Abstract
Background and PurposeSpinocerebellar ataxias (SCAs) are a family of chronic progressive neurodegenerative diseases, clinically and genetically heterogeneous, characterized by loss of balance and motor coordination due to degeneration of the cerebellum and its afferent and efferent connections. Unlike other motor disorders, the possible role of changes in the endocannabinoid system in the pathogenesis of SCAs has not been investigated.
Experimental ApproachThe status of cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2) receptors in the post-mortem cerebellum of SCA patients and controls was investigated using immunohistochemical procedures.
Key ResultsImmunoreactivity for the CB1 receptor, and also for the CB2 receptor, was found in the granular layer, Purkinje cells, neurons of the dentate nucleus and areas of white matter in the cerebellum of SCA patients at levels notably higher than controls. Double-labelling procedures demonstrated co-localization of CB1 and, in particular, CB2 receptors with calbindin, supporting the presence of these receptors in Purkinje neurons. Both receptors also co-localized with Iba-1 and glial fibrillary acidic protein in the granular layer and white matter areas, indicating that they are present in microglia and astrocytes respectively.
Conclusions and ImplicationsOur results demonstrate that CB1 and CB2 receptor levels are significantly altered in the cerebellum of SCA patients. Their identification in Purkinje neurons, which are the main cells affected in SCAs, as well as the changes they experienced, suggest that alterations in endocannabinoid receptors may be related to the pathogenesis of SCAs. Therefore, the endocannabinoid system could provide potential therapeutic targets for the treatment of SCAs and its progression.
Linked ArticlesThis article is part of a themed section on Cannabinoids 2013. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-6
Keywords: cannabinoids, endocannabinoid system, CB1 and CB2 receptors, cerebellum, Purkinje neurons, spinocerebellar ataxias
Introduction
During the last decade, robust preclinical evidence has accumulated demonstrating the neuroprotective potential of compounds that affect the activity of the endocannabinoid system, including both plant-derived cannabinoids and certain signalling lipids (reviewed in Fernández-Ruiz et al., 2010; 2011). This potential has been demonstrated for both acute and chronic brain damage and, in some cases (e.g. Huntington's disease), it is already being investigated at the clinical level (de Yébenes, 2010) based on preclinical results obtained with specific cannabinoid-based drugs (Sagredo et al., 2011; Valdeolivas et al., 2012). The evidence that cannabinoids are neuroprotective compounds is mostly derived from the pharmacological correction of alterations in the endocannabinoid communicating system observed in neurodegenerative disorders (reviewed in Fernández-Ruiz et al., 2007; 2010), alterations that several authors have related to the pathogenesis of these disorders (reviewed in Centonze et al., 2007; Fernández-Ruiz et al., 2010). These alterations are usually deficits, but increases in specific elements of this communicating system (i.e. ligands, receptors, enzymes) have also been found even in early and presymptomatic phases of these disorders, and have been characterized in experimental models and, to a lesser extent, in human subjects (reviewed in Centonze et al., 2007; Fernández-Ruiz et al., 2010). These changes could play a pivotal role in the pathogenesis of these conditions, presumably by aggravating the neuronal injury; although, in some cases, these alterations have been interpreted as being part of an endogenous protectant response against brain damage (reviewed in Fernández-Ruiz et al., 2010; Pacher and Mechoulam, 2011). For example, several studies have described an increased accumulation of arachidonoyl-ethanolamide, 2-arachidonoyl-glycerol or both in specific brain structures in experimental models of different neurodegenerative disorders including multiple sclerosis, amyotrophic lateral sclerosis, Parkinson's disease, ischaemia and brain trauma (Baker et al., 2001; Panikashvili et al., 2001; Witting et al., 2004). Data from patients are also available (Pisani et al., 2005). Experimental brain injury has also been found to be associated with an up-regulation of cannabinoid receptors, including cannabinoid receptor type 1 (CB1) receptors (Jin et al., 2000; Hansen et al., 2001), although the most pertinant data were obtained with the cannabinoid receptor type 2 (CB2) receptor. The CB2 receptor is mostly absent in the brain of healthy individuals but has been reported to be significantly up-regulated in many degenerative pathologies. These results were obtained mainly in animal models of disease but also from a few studies in human samples (reviewed in Fernández-Ruiz et al., 2007; Benito et al., 2008). This response occurs preferentially in activated astrocytes and, in particular, reactive microglial cells (note that resident microglial cells do not express CB2 receptors), which indicates that CB2 receptors may play a role in regulating the trophic and/or the cytotoxic influences of these cells on neurons (Fernández-Ruiz et al., 2007; Benito et al., 2008). The induction/up-regulation of CB2 receptors has also been observed in structures undergoing neuronal damage in patients and animal models of stroke (Ashton et al., 2007), Alzheimer's disease (Benito et al., 2003), Huntington's disease (Palazuelos et al., 2009; Sagredo et al., 2009), Parkinson's disease (Price et al., 2009; García et al., 2011), multiple sclerosis (Maresz et al., 2005; Benito et al., 2007), amyotrophic lateral sclerosis (Yiangou et al., 2006), simian immunodeficiency virus encephalitis (Benito et al., 2005), Down's syndrome (Núñez et al., 2008) and neuropathic pain (Zhang et al., 2003), but not in cases of spinocerebellar ataxias (SCAs), the pathology under investigation in the present study. Importantly, preclinical evaluations of different selective agonists of this receptor have generally demonstrated that these drugs delay the progression of brain damage in most of these disorders, thus indicating their potential to act as neuroprotectants (see below and some reviews in Fernández-Ruiz et al., 2007; Benito et al., 2008).
Autosomal-dominant SCAs are a group of inherited neurodegenerative disorders. The most prevalent cases belong to the family of polyglutaminopathies, which also includes Huntington's disease, and are primarily caused by excessive CAG repeats leading to the expansion of a polyglutamine tract in different recipient proteins (i.e. huntingtin in Huntington's disease, frequently ataxins but also other proteins in SCAs) (Klockgether, 2011). Despite its ubiquitous distribution, the mutant protein usually only affects specific structures within the CNS, the cerebellum being the key structure affected in SCAs, which explains the specificity of the neurological symptoms, that is motor incoordination and ataxia (Matilla-Dueñas et al., 2012). The age of onset of the clinical symptoms is typically between 30 and 50 years of age, although early onset in childhood, as well as cases in which the pathology initiates after 60 years, have been reported for specific SCA subtypes, frequently related to longer or shorter polyglutamine expansions (Durr, 2010). Like other polyglutamine diseases, SCAs are characterized by protein misfolding, failed or incomplete proteolysis and the deposition and formation of intracellular protein aggregates, which represents the key neuropathological characteristic of these disorders (Orr, 2012). Although there is some controversy as regards the role of these aggregates in neurotoxicity, they appear to play a key role in eliciting transcription dysregulation, mitochondrial failure, excitotoxicity, alterations in calcium homeostasis, oxidative stress and local inflammation, which ultimately lead to cell death in specific subpopulations of cerebellar neurons (Fratkin and Vig, 2012). Hence cannabinoid agonists that have been demonstrated to be effective in reducing excitotoxicity (e.g. selective CB1 agonists), oxidative injury (e.g. antioxidant cannabinoids) or inflammation (e.g. selective CB2 agonists) in various chronic neurodegenerative disorders (reviewed in Fernández-Ruiz et al., 2010) may also have a therapeutic effect in SCAs. However, it is also possible that cannabinoids could enhance autophagy, a cellular process responsible, among others, for eliminating the accumulation of toxic proteins, and this has been proposed as a possible therapeutic strategy for SCAs and other polyglutamine disorders, given that mutant proteins are autophagy substrates due to the failure of primary proteolytic processes (Williams et al., 2006). Interestingly, certain cannabinoid agonists have been demonstrated to stimulate cell autophagy in human glioma cells (Salazar et al., 2009), which may also have a therapeutic effect in polyglutamine disorders such as SCAs.
Whether cannabinoids can provide neuroprotection in SCAs as they do in preclinical models of other neurodegenerative disorders also needs to be investigated. However, before this can be done, the type of changes that the development of cerebellar degeneration in the different SCAs produces in particular elements of the endocannabinoid signalling system, specifically alterations in endocannabinoid receptors, needs to be clarified. Hence, we used immunohistochemical procedures to identify and quantify the major endocannabinoid receptors, that is CB1 and CB2 receptors, in the post-mortem cerebellum of SCA patients and control subjects.
Methods
Subjects
We used post-mortem human cerebellum from control subjects and from patients diagnosed with an autosomal dominant cerebellar ataxia and these samples were obtained from The Netherlands Brain Bank (NBB), Institute for Neuroscience, Amsterdam, the Netherlands. All material was collected from donors for and from whom a written informed consent for a brain autopsy and the use of material and clinical information for research purposes had been obtained from the NBB. The samples received were fixed, paraffinized, sliced (8 μm) and mounted on glass slides. Table 1 summarizes the major characteristics (age, gender and post-mortem delay) of the individuals (patients and controls) included in our analyses. Unfortunately, the number of SCA types in the NBB, as well as in other biobanks that we contacted, is rather small, so our experimental group had to be formed using only one subject of the most representative types of SCAs. However, the neuropathological data provided by the biobank confirmed that all the SCA patients exhibited a marked atrophy in the cerebellum and the pons (small size, presence of gliosis, and evidence of neuronal loss), but with no important changes in the remaining brain areas, for example, the substantia nigra was generally normal, the locus coeruleus was visible and the ventricular system was not dilated. We also did a histopathological evaluation of all the tissues received so as to confirm the data provided by the biobank (see Figures 1–3, and Results section). This evaluation was done before the tissues were used for the immunohistochemical analysis of CB1 and CB2 receptors. We also considered the pharmacological information (medication and intoxication history) of SCA patients and control subjects provided by the NBB. Most of SCA patients had been treated with benzodiazepines (e.g. clonazepam, temazepam, diazepam), GABA-acting substances (e.g. baclofen, GABApentine) and, in a few cases, tricyclic antidepressants (e.g. amytriptiline). They were also sporadically treated with antibiotics (e.g. amoxyciline), and all of them received morphine during the last days/hours before death. One patient (subject #7) was treated with nabilone 6 years before death but the treatment was not effective. Five of the patients with a genetic diagnosis of the most prevalent SCAs had to be discarded from the initial patient population (n = 10) because of a previous history of tobacco and/or alcohol addiction, or because they consumed cannabis; this was another reason why our sample size was small. Medication and intoxication history of control subjects was also available and used to select suitable control subjects. Some of the control subjects had been diagnosed with colon carcinoma and, therefore, also received morphine during the last days/hours before death.
Table 1.
Major characteristics of patients and control subjects whose post-mortem samples were used in this study (they were obtained from the Netherlands Brain Bank)
| Subject # | Age | Gender | Diagnosis | Post-mortem delay |
|---|---|---|---|---|
| 1 | 56 | Male | Control | 325 min |
| 2 | 62 | Male | Control | 480 min |
| 3 | 66 | Male | Control | 465 min |
| 4 | 83 | Female | Control | 315 min |
| 5 | 88 | Female | Control | 355 min |
| 6 | 54 | Male | SCA3 | 315 min |
| 7 | 59 | Male | SCA2 | 310 min |
| 8 | 61 | Male | SCA | 325 min |
| 9 | 82 | Female | SCA7 | 305 min |
| 10 | 86 | Female | SCA6 | 395 min |
Figure 1.
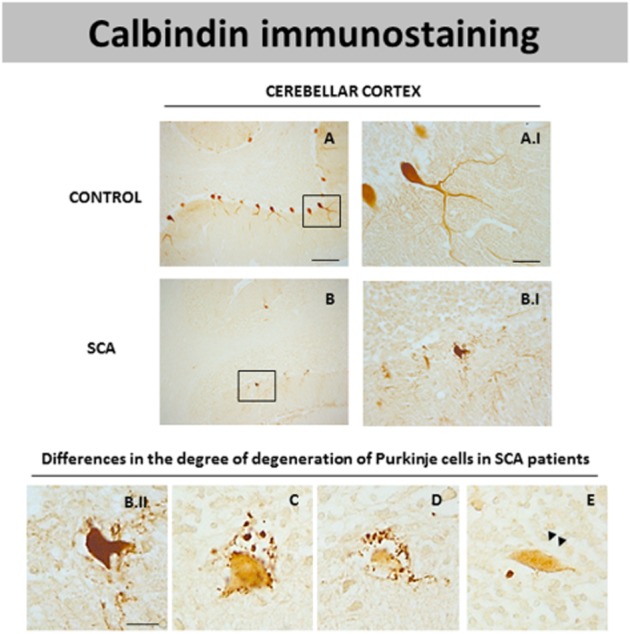
DAB immunostaining for calbindin, a marker of Purkinje cells, in the cerebellar cortex of SCA patients (B and B.I) and control subjects (A and A.I). Microphotographs shown in B.II, C, D and E correspond to details obtained in the cerebellar cortex of SCA patients proving the existence of Purkinje cells with different degrees of degeneration noted by loss in calbindin immunostaining (scale bars: A, B = 200 μm; A.I, B.I = 50 μm; B.II-E = 20 μm). Arrowheads indicate the presence of axonal torpedoes in surviving Purkinje cells.
Figure 3.
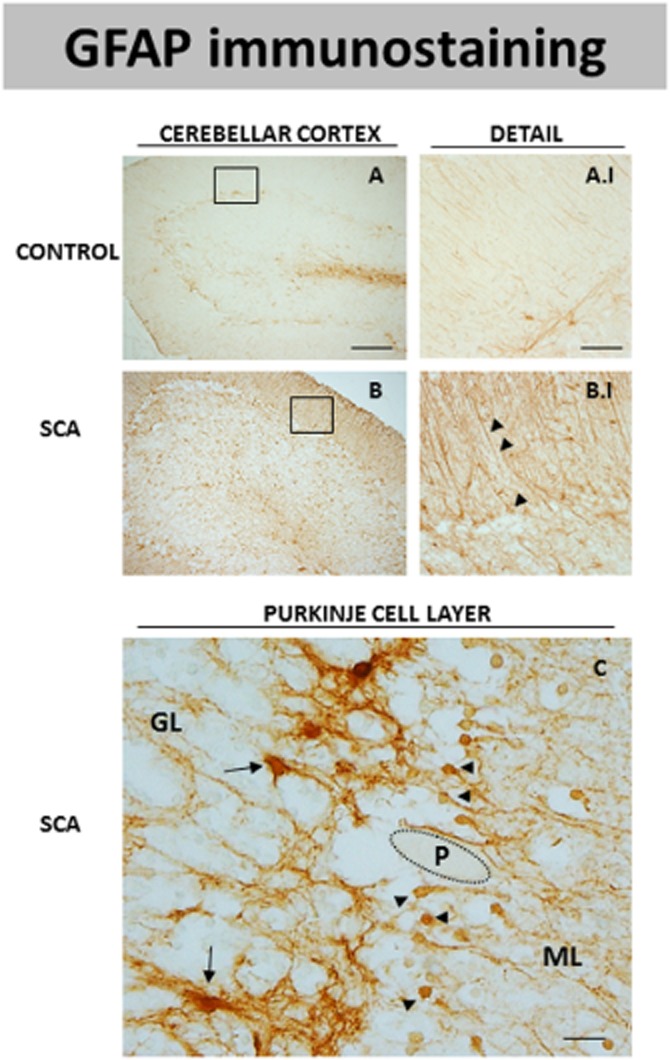
DAB immunostaining for GFAP, a marker of astrocytes, in the cerebellar cortex of SCA patients (B and B.I) and control subjects (A and A.I). The microphotograph shown in C corresponds to a detail of the Purkinje layer of SCA patients in which GFAP immunostaining was seen in two cell subpopulations that may correspond to protoplasmic astrocytes (marked with arrows) and Bergmann glia (marked with arrowheads). GL = granular layer; ML = molecular layer; P = Purkinje neurons (scale bars: A and B = 200 μm; A.I and B.I = 50 μm; C = 20 μm).
Histochemical techniques
Immunohistochemistry
The protocol used was as described previously (Tsou et al., 1998; Benito et al., 2005) with slight modifications. Tissue sections were deparaffinized and washed extensively in 50 mM, pH 8, potassium phosphate-buffered saline (KPBS). To obtain more efficient immunostaining, samples were subjected to an antigen retrieval procedure (Shi et al., 2001). Briefly, tissue sections were placed into a stainless steel pressure cooker containing Antigen Retrieval Solution pH 9 (Dako Cytomation, Glostrup, Denmark). After being heated under pressure for 2 min, samples were removed and washed extensively in KPBS. Then, endogenous peroxidase was blocked by 30 min incubation at room temperature in peroxidase blocking solution (Dako Cytomation). After several washes with KPBS, tissues were incubated with the primary antibody overnight at room temperature. Antibodies (see those used and specific details in Table 2) were diluted in KPBS containing 1% BSA (Sigma Chem., Madrid, Spain) and 1% Triton X-100 (Sigma Chem.). After the incubation, sections were washed in KPBS, followed by incubation with biotinylated goat anti-rabbit antibody (1:200), biotinylated horse anti-mouse antibody (1:200) or biotinylated horse anti-goat antibody (1:200) for 2 h at room temperature. Avidin–biotin complex (Vector Elite; Vector Laboratories, Burlingame, CA, USA) and a 3,3′-Diaminobenzidine (DAB) substrate–chromogen system (Dako Cytomation) were used to obtain a visible reaction product. Negative controls were obtained for each sample by omitting primary antibody as well as by using a blocking peptide. Sections were dehydrated, sealed and coverslipped. A Leica DMRB microscope (Leica, Wetzlar, Germany) and DFC300FX camera (Leica) were used for the observations and photography of the slides respectively.
Table 2.
List of antibodies used in immunohistochemical studies
| Antibody | Dilution (IHC) | Dilution (IF) | Class | Manufacturer |
|---|---|---|---|---|
| CB1 receptor | 1:300 | 1:100 | Polyclonal | Affinity BioReagents, Rockford, IL, USA |
| CB2 receptor | 1:100 | 1:50 | Monoclonal | R&D systems, Minneapolis, MN, USA |
| Calbindin D-28 K | 1:500 | 1:300 | Monoclonal | Sigma Chem., Madrid, Spain |
| Calbindin D-28 K | 1:250 | Polyclonal | Millipore, Billerica, MA, USA | |
| GFAP-Cy3 | 1:1500 | Monoclonal | Sigma Chem., Madrid, Spain | |
| GFAP | 1:500 | Monoclonal | AbD Serotec, Oxford, UK | |
| Iba-1 | 1:1000 | 1:1000 | Polyclonal | Wako, Osaka, Japan |
| Iba-1 | 1:75 | Polyclonal | Abcam, Cambridge, UK | |
| Cd68 | 1:50 | Monoclonal | Dako Cytomation, Glostrup, Denmark |
GFAP, glial fibrillary acidic protein; IF, immunofluorescence; IHC, immunohistochemistry.
Quantification of CB1 and CB2 immunostaining was carried out on high-resolution digital microphotographs that were taken with the 20× objective and under the same conditions of light and brightness/contrast. The analysis software ImageJ (NIH, USA) was used to measure the mean density of labelling in a selected area, in the case of the granular layer and the white matter regions, or the mean density of labelling in individual cells in the case of the Purkinje cells (approximately 10 cells per sample in patients and 25 cells per sample in control subjects) and the neurons of the dentate nucleus (approximately 25 cells per sample in both groups). Five images were obtained for each sample. We carried out separate densitometrical analysis for each region. All data are expressed in arbitrary units.
Immunofluorescence
To identify specific cell populations, we performed co-localization studies with immunofluorescence combining the anti-CB1 or the anti-CB2, antibodies with antibodies for specific markers for Purkinje cells (i.e. calbindin), macrophages (i.e. Cd68), microglia (i.e. Iba-1) and astrocytes and Bergmann glia [i.e. glial fibrillary acidic protein (GFAP)]. After the antigen retrieval procedure, tissue sections were washed with pH 7.5 Tris-buffered saline (TBS) before overnight incubation at room temperature with the antibodies used for identification of the cell types followed by incubation with an Alexa 546 secondary antibody conjugate (Invitrogen, Carlsbad, CA, USA) at 37°C for 2 h, rendering red fluorescence. In the case of the astrocyte marker GFAP, the tissue was incubated with GFAP-Cy3 antibody (Sigma Chem.) at 37°C for 2 h, rendering red fluorescence too. Afterwards, the primary antibodies for CB1 or CB2 were incubated overnight at room temperature after extensive washes in TBS, followed by incubation with Alexa 488 secondary antibody conjugate (Invitrogen), rendering green fluorescence. Sections were counter-stained with nuclear stain TOPRO-3-iodide (Molecular Probes, Eugene, OR, USA) to visualize cell nuclei. To quench endogenous autofluorescence, tissue sections were treated with 1% Sudan Black (Merck, Darmstadt, Germany) in 70% ethanol for 5 min and differentiated with 70% ethanol (Schnell et al., 1999). Sections were mounted onto glass slides with aqueous solution (Vectashield; Vector Laboratories, Burlingame, CA, USA).
Confocal microscopy analysis
Samples were viewed on a Leica TCS SP5 microscope system using argon and helium-neon lasers. Differential visualization of the fluorophores Alexa 488 (excitation 496 nm and emission 519 nm), Alexa 546 (excitation 556 nm and emission 573 nm), Cy3 (excitation 550 nm and emission 565 nm) and Topro-3 (excitation 650 nm and emission 667 nm) was accomplished through the use of specific filter combinations. Samples were scanned sequentially to avoid any potential for bleed through of fluorophores. In some cases, images were processed with the Leica LAS AF software to obtain three-dimensional orthogonal projections.
Statistics
Data were assessed by Student's t-test using the GraphPad software (version 5.0).
The receptor nomenclature conforms to BJP's Concise guide to PHARMACOLOGY (Alexander et al., 2013).
Results
Evidence of neuronal damage and glial activation in the cerebellum of SCA patients compared to control subjects
Before using the post-mortem samples for the analysis of endocannabinoid receptors, we analysed several parameters to confirm the presence of histopathological signs of dysfunction/degeneration in the patients affected by different types of SCAs. Our analyses have always concentrated in the cerebellum which is the main structure affected in this disease. As expected, the cerebellum of SCA patients had a notable loss of Purkinje cells (Figure 1B and B.I) compared to control subjects (Figure 1A and A.I), shown using immunolabelling with calbindin, a marker of these neurons. In addition, the immunostaining with calbindin also indicated that the surviving Purkinje cells in SCA patients showed a rare morphology, for example, they have a shrunken cell body and a notable reduction in the dendritic tree (Figure 1B.I). The surviving Purkinje cells present axonal swellings also termed axonal torpedoes (see arrowheads in Figure 1E). These structural alterations of Purkinje cells are evident to different extents in the cerebellum of each SCA patient, some have cells with strong calbindin immunostaining and others cells with a complete loss of immunostaining for this marker (Figure 1B.II, C and D). This pattern has also been observed previously (Ishikawa et al., 1995; Seidel et al., 2012). In addition to the loss of Purkinje neurons, there was also a marked degeneration of neurons located in the dentate nucleus in the cerebellum of SCA patients. These results were obtained using immunostaining with another marker such as neuron-specific enolase (data not shown), which was used to determine neuronal damage in other cerebellar disorders (Koeppen et al., 2011).
We also observed significant microgliosis in the cerebellum of patients with SCAs (Figure 2D–F) compared with the control subjects (Figure 2A–C). The microglial activation was observed using immunolabelling with the microglial marker Iba-1 and was particularly evident in the white matter of the folia (Figure 2E; see inside the ellipse) and in that surrounding the dentate nucleus (Figure 2F), as well as in the granular layer (Figure 2D). The microglial cells identified in SCA patients presented a typical morphology of reactive microglia (i.e. reduction of processes and increase in the cell body size; see Figure 2D.I and F.I). There was also an increase in the perivascular microglia in SCA patients (Figure 2F.II).
Figure 2.
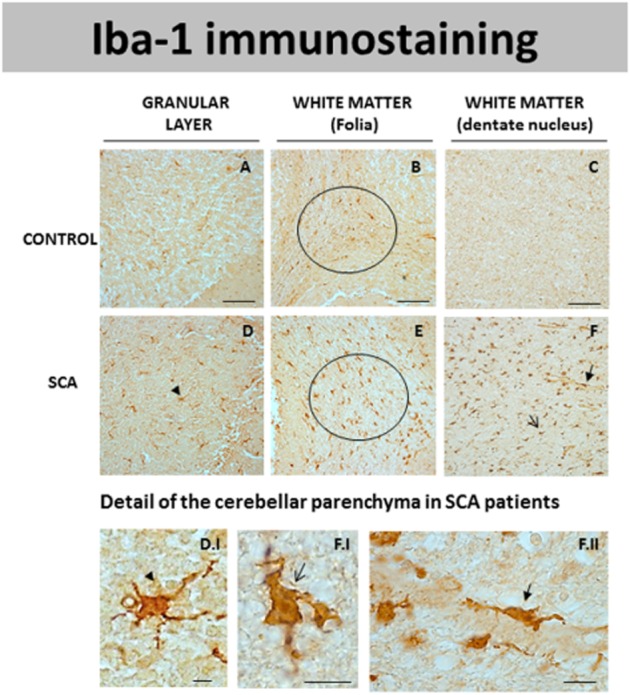
DAB immunostaining for Iba-1, a marker of microglial cells, in different areas of the cerebellum of SCA patients (D–F) and control subjects (A–C). Microphotographs shown in D.I, F.I and F.II correspond to details obtained in the cerebellar parenchyma of SCA patients proving the presence of Iba-1-positive cells probably corresponding to microglial cells (marked with arrows and arrowheads) (scale bars: A–F = 100 μm; D.I, F.I and F.II = 10 μm).
Lastly, there was also marked astrogliosis, demonstrated by an increase in GFAP immunostaining, in the cerebellum of SCA patients compared to controls (see Figure 3). This was observed in the granular layer as well as in the white matter of the folia or that surrounding the dentate nucleus (data not shown), but it was particularly evident in the cerebellar cortex in which a notable difference in GFAP immunostaining was found in SCA patients (Figure 3B and B.I) compared to controls (Figure 3A and A.I). The increase in GFAP immunostaining in this structure in SCA patients may reflect the increase experienced by the Bergmann glia, a characteristic astrocyte subtype present in the cerebellum (marked with arrowheads in Figure 3B.I). The increase corresponded to both a higher number of these glial cells and also an increase in the number of cell processes. Bergmann gliosis was found close to the Purkinje cells, as these glial cells surround the Purkinje cells and their processes reach the molecular layer (see arrowheads in Figure 3C). In addition to Bergmann glial cells, other GFAP-positive cells were also identified close to the Purkinje cells. They corresponded to protoplasmic astrocytes that form a barrier between the granular layer and the Purkinje cells (marked with arrows in Figure 3C). These changes to astrocytes in SCA patients have been observed previously (Robitaille et al., 1995).
Immunohistochemical study of CB1 receptors in the cerebellum of SCA patients
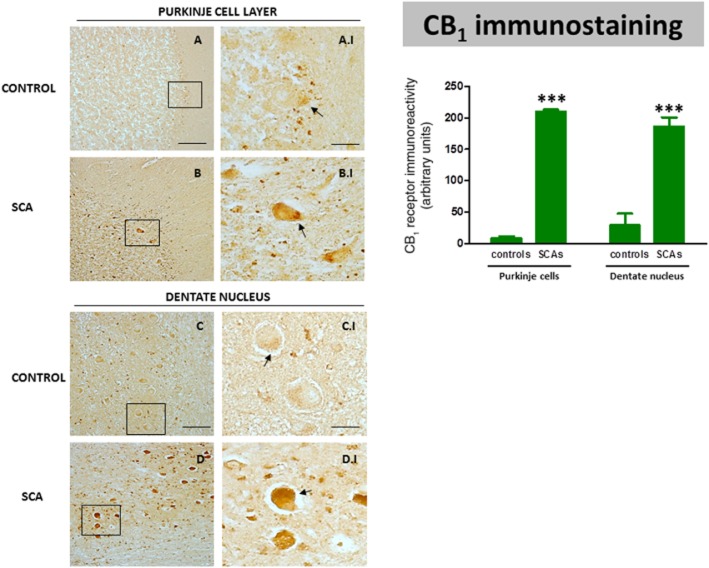
As regards changes in endocannabinoid receptors, we first analysed the immunoreactivity for the CB1 receptor, the major cannabinoid receptor present in neurons within the CNS. We found immunolabelling for this receptor in the Purkinje layer and also in the dentate nucleus (Figure 4). In control subjects, CB1 receptors were apparent in Purkinje neurons; the immunolabelling was found in the basal region of these cells, in the so-called pinceau formations, which might indicate that the immunolabelling corresponds to the axons of basket cells that contact Purkinje cells (Figure 4A; see arrows in A.I). Similar findings have been obtained in the cerebellum of laboratory animals (Tsou et al., 1998; Suárez et al., 2008), although CB1 receptor immunostaining has also been identified in the soma of Purkinje cells in the rat cerebellum (Moldrich and Wenger, 2000). In contrast, in all the SCA patients indicated the immunolabelling was much more evident in these pinceau formations but also in the remaining cell body of Purkinje cells (Figure 4B; see arrows in B.I). CB1 immunostaining was also extremely high in neurons of the dentate nucleus in all SCA patients (Figure 4D; see arrows in D.I.), whereas it was rather low in control subjects (Figure 4C; see arrows in C.I). As an additional measure to determine the relevance of these observations, we quantified the CB1 receptor immunolabelling in the Purkinje cells and in neurons of the dentate nucleus following the procedure described in Methods. These results confirmed that CB1 receptor immunolabelling was strongly elevated in each surviving neuron of SCA patients compared to controls and this occurred in both structures (Figure 4), although it is important to note that, given the marked neuronal loss found in the Purkinje layer and the dentate nucleus, the immunolabelling for the CB1 receptor in SCA patients would be lower than controls if the whole structure is considered rather than the labelling in individual cells. The quantified results also confirmed that the increase seen in individual cells occurred in all patients, even though they corresponded to different SCA types, and that the differences among the different SCA patients were not significant in either structure (Figure 4).
Figure 4.
DAB immunostaining for the CB1 receptor in the Purkinje layer and dentate nucleus of the cerebellum of SCA patients (B, B.I, D and D.I) and control subjects (A, A.I, C and C.I). The arrows indicate the CB1 receptor-positive cells (scale bars: A–D = 100 μm; A.I–D.I = 25 μm). The microphotographs of SCA cases correspond to the subject #9 (SCA7) for the Purkinje layer and to subject #10 (SCA6) for the dentate nucleus. The bar panel corresponds to the quantification of CB1 receptor immunostaining in the two structures of SCA patients and control subjects. Values correspond to the mean density of labelling in individual cells and are expressed as means ± SEM of five subjects per group. Data were assessed by Student's t-test (***P < 0.001).
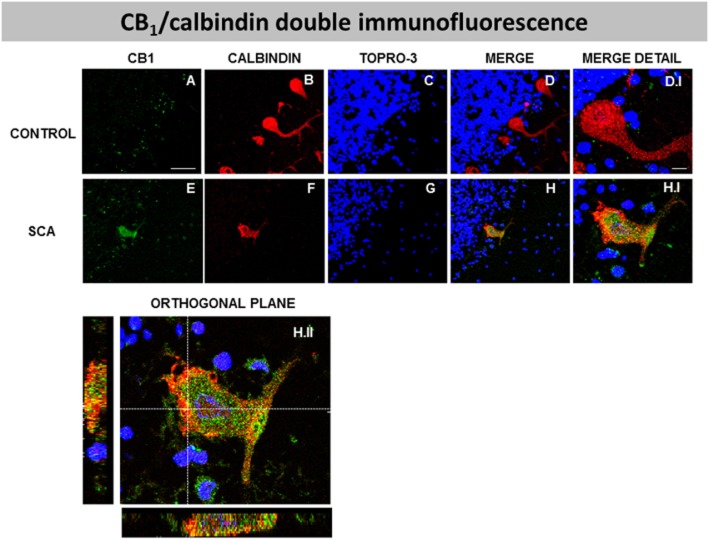
Double-labelling studies using calbindin as marker of Purkinje neurons indicated that CB1 receptors and calbindin were co-localized in SCA patients (see Figure 5E, F, G, H and H.I); this was confirmed by orthogonal reconstruction (see Figure 5H.II). This strongly supports the presence of CB1 receptors in Purkinje cells in SCA patients, but not, apparently, in the control subjects (see Figure 5A, B, C, D and D.I), probably due to the extremely low CB1 receptor immunofluorescence detected in the controls' samples. In this sense, our data confirmed the increase in CB1 receptors in the Purkinje layer of SCA patients found with DAB immunostaining (Figure 4), as it was evident that the CB1 receptor immunolabelling was lower in cells containing high calbindin immunoreactivity (poorly degenerated cells), while it was higher when calbindin immunoreactivity was lower (highly degenerated cells) (data not shown).
Figure 5.
Double-labelling immunofluorescence using antibodies for the CB1 receptor and calbindin, and TOPRO-3 staining, in the Purkinje layer of the cerebellum of SCA patients (E–H and H.I) and control subjects (A-D, D.I), showing co-localization of CB1 receptors and calbindin in SCA patients but not in control subjects (scale bars: A–H = 50 μm; D.I and H.I = 10 μm). The H.II panel corresponds to the orthogonal reconstruction from confocal z-series in x-z (below) and y-z (left) planes. This reconstruction confirms the co-localization of CB1 receptors and calbindin in SCA patients (scale bar: H.II = 10 μm). The microphotographs of SCA cases correspond to subject #7 (SCA2).
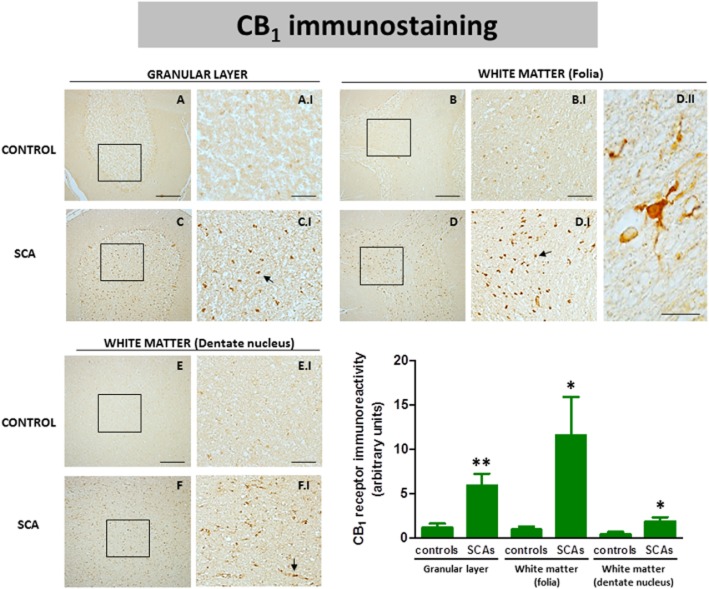
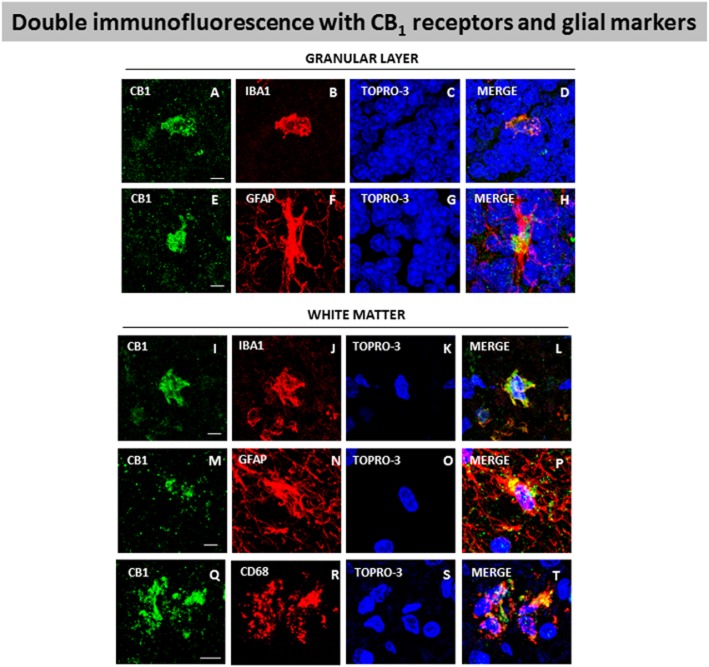
Using DAB immunostaining, we also identified CB1 receptors in the granular layer and in the white matter areas (folia and dentate nucleus) (see Figure 6). We found that the immunostaining was much more marked in the granular layer in all SCA patients (Figure 6C and C.I) compared to control subjects (Figures 6A and A.I). We also found a stronger immunostaining in the white matter of the cerebellar folia of SCA patients (Figure 6D and D.I) compared with controls (Figure 6B and B.I), and similarly with the white matter surrounding the dentate nucleus, although the differences in labelling of SCA patients (Figure 6F and F.I) compared with the controls (Figure 6E and E.I) were much more moderate in this structure. As in the case of the Purkinje layer and the dentate nucleus, we also quantified the CB1 receptor immunolabelling in these structures (this time using a defined area, not labelling in individual cells) as an additional measure to strengthen the relevance of the above observations. Again, we confirmed that immunolabelling was higher in SCA patients compared to controls in the three structures, and that this increase occurred in all SCA patients despite the fact that they corresponded to different SCA types (Figure 6). However, there was one exception, the white matter of the folia of one of the SCA patients (subject #8) showed a significantly higher value compared to the others, which explains the high SEM found in the quantification of SCA patients in this structure (Figure 6). On the other hand, the CB1 receptor-positive cells located in the white matter of the folia in SCA patients have a similar morphology to microglial cells (Figure 6D.II). To investigate this, we also conducted double-labelling experiments in these structures, using specific markers for glial elements (i.e. Iba-1 for microglia and GFAP for astrocytes) as well as for infiltrated macrophages (i.e. Cd68). Our data indicated the presence of CB1 receptors in Iba-1- and GFAP-positive cells in both the granular layer (Figure 7A–H) and the white matter structures (Figure 7I-P), while the presence of CB1 receptors in Cd68-positive cells was found only in the white matter areas (Figure 7Q–T) but not in the granular layer (data not shown).
Figure 6.
DAB immunostaining for the CB1 receptor in the granular layer and the white matter areas of the cerebellum of SCA patients (C, C.I, D, D.I, F and F.I) and control subjects (A, A.I, B, B.I, E and E.I). The arrows indicate the CB1 receptor-positive cells. The microphotograph shown in D.II corresponds to a detail of the CB1 receptor-positive cells in the white matter of the folia of SCA patients (scale bars: A–F = 200 μm; A.I-F.I = 50 μm; D.II = 10 μm). The microphotographs of SCA cases correspond to subject #9 (SCA7) for the granular layer and the white matter of the folia, and to subject #10 (SCA6) for the white matter surrounding the dentate nucleus. The bar panel corresponds to the quantification of CB1 receptor immunostaining in the three structures of SCA patients and control subjects. Values correspond to the mean density of labelling in a selected area and are expressed as means ± SEM of five subjects per group. Data were assessed by Student's t-test (*P < 0.05; **P < 0.01).
Figure 7.
Double-labelling immunofluorescence using antibodies for the CB1 receptor and markers of glial elements (Iba-1 for microglia and GFAP for astrocytes) or infiltrated macrophages (Cd68), and TOPRO-3 staining, in the granular layer and white matter of the cerebellum of SCA patients. The microphotographs showed co-localization of CB1 receptors with Iba-1 in the granular layer (A–D) and the white matter (I–L), with GFAP in the granular layer (E–H) and the white matter (M–P), and with Cd68 in the white matter (Q–T) (scale bars: A–T = 20 μm). The microphotographs of SCA cases correspond to subject #8 (SCA), except in the case of Cd68 immunostaining that corresponds to subject #9 (SCA7).
Immunohistochemical study of CB2 receptors in the cerebellum of SCA patients
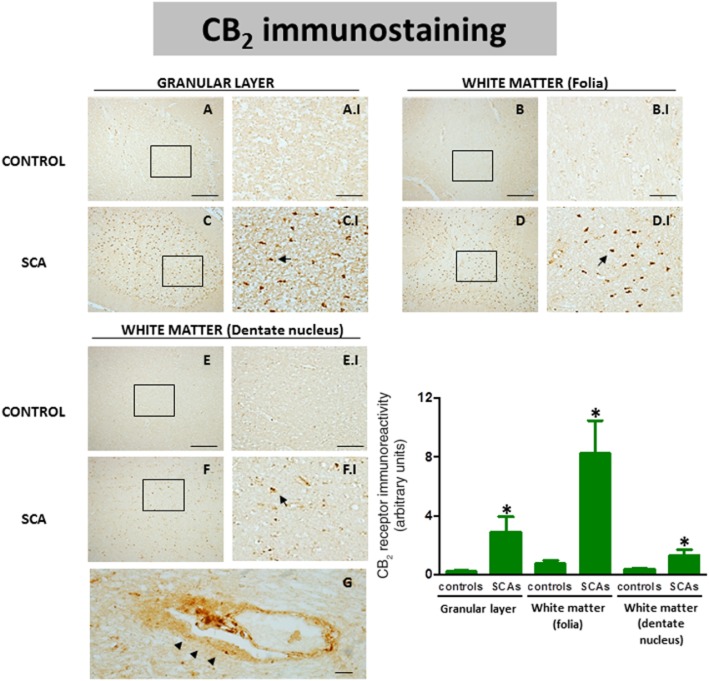
CB2 receptor expression in the CNS is, in general, relatively limited and frequently associated with glial elements, in particular, those recruited to lesioned structures in pathological conditions (Fernández-Ruiz et al., 2007). Our data indicated that in SCAs this receptor is up-regulated, as found previously for other neurodegenerative disorders (see specific references in the Introduction). Thus, we observed a more marked immunostaining for the CB2 receptor in the granular layer (Figure 8C; see arrows in C.I), in the white matter surrounding the dentate nucleus (Figure 8F; see arrows in F.I) and, in particular, in the white matter of the folia (Figure 8D; see arrows in D.I) of all SCA patients compared to control subjects (see Figure 8A and A.I, B and B.I, and E and E.I, respectively). Given the frequent problems observed with the anti-CB2 receptor antibodies used so far (reviewed in Atwood and Mackie, 2010), we were extremely careful to choose an appropriate antibody for these immunostainings as well as using appropriate internal controls (by omitting the primary antibody or by using a blocking peptide; the antibody is only for human tissue, so the option to check its specificity in CB2 receptor knockout mice is not valid as it does not label tissues from wild-type mice) to ensure the specificity of the signal found. Again, the quantification of this immunolabelling confirmed it was higher in SCA patients compared to controls in the three structures, and that this increase occurred in all patients despite the fact that they corresponded to different SCA types. The quantification data also confirmed that the differences among the different SCA patients were not significant in these structures, with the exception of the white matter of the folia, in which the SCA3 patient showed a significantly lower value compared to the others; again this explains the high SEM found in this structure for SCA patients (Figure 8).
Figure 8.
DAB immunostaining for the CB2 receptor in the granular layer and the white matter areas of the cerebellum of SCA patients (C, C.I, D, D.I, F and F.I) and control subjects (A, A.I, B, B.I, E and E.I). The arrows indicate the CB2 receptor-positive cells. The microphotographs of SCA cases correspond to subject #10 (SCA6). The microphotograph shown in G represents a detail of the CB2 receptor-positive cells (marked with arrowheads) in perivascular zones of the white matter of SCA patients (scale bars: A–F = 200 μm; A.I-F.I = 50 μm; G = 10 μm). It corresponds to the subject #8 (SCA). The bar panel corresponds to the quantification of CB2 receptor immunostaining in the three structures of SCA patients and control subjects. Values correspond to the mean density of labelling in a selected area and are expressed as means ± SEM of five subjects per group. Data were assessed by the Student's t-test (*P < 0.05).
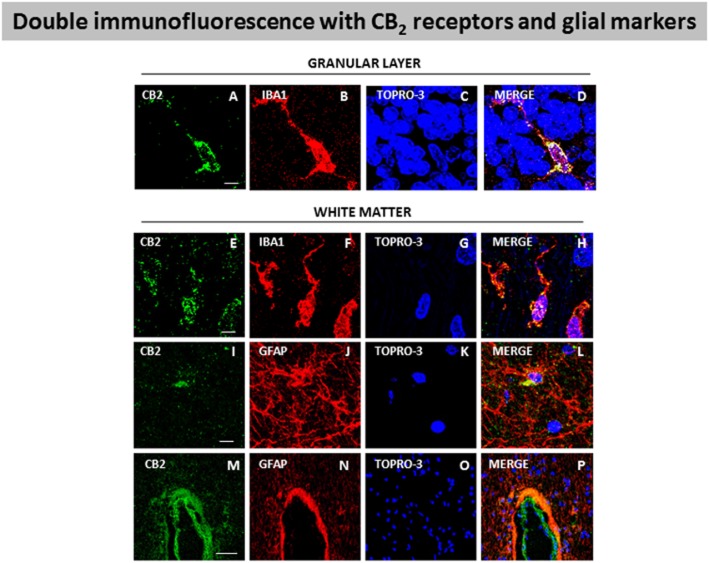
Our DAB immunostaining results indicate that the increase in CB2 receptors in SCA patients occurs in reactive microglial cells and possibly in astrocytes. The astrocytes observed in these structures in SCA patients did not develop the same hypertrophic profile as those located in the Purkinje and granular layers (see below). In the white matter areas, the most evident change experienced by these astrocytes was the increase in the number of processes (data not shown). Double-labelling studies using antibodies against CB2 receptors and GFAP confirmed that these receptors were located in astrocytes in white matter areas (see Figure 9I–L), while similar studies using Iba-1 to identify microglial cells also supported the presence of CB2 receptors in these cells in both the granular layer (see Figure 9A–D) and areas of the white matter (see Figure 9E–H). Lastly, DAB immunostaining also revealed the presence of immunoreactivity for the CB2 receptor in perivascular zones, although this only occurred in SCA patients (see Figure 8G). Double-labelling studies, using antibodies for GFAP and the CB2 receptor, demonstrated that the labelling corresponds to astrocytes (see Figure 9M–P). This co-localization did not exist in control subjects (data not shown).
Figure 9.
Double-labelling immunofluorescence using antibodies for the CB2 receptor and markers of glial elements (Iba-1 for microglia and GFAP for astrocytes), and TOPRO-3 staining, in the granular layer and white matter of the cerebellum of SCA patients. The microphotographs showed co-localization of CB2 receptors with Iba-1 in the granular layer (A–D) and the white matter (E–H), and with GFAP in the white matter (I–L) and also in perivascular zones (M–P) (scale bars: A–L = 20 μm; M–P = 50 μm). The microphotographs of SCA cases correspond to subject #9 (SCA7) for Iba-1 immunostaining and to subject #10 (SCA6) for GFAP immunostaining.
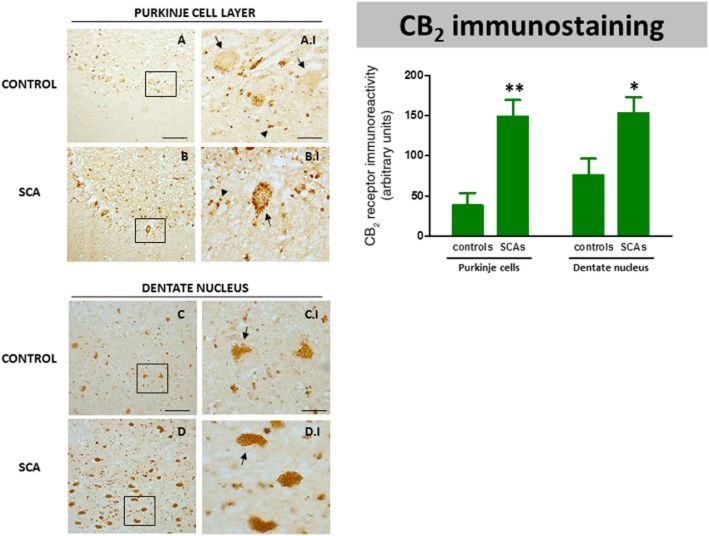
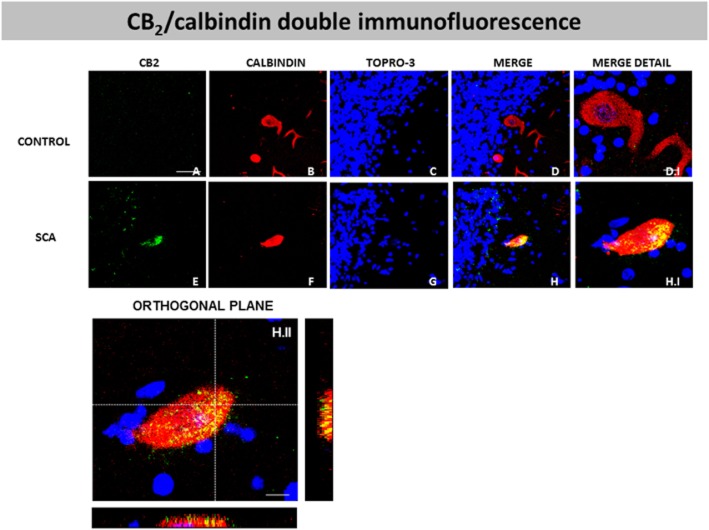
However, unexpectedly, the CB2 receptor was identified not only in glial cells but also in neurons, particularly in Purkinje cells (Figure 10A and B), and, as in the case of the CB1 receptor, this was apparent in all SCA patients but not in control subjects. In controls, the immunoreactivity for the CB2 receptor was found only in a few Purkinje cells (marked with arrows in Figure 10A.I), and, in the stained-cells, the labelling was found only in the basal region of the cell (marked with arrows in Figure 10A.I). This observation suggests that this labelling corresponds to terminals of basket cells rather than to Purkinje cells, as has been proposed in previous studies (Suárez et al., 2008). However, the immunoreactivity for the CB2 receptor seen in all SCA patients in Purkinje cells was much more intense, with most of the cells being immunolabelled and the immunoreactivity occupying most of the cell body (see Figure 10B.I). We next conducted double-labelling studies using antibodies against the CB2 receptor and calbindin to label Purkinje cells. We found that the CB2 receptors strongly co-localized with calbindin in all SCA patients (see Figure 11E, F, G, H and H.I), and this was confirmed by orthogonal reconstruction (see Figure 11H.II).
DAB immunostaining for the CB2 receptor in the Purkinje layer and dentate nucleus of the cerebellum of SCA patients (B, B.I, D and D.I) and control subjects (A, A.I, C and C.I). The arrows indicate the Purkinje cells that are CB2 receptor-positive, whereas the arrowheads indicate other CB2 receptor-positive cells that presumably are Bergmann glial cells (scale bars: A–D = 100 μm; A.I-D.I = 25 μm). The microphotographs of SCA samples correspond to subject #9 (SCA7) for the Purkinje layer and to subject #10 (SCA6) for the dentate nucleus. The bar panel corresponds to the quantification of CB2 receptor immunostaining in the two structures of SCA patients and control subjects. Values correspond to the mean density of labelling in individual cells and are expressed as means ± SEM of five subjects per group. Data were assessed by Student's t-test (*P < 0.05; **P < 0.01).
Figure 11.
Double-labelling immunofluorescence using antibodies for the CB2 receptor and calbindin, and TOPRO-3 staining, in the Purkinje layer of the cerebellum of SCA patients (E–H and H.I) and control subjects (A–D, D.I), showing co-localization of CB2 receptors and calbindin in SCA patients but not in control subjects (scale bars: A–H = 50 μm; D.I and H.I = 10 μm). The H.II panel corresponds to the orthogonal reconstruction from confocal z-series in x-z (below) and y-z (right) planes. This reconstruction confirms the co-localization of CB2 receptor and calbindin in SCA patients (scale bars: H.II = 10 μm). The microphotographs of SCA samples correspond to subject #9 (SCA7).
CB2 receptor immunolabelling was also found in the Purkinje layer in cells that do not appear to be neurons and that presumably could correspond to Bergmann glial cells (see Figure 10A.I and B.I). This immunoreactivity was found in control subjects (Figure 10A.I; indicated by arrowheads) but to a greater extent in all SCA patients (Figure 10B.I; indicated by arrowheads). As mentioned above, the Bergmann glial cells are activated (hypertrophic) in SCA patients (Robitaille et al., 1995); we also observed this (see Figure 3). We tried to identify these CB2 receptor-positive cells using double-labelling studies with L1-NCAM, vimentin or GFAP as glial markers, but our results were not conclusive (data not shown). Lastly, CB2 receptor immunoreactivity was also identified in neuronal elements in the dentate nucleus (Figure 10C and D), and, as with the CB1 receptors, the immunoreactivity was much more intense in all SCA patients (Figure 10D; see arrows in D.I) than in control subjects (Figure 10C; see arrows in C.I). We also quantified the CB2 receptor immunolabelling in this nucleus and in the Purkinje cells and, in both cases, we confirmed that the immunolabelling was higher in SCA patients compared to controls, and that this increase occurred in all patients despite them corresponding to different SCA types, and that the differences among the different SCA patients were not significant in either structure (Figure 10). As with the CB1 immunostaining, this increase was seen in surviving neurons when they were analysed individually, but the labelling would be lower in SCA patients compared to controls if the whole structure was considered instead of the labelling in individual cells, due to the marked loss of neurons seen in these two structures in SCA patients (Figure 10).
Discussion
The data obtained in this study support the idea that key elements of the endocannabinoid system, that is the cannabinoid CB1 and CB2 receptors, are significantly altered in the cerebellum of SCA patients compared to control subjects, as has been found in other neurodegenerative disorders (reviewed in Fernández-Ruiz et al., 2010). Our data were obtained from post-mortem samples from patients with different types of SCAs. However, it is important to note that, due to the difficulties in recruiting a sufficient number of samples for each type of SCA (see Methods), our experimental group was formed using only one subject from each of the most representative types of SCAs. A priori this might represent a problem with reliability of findings, given that these SCAs have different pathologies with regard to the specific neuronal subpopulation that is most affected. However, losses of Purkinje cells, as well as of other cerebellar neuronal subpopulations, have been documented in all the SCA types included in this study (Matilla-Dueñas et al., 2012), and the neuropathological information provided by the biobank and our preliminary examination of all the post-mortem samples received allowed us to confirm these neuronal losses. In addition, despite the specific characteristics of each type of SCA, we found the same responses for CB1 and CB2 receptors and, in general, to a similar extent in all SCA types included in our study, which support the validity of our experimental approach. Of course, it would have been better to concentrate on only one SCA type but, at present, it is difficult to collect a sufficient number of subjects of the same SCA type that are appropriately matched for gender, age and post-mortem delay, or that do not have to be excluded as a result of their medication and intoxication histories. We were extremely careful in the selection of SCA cases and control subjects and, in fact, only 50% of the samples available were used (see Methods). In this regard, we excluded all cases of cannabis, alcohol or tobacco addiction and confirmed the SCA group contained samples from patients that had received standard medications for this condition. Within the cases selected, we found two potential problems, a patient that had been treated with nabilone, and the use of morphine in all SCA patients days or hours before death. However, we concluded that these two factors had no significant effect on our results; the nabilone treatment occurred 6 years before death in this patient and palliative treatment with morphine (or fentanyl) was also administered to patients from the control group that had died from colon carcinoma, and no changes in the cerebellar levels of CB1 and CB2 receptors were observed in samples from the control group.
Our immunohistochemical analyses revealed that the level of CB1 receptors was increased in the SCA group in all the cerebellar structures investigated, that is the Purkinje and granular layers, dentate nucleus and white matter areas. However, the physiopathological significance of this up-regulatory response deserves some comments. For example, the increase in CB1 receptor immunolabelling observed in neurons, that is Purkinje cells and neurons of the dentate nucleus, of SCA patients was seen in those cells that survived the degeneration, but, as the number of neurons in these structures was significantly lower than the controls, the labelling in the whole structures was rather reduced compared to control subjects. As this up-regulation of CB1 receptors was seen in those neurons that had survived the degeneration, it is possible that this overexpression of CB1 receptors may serve as a marker of those cells that are going to degenerate or even that this response may contribute to the cerebellar dysfunction/degeneration during SCA pathogenesis. Indeed, activation of CB1 receptors has been associated with ataxia in some animal species (DeSanty and Dar, 2001; Patel and Hillard, 2001), so it is possible that the increase in CB1 receptors observed in the cerebellum of SCA patients, in particular in Purkinje cells, may be related to the occurrence of ataxia in these subjects. This would be similar to the scenario in Parkinsonian patients where an increase in CB1 receptor binding and signalling occurs in parallel with onset of rigidity and bradykinesia in these patients (Fernández-Ruiz, 2009). However, in Parkinson's disease the up-regulation of neuronal CB1 receptors occurs in neurons that do not degenerate and as a consequence of an adaptive response (Fernández-Ruiz, 2009). In contrast, in SCAs the overexpression of neuronal CB1 receptors occurs in those subpopulations that degenerate, thus making it unlikely that these CB1 receptor changes are the cause of the ataxic gait. Alternatively, as it has been well-demonstrated that cannabinoid receptors can have a neuroprotective function (Fernández-Ruiz et al., 2010), therefore, it is possible that the overexpression of CB1 receptors in degenerating cerebellar neurons may represent a protective response elicited to attenuate the cerebellar dysfunction. This may also apply to the response of CB1 receptors in the other cerebellar areas studied. The increase in CB1 receptors observed in the granular layer and white matter areas in SCA patients was not influenced by losses in cells containing this receptor, as, in these regions the CB1 receptor is located in glial elements and its increase is probably related to the reactive states exhibited by these cells during the pathology of SCA. If the overexpression of CB1 receptors is associated with a protective response in SCA, then, this could be pharmacologically exploited to develop possible novel disease-modifying therapies based on the pharmacological activation of this receptor type (see below).
We also studied the response of the other major receptor type for the endocannabinoid system, the CB2 receptor, in the cerebellum of SCA patients. Similar to the CB1 receptors, an overexpression of CB2 receptors was apparent in all the cerebellar structures investigated, that is the Purkinje and granular layers, dentate nucleus and white matter areas. An overexpression of this receptor typically occurs in glial elements, such as astrocytes and, in particular, reactive microglia, in most neurodegenerative disorders (see the Introduction) and we obtained similar results in the granular layer and the white matter regions of our SCA patients. As with the other pathologies (reviewed in Fernández-Ruiz et al., 2007; 2010; Benito et al., 2008), these findings suggest that the CB2 receptor may act as a controller of the glial influences on neurons, in particular, by enhancing the trophic support by astrocytes and by reducing the cytotoxic effects exerted by reactive microglial cells. However, we also observed an overexpression of CB2 receptors in Purkinje neurons, as well as in neurons of the dentate nucleus, in all SCA patients similar to the results found for the CB1 receptor. In fact, this overexpression also occurred in neurons that had survived the degenerative process, so as with the CB1 receptor, the levels of this receptor in the whole structures were reduced in the SCA patients compared to controls due to the loss of these neurons during the pathogenesis. Therefore, the same issues that have been discussed with regard to the physiopathological meaning of the overexpression of neuronal CB1 receptors (i.e. is it a marker of those neurons that are going to degenerate, does it contribute to the cerebellar dysfunction/degeneration, or is it a part of a protective response?) may be also applied to the overexpression of the neuronal CB2 receptor in those cells that degenerate in SCAs. The therapeutic implications of these responses are particularly challenging, not only for SCAs but also for other neurodegenerative disorders, such as Parkinson's disease where CB2 receptors have also been identified in degenerating neurons (unpublished data). If these observations are confirmed, the pharmacological scenario may be relatively similar in both of these disorders, and this should be further investigated by targeting the two key cannabinoid receptors located in the various types of neural cells.
In conclusion, our study demonstrates that the endocannabinoid system is significantly altered in the cerebellum of SCA patients. These changes included an increase in CB1 and CB2 receptors in glial elements in the granular layer and the white matter areas, which may be part of an endogenous protective response and could be used to develop novel pharmacological therapies based on targeting these receptors to control glial influences on cerebellar neurons. However, we also demonstrated that CB1 and CB2 receptors are overexpressed in those neurons that degenerate in this disease, in particular in Purkinje neurons, a fact that, in addition to its therapeutic implications, may be related to the pathogenesis of SCA. We are presently engaged in additional experiments aimed at confirming that the changes observed in the cerebellum of SCA patients can be reproduced in experimental models of SCAs (i.e. Tg-SCA3 mice) and to determine the timing/sequence of these alterations. We also plan to develop additional pharmacological experiments aimed at identifying the pharmacological profile of those cannabinoid compounds able to arrest/delay disease progression in these transgenic mouse models.
Acknowledgments
This study has been supported by MICINN (SAF2009-11847 and SAF2010-16706), CIBERNED (CB06/05/0089) and Fundación Eugenio Rodríguez Pascual. Carmen Rodríguez-Cueto is a predoctoral fellow supported by FPI Program-Ministry of Science, and Cristina Benito is a Juan de la Cierva postdoctoral fellow also supported by the Ministry of Science. Authors are indebted to Yolanda García-Movellán for administrative support.
Glossary
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- DAB
3,3′-Diaminobenzidine
- GFAP
glial fibrillary acidic protein
- KPBS
potassium phosphate-buffered saline
- SCA
spinocerebellar ataxia
- NBB
The Netherlands Brain Bank
- TBS
Tris-buffered saline
Conflicts of interest
Authors declare that they have no conflict of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–302. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- Benito C, Núñez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Kim WK, Chavarría I, Hillard CJ, Mackie K, Tolón RM, et al. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci. 2005;25:2530–2536. doi: 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Romero JP, Tolón RM, Clemente D, Docagne F, Hillard CJ, et al. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci. 2007;27:2396–2402. doi: 10.1523/JNEUROSCI.4814-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Tolón RM, Pazos MR, Núñez E, Castillo AI, Romero J. Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol. 2008;153:277–285. doi: 10.1038/sj.bjp.0707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Finazzi-Agrò A, Bernardi G, Maccarrone M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol Sci. 2007;28:180–187. doi: 10.1016/j.tips.2007.02.004. [DOI] [PubMed] [Google Scholar]
- De Yébenes J. Phase II-clinical trial on neuroprotection with cannabinoids in Huntington's disease (SAT-HD) EudraCT. 2010 2010-024227-24. [Google Scholar]
- DeSanty KP, Dar MS. Cannabinoid-induced motor incoordination through the cerebellar CB1 receptor in mice. Pharmacol Biochem Behav. 2001;69:251–259. doi: 10.1016/s0091-3057(01)00539-1. [DOI] [PubMed] [Google Scholar]
- Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894. doi: 10.1016/S1474-4422(10)70183-6. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol. 2009;156:1029–1040. doi: 10.1111/j.1476-5381.2008.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Romero J, Velasco G, Tolón RM, Ramos JA, Guzmán M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival. Trends Pharmacol Sci. 2007;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, García C, Sagredo O, Gómez-Ruiz M, de Lago E. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets. 2010;14:387–404. doi: 10.1517/14728221003709792. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Moreno-Martet M, Rodríguez-Cueto C, Palomo-Garo C, Gómez-Cañas M, Valdeolivas S, et al. Prospects for cannabinoid therapies in basal ganglia disorders. Br J Pharmacol. 2011;163:1365–1378. doi: 10.1111/j.1476-5381.2011.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratkin JD, Vig PJ. Neuropathology of degenerative ataxias. Handb Clin Neurol. 2012;103:111–125. doi: 10.1016/B978-0-444-51892-7.00005-X. [DOI] [PubMed] [Google Scholar]
- García C, Palomo-Garo C, García-Arencibia M, Ramos J, Pertwee R, Fernández-Ruiz J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson's disease. Br J Pharmacol. 2011;163:1495–1506. doi: 10.1111/j.1476-5381.2011.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HH, Schmid PC, Bittigau P, Lastres-Becker I, Berrendero F, Manzanares J, et al. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J Neurochem. 2001;78:1415–1427. doi: 10.1046/j.1471-4159.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Mizusawa H, Fujita T, Ohkoshi N, Doi M, Komatsuzaki Y, et al. Calbindin-D 28k immunoreactivity in the cerebellum of spinocerebellar degeneration. J Neurol Sci. 1995;129:179–185. doi: 10.1016/0022-510x(94)00279-w. [DOI] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Goldsmith PC, Greenberg DA. CB1 cannabinoid receptor induction in experimental stroke. Ann Neurol. 2000;48:257–261. [PubMed] [Google Scholar]
- Klockgether T. Update on degenerative ataxias. Curr Opin Neurol. 2011;24:339–345. doi: 10.1097/WCO.0b013e32834875ba. [DOI] [PubMed] [Google Scholar]
- Koeppen AH, Davis AN, Morral JA. The cerebellar component of Friedreich's ataxia. Acta Neuropathol. 2011;122:323–330. doi: 10.1007/s00401-011-0844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Matilla-Dueñas A, Corral-Juan M, Volpini V, Sanchez I. The spinocerebellar ataxias: clinical aspects and molecular genetics. Adv Exp Med Biol. 2012;724:351–374. doi: 10.1007/978-1-4614-0653-2_27. [DOI] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Núñez E, Benito C, Tolón RM, Hillard CJ, Griffin WS, Romero J. Glial expression of cannabinoid CB(2) receptors and fatty acid amide hydrolase are β-amyloid-linked events in Down's syndrome. Neuroscience. 2008;151:104–110. doi: 10.1016/j.neuroscience.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Orr HT. Cell biology of spinocerebellar ataxia. J Cell Biol. 2012;197:167–177. doi: 10.1083/jcb.201105092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system. Prog Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain. 2009;132:3152–3164. doi: 10.1093/brain/awp239. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Cannabinoid CB1 receptor agonists produce cerebellar dysfunction in mice. J Pharmacol Exp Ther. 2001;297:629–637. [PubMed] [Google Scholar]
- Pisani A, Fezza F, Galati S, Battista N, Napolitano S, Finazzi-Agrò A, et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson's disease patients. Ann Neurol. 2005;57:777–779. doi: 10.1002/ana.20462. [DOI] [PubMed] [Google Scholar]
- Price DA, Martinez AA, Seillier A, Koek W, Acosta Y, Fernandez E, et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Eur J Neurosci. 2009;29:2177–2186. doi: 10.1111/j.1460-9568.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille Y, Schut L, Kish SJ. Structural and immunocytochemical features of olivopontocerebellar atrophy caused by the spinocerebellar ataxia type 1 (SCA-1) mutation define a unique phenotype. Acta Neuropathol. 1995;90:572–581. doi: 10.1007/BF00318569. [DOI] [PubMed] [Google Scholar]
- Sagredo O, González S, Aroyo I, Pazos MR, Benito C, Lastres-Becker I, et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for Huntington's disease. Glia. 2009;57:1154–1167. doi: 10.1002/glia.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagredo O, Pazos MR, Satta V, Ramos JA, Pertwee RG, Fernández-Ruiz J. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington's disease. J Neurosci Res. 2011;89:1509–1518. doi: 10.1002/jnr.22682. [DOI] [PubMed] [Google Scholar]
- Salazar M, Carracedo A, Salanueva IJ, Hernández-Tiedra S, Lorente M, Egia A, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rüb U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21. doi: 10.1007/s00401-012-1000-x. [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Taylor CR. Antigen retrieval techniques: current perspectives. J Histochem Cytochem. 2001;49:931–937. doi: 10.1177/002215540104900801. [DOI] [PubMed] [Google Scholar]
- Suárez J, Bermúdez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, et al. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509:400–421. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Valdeolivas S, Satta V, Pertwee RG, Fernández-Ruiz J, Sagredo O. Sativex-like combination of phytocannabinoids is neuroprotective in malonate-lesioned rats, an inflammatory model of Huntington's disease: role of CB1 and CB2 receptors. ACS Chem Neurosci. 2012;3:400–406. doi: 10.1021/cn200114w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, Ravikumar B, et al. Aggregate-prone proteins are cleared from the cytosol by autophagy: therapeutic implications. Curr Top Dev Biol. 2006;76:89–101. doi: 10.1016/S0070-2153(06)76003-3. [DOI] [PubMed] [Google Scholar]
- Witting A, Weydt P, Hong S, Kliot M, Moller T, Stella N. Endocannabinoids accumulate in spinal cord of SOD1 G93A transgenic mice. J Neurochem. 2004;89:1555–1557. doi: 10.1111/j.1471-4159.2004.02544.x. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]