis (Rajesh et al., 2007; Lehmann et al., 2012; Ramirez et al., 2012). This difference in our results may be due to different potencies of HU308 in inhibiting adhesion molecules compared with that in decreasing leukocyte-endothelial adhesion in the eye. The doses of the agonist we chose were based on the IC50 of topical HU308 for inhibition of leukocyte-endothelial adhesion and the modulation of the levels of inflammatory mediators.
With the exception of TNF-α, IL-10 and CXCL2, treatment of animals with the CB2 receptor antagonist, AM630, did not alter the release of inflammatory mediators during EIU. A similar observation was reported by Roche et al. (2008), in which AM630 reduced circulating TNF-α in rats that had received systemic LPS. However, AM630 increased leukocyte-endothelial adhesion despite the observed decreases in TNF-α. Given that leukocyte recruitment and adhesion are dependent on many factors, including mediators measured in this study (Kumar et al., 2004; Greineisen and Turner, 2010), it would seem reasonable that a reduction in TNF-α alone might not be enough to prevent leukocyte recruitment and adhesion in this model of LPS-induced EIU.
Effects of CB2 receptor ligands on mRNA for NF-κB and AP-1 during ocular inflammation
LPS-induced increases in NF-κB are well recognised (Tak and Firestein, 2001; Akira and Takeda, 2004)and our results demonstrated that the CB2 receptor agonist, HU308, decreased LPS-induced NF-κB in EIU. These findings support a previous report by Rajesh et al. (2007) that HU308 attenuated NF-κB in a human coronary artery endothelial cell line stimulated with TNF-α. The decrease in NF-κB observed in our study following activation of CB2 receptors could account for the attenuated levels of cytokines, such as TNF-α, IL-1β, IL-6, as well as chemokines and adhesion molecules, as NF-κB is a known transcription factor for pro-inflammatory genes (Tak and Firestein, 2001). In keeping with our IVM findings, treatment with AM630 increased levels of NF-κB in EIU, when compared with LPS alone. Although this increase in NF-κB did not correlate with an increase in any of the pro-inflammatory mediators measured in this study, elevated levels of other mediators, such as COX-2, which are also associated with activation of NF-κβ may have occurred (Tak and Firestein, 2001). An increase in COX-2 metabolites, such as leukotrienes and PGs, could account for the increase in inflammation (Prusakiewicz et al., 2002; Mestre et al., 2006; Lowin et al., 2012). The combination of AM630 and HU308 significantly decreased the levels of NF-κB in comparison to LPS-only animals, which could account for the decreases of all three cytokines TNF-α, IL-1β and IL-6 (Tak and Firestein, 2001).
The mRNA for the transcription factor AP-1 was increased in EIU and this increase was reduced by HU308. LPS-induced increases in AP-1 have been previously reported (Johnson and Lapadat, 2002) and a decrease in the proteins that dimerize to form AP-1 was observed following treatment with HU308 during inflammation induced by a trail transaction in zebrafish (Liu et al., 2013). In our study, treatment with the CB2 receptor antagonist, AM630, had no effect on the levels of AP-1 mRNA in EIU and AM630 treatment did not block the effects of HU308 on AP-1 mRNA levels. As drug doses were selected based on leukocyte-endothelial adhesion and not ligand binding, it is possible that dose-dependent inhibition of HU308 effects on AP-1 might be observed with higher AM630 doses. AM630 may also activate different transcription factors which were not investigated in this study.
Taken together, our findings suggest that the anti-inflammatory actions of CB2 receptor activation in EIU involved a decrease in leukocyte-endothelial adhesion in the iridial microvasculature. Activation of CB2 receptors was associated with suppression of the levels of pro-inflammatory cytokines and chemokines important for chemotaxis of the leukocytes by a mechanism that involved modulation of the inflammatory transcription factors NF-κβ and AP-1. In other studies, CB2 receptors were robustly expressed by leukocytes and were associated with regulation of a number of leukocyte functions, including splenocyte proliferation, and B and T cell differentiation (Cencioni et al., 2010; Tanasescu and Constantinescu, 2010). CB2 receptor stimulation also inhibited the proliferation of macrophages, and the release of inflammatory mediators including, cytokines, chemokines, proteases, PGs and oxygen free radicals from these cells (Chang et al., 2001; Cabral et al., 2008; Pandey et al., 2009). CB2 receptor activation also suppressed macrophage and microglia phagocytosis (Pandey et al., 2009). Therefore, the anti-inflammatory effects of CB2 receptor activation in uveal tissue in EIU may involve CB2 receptor-mediated actions on tissue resident macrophages and dendritic cells. Macrophages and dendritic cells have been noted in non-inflamed iris and ciliary body tissue of mouse, rat, and human. These cells have also been reported to contribute to pro-inflammatory mediator release during experimental autoimmune uveoretinitis (McMenamin et al., 1994).
Immunomodulatory effects of clinical treatments
Dexamethasone, prednisolone and nepafenac are all used as treatments in inflammatory diseases such as uveitis (Hariprasad et al., 2009; Larson et al., 2011). Dexamethasone and prednisolone are corticosteroids that inhibit the inflammatory response by acting at the glucocorticoid receptor, which then binds to co-activators such as CREB binding protein and decreases the activation of transcription factors, such as NF-κB for pro-inflammatory genes (Barnes, 2006; LeHoang, 2012). Nepafenac is a non-steroidal anti-inflammatory COX 1 and COX 2 inhibitor that decreases PG synthesis (Gaynes and Onyekwuluje, 2008; Hariprasad et al., 2009). Different PG derivatives are active in the inflammatory process, and act on a variety of cells, including immune cells and endothelial cells, to increase blood flow and microvascular permeability and induce release of inflammatory cytokines (Ricciotti and FitzGerald, 2011).
In the acute model of ocular inflammation used in this study, the steroids, dexamethasone and prednisolone, and the NSAID, nepafanac, all failed to decrease leukocyte-endothelial adhesion in the iridial microvasculature during the 6 h observation period after LPS intravitreal injection. In contrast, in an experimental model of uveitis induced by systemic LPS injection, a similar dose of dexamethasone was effective in inhibiting ocular inflammation Kanai et al. (2012). There was a reduction in cellular infiltration and of protein in the aqueous humour, alon with improved histopathological grading score. These results, however, were obtained 24 h after initial LPS injection and treatments were given every 6 h for 24 h, including one dose before EIU was induced (Kanai et al., 2012). We also found the steroid prednisolone without effects on leukocyte-endothelial interactions or inflammatory mediators in our study. The dose of prednisolone used in our study (1%) was based on its relative anti-inflammatory potency and half-life in comparison to dexamethasone (LeHoang, 2012). As with dexamethasone, studies on the acute anti-inflammatory effects of prednisolone are limited. However, prednisolone, like dexamethasone, decreased cellular infiltrates in the aqueous humour, iris and ciliary body by 24 h (Medeiros et al., 2008). As with the steroid agents, nepafenac, a topical NSAID that reduced ocular inflammation in humans (Lane et al., 2007; Hariprasad et al., 2009), was also ineffective in reducing leukocyte-endothelial interactions and inflammation in our model of acute EIU. This finding was unexpected, as a previous study had reported that a single application of 0.1% topical nepafenac inhibited PG synthesis in the iris and ciliary body after paracentesis-induced ocular inflammation, although this study did not test for effects on immune cells (Gamache et al., 2000).
In conclusion, the present study has demonstrated that activation of CB2 receptor signalling attenuated leukocyte-endothelial adhesion within the iridial microvasculature in an acute model of EIU. Activation of CB2 receptor signalling was associated with a decrease in inflammatory mediators in uveal tissue including the pro-inflammatory cytokines, TNF-α, IL-1β, IL-6, INF-γ, as well as the chemokines, CCL5 and CXCL2. In contrast, antagonism of CB2 receptors resulted in an amplified inflammatory response in ocular tissue suggesting that endogenous activation of these receptors during ocular inflammation is anti-inflammatory. This study also demonstrated that CB2 receptor stimulation during acute ocular inflammation by the CB2 receptor agonist, HU308, exhibited greater anti-inflammatory effects compared with other anti-inflammatory agents including the COX inhibitor nepafenac and the corticosteroids, dexamethasone and prednisolone. While further research is required to elucidate the full spectrum of CB2 receptor-mediated anti-inflammatory effects in the eye, this study indicates that CB2 receptors should be considered as a potential target for the development of novel drugs for ocular inflammation.
Acknowledgments
The authors would like to thank Dr. George Robertson for the use of the Bio-Rad 200 instrument, Quinton Jones for his expert technical assistance and Elizabeth Cairns for her excellent editorial skills. This work was supported by grants from the Canadian Institutes of Health Research (M. E. M. K.), the Canadian Foundation for Innovation (C. L.) and the Nova Scotia Health Research Foundation (C. L.).
Glossary
- AEA
N-arachidonoylethanolamine, anandamide
- EIU
endotoxin-induced uveitis
- ICAM
intracellular adhesion molecule
- IVM
intravital microscopy
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- NSAID
non-steroidal anti-inflammatory drug
- sVCAM
soluble vascular cell adhesion molecule
- TLR
Toll-like receptor
Conflict of interest
The authors state no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site: http://dx.doi.org/10.1111/bph.12545
Representative histological cross section of the eye stained with H&E: (A) Control animal cornea, (B) LPS treated animal cornea, (C) Control animal iris, (D) LPS treated animal iris. (E) Histogram of inflammatory cells infiltrating the anterior chamber of the eye. ESS = anterior corneal epithelial stratified squamous; CS = corneal stroma; K = keratocyte; PE = posterior endothelium; AC = anterior chamber; IIC = infiltrating inflammatory cell; PC = posterior chamber. Magnification 10×. Scale bar = 100 μm.
Dose-response curve for the topical administration (0.1–1.5%) of,the CB2 receptor agonist, HU308, on leukocyte adhesion in iridial venules. Values are represented as percentage change in leukocyte adhesion to endothelium and normalised to the minimal response of control animals and maximum of those receiving only LPS. IC50 for HU308- mediated decrease in leukocyte adhesion is 0.7867%.
Haemodynamics including (A) Mean arterial pressure (mmHg) (B). Heart rate (beats per minute) in the following treatment groups: Control (n = 6), LPS (n = 4), LPS + 1.5% HU308 (n = 4), LPS + 2.5 mg·kg-1 AM630 (n = 6), LPS+ HU308 + AM630 (n = 5), LPS + 0.1% dexamethasone (n = 8), LPS + 0.1% nepafenac (n = 4). P < 0.05; compared to the LPS group. Values represent mean ± SEM.
Oligonucleotide primers used in this study. NF-κB, TNF-α; AP-1, activator protein 1; HPRT, hypoxanthineguanine phosphoribosyltransferase.
References
- Akira S, Takeda K. Toll–like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B, et al. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Invest. 2009;119:224–231. doi: 10.1172/JCI36411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aomatsu K, Kato T, Fujita H, Hato F, Oshitani N, Kamata N, et al. Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases. Immunology. 2008;123:171–180. doi: 10.1111/j.1365-2567.2007.02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 2006;148:245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MD, Nobiling R, Planck SR, Rosenbaum JT. Digital video-imaging of leukocyte migration in the iris?: intravital microscopy in a physiological model during the onset of endotoxin-induced uveitis. J Immunol Methods. 2000;240:23–37. doi: 10.1016/s0022-1759(00)00165-4. [DOI] [PubMed] [Google Scholar]
- Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42:2563–2566. [PubMed] [Google Scholar]
- Bellingan G. Leukocytes: friend or foe. Intensive Care Med. 2000;26(Suppl 1):S111–S118. doi: 10.1007/s001340051127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. Br J Pharmacol. 2008;153:240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi RR. Springer Seminars in Immunopathology Immune mechanisms in uveitis. Springer Semin Immunopathol. 1999;21:113–124. doi: 10.1007/BF00810244. [DOI] [PubMed] [Google Scholar]
- Caspi RR. Ocular autoimmunity: the price of privilege? Immunol Rev. 2006;213:23–35. doi: 10.1111/j.1600-065X.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- Cencioni MT, Chiurchiù V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, et al. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS ONE. 2010;5:e8688. doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002;10:263–279. doi: 10.1076/ocii.10.4.263.15592. [DOI] [PubMed] [Google Scholar]
- Chang YH, Lee ST, Lin WW. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J Cell Bochem. 2001;81:715–723. doi: 10.1002/jcb.1103. [DOI] [PubMed] [Google Scholar]
- Cheng CK, Berger AS, Pearson PA, Ashton P, Jaffe GJ. Intravitreal sustained-release dexamethasone device in the treatment of experimental uveitis. Invest Ophthalmol Vis Sci. 1995;36:442–453. [PubMed] [Google Scholar]
- Cho IJ, Kim GS. A novel mitogen-activated protein kinase phosphatise-1 and glucocorticoid receptor (GR) interacting protein-1-dependent combinatorial mechanism of gene transrepression by GR. Mol Endocrinol. 2009;23:86–99. doi: 10.1210/me.2008-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock TL, Decory HH. Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. Int J Inflam. 2012;2012:789623. doi: 10.1155/2012/789623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- De Filippo K, Dudeck A, Hasenberg M, Nye E, Van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract Res Clin Endocrinol Metab. 2009;23:1–15. doi: 10.1016/j.beem.2008.10.013. [DOI] [PubMed] [Google Scholar]
- De Vos AF, Klaren VN, Kijlstra A. Expression of multiple cytokines and IL-1RA in the uvea and retina during endotoxin-induced uveitis in the rat. Invest Ophthalmol Vis Sci. 1994;35:3873–3883. [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation. 2000;24:357–370. doi: 10.1023/a:1007049015148. [DOI] [PubMed] [Google Scholar]
- Gaynes BI, Onyekwuluje A. Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension. Clin Ophthalmol. 2008;2:355–368. doi: 10.2147/opth.s1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano EA. Nonsteroidal anti-inflammatory drugs in veterinary ophthalmology. Vet Clin North Am Small Anim Pract. 2004;34:707–723. doi: 10.1016/j.cvsm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Greineisen WE, Turner H. Immunoactive effects of cannabinoids: considerations for the therapeutic use of cannabinoid receptor agonists and antagonists. Int Immunopharmacol. 2010;10:547–555. doi: 10.1016/j.intimp.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz, et al. HU-308: a specific agonist for CB2R, a peripheral cannabinoid receptor. Proc Natl Acad Sci U S A. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariprasad SM, Akduman L, Clever JA, Ober M, Recchia FM, Mieler WF. Treatment of cystoid macular edema with the new-generation NSAID nepafenac 0.1% Clin Opthalmol. 2009;3:147–154. doi: 10.2147/opth.s4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kanai K, Ito Y, Nagai N, Itoh N, Hori Y, Chikazawa S, et al. Effects of instillation of eyedrops containing disulfiram and hydroxypropyl-β-cyclodextrin inclusion complex on endotoxin-induced uveitis in rats. Curr Eye Res. 2012;37:124–131. doi: 10.3109/02713683.2011.622853. [DOI] [PubMed] [Google Scholar]
- Kawamura H, Kawamura T, Kanda Y, Kobayashi T, Abo T. Extracellular ATP-stimulated macrophages produce macrophage inflammatory protein-2 which is important for neutrophil migration. Immunology. 2012;136:448–458. doi: 10.1111/j.1365-2567.2012.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44:2184–2191. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SS, Modi SS, Lehmann RP, Holland EJ. Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery. J Cataract Refract Surg. 2007;33:53–58. doi: 10.1016/j.jcrs.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Larson T, Nussenblatt RB, Sen HN. Emerging drugs for uveitis. Expert Opin Emerg Drugs. 2011;16:309–322. doi: 10.1517/14728214.2011.537824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läubli H, Spanaus KS, Borsig L. Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood. 2009;114:4583–4591. doi: 10.1182/blood-2008-10-186585. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Kianian M, Zhou J, Vladimir C, Kelly M. The endocannabioid system in sepsis – a potential target to improve microcirculation. Signa Vitae. 2011;6:7–13. [Google Scholar]
- Lehmann C, Kianian M, Zhou J, Küster I, Kuschnereit R, Whynot S, et al. Cannabinoid receptor 2 activation reduces intestinal leukocyte recruitment and systemic inflammatory mediator release in acute experimental sepsis. Crit Care. 2012;16:R47. doi: 10.1186/cc11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeHoang P. The gold standard of noninfectious uveitis: corticosteroids. Dev Ophthalmol. 2012;51:7–28. doi: 10.1159/000336676. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Fan HB, Jin Y, Ren CG, Jia XE, Wang L, et al. Cannabioid receptor 2 suppresses leukcoyte inflammatory migration by modulating the JNK/c-Jun/Alox5 pathway. J Biol Chem. 2013;288:13551–13562. doi: 10.1074/jbc.M113.453811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin T, Zhu W, Dettmer-Wilde K, Straub RH. Cortisol-mediated adhesion of synovial fibroblasts is dependent on the degradation of anandamide and activation of the endocannabinoid system. Arthritis Rheum. 2012;64:3867–3876. doi: 10.1002/art.37684. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, et al. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG, Crewe J, Morrison S, Holt PG. Immunomorphologic studies of macrophages and MHC class II-positive dendritic cells in the iris and ciliary body of the rat, mouse, and human eye. Invest Ophthalmol Vis Sci. 1994;35:3234–3250. [PubMed] [Google Scholar]
- Medeiros R, Bu G, Figueiredo P, Grumman A, Menezes-de-lima O, Passos GF. Molecular mechanisms of topical anti-inflammatory effects of lipoxin A4 in endotoxin-induced uveitis. Mol Pharmcol. 2008;74:154–161. doi: 10.1124/mol.108.046870. [DOI] [PubMed] [Google Scholar]
- Mestre L, Correa F, Docagne F, Clemente D, Guaza C. The synthetic cannabinoid WIN 55,212-2 increases COX-2 expression and PGE2 release in murine brain-derived endothelial cells following Theiler's virus infection. Biochem Pharmacol. 2006;72:869–880. doi: 10.1016/j.bcp.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Michler T, Storr M, Kramer J, Ochs S, Malo A, Reu S, et al. Activation of cannabinoid receptor 2 reduces inflammation in acute experimental pancreatitis via intra-acinar activation of p38 and MK2-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol. 2013;304:G181–G192. doi: 10.1152/ajpgi.00133.2012. [DOI] [PubMed] [Google Scholar]
- Murray PI, Hoekzema R, Haren MAC, De Von Hon FD, Kijlsrra A. Aqueous humor interleukin-6 levels in uveitis. Invest Ophthalmol Vis Sci. 1990;31:917–920. [PubMed] [Google Scholar]
- Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- Olszewski MB, Groot AJ, Dastych J, Knol EF. TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J Immunol. 2007;178:5701–5709. doi: 10.4049/jimmunol.178.9.5701. [DOI] [PubMed] [Google Scholar]
- Ooi KGJ, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis: is there a correlation with clinical phenotype? Clin Med Res. 2006;4:294–309. doi: 10.3121/cmr.4.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R, Mousawy K, Nagarkatti M, Nagarkatti P. Endocannabinoids and immune regulation. Pharmacol Res. 2009;60:85–92. doi: 10.1016/j.phrs.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Prusakiewicz JJ, Kingsley PJ, Kozak KR, Marnett LJ. Selective oxygenation of N-arachidonylglycine by cyclooxygenase-2. Biochem Biophys Res Commun. 2002;296:612–617. doi: 10.1016/s0006-291x(02)00915-4. [DOI] [PubMed] [Google Scholar]
- Raborn ES, Marciano-Cabral F, Buckley NE, Martin BR, Cabral GA. The cannabinoid delta-9-tetrahydrocannabinol mediates inhibition of macrophage chemotaxis to RANTES/CCL5: linkage to the CB2 receptor. J Neuroimmune Pharmacol. 2008;3:117–129. doi: 10.1007/s11481-007-9077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Bátkai S, Haskó G, Liaudet L, Huffman JW, et al. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol. 2007;293:H2210–H2218. doi: 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, Haskó J, Skuba A, Fan S, Dykstra H, McCormick R, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32:4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizman M. Corticosteroid therapy of the eye disease. Arch Ophthalmol. 1996;114:1000–1001. doi: 10.1001/archopht.1996.01100140208016. [DOI] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, Kelly JP, O'Driscoll M, Finn DP. Augmentation of endogenous cannabinoid tone modulates lipopolysaccharide-induced alterations in circulating cytokine levels in rats. Immunology. 2008;125:263–271. doi: 10.1111/j.1365-2567.2008.02838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- Scotter EL, Abood ME, Glass M. The endocannabiod system as a target for the treatment of neurodegenerative disease. Br J Pharmacol. 2010;160:480–498. doi: 10.1111/j.1476-5381.2010.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvi E, Lorenzini S, Garcia-Gonzalez E, Maggio R, Lazzerini PE, Capecchi PL, et al. Inhibitory effect of synthetic cannabinoids on cytokine production in rheumatoid fibroblast-like synoviocytes. Clin Exp Rheumatol. 2008;26:574–581. [PubMed] [Google Scholar]
- Siddique S, Shah R. Road to remission: a comprehensive review of therapy in uveitis. Expert Opin Investig Drugs. 2011;20:1497–1515. doi: 10.1517/13543784.2011.617741. [DOI] [PubMed] [Google Scholar]
- Silverman MD, Zamora DO, Pan Y, Texeira PV, Planck SR, Rosenbaum JT. cell adhesion molecule expression in cultured human iris endothelial cells. Invest Ophthalmol Vis Sci. 2001;42:2861–2866. [PubMed] [Google Scholar]
- Smith JR, Hart PH, Williams KA. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol Cell Biol. 1998;76:497–512. doi: 10.1046/j.1440-1711.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- Straiker AJ, Maguire G, Mackie K, Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci. 1999;40:2442–2448. [PubMed] [Google Scholar]
- Suetsugu A, Osawa Y, Nagaki M, Saji S, Moriwaki H, Bouvet M, et al. Imaging the recruitment of cancer-associated fibroblasts by liver-metastatic colon cancer. J Cell Biochem. 2011;112:949–953. doi: 10.1002/jcb.23011. [DOI] [PubMed] [Google Scholar]
- Szczesniak AM, Maor Y, Robertson H, Hung O, Kelly MEM. Nonpsychotropic cannabinoids, abnormal cannabidiol and canabigerol-dimethyl heptyl, act at novel cannabinoid receptors to reduce intraocular pressure. J Ocul Pharmacol Ther. 2011;27:1988–1992. doi: 10.1089/jop.2011.0041. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-κβ: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunobiology. 2010;215:588–597. doi: 10.1016/j.imbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Tomida I, Pertwee RG, Azuara-Blanco A. Cannabinoids and glaucoma. Br J Ophthalmol. 2004;88:708–713. doi: 10.1136/bjo.2003.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöp J, Kasten KR, Nogueiras R, Goetzman HS, Cave CM, England LG, et al. The cannabinoid receptor 2 is critical for the host response to sepsis. J Immunol. 2009;183:499–505. doi: 10.4049/jimmunol.0900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Sun D, Schering A, Nakao S, Zandi S, Liu P, et al. Novel molecular imaging approach for subclinical detection of iritis and evaluation of therapeutic success. Am J Pathol. 2010;177:39–48. doi: 10.2353/ajpath.2010.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Cheng CL, Chen M, Manivannan A, Cabay L, Pertwee RG, et al. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J Leukoc Biol. 2007;82:532–541. doi: 10.1189/jlb.0307159. [DOI] [PubMed] [Google Scholar]
- Zhong L, Geng L, Njie Y, Feng W, Song Z-H. CB2 cannabinoid receptors in trabecular meshwork cells mediate JWH015-induced enhancement of aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2005;46:1988–1992. doi: 10.1167/iovs.04-0651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative histological cross section of the eye stained with H&E: (A) Control animal cornea, (B) LPS treated animal cornea, (C) Control animal iris, (D) LPS treated animal iris. (E) Histogram of inflammatory cells infiltrating the anterior chamber of the eye. ESS = anterior corneal epithelial stratified squamous; CS = corneal stroma; K = keratocyte; PE = posterior endothelium; AC = anterior chamber; IIC = infiltrating inflammatory cell; PC = posterior chamber. Magnification 10×. Scale bar = 100 μm.
Dose-response curve for the topical administration (0.1–1.5%) of,the CB2 receptor agonist, HU308, on leukocyte adhesion in iridial venules. Values are represented as percentage change in leukocyte adhesion to endothelium and normalised to the minimal response of control animals and maximum of those receiving only LPS. IC50 for HU308- mediated decrease in leukocyte adhesion is 0.7867%.
Haemodynamics including (A) Mean arterial pressure (mmHg) (B). Heart rate (beats per minute) in the following treatment groups: Control (n = 6), LPS (n = 4), LPS + 1.5% HU308 (n = 4), LPS + 2.5 mg·kg-1 AM630 (n = 6), LPS+ HU308 + AM630 (n = 5), LPS + 0.1% dexamethasone (n = 8), LPS + 0.1% nepafenac (n = 4). P < 0.05; compared to the LPS group. Values represent mean ± SEM.
Oligonucleotide primers used in this study. NF-κB, TNF-α; AP-1, activator protein 1; HPRT, hypoxanthineguanine phosphoribosyltransferase.


