Abstract
Background and Purpose
H2S induces vasodilatation by opening KATP channels but it may also affect other ion channels. The aim of this study was to investigate the effect of H2S on intestinal motility in rats and its underlying mechanism.
Experimental Approach
The tension of intestinal muscle strips, afferent firing of intestinal mesenteric nerves, length of duodenal smooth muscle cells and whole-cell membrane potential of dorsal root ganglion (DRG) neurons were monitored. H2S-producing enzymes were located by immunofluorescence staining.
Key results
NaHS exerted early transient excitation and late long-lasting inhibition on the intestinal contraction. The excitation was attenuated by TRPV1 antagonists capsazepine, A784168, SB-366791 and NK1 receptor antagonist L703606, while the inhibition was attenuated by glibenclamide. NaHS increased duodenal afferent nerve firing and depolarized DRG neurons. These effects were reduced by capsazepine and A784168. NaHS relaxed isolated duodenal smooth muscle cells. The KATP channels were expressed in smooth muscle cells. Cystathionine β-synthase and cystathionine γ-lyase were expressed in rat duodenal myenteric neurons. L-cysteine and S-adenosyl-L-methionine increased the contraction of duodenal muscle strips, an effect attenuated by capsazepine and L703606.
Conclusions and Implications
NaHS induces biphasic effects on intestinal motility in rats while endogenous H2S only exerts an excitatory effect. This transient excitatory effect might be mediated by activation of TRPV1 channels in sensory nerve terminals with the consequent release of substance P. The long-lasting inhibitory effect might be mediated by activation of KATP channels in the smooth muscle cells. These findings reveal a novel mechanism for the excitatory effect of H2S on gastrointestinal motility.
Keywords: H2S, duodenum, motility, TRPV1, KATP
Introduction
Recent studies have established that H2S is a biologically relevant signalling molecule in mammals. H2S is generated in mammalian cells mainly by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), using L-cysteine as the main substrate. CBS and CSE are expressed in many organs, such as the brain, the liver, the kidney and the enteric nervous system (ENS) (Stipanuk and Beck, 1982; Swaroop et al., 1992; Pitcher et al., 2000; Wang, 2002; Kamoun, 2004; Schicho et al., 2006; Martin et al., 2010). H2S acts as a physiological vasorelaxant and dilates vascular smooth muscle mainly by opening ATP-sensitive K+ (KATP) channels (Zhao et al., 2001; Cheng et al., 2004; Bhatia, 2005; Fiorucci et al., 2005; Yang et al., 2008). In addition, H2S opens KATP channels in cardiomyocytes, pancreatic beta cells, neurons and gastrointestinal smooth muscle cells, thereby regulating myocardial contractility, insulin secretion, neurotransmission and gastrointestinal contractility (Tang et al., 2010).
Although it is well recognized that H2S acts as a vasodilator by opening KATP channels, its effects on other ion channels have just begun to be addressed. H2S stimulates transient receptor potentials vanilloid 1 (TRPV1) channels in rat urinary bladder and guinea-pig airway, which cause bladder constriction and airway contraction through a neurogenic inflammation mechanism (Patacchini et al., 2004; Trevisani et al., 2005). Moreover, Schicho et al. demonstrated that NaHS, the donor of H2S, caused secretion in the guinea-pig and human colon by activating TRPV1 receptors located in afferent nerves, which resulted in the local release of substance P (SP) (Schicho et al., 2006). However, whether a similar mechanism is involved in the regulation of gastrointestinal motility induced by H2S is still unclear.
In our preliminary experiments, we found that NaHS exerted a biphasic effect on rat duodenal motility, inducing early transient excitation and late long-lasting inhibition. We hypothesized that the early transient excitation of the duodenal motility might be mediated by the opening TRPV1 channels in the sensory nerve fibres followed by the release of SP and the late long-lasting inhibition was caused by the opening KATP channels in smooth muscle.
In order to test our hypothesis, we monitored the isometric tension of intestinal muscle strips and the length of the isolated smooth muscle cells of duodenum to investigate the role of NaHS and endogenous H2S in regulating duodenal motility. The KATP channels, CBS and CSE were located by immunofluorescence staining. The effect of NaHS on afferent nerve activity and dorsal root ganglion (DRG) neurons were monitored by nerve recording and whole-cell patch clamp recording. The results support our hypothesis and reveal a new mechanism for H2S-induced regulation of intestinal motility.
Methods
All drug/molecular target nomenclature used (e.g. receptors, ion channels and so on) conforms to BJP's Concise Guide to PHARMACOLOGY (Alexander et al., 1999).
Animals
Wistar male rats (250–300 g) were provided by the Experimental Animal Center of Shandong University. All the procedures described were approved by the Ethics Committee for Research on Animals, Shandong University School of Medicine. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Recording of the tension of intestinal muscle strips in vitro
Rats were killed by cervical dislocation and segments of the duodenum, jejunum, ileum and colon were immediately removed and put into a glass beaker containing 4°C Krebs solution. The segments were cut along the mesenteric border and the content of the segments was washed away. The mucosal and submucosal layers were striped carefully using fine forceps under a microscope (SMZ-B4, Optec Instrument, Chongqing, China) in a silica gel dish filled with oxygenated Krebs solution. The longitudinal muscle strips (4 mm wide, 10 mm long) were prepared by cutting parallel to the longer axis of the intestinal segments. The isolated smooth muscle strips were suspended in tissue chambers containing 5 mL Krebs solution bubbled with 5%CO2/95%O2. The chambers were maintained at 37°C using a water pump (ZH-Z, Zhenghua Biological Equipment Ltd., Co., Huaibei, China). One end of the muscle strip was connected to a fixed hook and the other end was attached to an external isometric force transducer (JH-2B, Chengdu Instrument Factory, Chengdu, China). A polygraph system (ML785-PowerLab, ADI, Sydney, Australia) was used to record the tension of the muscle strips. The muscle strips were incubated at a 0.5–1.0 g preload. Once the spontaneous contraction was stable for more than 15 min, NaHS (0.1, 0.5, 1.0 and 2.0 mM), L-cysteine (0.1, 0.5 and 1.0 mM) or S-adenosyl-L-methionine (SAM, 0.1 mM, 0.5 mM and 1.0 mM) were administrated to the chambers. Each muscle strip was exposed to the reagent only once. In other experiments, the muscle strips were pretreated with Carbachol (2 μM), capsazepine (10 μM), L703606 (10 μM), glibenclamide (Glib, 10 μM), tetrodotoxin (TTX, 10 μM), L-NG nitroarginine methyl ester (L-NAME, 0.1 mM), A784168 (0.3 μM), SB-366791 (1 μM), D, L-propargylglycine (PAG, 0.5 mM) or amino-oxyacetic acid (AOAA, 0.05 mM) 10–20 min before NaHS, L-cysteine or SAM administration. Control strips were exposed to normal saline (NS) or DMSO for the same time period.
Mesenteric afferent nerve recording
Rats were anaesthetized by i.p. administration of pentobarbitone sodium (60 mg kg−1), a segment of duodenum with a clear artery projection and the mesentery attached was excised carefully and immersed in Krebs solution quickly. The tissue was moved to a purpose-built organ bath and fixed the both ends to the organ chamber, which was continuously perfused with Krebs solution bubbled with 5% CO2/95%O2 at a rate of 10 mL min−1 at 34°C. Intraluminal pressure was recorded by a pressure transducer. The mesenteric bundle was pinned out onto the sylgard base of the recording chamber and one of the two mesenteric nerves was carefully dissected out of the neurovascular mesenteric bundle. The nerve bundle was wrapped around one arm of a bipolar wire platinum recording electrode, while one strip of connective tissue was attached to the other electrode. The signal was amplified 10 000 times and filtered with a bandwidth of 100–1000 Hz via a single channel 1902 preamplifier/filter (Cambridge Electronic Design, Cambridge, UK). Signals of the pressure transducer were transmitted to another single channel 1902 preamplifier/filter. The raw signals from 1902 were sent to a power Micro 1401 interface system (Cambridge Electronic Design) and stored in a computer using Spike 2 software (Cambridge Electronic Design).
Cell culture and whole-cell patch-clamp recordings of DRG neurons
Rats were killed by cervical dislocation and the entire spinal columns were removed, transferred to a beaker containing ice-cold sterile Krebs solution, and bisected longitudinally. Bilateral DRG (T7–L5) were dissected and washed twice with L-15 medium (Gibco, Gaithersburg, MD, USA), and then incubated for 50 min in collagenase type 1 (1 mg mL−1, Sigma-Aldrich, St. Louis, MO, USA) and 0.5 mL trypsin (0.25%, Gibco) in 20 mL L-15 medium at 37°C. DRG were then washed twice and transferred to 2 mL L-15 medium containing 10% FBS. A single-cell suspension was subsequently obtained by repeated trituration using a fire-polished Pasteur pipe. The cells were then cultured at 37°C in a 5% CO2 incubator (Thermo Forma, Hamilton, NJ, USA).
Whole-cell patch-clamp recordings were performed using an Axon Instruments Multiclamp 700B amplifier (Molecular Devices, New York, NY, USA) interfaced to Digidata 1440A with the pClamp 10.2 software (Molecular Devices). Glass pipettes filled with an intracellular saline had a resistance of 5–7 MΩ. The external solution was Krebs saline. All recordings were conducted at 30°C. In this study, we used relatively small DRG neurons (17–27 μm in diameter) that are considered to transmit visceral sensory information (Scroggs and Fox, 1992).
Measurement of the length of isolated smooth muscle cells
Smooth muscle cells were prepared from duodenum as described by Zhao et al. with some modifications (Qin et al., 2009; Zhao et al., 2009). Briefly, the muscle strips were placed into Ca2+-free physiological saline solution (PSS), cut into small segments (2 × 2 mm), and then digested at 37°C in 1 mL Ca2+-free solution which contained 1.7–1.9 mg collagenase II (Worthington Biochemicals, Lakewood, NJ, USA), 500–700 μg papain (Sigma-Aldrich), 1 mg dithiothreitol (Sigma-Aldrich), 0.5 mg trypsin inhibitor (Sigma-Aldrich), 1 mg BSA (Roche Diagnostics GmbH, Mannhein, Germany) for 20–30 min. Next, the muscle segments were washed several times with the modified Kraft–Bruhe solution (KB solution) followed by gentle trituration using a wide-bore fire-polished glass pipette (2 mm bore diameter) to obtain a cell suspension. Drops of the cell suspension were transferred to a dish and an inverted microscope (IX51, Olympus, Tokyo, Japan) was used to observe the smooth muscle cells and take photographs. Image Pro Plus 6.0 (Media Cybernetics, Leiden, The Netherlands) was used to measure the length of smooth muscle cells.
Immunofluorescence in longitudinal muscle myenteric plexus
Longitudinal muscle myenteric plexus (LMMP) was prepared according to our previous report (Che et al., 2012). In the double-labelling experiments, two proteins were incubated with the myenteric plexus; these included CBS and neuronal nuclei (NeuN; neuron-specific nuclear protein), CSE and NeuN, CBS and CSE. The LMMP were incubated in Krebs solution containing papain (10 mg mL−1) for 50 min to digest the smooth muscle cells. Thereafter, the specimen was stretched in order to expose the myenteric plexus more clearly. The preparations were washed three times with PBS and soaked in 4% paraformaldehyde for 30 min. Subsequently, they were incubated with hydrogen peroxide (3%) for 15 min to quench endogenous peroxidase activity, tritonX-100 (0.2%) for 20 min to improve permeability of cell membrane and donkey serum (10%) for 1 h to block non-specific binding. All these procedures were conducted at room temperature. For KIR6.2 (a subunit of KATP channels) and SM22 (a smooth muscle marker) double staining, the tissues were incubated with a primary antibody mixture containing rabbit polyclonal anti-KIR6.2 antibody (1:500, Abcam, Cambridge, UK) and goat polyclonal anti-SM22 antibody (1:200, Abcam) at 4°C overnight. For KIR6.1 (a subunit of KATP channels) and SM22 double staining, the tissues were incubated with a primary antibody mixture containing rabbit polyclonal anti-KIR6.1 antibody (1:300, Abcam) and goat polyclonal anti-SM22 antibody (1:200, Abcam) at 4°C overnight. In triple-labelling studies, the LMMP specimens were incubated with either rabbit anti-CBS (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit anti-CSE (1: 200, Abcam) polyclonal antibody mixed with mouse monoclonal anti-NeuN (1:200, Chemicon, Temecula, CA, USA) and goat polyclonal anti-SM22 antibody (1:200, Abcam) at 4°C overnight. In the double-staining experiment of CBS and NeuN, CSE and NeuN, SM22 antibody was omitted from the antibody mixture, as mentioned earlier. In the double staining of CBS and NeuN, rabbit anti-CBS (1:200, Abcam) polyclonal antibody was also used. In the experiment of double staining of CBS and CSE, the tissues were incubated with rabbit anti-CBS (1:100, Santa Cruz Biotechnology) and goat anti-CSE (1: 100, Santa Cruz Biotechnology) polyclonal antibody.
After several washes for 10 min each in PBS, the tissues were incubated with corresponding secondary antibodies, Alexa Fluor 350-conjugated donkey anti-goat (1:300, Invitrogen, Carlsbad, CA, USA), Alexa Fluor 488-conjugated donkey anti-mouse (1:300, Invitrogen), Alexa Fluor 488-conjugated donkey anti-goat (1:300, Invitrogen) and Alexa Fluor 568-conjugated donkey anti-rabbit (1:300, Invitrogen) IgG for 2 h at room temperature followed by three washes with PBS. CBS and CSE antibodies were tested for their specificity by pre-absorption controls with human CBS full-length recombinant protein (1:5, Abnova, Taipei, Taiwan) and human recombinant CSE protein (1:5, Santa Cruz Biotechnology). Staining was absent after the antibodies were pre-absorbed with the respective recombinant proteins. The specimens were examined under confocal microscopy (LSM780; Carl Zeiss, Jena, Germany). The protocol for localization of KIR6.2 and SM22 in smooth muscle cells was same of that for LMMP. The smooth muscle cells used in this experiment were purchased from Chi Scientific (Jiangsu, China). Negative controls were performed during the staining procedure by omitting the primary antibodies from the incubation solution and no positive staining was found in these specimens.
Chemicals
NaHS, L-cysteine, carbachol, DMSO, L703606, Glib, L-NAME, SB-366791, capsaicin, PAG and AOAA were purchased from Sigma (Sigma-Aldrich). A784168 was purchased from Axon (Axon Medchem, Groningen, The Netherlands). Capsazepine was provided by Tocris (Tocris Cookson, Ellisville, MO, USA). TTX was purchased from RuiFang Biotechnology Ltd., Co. (Dalian, China). SAM was purchased from SenFu Biotechnology limited company (Xian, China). Glib, L703606, capsazepine, A784168, capsaicin and SB-366791 were dissolved in DMSO, with final DMSO concentration less than 0.1%. L-cysteine, PAG, AOAA and SAM were dissolved in NS and adjusted to pH 7.4 with NaOH or HCl. NaHS was dissolved in Krebs solution and was freshly prepared just before use. The stock solutions were directly added into the bath solution to achieve the final concentration.
Solutions
The Krebs solution was composed of the following reagents (in mM): NaCl 120.6, KCl 5.9, CaCl2 2.5, KH2PO4 1.2, MgCl2 1.2, NaHCO3 15.4 and glucose 11.5. The intracellular saline was composed of the following reagents (in mM): KMeSO4 110–115, NaCl 9, CaCl2 0.09, MgCl2 1.0, HEPES 10, Na3GTP 0.2 and BAPTA 0.2 and adjusted to pH 7.3 with KOH (Ma et al., 2009). The modified KB solution contained (in mM) EGTA 0.5, HEPES 10, MgCl2 3, KCl 50, glucose 10, KH2PO4 20, taurine 20 and glutamic acid 50, adjusted to pH 7.4 with KOH. The PSS contained (in mM) NaCl 135, KCl 5, MgCl2 1.2, CaCl2 2, glucose 10 and HEPES 10, adjusted to pH 7.4 with Tris. CaCl2 was removed in the Ca2+-free PSS.
Data analysis
Contraction of muscle strips
The motility index was calculated by integrating the area under the recording traces of the muscle strip tension by a software programme (Chart 5, ADInstrument, Sydney, Australia). The mean motility index for 3 min before treatment with NaHS, L-cysteine or SAM was taken as baseline and the values at different time points after each reagent (NaHS, L-cysteine or SAM) treatment were normalized to R by dividing by the baseline. The R value before the reagent treatment was equal to 1. The basal tension of muscle strips was measured by chart 5. The average basal tension for 3 min before NaHS treatment was taken as baseline. The change in basal tension after NaHS treatment was calculated by subtracting the baseline, so the change in basal tension before NaHS application was equal to 0 g.
Afferent nerve discharge
Raw multiunit nerve activity was quantified using wavemark analysis, which counted the number of action potentials crossing a pre-set threshold. A threshold level for spike counting was set at twice the baseline noise level. The mean frequency of the spikes (imp s−1) was calculated by dividing the sum of spikes by 60 in 1 min. In order to quantify the change in mean discharge rate, the mean spike frequency before NaHS administration (baseline) was subtracted from that after NaHS administration.
Patch clamp recording
The membrane potentials and currents before treatment with NaHS were taken as the baseline. The change of membrane potential and current after NaHS treatment was calculated by subtracting the baseline, so the change in each experiment before NaHS application was equal to 0 mV or 0 pA.
Length of isolated smooth muscle cells
The cell length before NaHS treatment was taken as baseline. The change of cell length after NaHS treatment was calculated by subtracting the baseline, so the change in each experiment before NaHS application was equal to 0 μm.
Values are expressed as mean ± SEM. One-way anova followed by Dunnett's test was used to compare several treatment groups with one control group. Student's t-test was used to determine the difference between two groups. Statistical analysis and curve fitting were carried out using Sigma Plot 10 (Systat Software Inc, Germany). P < 0.05 was considered to be significant. The n value = the number of rats or the number of cells in a group.
Results
Effects of NaHS on the spontaneous contraction of intestinal muscle strips
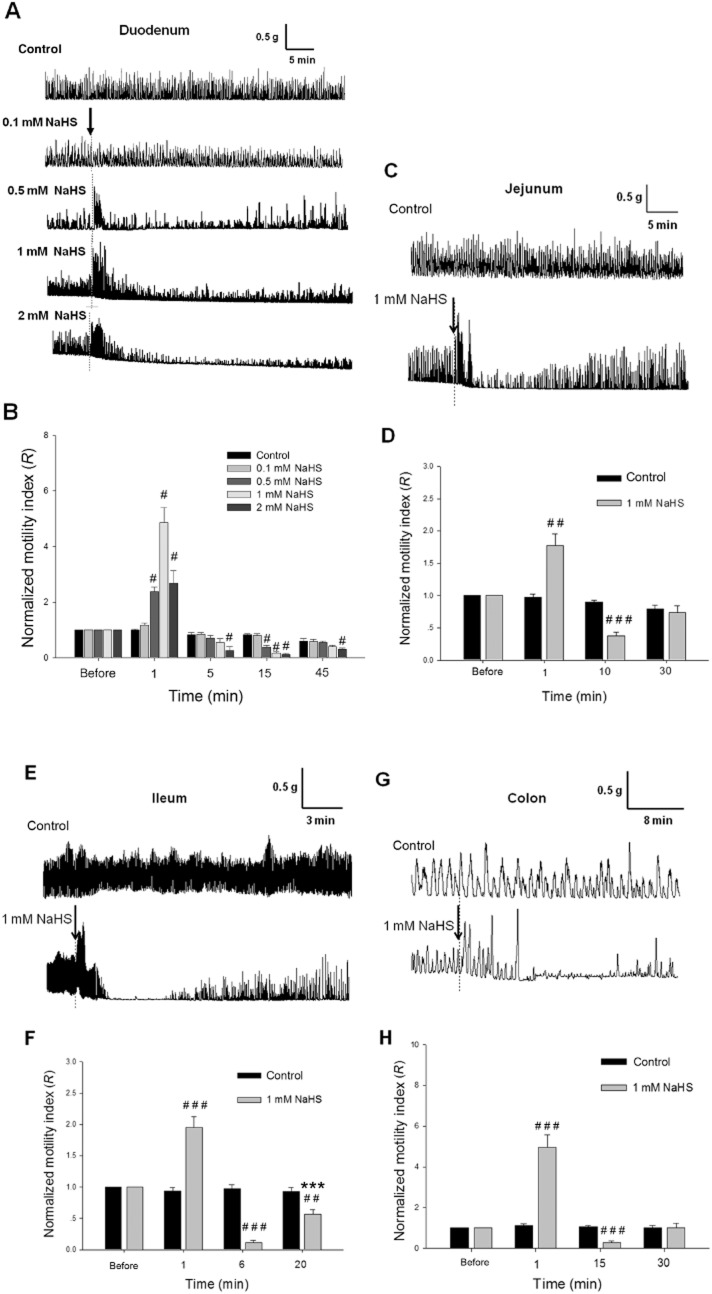
NaHS (0.5, 1.0 and 2.0 mM) caused a marked biphasic effect on the spontaneous contraction of the duodenal longitudinal muscle strips of rats (Figure 1A). Shortly after NaHS (1 mM) administration, the contraction of muscle strips was increased significantly, reached the highest level at 1–2 min and returned to normal within 5 min (Figure 1A and B). Immediately after NaHS (1 mM) administration, the R value (normalized motility index) was increased (n = 10), much bigger than that of the control group (n = 6, P < 0.05) (Figure 1B). Following this excitatory effect, there was a long-lasting (more than 30 min) inhibition of the contraction of muscle strips (Figure 1A). Fifteen minutes after NaHS application, the R value was decreased (n = 10) and became much smaller than that of the control group (n = 6, P < 0.05). The contractile activities of the muscle strips partially recovered at 45 min (Figure 1A and B).
Figure 1.
Effects of NaHS on the contraction of intestinal longitudinal muscle strips from male rats in vitro. (A) Representative recordings of the effects of NaHS (0.1, 0.5, 1.0 and 2.0 mM) on the contraction of duodenal muscle strips. (B) The time course and concentration-response of the effects of NaHS on the normalized motility index (R) of duodenal muscle strips. #P < 0.05 versus control. Representative recordings of the effects of NaHS (1 mM) on the contraction of longitudinal muscle strips from rat jejunum, ileum and colon are shown in (C), (E) and (G) respectively. The summarized data of the changes in the normalized motility index (R) at different time points after treatment with NaHS (1 mM) are shown in (D) (jejunum), (F) (ileum) and (H) (colon). ##P < 0.01 versus control, ###P < 0.001 versus control, ***P < 0.001 versus R at 6 min. The arrows indicate NaHS treatment.
NaHS (1 mM) exerted similar biphasic effects on the spontaneous contraction of the longitudinal muscle strips of rat jejunum, ileum and colon (Figure 1C, E and G). Immediately after NaHS (1 mM) administration, the R values were increased in the jejunum (n = 8), ileum (n = 6) and colon (n = 6), and were much bigger than that of the control groups (P < 0.01), but the R values were reduced in the jejunum (P < 0.001) at 10 min, in the ileum (P < 0.001) at 6 min and in the colon (P < 0.001) at 15 min after NaHS administration (Figure 1 D, F and H)
Effect of NaHS on carbachol-induced contraction of the duodenal longitudinal muscle strips
Carbachol (2 μM) induced a long-lasting increase in the tension of the muscle strips. This effect persisted for more than 20 min. NaHS was administered 10 min after carbachol administration. Immediately after NaHS treatment, the basal tension of the muscle strips was decreased in a concentration-dependent manner (Figure 2A). Five minutes after NaHS (0.2, 0.5 and 1.0 mM) administration, the basal tension of the muscle strips was reduced and was significantly different from that of the control group (n = 6, P < 0.05) (Figure 2B). The lowest concentration of NaHS added (0.05 mM) did not exert a significant effect (Figure 2).
Figure 2.
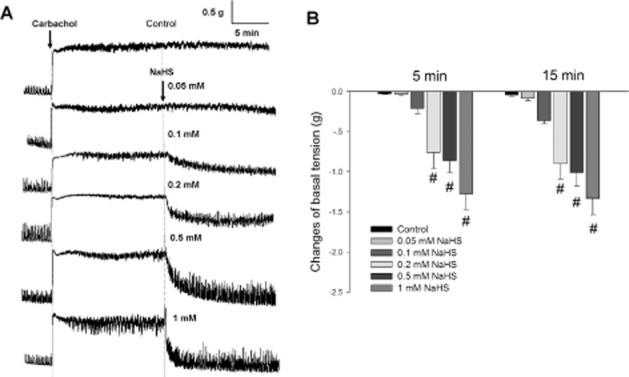
Effects of NaHS on the carbachol-induced increase in basal tension of duodenal longitudinal muscle strips. (A) Representative recordings of the effects of NaHS on the carbachol-induced contraction. Carbachol (2 μM)-induced increase in basal tension was reversed by administration of NaHS (0.2, 0.5 and 1.0 mM). The left arrow indicates the administration of carbachol and the right arrow indicates NaHS treatment. (B) The summarized data of the changes in basal tension at different time points after treatment with different concentrations of NaHS. #P < 0.05 versus control.
Involvement of TRPV1 channels and SP in NaHS-induced transient excitatory effect on the duodenal muscle strips
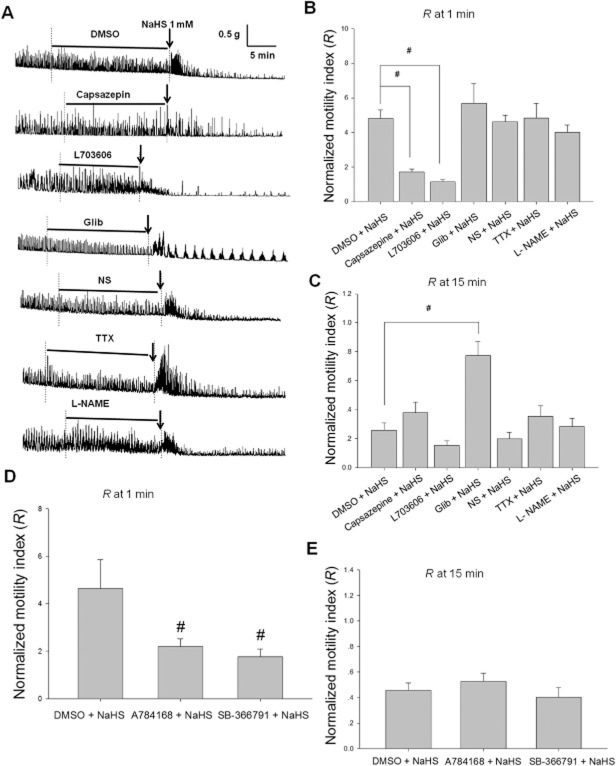
Both capsazepine (10 μM), the TRPV1 antagonist (Schicho et al., 2006), and L703606 (10 μM), the NK1 receptor antagonist (Shanmugam and Bhatia, 2010), attenuated the excitatory effect of NaHS (1 mM) on the contraction of duodenal muscle strips (Figure 3A). One minute after NaHS administration, the R values for the capsazepine + NaHS and L703606 + NaHS groups were much smaller (n = 10 and 8, respectively; P < 0.05) than that of the DMSO + NaHS group (n = 11) (Figure 3B). However, the excitatory effect of NaHS on the contraction of the muscle strips was not altered by pretreatment with Glib (10 μM), a KATP channel blocker, L-NAME (0.1 mM), the inhibitor of NO synthase or TTX (10 μM), a blocker of voltage-sensitive sodium channels on nerve fibres (Figure 3B). In order to further determine the role of TRPV1 channels, two other TRPV1 antagonists, A784168 (0.3 μM) and SB-366791 (1 μM), were used. Both A784168 and SB-366791 attenuated the excitation induced by NaHS (0.6 mM). One minute after NaHS administration, the R values in the A784168 + NaHS and SB-366791 + NaHS groups were much smaller (n = 13 and 8, respectively, P < 0.05) than that of the DMSO + NaHS group (n = 10) (Figure 3D).
Figure 3.
Pharmacological manipulation of NaHS-induced responses in rat duodenal muscle strips. (A) Representative recordings of the effects of different pretreatments on the responses of duodenal muscle strips to NaHS (1 mM). DMSO or NS acted as the vehicle control for the different chemicals. The horizontal lines indicate different chemical pretreatment and the arrows indicate NaHS treatment. The summarized data of the effects of the blockers, capsazepine (10 μM), L703606 (10 μM), Glib (10 μM), TTX (10 μM) and L-NAME (0.1 mM), on the biphasic responses to NaHS of duodenal muscle strips are shown in (B) and (C). (B) The normalized motility index (R) of the muscle strips at 1 min following NaHS administration. Capsazepine and L703606 pretreatment attenuated the excitatory effect of NaHS on the spontaneous contractions but TTX, L-NAME and Glib did not. (C) Summarized data of R at 15 min after application of NaHS. (D) and (E) Summarized data of R at 1 min and 15 min after application of NaHS respectively. A784168 (0.3 μM) and SB-366791 (1 μM) pretreatment attenuated the excitatory effect of NaHS (0.6 mM) on the spontaneous contractions. #P < 0.05 versus DMSO.
Involvement of KATP channels in NaHS-induced long-lasting inhibition on the contraction of muscle strips
Although Glib did not influence the early excitatory effect of NaHS, it attenuated the late long-lasting inhibition. At 15 min after NaHS administration, the R value in the Glib + NaHS group was much greater (n = 8, P < 0.05) than that of the control group (n = 11) (Figure 3C). Capsazepine, L703606, TTX, L-NAME, A784168 and SB-366791 did not influence the long-lasting inhibitory effect of NaHS on the contraction of muscle strips (Figure 3).
Effect of NaHS on duodenal extrinsic afferent nerves
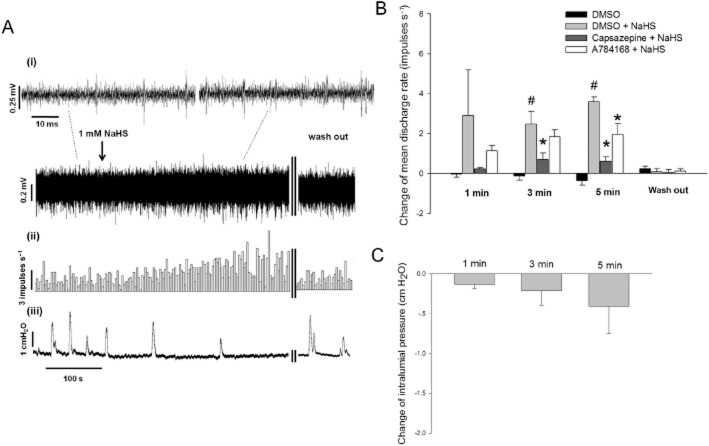
Serosal application of NaHS (1 mM) caused an increase in duodenal afferent nerve activity (Figure 4A). The mean discharge rate of afferent nerves before NaHS treatment was 3.9 ± 0.9 impulses s−1. Five minutes after NaHS application, the change in mean discharge rate of afferent nerves was increased (n = 6, P < 0.001), while the control group was slightly decreased (n = 6). The afferent response to 1 mM NaHS was significantly reduced in the presence of 10 μM capsazepine (Figure 4B). A784168 (0.3 μM) also partly attenuated the afferent response to NaHS at 5 min (Figure 4B).
Figure 4.
Effect of NaHS on duodenal mesenteric afferent nerves. (A) Representative recording of the afferent discharge (i) histogram of whole-nerve afferent activity (ii) and the intraluminal pressure (iii). (B) Summarized data of the changes in mean discharge rate. At 3 min and 5 min after NaHS (1 mM) administration, the rate of afferent discharge was significantly increased. This effect was attenuated by capsazepine (10 μM) or A784168 (0.3 μM). (C) Summarized data of the changes in intralumial pressure of the duodenum. #P < 0.05 versus DMSO, *P < 0.05 versus DMSO + NaHS.
The intraluminal pressure was not increased within 5 min following NaHS administration (Figure 4C), so we can exclude the possibility that the increase of mesenteric nerve discharge evoked by NaHS was caused by changes in intraluminal pressure.
Effect of NaHS on DRG neurons
The resting membrane potential of DRG neurons was –54.6 ± 5.1 mV (n = 30). Immediately after NaHS (1 mM) administration, the membrane potential was depolarized significantly, reaching a maximal effect at 3 min and returning to normal after washing the cells with extracellular saline (Figure 5A). Three minutes after administration of NaHS, the membrane potential was significantly depolarized (n = 7, P < 0.001) compared to that of the control group (n = 6) (Figure 5B). The response to 1 mM NaHS was significantly reduced by 10 μM capsazepine pretreatment (Figure 5B).
Figure 5.
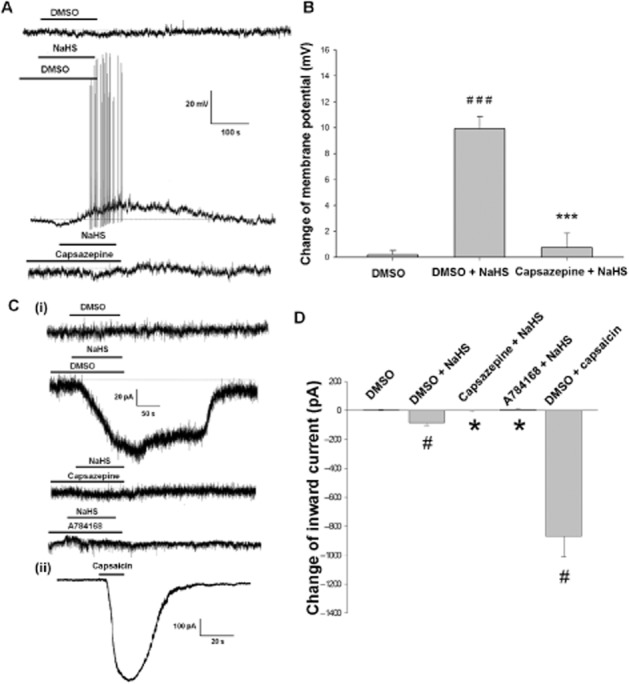
Effect of NaHS on DRG neurons. Panel A shows the representative recording of the effect of NaHS (1 mM) on the membrane potential of DRG neurons. Panel B is the summarized data of the change of membrane potential at 3 min after NaHS administration. C (i) shows at a holding potential of −60 mV, NaHS evoked an inward current of DRG neurons and pre-incubation of Capsazepine (10 μM) or A784168 (0.3 μM) blocked the NaHS-evoked inward current. C (ii) shows capsaicin (10 μM) evoked an inward current of DRG neurons. Panel D is the summarized data of the change of inward current. ###P < 0.001 versus DMSO, ***P < 0.001 versus DMSO + NaHS, #P < 0.05 versus DMSO, *P < 0.05 versus DMSO + NaHS.
At a holding potential of −60 mV, administration of NaHS (5 mM) evoked inward currents of (n = 8) in DRG neurons (compared with DMSO group, n = 6, P < 0.05), which were significantly inhibited by capsazepine (10 μM) and A784168 (0.3 μM). Capsaicin (10 μM), the agonist of TRPV1 receptors, evoked inward currents (n = 6) about 10 times of that of NaHS (5 mM) in DRG neurons (Figure 5C and D).
Expression of KATP channels in the smooth muscle of rat duodenum
SM22 is a marker of smooth muscle (Steitz et al., 2001), while KIR6.1 and KIR6.2 are subunits of KATP channels (Aguilar-Bryan and Bryan, 1999). Double labelling of KIR6.2 and SM22 in isolated smooth muscle cells (Figure 6A) showed that the KIR6.2 subunit was expressed in smooth muscle cells. Moreover, double labelling of KIR6.2 and SM22, KIR6.1 and SM22 in LMMP (Figure 6B and C) showed that both KIR6.2 and KIR6.1 of KATP channels were expressed in smooth muscle cells.
Figure 6.
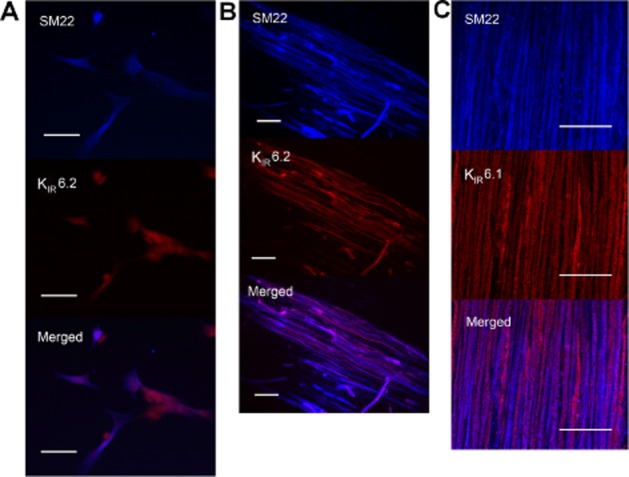
Localization of KATP channels in the duodenum of rats by immunofluorescence. (A) Representative photos of the co-localization of KIR6.2 and SM22 in the isolated smooth muscle cells from duodenum. All the SM22-immunoreactive smooth muscle cells were KIR6.2-immunoreactive. (B) and (C) Representative photos of the double labelling of KIR6.2 and SM22, KIR6.1 and SM22 in the LMMP. KIR6.2 or KIR6.1 was co-expressed with SM22 in smooth muscles. Scale bars are 50 μm.
The direct effect of NaHS on the isolated smooth muscle cells
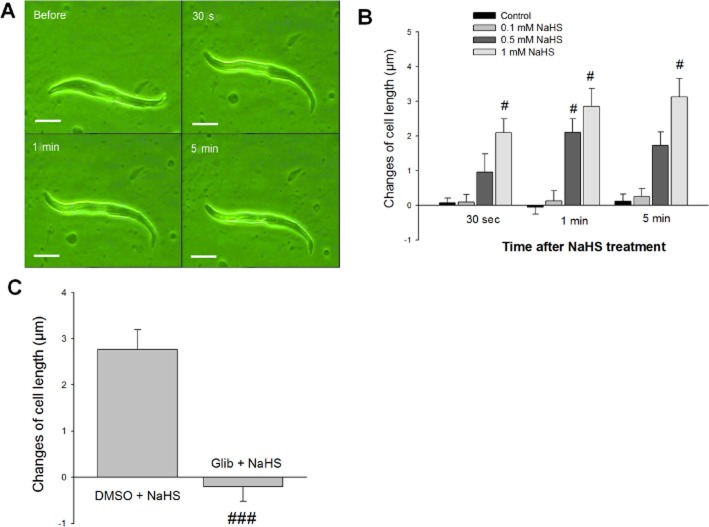
To provide further support for the hypothesis that NaHS may directly relax the smooth muscle by opening KATP channels, we investigated the effect of NaHS on isolated smooth muscle cells. The normal length of the smooth muscle cells from duodenum ranged from 50 to 150 μm. Administration of NaHS (0.5 and 1.0 mM) induced a prompt relaxation of the cells and the effect lasted for more than 5 min (Figure 7A). A low concentration of NaHS (0.1 mM) had no effect on the cell length. Five minutes after administration of NaHS (1 mM), the change of cell length was increased (n = 18, P < 0.05) when compared to the control group (Figure 7B). The relaxation induced by NaHS (1 mM) was completely abolished by pretreatment with Glib (10 μM) (Figure 7C).
Figure 7.
Effects of NaHS on the isolated smooth muscle cells from rat duodenum. (A) A smooth muscle cell at different time points before and after treatment with NaHS (1 mM). The length of this smooth muscle cell was increased following NaHS administration. Scale bars are 25 μm. (B) The concentration-response and time course of the changes in cell length following NaHS administration. #P < 0.05 versus control. (C) The effects of Glib (10 μM) pretreatment on NaHS-induced relaxation of duodenal smooth muscle cells. ###P < 0.001 versus DMSO + NaHS.
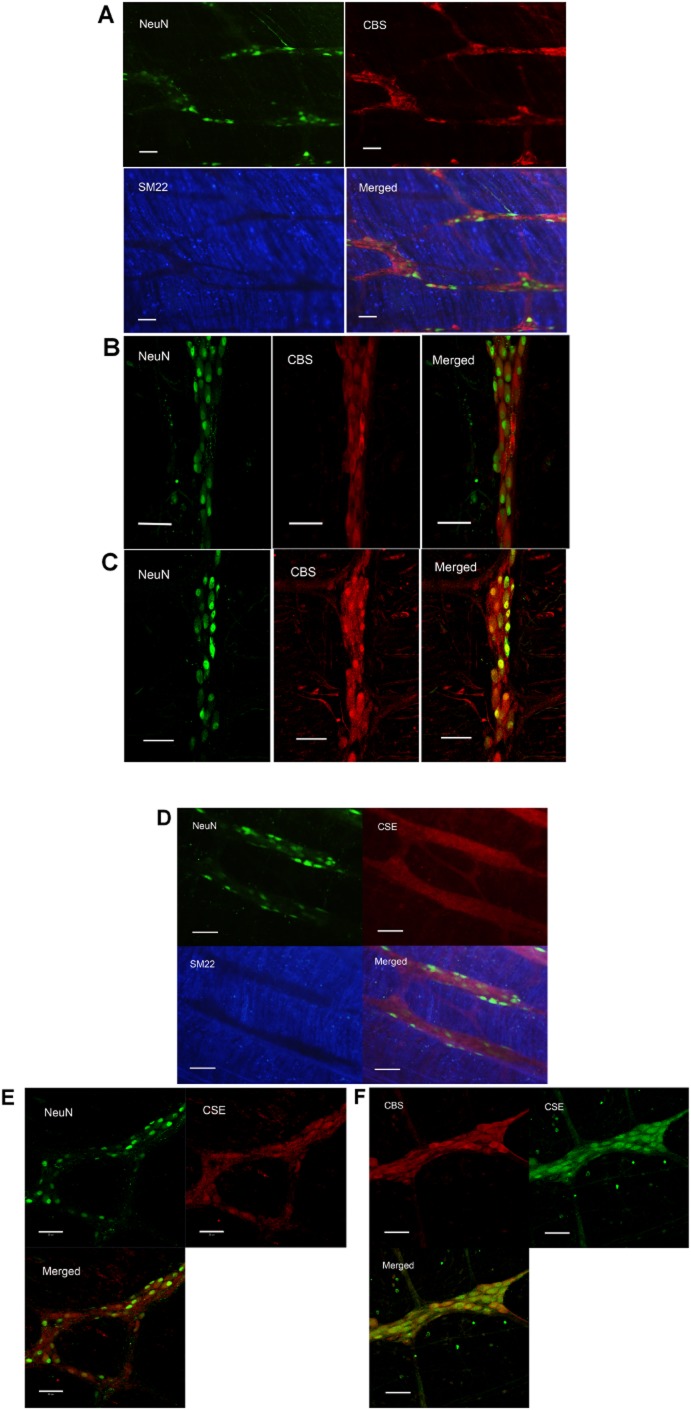
Expression of H2S-producing enzymes in rat duodenal ENS
The neurons and smooth muscle cells were marked by NeuN and SM22. Triple-labelling of CBS or CSE, NeuN and SM22 in LMMP showed that both CBS and CSE were present in the duodenal myenteric plexus while neither CBS nor CSE was expressed in smooth muscle cells (Figure 8A and D). In order to expose the cells in the myenteric plexus more clearly, LMMP was digested by papain to strip the smooth muscle cells chemically. The double staining performed on this specimen revealed that most CBS and CSE were located on the NeuN-immunoreactive cells in myenteric plexus. All NeuN-immunoreactive cells in the myenteric plexus were CBS-positive and CSE-positive (Figure 8B, C and E), but there were a few cells that were not NeuN-immunoreactive that also expressed CBS (Figure 8B). CSE-immunoreactive cells were also positive for CBS in rat myenteric plexus, indicating that both H2S-producing enzymes were expressed in the same cells (Figure 8F).
Figure 8.
Localization of H2S-producing enzymes in the myenteric plexus of rat duodenum. (A) The triple-labelling of NeuN, CBS and SM22 of LMMP. CBS was expressed in NeuN-positive neurons but not SM22-immunoreactive smooth muscle cells. (B) With CBS antibody from Santa Cruz and (C) CBS antibody from Abcam, show the photos taken of the myenteric plexus. It is clear that in the myenteric plexus, all NeuN-immunoreactive cells were CBS-positive. (D) The triple-labelling of NeuN, CSE and SM22 of LMMP. CSE was expressed in NeuN-positive neurons but not in the smooth muscles. (E) The double labelling of NeuN and CSE of the myenteric plexus (CSE antibody from Abcam). All NeuN-positive cells expressed CSE. (F) A myenteric ganglion with double labelling of CBS and CSE (CSE antibody from Santa Cruz). In this ganglion, all the CSE-positive cells were also CBS-positive. Scale bars are 50 μm.
Effects of L-cysteine and SAM on the contraction of duodenal muscle strips
L-cysteine (0.5 and 1.0 mM), a substrate of the H2S-producing enzymes, significantly increased the motility of the muscle strips in a concentration-dependent manner (Figure 9A). After L-cysteine (1 mM) administration, the motility of muscle strips was increased significantly, reaching the highest level at 2 min, and returned to normal within 15 min (Figure 9A and B). Two minutes after L-cysteine (1 mM) administration, the R value was increased (n = 9) and was much bigger than that of the control group (n = 6, P < 0.05). Pretreatment with PAG (0.5 mM, an inhibitor of CSE) and AOAA (0.05 mM, an inhibitor of CBS) significantly attenuated the excitatory effect of L-cysteine (1 mM) on the contraction of muscle strips. The excitatory effect was also blocked by capsazepine (10 μM, n = 11) or L703606 (10 μM, n = 6) pretreatment. (Figure 9B and C).
Figure 9.
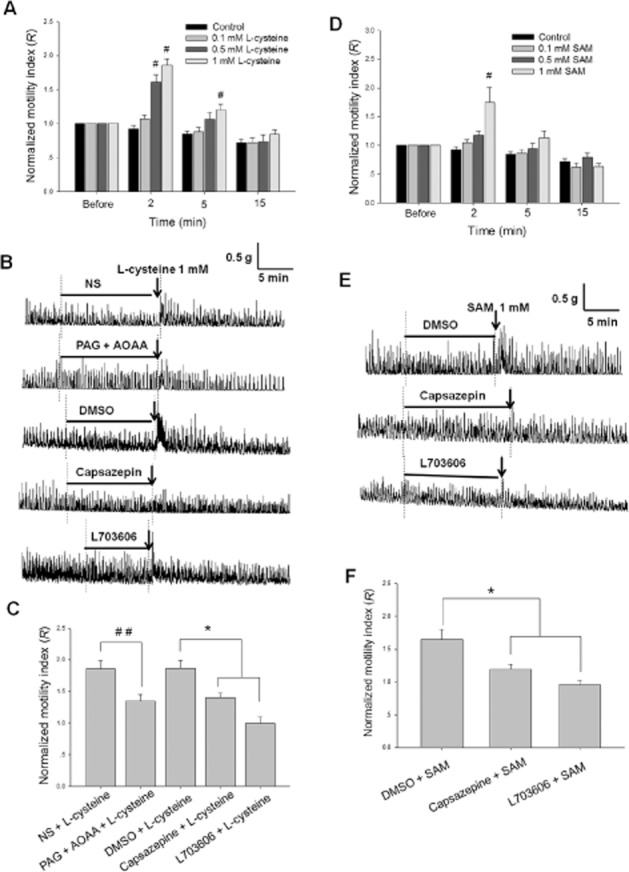
Effects of L-cysteine and SAM on the contraction of duodenal longitudinal muscle strips. (A) and (D) The summarized data of the effects of different concentrations of L-cysteine and SAM on the normalized motility index (R) of the contractions of muscle strips. #P < 0.05 versus control. (B) and (E) The representative recordings of the effects of L-cysteine and SAM on the contraction of muscle strips with or without pretreatment with the blockers, including PAG (0.5 mM), AOAA (0.05 mM), capsazepine (10 μM) and L703606 (10 μM). The horizontal lines indicate the different chemical pretreatments and the arrows indicate L-cysteine or SAM treatment. (C) and (F) The summarized data of the effects of different blockers on the excitatory responses evoked by L-cysteine or SAM. ##P < 0.01 versus NS, *P < 0.05 versus DMSO.
SAM (1 mM), a CBS activator, increased the contraction of duodenal muscle strips, but lower concentrations of SAM (0.1 and 0.5 mM) had no effect. The manner of the excitatory effect of SAM was similar to that of L-cysteine. Two minutes after SAM (1 mM) administration, the R value of motility index was increased (n = 12, P < 0.05) and greater than that of the control group (n = 6) (Figure 9D). The response to SAM (1 mM) was significantly reduced in the presence of capsazepine (10 μM, n = 8) or L703606 (10 μM, n = 6) (Figure 9E and F).
Unlike NaHS, neither L-cysteine nor SAM inhibited the contraction of muscle strips at 15 min after their administration (Figure 9).
Discussion
Most studies have shown that H2S inhibits gastrointestinal motility (Teague et al., 2002; Gallego et al., 2008; Dhaese and Lefebvre, 2009; Kasparek et al., 2012; Nagao et al., 2012; ), but there is a report demonstrating that NaHS (0.1–0.3 mM) excites gastric motility in mice by inhibiting the voltage-gated K+ channels (Zhao et al., 2009). However, in this study, we found that NaHS had dual effects on the motility of duodenum, jejunum, ileum and colon (Figure 1). These differences between our present study and previous reports might be attributed to the different species and tissues used.
The TRPV1 channel is abundantly expressed in primary afferent nerve endings (Nagy et al., 2004). Capsaicin, the agonist of TRPV1 receptors, excites intestinal motility by activating the local efferent function of sensory nerves, this effect might be mediated by tachykinins (most likely SP) released from afferent nerves (De Man Et Al., 2008). In our study, we found that capsazepine, A784168 and SB-366791, TRPV1 channel antagonists, and L703606, a NK1 receptor antagonist, significantly attenuated the excitatory responses evoked by NaHS (Figure 3). This result indicates that NaHS might activate TRPV1 channels in the afferent nerve fibres with the consequent release of SP. Because NK1 receptors are present in smooth muscle cells and interstitial cells of Cajal (ICC) (Holzer and Holzer-Petsche, 2001), it is possible that H2S-induced release of SP influences both the amplitude and frequency of the muscle contraction. A study in the rat urinary bladder (Patacchini et al., 2004) provides further support to our hypothesis that H2S stimulates capsaicin-sensitive primary afferent nerves.
Schicho et al. reported that H2S activates TRPV1 receptors on extrinsic primary afferent terminals, which in turn activate enteric neurons resulting in mucosal Cl– secretion and this effect is blocked by TTX (Schicho et al., 2006). In the present study, we found that the excitatory effect of NaHS on the muscle contraction was TTX-insensitive. The difference between these two studies might be due to the different mechanisms of the effect of H2S on intestinal secretion and motility. Unlike the effect on secretion, which is dependent of the enteric neurons, the excitatory effect of NaHS on muscle contraction might be caused by the release of SP from extrinsic afferent endings following TRPV1 activation, which is TTX-insensitive (de Man et al., 2008).
Apart from the local efferent-like effect induced by the release of tachykinins from their nerve endings in the gastrointestinal tract, afferent nerves also transmit information to the CNS (Holzer, 1988). In our study, NaHS (1 mM) increased the activity of duodenal extrinsic afferent nerves and depolarized DRG neurons. These effects evoked by NaHS were significantly attenuated by capsazepine or A784168 pretreatment (Figure 4 and Figure 5). These results provide further supports for our hypothesis that H2S stimulates TRPV1 receptors in afferent nerve fibres. It seems strange that NaHS increased the motility index of the longitudinal muscle strips while decreasing the intraluminal pressure. There might be two reasons for this. Firstly, the intestinal pressure is mainly controlled by the contraction of circular muscle, whereas the tension of longitudinal muscle strips was recorded in our study. Secondly, the preparations used for the afferent nerve recordings were different from those used in the muscle strip experiments. The former were whole thick segments of duodenum, while the latter were muscle strips in which the mucous and submucous layers were removed.
The most prevalent mechanism for the relaxant effect of H2S on smooth muscles is opening KATP channels. In this study, we found that the inhibitory effect of NaHS on duodenal motility was mainly attenuated by Glib pretreatment (Figure 3A and C) and the KATP channels were expressed in duodenal smooth muscle cells (Figure 6). NaHS induced a prompt relaxation of the smooth muscle cells isolated from duodenum and this effect was completely abolished by Glib pretreatment (Figure 7). These results support our hypothesis that the inhibitory effect of H2S was mediated by the opening of KATP channels in the smooth muscle cells.
It has been reported that H2S increases mucosal HCO3- secretion in the rat duodenum and this effect is partly mediated by NO (Ise et al., 2011). However, it seems that the NO pathway was not involved in the effect of H2S on duodenal motility, because L-NAME, an inhibitor of NO synthase, did not influence the biphasic effect of H2S on duodenal muscle strips (Figure 3).
Both CBS and CSE have been detected in the ENS of guinea-pig and human colon (Schicho et al., 2006). In this study, we found that these two enzymes were also expressed in the neurons of rat duodenal myenteric plexuses. So it is possible that the duodenal enteric neurons in rats synthesize endogenous H2S. Furthermore, the co-expression of CBS and CSE in myenteric plexuses neurons indicates that both of the two enzymes were involved in the production of endogenous H2S, unlike in the brain where CBS is the predominant source (Heinonen, 1973; Ishii et al., 2004).
L-cysteine has been used previously to generate endogenous H2S (Hosoki et al., 1997). In this study we found that L-cysteine also increased the motility of duodenal muscle strips and this effect was reduced by the inhibitors of CBS and CSE (Figure 9). SAM, the activator of CBS, exerted a similar effect with L-cysteine. Capsazepine and L703606 significantly attenuated the excitatory responses evoked by L-cysteine or SAM (Figure 9). These results indicate that both endogenous H2S (from L-cysteine or SAM) and exogenous H2S (from NaHS) exerted an excitatory effect on duodenal motility through a similar mechanism of action. However, unlike NaHS, neither L-cysteine nor SAM inhibited the contraction of duodenal muscle strips. This might be the difference between the effects of endogenous and exogenous H2S. Although both endogenous and exogenous H2S activated TRPV1 channels in the sensory fibres in duodenum, the concentration of endogenous H2S from enteric neurons might be too low to directly open the KATP channels in the smooth muscle cells. That is, the long-lasting inhibitory effect of NaHS on the duodenal muscle strips might be pharmacological, while the early transient effect might be physiological.
In summary, our results suggest that both NaHS and endogenous H2S excite duodenal spontaneous contraction and this effect is mediated by activating TRPV1 channels in afferent nerve terminals with the consequent release of SP, which binds to NK1 receptors in duodenal smooth muscle and ICC. H2S might be a signal molecule that transmits information to the CNS via extrinsic sensory fibres and is involved in the efferent-like effect of capsaicin-sensitive afferent nerve terminals. This might be a novel mechanism by which H2S induces an excitatory effect and regulates gastrointestinal motility in rats.
Acknowledgments
This project was supported by the Natural Scientific Foundation of China (Nos. 31100837 and 31271237) and the Shandong Natural Funds for Distinguished Young Scientists (JQ-2011-20).
Glossary
- AOAA
amino-oxyacetic acid
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- DRG
dorsal root ganglion
- ENS
enteric nervous system
- Glib
glibenclamide
- H2S
hydrogen sulfide
- ICC
interstitial cells of Cajal
- L-NAME
L-NG nitroarginine methyl ester
- LMMP
longitudinal muscle myenteric plexus
- NeuN
neuronal nuclei
- NS
normal saline
- PAG
D, L-propargylglycine
- SAM
S-adenosyl-L-methionine
- SP
substance P
- TTX
tetrodotoxin
Conflict of interests
None.
References
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev. 20:101–135. doi: 10.1210/edrv.20.2.0361. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 1999;170:1449–1867. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life. 2005;57:603–606. doi: 10.1080/15216540500217875. [DOI] [PubMed] [Google Scholar]
- Che T, Sun H, Li J, Yu X, Zhu D, Xue B, et al. Oxytocin hyperpolarizes cultured duodenum myenteric intrinsic primary afferent neurons by opening BK(Ca) channels through IP(3) pathway. J Neurochem. 2012;121:516–525. doi: 10.1111/j.1471-4159.2012.07702.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- Dhaese I, Lefebvre RA. Myosin light chain phosphatase activation is involved in the hydrogen sulfide-induced relaxation in mouse gastric fundus. Eur J Pharmacol. 2009;606:180–186. doi: 10.1016/j.ejphar.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- Gallego D, Clave P, Donovan J, Rahmati R, Grundy D, Jimenez M, et al. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008;20:1306–1316. doi: 10.1111/j.1365-2982.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- Heinonen K. Studies on cystathionase activity in rat liver and brain during development. Effects of hormones and amino acids in vivo. Biochem J. 1973;136:1011–1015. doi: 10.1042/bj1361011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P, Holzer-Petsche U. Tachykinin receptors in the gut: physiological and pathological implications. Curr Opin Pharmacol. 2001;1:583–590. doi: 10.1016/s1471-4892(01)00100-x. [DOI] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Ise F, Takasuka H, Hayashi S, Takahashi K, Koyama M, Aihara E, et al. Stimulation of duodenal HCO(3)(–) secretion by hydrogen sulphide in rats: relation to prostaglandins, nitric oxide and sensory neurones. Acta Physiol (Oxf) 2011;201:117–126. doi: 10.1111/j.1748-1716.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- Ishii I, Akahoshi N, Yu XN, Kobayashi Y, Namekata K, Komaki G, et al. Murine cystathionine gamma-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J. 2004;381(Pt 1):113–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- Kasparek MS, Linden DR, Farrugia G, Sarr MG. Hydrogen sulfide modulates contractile function in rat jejunum. J Surg Res. 2012;175:234–242. doi: 10.1016/j.jss.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Mao YK, Wang B, Huizinga JD, Bienenstock J, Kunze W. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am J Physiol Gastrointest Liver Physiol. 2009;296:G868–G875. doi: 10.1152/ajpgi.90511.2008. [DOI] [PubMed] [Google Scholar]
- de Man JG, Boeckx S, Anguille S, de Winter BY, de Schepper HU, Herman AG, et al. Functional study on TRPV1-mediated signalling in the mouse small intestine: involvement of tachykinin receptors. Neurogastroenterol Motil. 2008;20:546–556. doi: 10.1111/j.1365-2982.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- Martin GR, McKnight GW, Dicay MS, Coffin CS, Ferraz JG, Wallace JL. Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig Liver Dis. 2010;42:103–109. doi: 10.1016/j.dld.2009.05.016. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Duenes JA, Sarr MG. Role of hydrogen sulfide as a gasotransmitter in modulating contractile activity of circular muscle of rat jejunum. J Gastrointest Surg. 2012;16:334–343. doi: 10.1007/s11605-011-1734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I, Santha P, Jancso G, Urban L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500:351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Patacchini R, Santicioli P, Giuliani S, Maggi CA. Hydrogen sulfide (H2S) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol. 2004;142:31–34. doi: 10.1038/sj.bjp.0705764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher MC, Beatty ER, Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000;46:64–72. doi: 10.1136/gut.46.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Feng M, Wang C, Ye Y, Wang PS, Liu C. Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats. Neurogastroenterol Motil. 2009;21:430–438. doi: 10.1111/j.1365-2982.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, et al. Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Scroggs RS, Fox AP. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol. 1992;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam MK, Bhatia M. The role of pro-inflammatory molecules and pharmacological agents in acute pancreatitis and sepsis. Inflamm Allergy Drug Targets. 2010;9:20–31. doi: 10.2174/187152810791292881. [DOI] [PubMed] [Google Scholar]
- Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop M, Bradley K, Ohura T, Tahara T, Roper MD, Rosenberg LE, et al. Rat cystathionine beta-synthase. Gene organization and alternative splicing. J Biol Chem. 1992;267:11455–11461. [PubMed] [Google Scholar]
- Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol. 2010;37:753–763. doi: 10.1111/j.1440-1681.2010.05351.x. [DOI] [PubMed] [Google Scholar]
- Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, et al. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol. 2005;145:1123–1131. doi: 10.1038/sj.bjp.0706277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter. FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Huang X, Wang ZY, Qiu ZX, Han YF, Lu HL, et al. Dual effect of exogenous hydrogen sulfide on the spontaneous contraction of gastric smooth muscle in guinea-pig. Eur J Pharmacol. 2009;616:223–228. doi: 10.1016/j.ejphar.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]