Abstract
Background and Purpose
A new imidazoline I2 receptor ligand, CR4056, is effective for chronic inflammatory pain and diabetic neuropathy. However, it is unclear whether other I2 receptor ligands have similar effects and whether antinociceptive tolerance develops with repeated treatment.
Experimental Approach
The Von Frey filament test was used to measure mechanical hyperalgesia and the plantar test to measure thermal hyperalgesia in rats injected with complete Freund's adjuvant (CFA) treatment or had undergone surgery to induce chronic constriction injury (CCI), models of inflammatory pain and peripheral neuropathic pain respectively. The effects of morphine and I2 receptor ligands, 2-BFI, BU224, tracizoline and CR4056, 3.2–32 mg·kg−1, i.p., on hyperalgesia or affective pain (as measured by a place escape/avoidance paradigm) were studied in separate experiments.
Key Results
Morphine and the I2 receptor ligands (2-BFI, BU224 and tracizoline) all dose-dependently attenuated mechanical and thermal hyperalgesia in CFA-treated rats. The anti-hyperalgesic effects of 2-BFI in CFA-treated and CCI rats were attenuated by the I2 receptor antagonist idazoxan. The combination of 2-BFI and morphine produced additive effects against mechanical hyperalgesia in CFA-treated rats. Repeated treatment (daily for 7–9 days) with 2-BFI or CR4056 did not produce antinociceptive tolerance in CFA-treated or CCI rats. Morphine and the I2 receptor ligands (2-BFI, BU224 and CR4056) were all effective at attenuating place escape/avoidance behaviour in CFA-treated rats.
Conclusions and Implications
Imidazoline I2 receptor ligands have antihyperalgesic effects in rat models of inflammatory and neuropathic pain and may represent a new class of pharmacotherapeutics for the management of chronic pain.
Keywords: imidazoline I2 receptor, von Frey test, plantar test, complete Freund's adjuvant, chronic constriction injury, hyperalgesia, affective pain
Introduction
Pain remains the leading factor that causes patients to live with disability and continues to impart high health cost and economic loss to society (Itoh et al., 2013; Neogi, 2013). Currently, clinically available analgesics are not adequate to meet all the needs and there remains a large population with undertreated pain. For example, whereas opioids remain the most effective analgesics for many painful conditions, clinical use of opioids is limited largely due to their various unwanted effects, such as constipation, physical dependence, abuse and overdose. In the past half a century, pharmacotherapy of pain has seen great progress; however, a recent analysis of the analgesics marketed during this period revealed a lack of clinically significant advances (Kissin, 2010). This clinical reality substantiates the urgent need to develop novel and effective pharmacotherapies.
Emerging literature suggests that drugs that target imidazoline I2 receptors could be a novel class of analgesics, particularly for chronic pain (Ferrari et al., 2011; Li and Zhang, 2011; 2012,; Meregalli et al., 2012). For example, an earlier study found that several selective I2 receptor ligands alone have no antinociceptive effect but enhance the effects of morphine in a mouse hot water tail immersion test (Sanchez-Blazquez et al., 2000). We also reported that I2 receptor ligands only have modest antinociceptive effect in a rat warm water tail withdrawal test when the nociceptive stimulus (water temperature) is mild, but enhance the antinociceptive effects of morphine and tramadol (Thorn et al., 2011; Sampson et al., 2012). Moreover, the antinociceptive effect appears to be assay dependent because I2 receptor ligands, such as 2-BFI and BU224 are effective in a writhing assay (Li et al., 2011b). Nevertheless, many questions remain unclear. For instance, not all I2 receptor ligands are antinociceptive. In an animal model of chronic inflammatory pain, the highly selective I2 receptor ligand, RS45041, is either ineffective or pronociceptive (Houghton and Westlund, 1996; Jett et al., 1999). So far, little is known of the pharmacological or pathophysiological determinants of I2 receptor ligand-induced antinociception. Recently, a selective I2 receptor ligand, CR4056, has been shown to be effective in animal models of chronic inflammatory and neuropathic pain (Ferrari et al., 2011; Meregalli et al., 2012). These data suggest that CR4056 may be particularly effective for chronic pathological pain conditions. However, the generality of these findings on CR4056 to other I2 receptor ligands is unclear. Because the treatment of chronic pain demands repeated dosing, the functional consequences of repeated drug treatment are also unclear.
This study examined the anti-hyperalgesic effects of three highly selective I2 receptor ligands (2-BFI, BU224 and tracizoline) in a rat model of complete Freund's adjuvant (CFA)-induced inflammatory pain. The anti-hyperalgesic effects of repeated treatment with two I2 receptor ligands (2-BFI and CR4056) were also studied. In addition, we examined the effects of acute and repeated treatment with 2-BFI or CR4056 in rat models of chronic constriction injury (CCI)-induced neuropathic pain and CFA-induced inflammatory pain. Because affective pain has been receiving increasing interest and is an important component of the overall pain experience that is dissociable from sensory pain (Uhelski et al., 2012), we also examined the effects of several I2 receptor ligands in rats with CFA-induced inflammatory pain in a place escape/avoidance paradigm, a procedure that is thought to measure the affective component of pain (Fuchs and McNabb, 2012; Li, 2013).
Methods
Subjects
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) were housed individually on a 12/12 h light/dark cycle (behavioural experiments were conducted during the light period) with free access to water and food except during experimental sessions. The total number of rats used was 90. Animals were maintained and experiments were conducted in accordance with the guidelines of the International Association for the Study of Pain (Zimmermann, 1983) and were approved by the Institutional Animal Care & Use Committee, University at Buffalo, the State University of New York, and with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington, DC). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Induction of inflammatory and neuropathic pain
Inflammatory pain was induced by the CFA inoculation in the rats. Briefly, 0.1 mL of CFA (Difco, Detroit, MI, USA) that contains 0.05 mg Mycobacterium butyricum dissolved in paraffin oil was injected in the right foot pad (hind paw) of the rats under isoflurane anaesthesia (2% isoflurane mixed with 100% oxygen at a flow rate of 5 L min-1). The level of anaesthesia was assessed by loss of righting reflex. The rats serving as controls were injected with 0.1 mL of saline.
Neuropathic pain was induced by chronic constriction injury (CCI) procedure (Bennett and Xie, 1988). Briefly, rats were anaesthetized with a mixture of ketamine (60 mg·kg−1) and xylazine (15 mg·kg−1) i.p. prior to surgery. The right sciatic nerve was exposed and four ligatures (4.0 chromic gut; Roboz Surgical Instrument Co. Inc., Rockville, MD, USA) were placed around the nerve (around 1 mm apart) proximal to the trifurcation. Ligatures were loosely tied so that circulation through the epineural vasculature was uninterrupted. In sham procedures, the nerve was similarly exposed but no ligatures were placed. The incisions were closed with surgical clips.
Mechanical hyperalgesia
Mechanical hyperalgesia was measured using the electronic von Frey filament test (Bioseb®, Chaville, France) consisting of hand-held force transducer fitted with a plastic tip. Rats were placed in elevated plastic boxes with a wire mesh floor (IITC Life Science Inc., Woodland Hills, CA, USA) 10 min before the test. The plastic tip was then applied perpendicularly to the medial plantar surface of hind paw from below the mesh floor, until a clear withdrawal was observed. Mechanical thresholds (expressed in g), corresponding to the force that elicits a behavioural response (withdrawal of the hind paw), were automatically registered by the electronic device. Two measurements were taken at 1 min interval and averaged for each animal and the responses of both the left and the right paws were measured.
Thermal hyperalgesia
Thermal hyperalgesia was measured using a plantar test apparatus (IITC Life Science Inc.), wherein the paw withdrawal latency (PWL) to a thermal stimulus was measured, as described previously (Hargreaves et al., 1988), by observers blinded to the treatments. The apparatus used a test unit containing a heat source that radiated a light beam. An adjustable angled mirror on the test unit was used to locate the correct targeting area on the paw. The beam source was set with an active intensity of 40%, an idle intensity of 10% and a cut-off time of 20 s. PWL comprised the time from the start of the beam light until the animal withdrew the paw from the heat stimulus (reaction time was measured to 0.01 s). An acrylic six-chamber container was used to separate the rats that were placed on the glass base. The baseline PWL values were close to 10 s when the current parameters were used. Measurements were taken in duplicate at least 1 min apart, and the average was used for statistical analysis. Behavioural responses of both left and right paws were measured.
Place escape/avoidance paradigm
The place escape/avoidance paradigm has recently been used as a non-reflex measurement of the affective-emotional component of pain (LaBuda and Fuchs, 2000b; Fuchs and McNabb, 2012). For this paradigm, a rat was placed into an inverted rat cage (26 × 47 × 22 cm) that was painted half white and half black. The cage was placed on an elevated metal grid (IITC Life Science Inc.) to enable the experimenter to determine the location of the rat as well as to stimulate the plantar surface of the hind paw with a 60 g von Frey hair every 15 s over a 30 min session. When the rat was in the white side of the chamber, the non-inflamed left paw was mechanically stimulated. When the rat was in the black side of the chamber, the inflamed right paw was mechanically stimulated. The mechanical stimulation was enough to elicit a reflex response in non-inflamed rats [average paw withdrawal threshold (PWT) in non-inflamed rats was 24.7 ± 1.2 g in our studies]. Location in the chamber (black vs. white) at the time of stimulation was noted. Percentage of the time the rats spent on the white side of the chamber was calculated from observations (location in the chamber when stimulated) every 15 s during each 5 min period and was used as the indication of escape/avoidance learning (observations that rats stay at the white side/total number of observations × 100). To examine the effects of the drugs on the place escape/avoidance learning behaviours, drug or vehicle was administered 10 min before the behavioural testing sessions.
Paw thickness measurement
In rats that received CFA injection, the paw thickness was used as an index of the magnitude of CFA-provoked inflammation. The paw thickness was measured after the behavioural test by two investigators who were blinded to treatment assignments using a standard caliper (Pittsburgh 6 in. digital caliper, accuracy of 0.02 mm).
Experimental design
For acute effects of morphine and I2 receptor ligands (2-BFI, BU224 and tracizoline), different groups (n = 6 per group) of rats were used and mechanical hyperalgesia was measured 24 h after CFA treatment, whereas thermal hyperalgesia was measured 48 h after CFA treatment in the same animals. Because rats developed significant and reliable mechanical hyperalgesia 2 days after CCI procedure (see the Results section), the effects of 2-BFI were studied 2 days after CCI surgery. Dose–effect relationships of the drugs were determined using a cumulative dosing procedure with 0.25 or 0.5 log unit increments. For this dosing procedure, a baseline behavioural test was conducted, which was immediately followed by a vehicle injection. Thirty minutes after the vehicle injection, a behavioural test was conducted and this was immediately followed by the injection of the first dose of the drug. The same protocol continued with increasing doses of the drugs being administered until the last dose was given. Thirty minutes after the last dose injection, the behavioural test was conducted one last time to conclude the study. This cumulative dosing procedure is a widely used procedure in behavioural pharmacological studies, including the studies of pain and has high efficiency and proven reproducibility (Wenger, 1980; Li et al., 2008; 2011b,). For antagonist studies, the antagonist (idazoxan) was administered 10 min before vehicle injection.
For repeated dosing studies, selected doses of 2-BFI and CR4056 were studied daily. After the daily baseline determination, the drugs were administered to the rats and their effects were determined 30 min after the injections. Control groups of rats that received manipulations (CFA injection or CCI surgery) or sham treatments were also studied to confirm the duration and presence of chronic hyperalgesia throughout the course of the repeated dosing studies.
For place escape/avoidance studies, vehicle or selected doses of morphine and I2 receptor ligands that demonstrated significant antinociceptive effects in the acute dosing studies were examined in different groups of rats (n = 6 per group). Drugs or vehicle was administered 30 min before the test sessions. This pretreatment time was chosen because our pilot studies showed that 30 min was adequate for these test drugs to produce significant anti-hyperalgesic effects.
Data analyses
CFA treatment and CCI surgery-induced mechanical hyperalgesia are presented as PWT (in g) in the electronic von Frey filament test and thermal hyperalgesia is presented as PWL (in s) in the plantar test. The mean values (±SEM) were calculated from individual animals for all behavioural tests and one-way or two-way repeated measurements anova (Time × Treatment or Dose × Treatment) followed by post hoc Bonferroni's test were used to determine the statistical significances. P < 0.05 was considered statistically significant for all tests.
Antihyperalgesic effects of the drugs studied were quantified for each animal as % maximal possible effect (MPE) for each drug dose. The following formula was used to quantify % MPE: % MPE = [(Post-drug value for a behavioural response (g or s) − Pre-drug value for a behavioural response/(Pre-manipulation [CFA or CCI] value – Pre-drug value for a behavioural response) × 100. When necessary, ED50 (±SEM) values were determined from the % MPE of each drug.
For the study that examined the interactions between morphine and 2-BFI, a fixed proportion dose-addition analysis method was used as described previously (Li et al., 2011a,b2011b; An et al., 2012). For this analysis, two drugs were combined in a fixed proportion (1:1 in this study) and administered using a cumulative dosing procedure. Briefly, the dose–effect curve of the drug mixture was determined and the individual ED50 values of the two drugs in the mixture were calculated based upon the shared dose–response curves. Isobolograms were constructed to visually represent the nature of the drug interactions as additive, supra-additive or infra-additive. An isobologram plots equieffective doses (e.g. ED50 ± SEM) of one drug in the presence of a second drug. If the effects of the two drugs are additive, then the ED50 values (±SEM) for the drug combination should overlap with the diagonal line between the ED50 values (±SEM) for the two drugs alone (line of additivity). If the ED50 values (±SEM) fall below the limits of the line of additivity, then the effects of the two drugs are considered supra-additive (i.e. in the presence of one drug, smaller than predicted doses of a second drug are needed to produce the same effect). If the ED50 values (±SEM) fall above the limits of the line of additivity, then the effects of the two drugs are considered infra-additive (i.e. in the presence of one drug, larger than predicted doses of a second drug are needed to produce the same effect).
Drugs
2-BFI hydrochloride, BU224 hydrochloride, tracizoline oxalate and CR4056 were synthesized according to standard procedures (Jarry et al., 1997; Ishihara and Togo, 2007). Idazoxan hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA). Morphine sulfate was provided by Research Technology Branch, National Institute on Drug Abuse, National Institutes of Health (Rockville, MD, USA). Unless otherwise noted, all drugs were dissolved in physiological saline and administered i.p. CR4056 was dissolved in 20% DMSO with saline and a drop of HCl. Doses are expressed as mg of the form indicated earlier kg−1 body weight. Injection volumes were 1 mL·kg−1.
Results
Under control conditions, rats lifted their hind paws when the pressure that applied to the plastic tip increased to nearly 75 g. This pressure threshold was not different between the two hind paws and remained stable during repeated testing across a period of 16 days (Figure 1). However, CFA injection markedly and significantly decreased the PWT to nearly 30 g. The decrease began 2 h after CFA injection and remained stable throughout the 16 day period of measurement (Figure 1). In addition, the right hind paw of the rats that received CFA injection became swollen and reddish 2 h after CFA injection as demonstrated by a significant increase in the paw thickness (Figure 1). As compared to the left hind paw (no CFA injection), the paw thickness of the right hind paw remained significantly greater throughout the period of 16 days. Thus, the increased paw thickness and decreased PWT (mechanical hyperalgesia) paralleled throughout the period of study. In rats that received CCI surgery, the PWT of the right hind paw significantly decreased to ∼25 g (mechanical hyperalgesia) 2 days after the surgery as compared to the left hind paw that did not receive CCI surgery and remained stable throughout the period of 10 days (Figure 1). In contrast, the PWT of the left hind paw remained unchanged during repeated testing.
Figure 1.
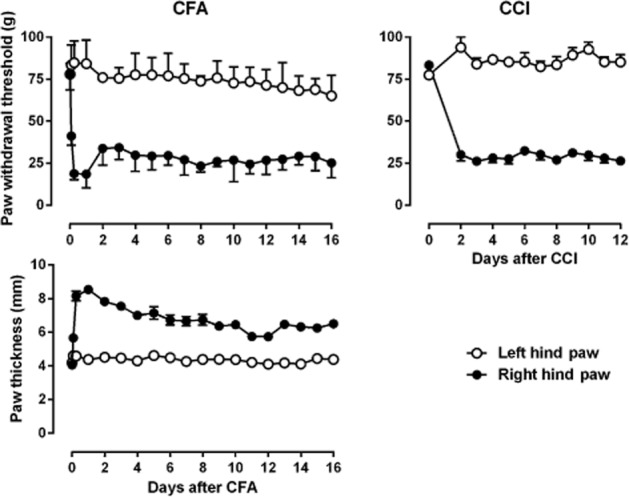
CFA-induced mechanical hyperalgesia (top left) and increased paw thickness (bottom left) and CCI-induced mechanical hyperalgesia (right) in rats. Ordinates: top, paw withdrawal threshold (g) measured by electronic von Frey filament (top); bottom, paw thickness (mm) measured by electronic caliper. Abscissa: days after CFA injection or CCI surgery. For filled circles, all the data points were significantly different from the data point above ‘0’ as analysed by two-way anova and post hoc Bonferroni's analysis (n = 6 per group).
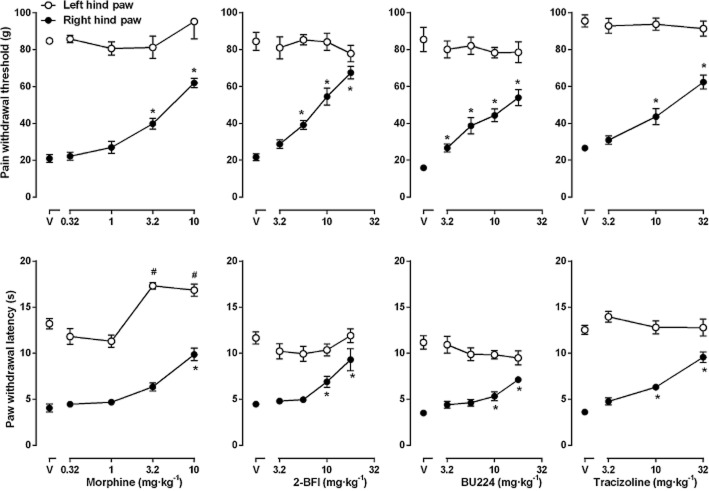
Repeated vehicle injection did not significantly change the PWT and PWL in the rats (data no shown). Morphine dose-dependently increased the PWT of the CFA-treated paw but did not change the PWT of the non-injured paw [one-way anova for right hind paw: F(4, 20) = 22.04, P < 0.0001] (Figure 2). Post hoc analyses indicated that 3.2 and 10 mg·kg−1 morphine significantly increased the PWT of the right hind paw (P < 0.05). In addition, morphine also dose-dependently increased the PWL of both CFA-treated and non-injured paws [one-way anova for left hind paw: F(4, 20) = 18.88, P < 0.0001; one-way anova for right hind paw: F(4, 20) = 5.29, P < 0.01] (Figure 2). Post hoc analyses indicated that 3.2 and 10 mg·kg−1 morphine significantly increased the PWL of the left hind paw (P < 0.05) and 10 mg·kg−1 morphine significantly increased the PWL of the right hind paw (P < 0.05). 2-BFI dose-dependently increased both the PWT and the PWL on the CFA-treated paw but had no effect on the non-injured paw [one-way anova for right hind paw: PWT, F(4, 32) = 31.59, P < 0.0001; PWL, F(4, 32) = 20.93, P < 0.0001]. Post hoc analyses indicated that 5.6–17.8 mg·kg−1 2-BFI significantly increased the PWT and 10–17.8 mg·kg−1 2-BFI significantly increased the PWL of the right hind (CFA-treated) paw (P < 0.05). BU224 dose-dependently increased both the PWT and the PWL of the CFA-treated paw but had no effect on the non-injured paw [one-way anova for right hind paw: PWT, F(4, 24) = 15.29, P < 0.0001; PWL, F(4, 24) = 3.73, P < 0.05]. Post hoc analyses indicated that 3.2–17.8 mg·kg−1 BU224 significantly increased the PWT and 10–17.8 mg·kg−1 BU224 significantly increased the PWL on the CFA-treated paw (P < 0.05). Tracizoline dose-dependently increased both the PWT and the PWL on the CFA-treated paw but had no effect on the non-injured paw [one-way anova for right hind paw: PWT, F(3, 15) = 24.27, P < 0.0001; PWL, F(3, 15) = 20.23, P < 0.05]. Post hoc analyses indicated that 10 and 32 mg·kg−1 tracizoline significantly increased both the PWT and the PWL on the CFA-treated paw (P < 0.05) (Figure 2).
Figure 2.
Effects of morphine, 2-BFI, BU224 and tracizoline on mechanical (top) and thermal hyperalgesia (bottom) in CFA-treated rats. Left hind (non-injured) paw; right hind (CFA-treated) paw. * P < 0.05 as compared to left hind paw above ‘V’; #P < 0.05 as compared to left hind paw above ‘V’ paw (n = 6 per group).
The anti-hyperalgesic effectiveness of the drugs was compared by converting the data into % MPE (Figure 3). Morphine produced a maximum of 69.3% MPE in the von Frey test and 76.8% MPE in the plantar test (ED50 ± SEM values: 2.67 ± 0.38 mg·kg−1 in von Frey test, 3.40 ± 0.70 mg·kg−1 in plantar test). 2-BFI produced a maximum of 81.5% MPE in the von Frey test and 82.8% MPE in the plantar test (ED50 values: 6.00 ± 0.97 mg·kg−1 in von Frey test, 8.31 ± 3.53 mg·kg−1 in plantar test). BU224 produced a maximum of 66.3% MPE in the von Frey test and 52.3% MPE in the plantar test (ED50 values: 8.09 ± 2.03 mg·kg−1 in von Frey test, 11.30 ± 3.46 mg·kg−1 in plantar test). Tracizoline produced a maximum of 62.1% MPE in the von Frey test and 72.0% MPE in the plantar test (ED50 values: 10.33 ± 1.96 mg·kg−1 in von Frey test, 10.42 ± 2.57 mg·kg−1 in plantar test). Thus, the potency orders of the drugs were similar in both tests: morphine > 2-BFI > tracizoline. BU224 was more potent in the von Frey test than in plantar test. Its potency was similar to that of 2-BFI in the von Frey test and similar to that of tracizoline in the plantar test.
Figure 3.
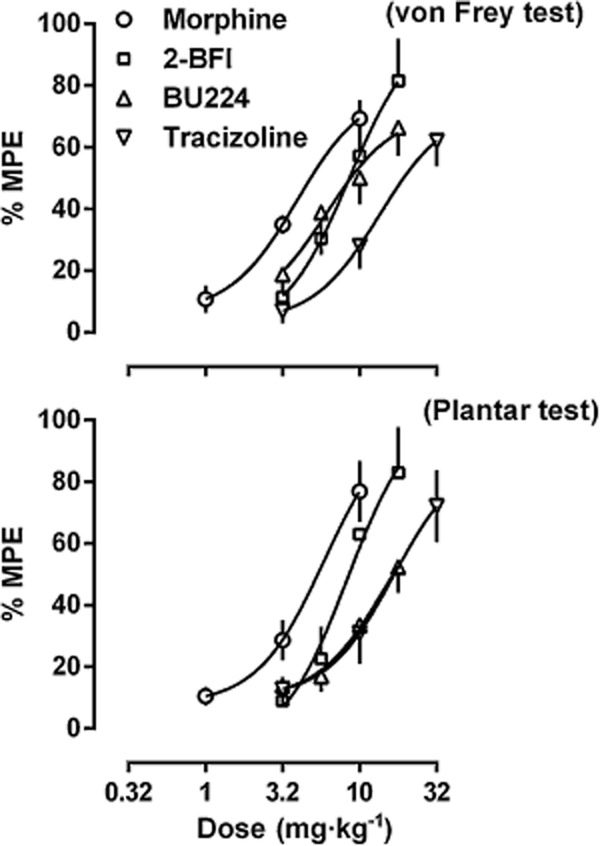
Antihyperalgesic effects of morphine, 2-BFI, BU224 and tracizoline on mechanical (top) and thermal hyperalgesia (bottom) in CFA-treated rats. Data are expressed as percentage of maximal possible effect and plotted as a function of drug dose; 100% MPE represents data from the baseline measure of the right hind paw before CFA treatment.
The imidazoline I2 receptor ligand idazoxan alone did not change the PWT in the von Frey test both in CFA-treated rats and in rats that received CCI surgery (square symbols above ‘V’, Figure 4). When studied as a combination, idazoxan significantly attenuated the anti-hyperalgesic effects of 2-BFI in both models of chronic pain. In CFA-treated rats, two-way anova revealed significant main effects of 2-BFI dose [F(2, 10) = 9.69, P < 0.01] and idazoxan treatment [F(1, 5) = 79.25, P < 0.001]. Post hoc analyses indicated that idazoxan significantly decreased the anti-hyperalgesic effects of 5.6, 10 and 17.8 mg·kg−1 2-BFI. In CCI rats, two-way anova revealed significant main effects of 2-BFI dose [F(1, 5) = 30.33, P < 0.01] and idazoxan treatment [F(1, 5) = 73.95, P < 0.001]. Post hoc analyses indicated that idazoxan significantly decreased the anti-hyperalgesic effects of 5.6 and 10 mg·kg−1 2-BFI.
Figure 4.
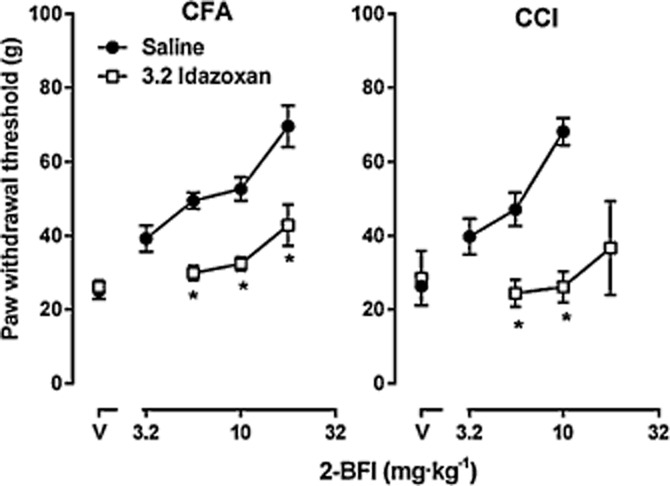
Effects of idazoxan on the antihyperalgesic activity of 2-BFI in CFA-treated (left) and CCI (right) rats. See Figure 2 for other details (n = 6 per group).
When morphine and 2-BFI were studied as a combination under a fixed proportion of 1:1, the dose–effect curve of 2-BFI in the drug mixture (when morphine was present) was shifted leftward as compared to 2-BFI alone (left panel, Figure 5). Thus, the ED50 value (±SEM) of 2-BFI was decreased from 6.00 ± 0.97 mg·kg−1 (2-BFI alone) to 3.03 ± 0.48 mg·kg−1 (2-BFI and morphine mixture) in the von Frey test, and the ED50 value (±SEM) of morphine was decreased from 2.67 ± 0.38 mg·kg−1 (morphine alone) to 1.38 ± 0.22 mg·kg−1 (2-BFI and morphine mixture). Isobolographical analysis indicated that the ED50 values (±SEM) of the drug mixture fell onto the line of additivity, suggesting a simple additive interaction between 2-BFI and morphine.
Figure 5.
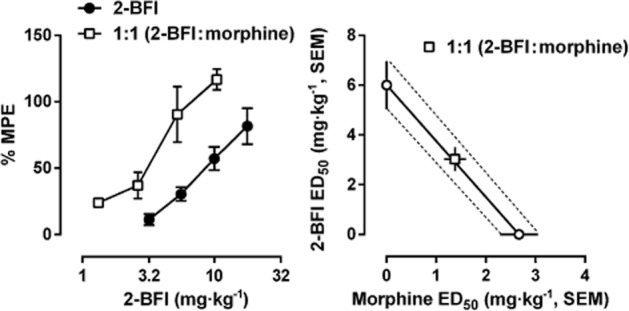
Effects of 2-BFI alone and in combination with morphine on CFA-induced mechanical hyperalgesia in rats. Left: dose–effect curves for 2-BFI alone and 2-BFI/morphine under a fixed proportion of 1:1. Right: isobologram for 2-BFI/morphine mixture. Abscissa scale: ED50 value of morphine. Ordinate scale, ED50 value of 2-BFI (n = 6 per group).
Daily treatment with 10 mg·kg−1 2-BFI produced a significant anti-hyperalgesic effect in rats receiving CCI surgery (Figure 6, left panel). Importantly, this antihyperalgesic effect did not significantly change over a period of 9 days of daily treatment. Two-way anova revealed the significant main effects of 2-BFI treatment [F(1, 5) = 89.26, P < 0.001] and days [F(9, 45) = 6.53, P < 0.0001]. Post hoc analyses indicated that 2-BFI significantly increased the PWT throughout the period of 9 days. However, when saline instead of 10 mg·kg−1 2-BFI was administered during a probe test, the PWT was not significantly different from the control animals that never received drug treatment, suggesting that daily 10 mg·kg−1 2-BFI does not accumulate and carry over 24 h later and also does not have a bona fide curative effect on CFA-induced inflammatory pain. Similarly, 10 mg·kg−1 2-BFI and 32 mg·kg−1 CR4056 also produced significant antihyperalgesic effects in CFA-treated rats and the effect did not significantly change over a period of 7 days of daily treatment (Figure 6, right panel). Two-way anova revealed significant main effects of drug treatment [F(1, 5) = 216.1, P < 0.0001] and days [F(14, 70) = 2.97, P < 0.01]. Post hoc analyses indicated that 2-BFI and CR4056 both significantly increased the PWT throughout the period of 14 days. Instead, saline treatment did not significantly alter the PWT, similar to rats receiving CCI surgery.
Figure 6.
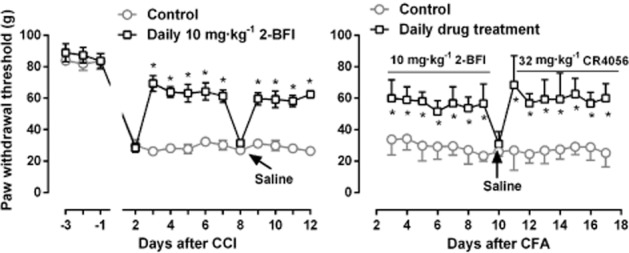
Effects of daily treatment with 10 mg·kg−1 2-BFI (left) or 10 mg·kg−1 2-BFI followed by 32 mg·kg−1 CR4056 (right) on CCI-(left) or CFA-(right) induced mechanical hyperalgesia in rats. Arrow indicates the day that the animals received saline instead of treatment drug. *P < 0.05 as compared to the control. See Figure 1 for other details (n = 6 per group).
The place escape/avoidance paradigm test was conducted 24 h after CFA treatment. Rats naturally preferred the black side over the white side of the test chamber such that the rats spent more than 80% of the 30 min test session in the black side (data not shown). However, when repetitive mechanical stimuli were applied, rats that only received saline treatment progressively switched their stay preference from the black side (where the CFA-treated hind paw was stimulated) to the white side (where the non-injured hind paw was stimulated) (Figure 7), demonstrating the place escape/avoidance behaviour. Morphine (10 mg·kg−1), 2-BFI (10 mg·kg−1), BU224 (10 mg·kg−1) and CR4056 (32 mg·kg−1) at doses that significantly attenuated mechanical hyperalgesia all markedly and almost completely prevented the place escape/avoidance behaviour (Figure 7). There was a treatment (CFA vs. saline) by time interaction [F(5, 50) > 5.74, P < 0.0001] and main effects of time [F(5, 50) > 6.49, P < 0.001] and treatment [F(1, 10) > 33.2, P < 0.001]. There were differences between CFA and saline rats during the last 20–25 min of the tests that approached maximum during the final 10 min (P < 0.05).
Figure 7.
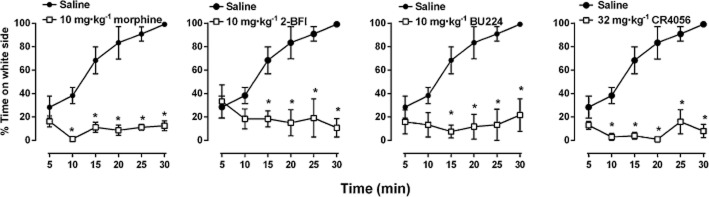
Mean percentage of time (± SEM) spent in the white side of the chamber in 5 min intervals over the 30 min session for CFA-treated rats following stimulation with a 60 g von Frey filament every 15 s. Rats were stimulated on the right paw (CFA-treated) on the black side of chamber and the left paw (untreated) on the white side of the chamber. *P < 0.05 versus saline-treated control rats.
Discussion
The primary findings of the current study were that selective imidazoline I2 receptor ligands have significant antihyperalgesic effects in rat models of inflammatory and neuropathic pain. More importantly, these effects did not show evidence of significant tolerance after repeated dosing. In addition, these I2 receptor ligands were also effective in a rat model designed to measure the affective component of pain. Taken together, these data extended the previous findings that CR4056 is effective against inflammatory pain and diabetic neuropathy (Ferrari et al., 2011) to showing that I2 receptor ligands are efficacious analgesics against both inflammatory and nerve injury-induced neuropathic pain without the propensity of developing analgesic tolerance.
CFA treatment is a well-characterized animal model of arthritis. Early studies injected CFA into the tail base of the rats and animals typically developed serious sickness and sometimes death (Colpaert et al., 1980). Later modifications of the method usually inject CFA into the foot pad of the hind paw. Following CFA injection into the hind paw, animals typically develop long-lasting inflammation accompanying mechanical and thermal hyperalgesia that last for weeks (Nagakura et al., 2003). Consistent with the literature, we found that 2 h after CFA injection animals began to develop significant mechanical and thermal hyperalgesia and swollenness in the right hind paw. We continued to monitor the mechanical hyperalgesia and paw thickness for 16 days after CFA injection and the chronic inflammatory symptoms remained relatively stable throughout the course of the study (Figure 1). CCI is a widely used animal model of nerve injury-induced neuropathic pain (Bennett and Xie, 1988). Our data were consistent with the literature that animals developed significant mechanical hyperalgesia after CCI that lasted for at least 12 days (Figure 1). Based on these data, subsequent acute drug studies were conducted 24 h after CFA treatment and 2 days after CCI surgery.
2-BFI, BU224 and tracizoline all produced dose-dependent anti-hyperalgesic effects both for mechanical and thermal hyperalgesia (Figure 2). The effectiveness of all the three I2 receptor ligands was similar (Figure 3). These results were in congruence with the findings that CR4056 was effective against mechanical hyperalgesia in animal models of CFA-induced inflammatory pain, although a different method for mechanical hyperalgesia measurement was used (von Frey test vs. Randall-Selitto method) (Ferrari et al., 2011). These anti-hyperalgesic effects were not due to general behavioural inhibition because within the dose ranges studied, all three drugs do not alter the general locomotor activity (Thorn et al., 2012). Similarly, morphine also dose-dependently attenuated the mechanical and thermal hyperalgesia (Figure 2), consistent with the literature (Nagakura et al., 2003; Soignier et al., 2011). Von Frey filament stimulation does not induce physiological nociception in rats, although a reflexive paw withdrawal can usually be provoked when a large enough pressure is applied to the paw. However, this reflexive response is not changed by analgesic doses of morphine and I2 receptor ligands, as seen in this study that the PWT of the left (non-injured) hind paw was not significantly changed across the dose range studied (Figure 2). In contrast, plantar test is used for the study of both thermal hyperalgesia (in animals with hyperalgesia) and thermal nociception (in healthy pain-free animals) (Hargreaves et al., 1988; Nagakura et al., 2003). As expected, morphine significantly increased the PWL on the left hind paw (Figure 2). However, none of the I2 receptor ligands produced thermal nociception on the left hind paw. These results were consistent with the previous studies that I2 receptor ligands alone have little effect in measures of acute thermal nociception in the hot water tail immersion assay both in rats and mice (Sanchez-Blazquez et al., 2000; Thorn et al., 2011). In fact, 2-BFI has antinociceptive effect only when the thermal stimulus is weak (46°C) but has no effect when the thermal stimulus is stronger (50°C) in the hot water tail immersion assay (Sampson et al., 2012).
All three I2 receptor ligands have high affinities at I2 receptors and previous functional studies confirmed their similar pharmacological activities (Thorn et al., 2012). For example, 2-BFI, BU224 and tracizoline all dose-dependently and significantly decreased the rectal temperature in rats and their effects were antagonized by the I2 receptor antagonist/α2 adrenoceptor antagonist idazoxan but not α2 adrenoceptor antagonist yohimbine, suggesting that the effects are mediated by I2 receptors (Thorn et al., 2012). In this study, we examined the I2 receptor mechanisms of 2-BFI-induced anti-hyperalgesia in CFA-treated and CCI rats. The anti-hyperalgesic effects of 2-BFI were significantly attenuated by idazoxan under both conditions. Because all three I2 receptor ligands have at least 1000-fold selectivity for I2 receptors over α2 adrenoceptors (Thorn et al., 2012), the observed attenuation was most likely due to I2 receptor blockade, consistent with the previous findings (Thorn et al., 2012).
In a previous study, we reported that 2-BFI and BU224 had significant antinociceptive effects in a 5% hypertonic saline-induced writhing test (Li et al., 2011b). Moreover, when 2-BFI and morphine were studied as a combination, a supra-additive interaction was found (Li et al., 2011b). The current study examined the interaction between 2-BFI and morphine for their anti-hyperalgesic effects in CFA-treated rats. Isobolographical analyses indicated a pure additive interaction between 2-BFI and morphine (Figure 5). This finding is different from the results of the writhing test, and the difference is likely due to the different pain models used. Writhing test is often used to model chemical irritant provoked visceral pain (Le Bars et al., 2001) and the neurobiology of visceral pain is different from inflammatory pain, which CFA treatment is designed to model (Almarestani et al., 2011; Gebhart and Bielefeldt, 2011). Another line of evidence also supports the critical role of pain models in determining the effects of imidazoline receptor ligands. The endogenous imidazoline receptor ligand agmatine is very effective in several animal models of chronic pain (see Li and Zhang, 2011 for a review). However, agmatine is not effective in the hot water tail immersion assay or in the 5% saline-induced writhing assay (Thorn et al., 2011; Li et al., 2011b).
Based on earlier findings and results from the current study, it seems that I2 receptor ligands are more effective in animal models of chronic persistent pain than acute nociception (Sanchez-Blazquez et al., 2000; Ferrari et al., 2011; Thorn et al., 2011; Li et al., 2011b; Meregalli et al., 2012; Sampson et al., 2012). Therefore, I2 receptor ligands may be particularly useful in the treatment of chronic pain. Chronic pain usually refers to persistent painful conditions that last for more than 3 months, and the pharmacotherapy of chronic pain almost always involve repeated dosing. One possible consequence of repeated dosing with analgesics is the progressive decrease of their analgesic effects (analgesic tolerance). So far, it is unclear whether repeated treatment with antinociceptive doses of I2 receptor ligands will lead to tolerance. In this study, we determined the effects of repeated treatment with an antinociceptive dose of 2-BFI (10 mg·kg−1) in CCI rats and 10 mg·kg−1 2-BFI, followed by an effective dose of CR4056 (32 mg·kg−1) in CFA-treated rats. In a limited period of daily treatment (7–9 days), the anti-hyperalgesic effects of 2-BFI and CR4056 showed no trend of developing tolerance. This is the first attempt to examine the antinociceptive effects of I2 receptor ligands under the condition of repeated treatment and the results support the beneficial effects of I2 receptor ligands for the management of chronic pain. It is noted that daily injection may not closely mimic the strategy of clinical chronic pain management which typically requires the analgesics to be taken two to three times a day or use controlled/slow release formulations to maintain around-the-clock analgesia. Future studies employing dosing strategies that more closely mimic clinical practice (e.g. multiple dosing per day or using subcutaneous minipumps to achieve around-the-clock antinociception) can better address the issue of antinociceptive tolerance (Alba-Delgado et al., 2012).
Pain experience comprises several dissociable and interplaying components (Fernandez and Turk, 1992; Melzack, 1999). Whereas the majority of pre-clinical pain studies remain focused on the study of sensory-discriminative pain, increasing efforts call for the study of both sensory and affective components of pain (Mogil, 2009; Li, 2013). This study utilized the place escape/avoidance paradigm to examine the effects of I2 receptor ligands on the aversive learning related to affective pain in CFA-treated rats. This procedure has been well characterized and is shown to measure a dissociable component of affective pain that is different from sensory pain (LaBuda and Fuchs, 2000a; Fuchs and McNabb, 2012; Uhelski et al., 2012). We found that at a dose that effectively attenuated hyperalgesia, 2-BFI, BU224 and CR4056 all markedly attenuated the place escape/avoidance behaviour, and these results can be interpreted that I2 receptor ligands are effective against affective component of pain (Fuchs and McNabb, 2012).
In summary, this study found that I2 receptor ligands are effective in two rat models of chronic pain: CFA-induced inflammatory pain and CCI-induced neuropathic pain. The antinociceptive effects are primarily mediated by imidazoline I2 receptors and repeated dosing study found little evidence of the development of antinociceptive tolerance. In addition, I2 receptor ligands are efficacious against both sensory-discriminative (as measured by von Frey test and plantar test) and affective (as measured by place escape/avoidance paradigm) components of pain. Taken together, these results support the notions that imidazoline I2 receptors represent a viable novel drug target for analgesics and that ligands targeting I2 receptors are a valuable class of drugs for the management of chronic pain.
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Award Nos. R01DA034806 and R21DA032837) and by National Natural Science Foundation of China (81373390). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- 2-BFI
2-(2-benzofuranyl)-2-imidazoline
- BU224
2-(4,5-dihydroimidazol-2-yl)quinolone
- CCI
chronic constriction injury
- CFA
complete Freund's adjuvant
- CR4056
2-phenyl-6-(1H-imidazol-1-yl)quinazoline
- PWL
paw withdrawal latency
- PWT
paw withdrawal threshold
- tracizoline
2-styryl-4,5-dihydro-lH-imidazole
Conflicts of interest
The authors declare no conflict of interest.
References
- Alba-Delgado C, Mico JA, Sanchez-Blazquez P, Berrocoso E. Analgesic antidepressants promote the responsiveness of locus coeruleus neurons to noxious stimulation: implications for neuropathic pain. Pain. 2012;153:1438–1449. doi: 10.1016/j.pain.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Almarestani L, Fitzcharles MA, Bennett GJ, Ribeiro-da-Silva A. Imaging studies in Freund's complete adjuvant model of regional polyarthritis, a model suitable for the study of pain mechanisms, in the rat. Arthritis Rheum. 2011;63:1573–1581. doi: 10.1002/art.30303. [DOI] [PubMed] [Google Scholar]
- An XF, Zhang Y, Winter JC, Li JX. Effects of imidazoline I(2) receptor agonists and morphine on schedule-controlled responding in rats. Pharmacol Biochem Behav. 2012;101:354–359. doi: 10.1016/j.pbb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, De Witte P, Maroli AN, Awouters F, Niemegeers CJ, Janssen PA. Self-administration of the analgesic suprofen in arthritic rats: evidence of Mycobacterium butyricum-induced arthritis as an experimental model of chronic pain. Life Sci. 1980;27:921–928. doi: 10.1016/0024-3205(80)90101-0. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Turk DC. Sensory and affective components of pain: separation and synthesis. Psychol Bull. 1992;112:205–217. doi: 10.1037/0033-2909.112.2.205. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, et al. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011;4:111–125. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PN, McNabb CT. The place escape/avoidance paradigm: a novel method to assess nociceptive processing. J Integr Neurosci. 2012;11:61–72. doi: 10.1142/S0219635212500045. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, Bielefeldt K. Visceral pain. In: Basbaum AI, Bushnell C, editors. Science of Pain. 1st edn. Oxford: Elsevier; 2011. pp. 543–569. [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Houghton AK, Westlund KN. An I2 imidazoline ligand, RS 45041, potentiates hyperalgesia in acute arthritis. Neuroreport. 1996;7:1497–1501. doi: 10.1097/00001756-199606170-00011. [DOI] [PubMed] [Google Scholar]
- Ishihara M, Togo H. Direct oxidative conversion of aldehydes and alcohols to 2-imidazolines and 2-oxazolines using molecular iodine. Tetrahedron. 2007;63:1474–1480. [Google Scholar]
- Itoh H, Kitamura F, Yokoyama K. Estimates of annual medical costs of work-related low back pain in Japan. Ind Health. 2013;51:524–529. doi: 10.2486/indhealth.2013-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry C, Forfar I, Bosc J, Renard P, Scalbert E, Guardiola B. 1997. 5-(Arloxymethyl)oxazoline US Patent 5,686,477, Adir e Compagnie.
- Jett MF, Hedley LR, Dillon MP, Eglen RM, Hunter JC. Behavioral effects of RS-45041-190, a selective I2 imidazoline ligand, in rats. Ann N Y Acad Sci. 1999;881:369–371. doi: 10.1111/j.1749-6632.1999.tb09383.x. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin I. The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth Analg. 2010;110:780–789. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol. 2000a;163:490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. Morphine and gabapentin decrease mechanical hyperalgesia and escape/avoidance behavior in a rat model of neuropathic pain. Neurosci Lett. 2000b;290:137–140. doi: 10.1016/s0304-3940(00)01340-9. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Li JX. The application of conditioning paradigms in the measurement of pain. Eur J Pharmacol. 2013;716:158–168. doi: 10.1016/j.ejphar.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Imidazoline I2 receptors: target for new analgesics? Eur J Pharmacol. 2011;658:49–56. doi: 10.1016/j.ejphar.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Emerging drug targets for pain treatment. Eur J Pharmacol. 2012;681:1–5. doi: 10.1016/j.ejphar.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Li JX, McMahon LR, France CP. Comparison of naltrexone, 6alpha-naltrexol, and 6beta-naltrexol in morphine-dependent and in nondependent rhesus monkeys. Psychopharmacology (Berl) 2008;195:479–486. doi: 10.1007/s00213-007-0914-9. [DOI] [PubMed] [Google Scholar]
- Li JX, Crocker C, Koek W, Rice KC, France CP. Effects of serotonin (5-HT)1A and 5-HT2A receptor agonists on schedule-controlled responding in rats: drug combination studies. Psychopharmacology (Berl) 2011a;213:489–497. doi: 10.1007/s00213-010-2136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Zhang Y, Winter JC. Morphine-induced antinociception in the rat: supra-additive interactions with imidazoline I(2) receptor ligands. Eur J Pharmacol. 2011b;669:59–65. doi: 10.1016/j.ejphar.2011.07.041. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R. From the gate to the neuromatrix. Pain. 1999;(Suppl 6):S121–S126. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- Meregalli C, Ceresa C, Canta A, Carozzi VA, Chiorazzi A, Sala B, et al. CR4056, a new analgesic I2 ligand, is highly effective against bortezomib-induced painful neuropathy in rats. J Pain Res. 2012;5:151–167. doi: 10.2147/JPR.S32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, et al. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther. 2003;306:490–497. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson C, Zhang Y, Del Bello F, Li JX. Effects of imidazoline I2 receptor ligands on acute nociception in rats. Neuroreport. 2012;23:73–77. doi: 10.1097/WNR.0b013e32834e7db3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Boronat MA, Olmos G, Garcia-Sevilla JA, Garzon J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br J Pharmacol. 2000;130:146–152. doi: 10.1038/sj.bjp.0703294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soignier RD, Taylor BK, Baiamonte BA, Lee FA, Paul D, Gould HJ., 3rd Measurement of CFA-induced hyperalgesia and morphine-induced analgesia in rats: dorsal vs plantar mechanical stimulation of the hindpaw. Pain Med. 2011;12:451–458. doi: 10.1111/j.1526-4637.2011.01066.x. [DOI] [PubMed] [Google Scholar]
- Thorn DA, Zhang Y, Peng BW, Winter JC, Li JX. Effects of imidazoline I(2) receptor ligands on morphine-and tramadol-induced antinociception in rats. Eur J Pharmacol. 2011;670:435–440. doi: 10.1016/j.ejphar.2011.09.173. [DOI] [PubMed] [Google Scholar]
- Thorn DA, An XF, Zhang Y, Pigini M, Li JX. Characterization of the hypothermic effects of imidazoline I(2) receptor agonists in rats. Br J Pharmacol. 2012;166:1936–1945. doi: 10.1111/j.1476-5381.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhelski ML, Davis MA, Fuchs PN. Pain affect in the absence of pain sensation: evidence of asomaesthesia after somatosensory cortex lesions in the rat. Pain. 2012;153:885–892. doi: 10.1016/j.pain.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Wenger GR. Cumulative dose-response curves in behavioral pharmacology. Pharmacol Biochem Behav. 1980;13:647–651. doi: 10.1016/0091-3057(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]