Abstract
OBJECTIVES
The aim of this study was to perform direct quantification of myocardial extracellular volume fraction (ECF) with T1-weighted cardiac magnetic resonance (CMR) imaging in patients suspected to have infiltrative heart disease.
BACKGROUND
Infiltrative heart disease refers to accumulation of abnormal substances within the myocardium. Qualitative assessment of late gadolinium enhancement (LGE) remains the most commonly used method for CMR evaluation of patients suspected with myocardial infiltration. This technique is widely available and can be performed in a reproducible and standardized manner. However, the degree of extracellular matrix expansion due to myocardial infiltration in the intercellular space has, to date, not been amenable to noninvasive quantification with LGE.
METHODS
We performed 3-T CMR in 38 patients (mean age 68 ± 15 years) who were referred for assessment of infiltrative heart disease and also in 9 healthy volunteers as control subjects. The T1 quantification by Look-Locker gradient-echo before and after contrast determined segmental myocardial partition coefficients. The ECF was obtained by referencing the tissue partition coefficient for gadolinium to the plasma volume fraction in blood, derived from serum hematocrit. Cine CMR and LGE imaging in matching locations were also performed.
RESULTS
Seventeen patients (45%) had cardiac amyloidosis (CA) (biopsy-confirmed or clinically highly probable), 20 (53%) had a non-amyloid cardiomyopathy, and 1 had lysosomal storage disease. Median global ECF was substantially higher in CA patients (0.49) compared with non-amyloid cardiomyopathy patients (0.33, p < 0.0001) and volunteers (0.24, p < 0.0001). The ECF strongly correlated with visually assessed segmental LGE (r = 0.80, p < 0.0001) and LV mass index (r = 0.69, p < 0.0001), reflecting severity of myocardial infiltration. In patients with CA, ECF was highest in segments with LGE, although it remained elevated in segments without qualitative LGE.
CONCLUSIONS
The CMR ECF quantification identified substantial expansion of the interstitial space in patients with CA compared with volunteers. Further studies using this technique for diagnosis and assessment of the severity of myocardial infiltration are warranted.
Keywords: amyloid, infiltrative cardiomyopathy, left ventricular mass, myocardial delayed enhancement, T1 mapping
Infiltrative heart disease refers to accumulation of abnormal substances within the myocardium (1). In imaging, some of these uncommon cardiomyopathies present with increased myocardial wall thickness, whereas others present with chamber dilation and wall thinning (1). Amyloidosis, Fabry disease (2), and mucopolysaccharidosis (3) are examples of conditions that present with increased left ventricular (LV) mass and wall thickness (1). Hypertensive heart disease might also mimic morphologic, functional, and electrocardiographic features of infiltrative heart disease. Hypertrophic cardiomyopathy is another important and common differential diagnosis adding to the challenge of recognizing infiltrative heart disease. Clinical history and electrocardiogram (ECG) might be helpful. Low ECG voltages are suggestive of cardiac amyloidosis (CA), but ECG voltages might be normal or increased in other infiltrative heart diseases (1). A definitive diagnosis usually relies on endomyocardial biopsy, which is invasive and subject to sampling error.
Growing evidence suggests that tissue characterization by cardiac magnetic resonance (CMR), in particular the use of late gadolinium enhancement (LGE) imaging, can provide evaluation of myocardial infiltration (1). Among infiltrative heart diseases, the pattern of LGE in CA has been most studied (4–9) and shown to have prognostic implications (6,7,10). In smaller patient series, LGE has proven useful for recognition of Fabry disease (2) and of Danon disease (11), among others. Myocardial fibrosis, identified by LGE, is also present in patients with LV hypertrophy, regardless of diagnosis (12).
Qualitative assessment of LGE remains the most commonly used method for CMR evaluation of patients with suspected myocardial infiltration. It is widely available and can be performed in a reproducible and standardized manner. However, the degree of extracellular matrix expansion due to myocardial infiltration in the intercellular space has, to date, not been amenable to noninvasive quantification with LGE. In the particular case of CA, LGE was significantly associated with fibrosis but not with the extent of amyloid infiltration in a histologic comparison study (5). In cases of myocardial infiltration, accumulation of gadolinium is thought to result from expansion of the extracellular matrix, not only by fibrotic replacement but mostly by accumulation of insoluble proteins (1,13). Such accumulation is diffuse and might be difficult to quantify by LGE imaging alone. There is no universal threshold that defines fibrosis or infiltration by LGE. Contrast relies on signal intensity differences between normal and abnormal myocardium, a difference that might not exist in a diffuse process. Signal intensity also depends on the selection of an inversion time that nulls the signal of a myocardial reference region.
T1 mapping techniques identify areas of myocardium where gadolinium accumulates by relying on its T1 shortening effect (14,15). Thornhill et al. (16,17) previously described that the linear relation-ship between the T1 relaxation of myocardium and blood pool reflects the distribution of gadolinium and allows calculation of the partition coefficient for gadolinium in myocardial infarction. Arheden et al. (18) further reported that myocardial extracellular volume fraction within infarcted tissue can be directly determined by normalizing the partition co-efficient to (1 – blood hematocrit). This technique has identified extracellular matrix expansion in the absence of LGE in dilated cardiomyopathy (19) and congenital heart disease (20). Another study using an equivalent technique provided correlation between CMR measurements and histological findings in aortic stenosis and hypertrophic cardiomyopathy (21). In CA, accumulation of gadolinium results from expansion of the myocardial extracellular matrix, either from fibrosis or interstitial accumulation of amyloid protein (4,5,13). Maceira et al. (4) have identified that subendocardial T1 was shorter in CA, corresponding to areas of interstitial expansion and of LGE. These abnormal gadolinium kinetics have been associated with worse prognosis in patients with CA (22).
In the current paper, our group performed direct quantification of myocardial extracellular volume fraction (ECF) by CMR in patients suspected to have infiltrative cardiomyopathy. The technique used in the current study extended the work of others by inclusion of up to 4 total time points, before and after administration of gadolinium contrast agent, to improve the fitting of linear relation-ship for the measurement of partition coefficient and subsequently ECF. We hypothesized that measurement of the ECF can provide a quantitative marker of disease burden in patients with proven and suspected infiltrative cardiomyopathy and aid in evaluating the magnitude of adverse tissue remodeling in comparison with healthy volunteers and in adjunct to LGE. We further hypothesized that ECF will be associated with markers of infiltrative heart disease, including LV mass index, LV systolic and diastolic dysfunction, and LGE.
METHODS
Patient subjects
We performed CMR on 38 consecutive patients referred for evaluation of infiltrative heart disease between December 2008 and September 2010. Patients were excluded from performing CMR in any of the following conditions: 1) decompensated heart failure; 2) hemodynamic instability; 3) severe claustrophobia despite use of sedatives; 4) presence of metallic hazards; and 5) estimated glomerular filtration rate ≤30 ml/min/1.73 m2 within 30 days before CMR. The CMR referral was based on the following reasons: 1) suspicion of infiltrative heart disease due to increased wall thickness by echocardiography and/or voltage criteria for LV hypertrophy by ECG (n = 26); 2) suspicion of myocardial infiltration in patients with known systemic infiltrative disease such as amyloidosis (n = 4); and 3) assessment of severity of cardiac involvement in patients with known CA (n = 8) (Table 1). Control subjects were 9 healthy volunteers who underwent 3.0-T CMR with identical imaging parameters in the setting of a prior study (20). Healthy volunteers had no history of heart disease, hypertension, or any other risk factors for coronary artery disease, and they did not undergo echocardiography and ECG. All subjects, cases and controls, gave informed consent to CMR study and administration of gadolinium. For patients, no gadolinium was administered for the sole purpose of this study. Retrospective review of clinical, imaging, and pathology records of patients was approved by the Partners Healthcare Institutional Review Board.
Table 1.
Indications for CMR and Final Diagnosis After Complete Clinical Evaluation in 38 Patients
| Indication for Referral to CMR | ||||
|---|---|---|---|---|
| Final Diagnosis | Known CA | Unexplained Thickened LV or ECG Voltage Criteria for LVH |
Systemic Infiltrative Disease |
Total |
| CA | ||||
| By biopsy | 7 | 7 | 0 | 14 (37%) |
| By clinical diagnosis | 1 | 1 | 1 | 3 (8%) |
| Non-amyloid CMP | 0 | 18 | 2 | 20 (53%) |
| Lysosomal storage disease | 0 | 0 | 1 | 1 (5%) |
| Total | 8 (17%) | 26 (55%) | 4 (9%) | 38 |
CA = cardiac amyloidosis; CMP = cardiomyopathy; CMR = cardiac magnetic resonance imaging; ECG = electrocardiogram; LVH = left ventricular hypertrophy; LV = left ventricular.
Clinical, electrocardiographic, and echocardiographic data
Clinical history was collected at the time of CMR imaging. The ECGs closest in time to the CMR were reviewed with regard to rhythm, presence of low voltages, presence of R-wave in lead V1–V3, and bundle branch block, blinded to all clinical and study data. Low voltages were defined as the sum of the amplitude of the S-wave in lead V1 and of R-wave in lead V5 or V6 <15 mm (23). Echocardiographic studies were reviewed offline for diastolic function assessed with the peak velocities of the mitral E and A waves and the pulmonary veins S and D waves by pulse-wave Doppler and of the E’-wave of the lateral and septal mitral annulus by tissue Doppler imaging. A decreasing peak velocity of the E’-wave of the mitral annulus by tissue Doppler imaging was used as an indicator of progressively more restrictive cardiomyopathy (24).
The final diagnosis for each patient was established after completion of all clinical evaluations and independently from ECF data. Final diagnoses fell in the following categories (Tables 1 and 2): 1) confirmed CA by endomyocardial biopsy; 2) highly probable CA; or 3) non-amyloid cardiomyopathy. For patients who underwent endomyocardial biopsy, the final diagnosis was established by the histopathologic interpretation of an experienced pathologist. Confirmed CA was based on endomyocardial biopsy evidence with Congo Red and sulphated Alcian Blue stains or immuno-peroxidase staining for transthyretin and immuno-fluorescence staining for immunoglobulins IgG, IgM, IgA, kappa, lambda, and protein A. For patients who did not undergo endomyocardial biopsy, the final diagnosis is the one retained by the treating cardiologist of the patient on the basis of symptoms, ECG, or echocardiographic or CMR findings. The diagnosis of highly probable CA was reached on the basis of accepted evidence of CA by clinical history, echocardiography, and laboratory testing (25) combined with a LGE pattern consistent with the description by Maceira et al. (4). Patients with no histopathologic evidence of amyloidosis on biopsy and without any evidence of myocardial infiltration by LGE imaging were considered to have non-amyloid cardiomyopathy. We have made every effort to clarify the diagnosis, but it could not be definitively established in 11 patients, even with an endomyocardial biopsy in 3 of them. The detailed final diagnoses of patients with non-amyloid cardiomyopathy are outlined in Table 2. No patient in this group could be considered to have a normal heart on the basis of symptoms, ECG, or echocardiographic or biopsy findings.
Table 2.
Final Diagnosis in Patients With Non-Amyloid Cardiomyopathy
| Frequency (%) | |
|---|---|
| Hypertensive cardiomyopathy | 9 (45%) |
| Cardiomyopathy of undefined etiology by clinical workup | 6 (30%) |
| Cardiomyopathy of undefined etiology by biopsy | 3 (15%) |
| Ventricular arrhythmias | 1 (5%) |
| Hypertrophic cardiomyopathy | 1 (5%) |
| Total | 20 |
CMR protocol
All patients were studied supine in a 3.0-T CMR system (Tim Trio, Siemens Medical Systems, Malvern, Pennsylvania). For LV size and cine function, steady-state free-precession sequence (typical repetition time: 3.4 ms; echo time: 1.2 ms; temporal resolution, 40 to 50 ms; in-plane spatial resolution 1.5 to 1.8 mm and 1.8 to 2.1 mm, on the basis of the field of view) was performed in multiple parallel short-axis planes (8-mm-thick without spacing). An inversion recovery fast gradient-echo sequence, triggered every other heartbeat, was used to assess for LGE in short-axis locations, matching those for cine imaging (8-mm-thick, 0-mm spacing) and 3 radial long-axis planes. Acquisition of LGE images began within 5 to 10 min after bolus administration of a cumulative dose of 0.15 mmol/kg of gadolinium diethylenetriamine pent-acetic acid (Magnevist, Berlex, Wayne, New Jersey). The T1 measurements were performed with a Look-Locker sequence (19,20) with a nonslice-selective adiabatic inversion pulse, followed by segmented gradient-echo acquisition for 17 cardiac phases/times after inversion, spread over 1 to 2 cardiac cycles (temporal resolution 80 ms pre-contrast, and 45 ms post-contrast, slice thickness 8 mm, repetition time >3 RR intervals pre-contrast and 2 RR intervals post-contrast). The Look-Locker sequence was repeated in the same mid LV short-axis slice once before and 3 additional times after the injection of gadolinium spanning a 30-min period. With model simulations, Jerosch-Herold et al. (19) showed that the apparent distribution volume of gadolinium agrees within 1% with the extracellular volume for times >3 min from the beginning of the contrast bolus injection, for myocardial blood flows above 0.5 ml/min/g. For that reason, the post-contrast T1 measurements were started no earlier than 3 min after contrast bolus injection.
Quantification of global and segmental ECF
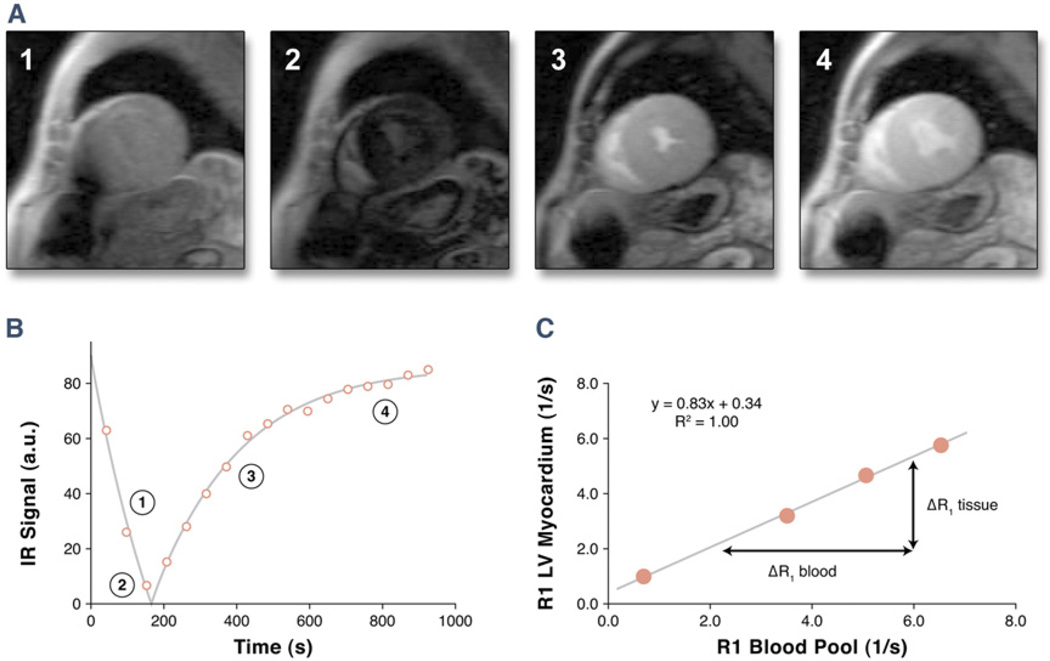
The ECF was quantified as reported previously and is illustrated in Figure 1 (19,20). For each Look-Locker sequence the endo- and epicardial borders of the LV were traced and divided into 6 standard segments (QMass MR 7.1 software, Medis, the Netherlands). The signal intensity versus time curves for each segment and the blood pool were used to determine segmental T1 through fitting to an analytical expression for the inversion recovery and correction for the radiofrequency pulse alteration of the inversion recovery. The reciprocal of T1 (R1 = 1/T1) was used to plot the myocardial R1 against the R1 in the blood pool and calculate the slope by linear regression, with all measurement points. The slope of the linear relationship defines the partition coefficient for gadolinium (λGd). The ECF values of all 6 myocardial segments were obtained by multiplying each of the segmental λGd by (1 – hematocrit in percent/100). Global ECF for each patient or healthy volunteer was then calculated by averaging the 6 myocardial segmental values.
Figure 1. ECF Quantification.
The method of myocardial extracellular volume fraction (ECF) quantification by the Look-Locker gradient-echo sequence with an adiabatic inversion pulse is illustrated. (A) Images from the mid left ventricular (LV) short-axis location are shown (only 4 phases displayed). (B) Determination of T1 and R1 by exponential fitting of the signal-intensity versus time curve for 1 myocardial segment. Numbers correspond to signal intensities obtained at time points displayed in (A). (C) Derivation of the partition coefficient by calculating the slope of the linear relationship between R1 for myocardium versus R1 for the blood pool from all R1 measurements (0.83). Each corresponding point is derived from a signal intensity versus time curve as displayed in (B). The ECF is then determined by multiplying the partition coefficient by (1 hematocrit). IR inversion recovery.
Cine CMR and LGE assessment
Manually traced epicardial and endocardial borders of matching short-axis cine locations at end-systole and end-diastole were used to determine the left ventricular ejection fraction (LVEF), LV end-diastolic volume index, LV end-systolic volume index, and LV myocardial mass (end-diastole only). The LVEF was measured by standard Simpson’s rule with summation of short-axis locations with no interslice spacing. The extent of LGE was assessed by assigning a score of 1 or 0 according to the presence or absence of LGE in the subendocardial and subepicardial halves of each myocardial segment, with the American Heart Association 17-segment model and blinded to ECF data. An LGE score was obtained by summing the score of each segment (0 or 1). The maximum possible score was 33, because the apical cap (segment 17) was not considered to have a subendocardial portion.
Statistical analysis
Continuous data were expressed as mean ± SD or median (interquartile range) when appropriate. Categorical data were expressed as frequency and proportions. Continuous data were compared between groups with t test or Wilcoxon rank sum test according to the distribution. Categorical data were compared with a Fisher exact test when possible and with a chi-square test otherwise. A Kruskall-Wallis test was used for comparison of ECF across diagnostic categories. To account for multiple between-group comparisons, an adjusted p value of 0.0125 was considered significant. When segmental ECF values were included in the analysis, a linear mixed effects model was used to account for the fact that segmental ECF values are correlated within each patient. Spearman rank correlation was used to obtain correlation coefficients between continuous variables of interest.
RESULTS
Baseline characteristics
We studied 39 patients who met the inclusion criteria and used CMR studies with ECF measurements from 9 healthy volunteers. One subject was excluded due to poor CMR image quality. The indications for referral to CMR and the final diagnoses after completion of clinical evaluation of the remaining 38 patients are outlined in Table 1. The most common indication for CMR was further investigation of unexplained increased LV wall thickness by echocardiography or voltage criteria for LV hypertrophy by ECG. In this cohort of 38 patients, 17 (45%) were diagnosed to have CA. Among them, 14 (37%) were biopsy-confirmed CA, and 3 (8%) were highly probable CA on the basis of clinical grounds. One patient was found to have cardiac involvement by a lysosomal storage disease (Hunter disease) on the basis of extracardiac manifestations and typical valvular infiltration; this patient did not undergo endomyocardial biopsy. No evidence of myocardial infiltration could be found in the remaining 20 (53%) patients in whom it was suspected (Tables 1 and 2). Clinical, electrocardiographic, echocardiographic, and CMR characteristics of the study population are outlined in Table 3. Compared with patients with non-amyloid cardiomyopathy and with healthy control subjects, patients with CA were older and had worse LV systolic function, higher LV mass index, and marked LGE burden in both the atria and ventricles. The LV E/A ratio and the septal wall E/E’ ratio were higher in patients with CA compared with patients with non-amyloid cardiomyopathy.
Table 3.
Baseline Characteristics of the Study Population
| CA (n = 17) |
Non-Amyloid Cardiomyopathy (n = 20) |
Healthy Volunteers (n = 9) |
|
|---|---|---|---|
| Age (yrs) | 79 ± 6* | 61 ± 13†‡ | 45 ± 11 |
| Female | 2 (12%)† | 7 (35%) | 6 (67%) |
| Hypertension | 10 (59%)† | 12 (60%)† | 0 |
| Hematocrit (%) | 40 ± 4 | 40 ± 5 | 41 ± 2 |
| ECG | |||
| Non-sinus rhythm | 8 (53%) | 5 (26%) | na |
| Low voltages | 8 (53%) | 3 (16%)§ | na |
| R-wave V1–V3 | 12 (80%) | 19 (100%) | na |
| Echocardiography | |||
| LV E/A ratio | 2.9 ± 1.9 | 1.2 ± 1.5§ | na |
| LV deceleration time (ms) | 188 ± 52 | 191 ± 98 | na |
| LV E′ lateral wall (cm/s) | 5.2 ± 1.0 | 7.9 ± 3.5 | na |
| LV E′ septal wall (cm/s) | 4.3 ± 1.2 | 6.2 ± 1.9 | na |
| E/E′ lateral wall | 14 ± 5 | 12 ± 3 | na |
| E/E′ septal wall | 17 ± 4 | 15 ± 5‖ | na |
| Cardiac magnetic resonance | |||
| LVEDV index (ml/m2) | 94 ± 24† | 91 ± 37¶ | 65 ± 8 |
| LVESV index (ml/m2) | 50 ± 22* | 47 ± 35† | 20 ± 6 |
| LVEF (%) | 48 ± 12* | 53 ± 15† | 69 ± 9 |
| LV mass index (g/m2) | 101 ± 29* | 72 ± 43†‖ | 42 ± 9 |
| LGE | |||
| Atria | 12 (71%)* | 0‡ | 0 |
| LV | 16 (94%)* | 3 (15%)‡ | 0 |
| RV | 12 (71%)* | 0‡ | 0 |
| LV LGE score | 17 ± 6* | 0.7 ± 1.6‡ | 0 |
Values are mean ± SD or frequency (%). Electrocardiograms were available in 15 patients with CA and in 19 patients with non-amyloid cardiomyopathy. The patient with Hunter’s disease is excluded. Echocardiograms were available in 11 patients with CA and in 16 patients with non-amyloid cardiomyopathy.
p < 0.001 for comparison with healthy volunteers;
p < 0.01 for comparison with healthy volunteers;
p < 0.001 for comparison with patients with CA;
p < 0.05 for comparison with patients with CA;
p < 0.01 for comparison with patients with CA;
p < 0.05 for comparison with healthy volunteers.
EDV = end-diastolic volume; ESV = end-systolic volume; LGE = late gadolinium enhancement; LVEF = left ventricular ejection fraction; na = not available; RV = right ventricle; CA = cardiac amyloidosis; CMP = cardiomyopathy; CMR = cardiac magnetic resonance imaging; ECG = electrocardiogram; LVH = left ventricular hypertrophy; LV = left ventricular.
ECF
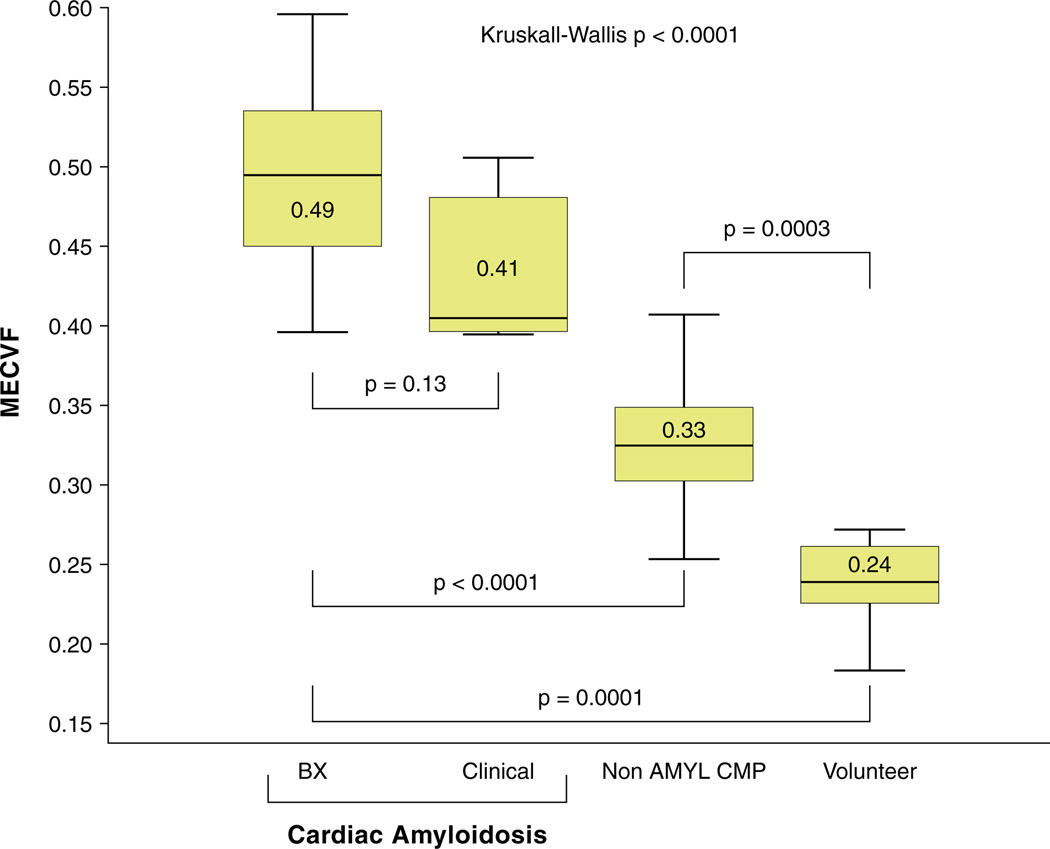
Figure 2 presents the median ECF values for each diagnostic group. The median ECF for healthy subjects was 0.24, whereas it was 0.33, 0.41, and 0.49 for patients with non-amyloid cardiomyopathy, highly probable CA, and confirmed CA, respectively. The ECF was not statistically different between patients with confirmed CA and those with highly probable CA (p = 0.13). We found wide margins of difference in median ECF between patients with CA, non-amyloid cardiomyopathy, and healthy control subjects (p < 0.0001 and p = 0.0001, respectively). The patient with Hunter’s disease was not included in this analysis, because this patient alone could not form a valid comparison group. However, it is noteworthy that the ECF in this patient (0.34) was higher than in healthy subjects (0.24) in absence of LGE.
Figure 2. Comparison of Median MECVF Between Diagnostic Groups.
Median myocardial extracellular volume fraction (MECVF) is highest in patients with cardiac amyloidosis (AMYL). There is a large margin of difference between diagnostic groups (Kruskall-Wallis p <0.0001). A p value <0.0125 is considered significant for between group comparisons after adjustment for multiple comparisons. For each box-and-whisker plot, the central box represents the values from the lower to upper quartile (25th to 75th percentile). The middle line represents the median. The vertical lines extend from the minimum to the maximum value. BX endomyocardial biopsy; CMP cardiomyopathy.
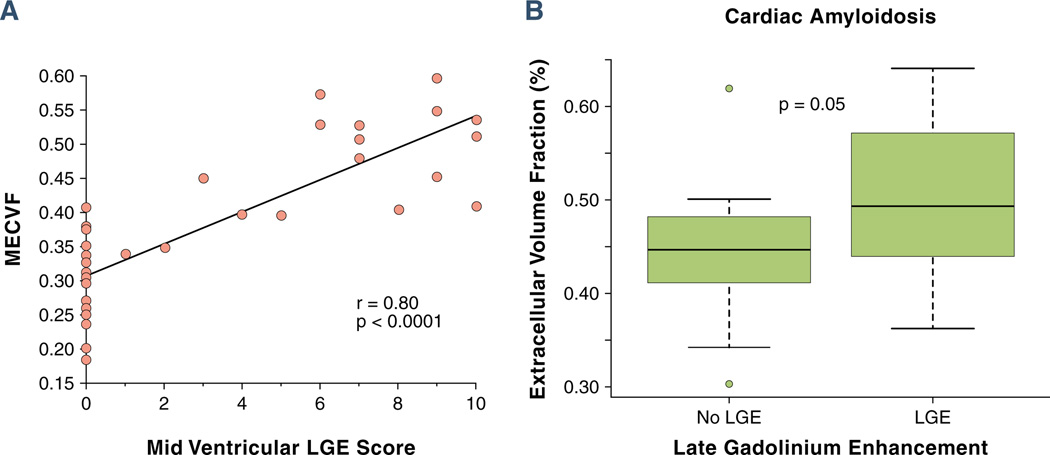
Global and segmental LGE
In all subjects (n = 47), ECF demonstrated a strong correlation with the LGE score in a corresponding mid-LV slice (r = 0.80, p < 0.0001) (Fig. 3A). However, examination of the vertical axis in Figure 3A showed that some subjects without LV myocardial LGE had an elevated ECF. The LV myocardial LGE was present in all patients with CA by qualitative evaluation (Table 3), indicating substantial expansion of the extracellular space. In the subgroup of patients with CA, ECF is highest in segments with LGE, but it also is elevated in myocardial segments without LGE (Fig. 3B).
Figure 3. Association Between Global and Segmental MECVF and LGE.
(A) Spearman rank correlation between global myocardial extracellular volume fraction (MECVF) and late gadolinium enhancement (LGE) score in corresponding mid left ventricular (LV) slices in all subjects (n = 47). Note that 23 (82%) of 28 patients with no LGE by qualitative interpretation (LGE score of 0) had an MECVF value >0.24. (B) Comparison of MECVF in myocardial segments with and without LGE in the subgroup of patients with cardiac amyloidosis (n = 17).
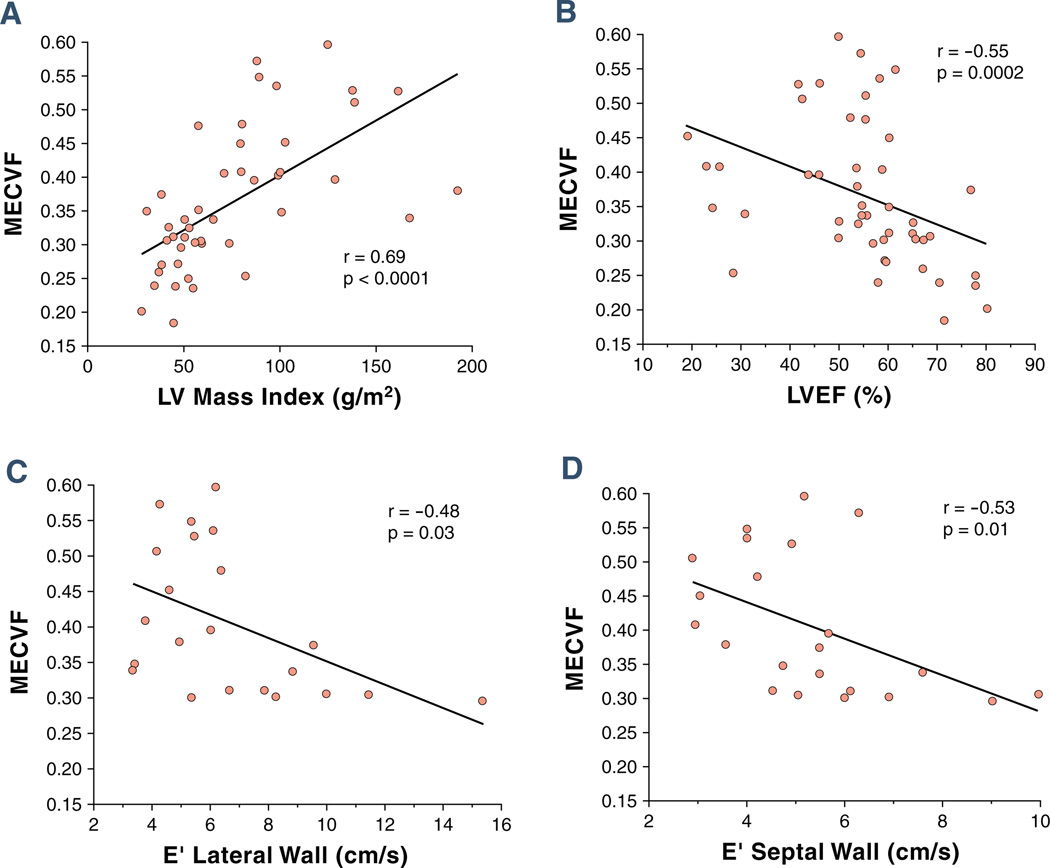
ECF and markers of cardiac infiltration
The ECF correlates strongly with LV mass index (r = 0.69, p < 0.0001) (Fig. 4A). It also correlates negatively with LVEF (r = −0.55, p = 0.0002) (Fig. 4B) and with the peak velocity of the E’-wave of both the lateral (Fig. 4C) and septal (Fig. 4D) wall by tissue Doppler echocardiography.
Figure 4. Correlations Between MECVF and Markers of Myocardial Infiltration.
Correlations between MECVF and (A) LV mass index, (B) LV ejection fraction, and (C) peak velocity of the E’ wave of the LV lateral wall and (D) of the LV septal wall by tissue Doppler echocardiography. Abbreviations as in Figure 3.
DISCUSSION
Summary of results
In this retrospective cross-sectional case-control study, we have measured the extracellular volume fraction in patients with known and suspected infiltrative heart disease. Most included patients either had CA or a non-amyloid cardiomyopathy, forming the 2 comparative patient groups in our study, along with healthy volunteers. Measurement of ECF indicates that patients with amyloidosis and cardiac involvement have the highest degree of myocardial extracellular matrix expansion, even in segments where LGE cannot be qualitatively seen. Flacke et al. (26) reported that the partition coefficient in patients with acute and chronic myocardial infarction averaged 0.91 and 0.78, in the acute infarct and chronic regions, respectively—which suggests an ECF of 0.55 and 0.47, respectively, assuming an average hematocrit of 40%. In patients with CA, the ECF approached similar levels as in acute myocardial infarction in regions with LGE but was lower in regions without LGE, although still significantly higher than in the patients with non-amyloid cardiomyopathy. These observations suggest that measurement of the ECF might allow a finer gradation of adverse tissue remodeling than is possible by qualitative assessment of LGE. Increasing values of ECF are associated with markers of increased myocardial infiltration, such as LV mass index and LVEF (25), and with indexes of restrictive cardiomyopathy, such as the E’ wave peak velocity (24). Greater ECF values are associated with higher LGE scores, both reflecting greater extracellular matrix expansion. The ECF also identifies abnormal characteristics in myocardium not displaying LGE. This allows appreciation of otherwise unrecognized infiltration and might lead to an earlier diagnosis.
Healthy volunteers
Study of healthy subjects allowed for establishment of the median ECF in that population (0.24) (20). A similar control group was used in the current study. This ECF value is comparable to that reported (range 18.4% to 29.1%) in another study of healthy subjects with a similar technique (27). The low ECF in healthy subjects corresponds to a longer post-contrast T1 relaxation time. Tightly packed normal cardiomyocytes do not allow for much accumulation of gadolinium in the interstitium.
Non-amyloid cardiomyopathy patients
The most common diagnosis among patients deemed not to have myocardial infiltration was hypertensive cardiomyopathy (Table 2), owing to its common presentation with LV hypertrophy. As patients age, hypertension is prevalent and is a frequent confounder when the clinical suspicion of infiltrative heart disease arises, because they both cause increased myocardial wall thickness. Late gadolinium enhancement can also occur in the hypertensive patient (12). However, our results suggest that the degree of ECF expansion is substantially lower than in CA. It is conceivable that similar observations could be made for hypertrophic cardiomyopathy patients, although our data do not allow further testing of this hypothesis. Flett et al. (21), however, reported extracellular matrix expansion in hypertrophic cardiomyopathy. The remainder of patients with non-amyloid cardiomyopathy presented with a suspicion of infiltrative heart disease, although no specific diagnosis could be reached after all clinically justifiable investigations, including endomyocardial biopsy in 3 patients. No convincing evidence of myocardial infiltration could be found in any of these patients. The presence of clinical heart failure, abnormal ECG or echocardiogram, or significant arrhythmias ruled out that these patients have a normal heart. A median ECF of 0.33 (range 0.25 to 0.41) in these 11 patients also supports that they have a cardiomyopathy, in comparison with healthy volunteers (median ECF = 0.24).
ECF quantification
In this study, we have applied a previously described technique to measure the expansion of myocardial extracellular matrix (19). This technique uses a repetition time equal to 3 RR intervals, making it very insensitive to variation in heart rate in the clinical setting. In fact, there was no significant association between the pre-contrast R1 values and the heart rate of the patient. The use of the partition coefficient also adjusts for the blood levels of gadolinium. Therefore, the estimate of T1 is independent of the dose of gadolinium and of its clearance from the blood pool, which will vary with renal function. The method also does not depend heavily on the exact timing of the T1 measurement after infusion of gadolinium, in contrast to LGE imaging, and the T1 measurement protocol proposed by Maceira et al. (4). In our protocol, T1 measurements are only acquired after a 3-min delay after gadolinium injection to allow for an equilibrium between gadolinium concentration in myocardium and blood. This can be repeated multiple times over the course of the CMR study to optimize the accuracy of the partition coefficient determination by linear regression analysis. A previous study, with kinetic parameters determined from a first pass perfusion imaging in each patient, used simulations (19) to conclude that equilibrium conditions for ECF estimation are met within 3 min for typical tissue perfusion and gadolinium clearance rates. A more recent study has shown that ECF did not change significantly between 12 and 50 min after a bolus injection (27) and left open the question of whether a 3-min delay is sufficient for contrast equilibrium to take place. We note that our findings of a significantly abnormal ECF in cardiomyopathy and amyloid become more marked if the first post-contrast T1 measurement, acquired as early as 3 min after injection, is eliminated from the determination of ECF. The ECF changed by +8% (p = 0.002) higher without this first post-contrast T1 measurement. This suggests that under some conditions—such as a markedly expanded extracellular space, as found in amyloid, or with reduced tissue perfusion—a delay of 3 min might be too short to achieve equilibrium. Overall, acquisition of 4 T1 measurements (1 before and 3 after gadolinium injection) adds between 5 and 15 min to the study, depending on whether a single slice or multiples levels are imaged.
The equivalent protocol proposed by Flett et al. (21) was based on only 2 T1 measurements. It requires an infusion of gadolinium lasting up to 90 min, during which the patient is removed from the scanner. Both the bolus and infusion methods were found to give stable ECF measures between 12 and 50 min after contrast injection, in a recent direct comparison (27). The study by Flett et al. (21) provided robust histological confirmation that the volume of distribution of gadolinium derived from T1 measurements matches the collagen volume fraction. Other techniques used in studies using T1 mapping (14,15) are subject to variability in measurements due to heart rate and to the dose, rate of injection, and washout of gadolinium. These techniques, however, can provide pixel-by-pixel T1 maps.
Late gadolinium enhancement
Late gadolinium enhancement has emerged as an important tool for the CMR diagnosis of infiltrative heart disease and of CA in particular (1,9). Late gadolinium enhance-ment was also associated with adverse survival outcomes in patients with CA (6,7,10); LGE is a robust and accessible technique that is a fundamental part of the CMR evaluation of patients with cardiomyopathy (28). However, in the particular case of CA, LGE imaging is faced with particular challenges. A pathologic study reported that the extent of amyloid deposition was not associated with LGE finding in any region (5). Late gadolinium enhancement corresponded more accurately to areas of fibrosis (5). Homogenous or diffuse deposition of amyloid protein in the myocardium can preclude automated detection of differences in signal intensity on the basis of an arbitrary threshold. For that reason, we have relied on qualitative assessment of LGE. Identification of extracellular matrix expansion in areas with questionable LGE can help resolve diagnostic dilemmas. Examples of the complementary use of LGE and ECF in diagnosis of cardiac infiltration are presented in Figure 5. Late gadolinium enhancement is also sensitive to selection of the inversion time and to observer interpretation. Selection of inversion time can be difficult in CA, due to the rapid myocardial uptake of gadolinium. This has even been reported as a diagnostic clue to the diagnosis of CA (8). Prognostic information associated with the presence of LGE has been obtained in patients with advanced disease (25). Maceira et al. (22) have reported a prognostic association between abnormal myocardial T1 mapping and survival in patients with CA. Late gadolinium enhancement was not associated with outcomes in their study (22). Therefore, a technique sensitive to changes in T1, such as ECF, might eventually be shown to better reflect the severity of cardiac involvement and might be associated with prognosis.
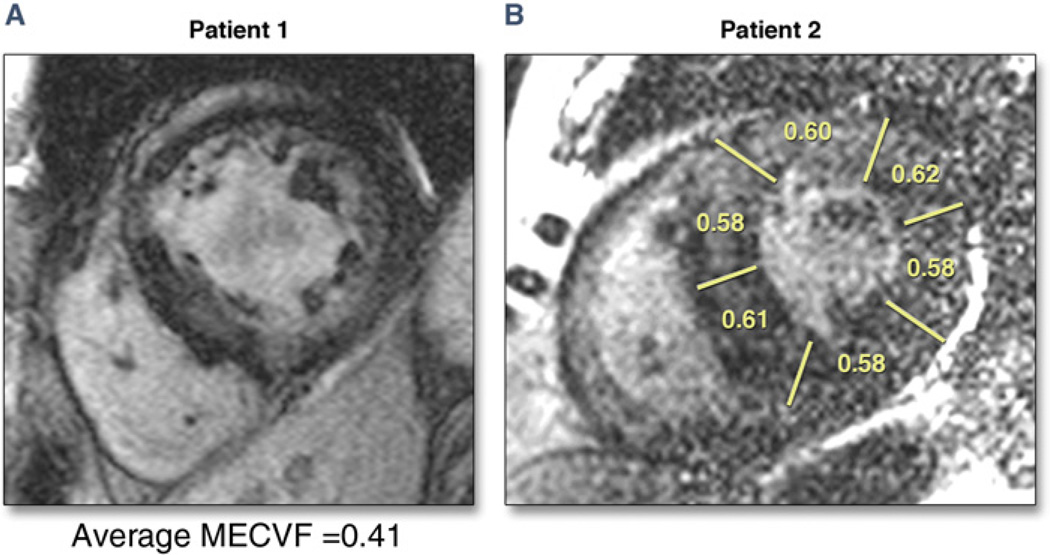
Figure 5. Complementary Use of LGE and MECVF in Patients With Cardiac Amyloidosis.
Late gadolinium enhancement (LGE) images obtained with a standard inversion recovery fast gradient echo sequence triggered every other heartbeat in 2 patients with biopsy proven cardiac amyloidosis. (A) Patient #1. Multiple areas of enhancement consistent with cardiac amyloidosis are present. Accordingly, myocardial extracellular volume fraction (MECVF) is elevated. (B) Patient #2. It is difficult to appreciate myocardial infiltration, if at all present. It seems that LGE is present in the anterior wall. However, segmental MECVF suggests marked infiltration in all segments (MECVF in healthy volunteers 0.24).
Study limitations
Our study is limited by a small sample size, which is largely due to the rarity of infiltrative heart disease. Because CMR has become part of routine assessment of CA, the group of patients with infiltrative heart disease consisted mostly of patients with advanced, clinically evident CA. This potentially reflects a referral bias influenced by the current diagnostic need and increased usage of CMR in the clinical diagnosis of infiltrative heart disease. We cannot assess the degree of myocardial extracellular matrix expansion in patients at an earlier stage of CA. A subset of 11 patients in this study were left with a nonspecific diagnosis, even after endomyocardial biopsy in 3 of them. Endomyocardial biopsies were not obtained for all patients, reflecting a parsimonious and diminishing use of this technique in the clinical work-up of patients suspected to have myocardial infiltration. When available, histological diagnosis of infiltrative cardiomyopathy relied on clinically obtained right ventricular endomyocardial biopsies. Biopsies of the LV myocardium studied with ECF were not available. However, infiltrative heart diseases are diffuse processes. For that reason, we have focused ECF measurement to a middle short-axis slice and not to the entire myocardium. The ECF quantification requires administration of gadolinium, which can be limited by renal insufficiency, which complicates primary and secondary forms of amyloidosis (25). The processing of CMR images to derive ECF remains labor intensive, but the technique might resolve diagnostic uncertainty and eventually obviate the need for further testing. The determination of ECF through pre- and post-contrast T1 measurements requires the establishment of an equilibrium state for the contrast distribution between blood and tissue. This imposes minimum delay for the first post-contrast T1 measurement after contrast injection. Because contrast clears rapidly from the blood pool in patients with amyloidosis, a delay of >3 min was chosen for this protocol to allow observation of large R1 changes relative to the native R1. If the earliest post-contrast measurement is excluded from the myocardial ECF estimation, then we obtain a myocardial ECF that is on average 6% to 8% higher. This difference did not alter the main results when the patient groups were compared. Finally, LGE was quantified as present or absent in the subendocardial and subepicardial halves of each myocardial segment. Due to the diffuse distribution of LGE, formal quantification of LGE on the basis of signal intensity could not be used.
CONCLUSIONS
Extracellular volume fraction measured by contrast-enhanced CMR is elevated in patients with clinically suspected myocardial infiltration. The highest extracellular volume expansion was found in patients with CA. This technique has the potential to provide a quantitative assessment of myocardial infiltration with CMR beyond qualitative interpretation. Further validation of the diagnostic utility of this technique in a larger subset of patients and at an earlier stage of infiltration is warranted. Measurement of ECF before and after treatment would also be worthy of further study.
Acknowledgments
Dr. Mongeon is supported by the Bourse du Coeur 2009 scholarship and receives research funding from Montreal Heart Institute Foundation, Montreal, Canada. Dr. Jerosch-Herold is supported in part by a research grant from the National Institutes of Health (R01HL090634-01A1). Dr. Kwong receives salary support from a research grant from the National Institutes of Health (NIH R01HL091157).
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Seward JB, Casaclang-Verzosa G. Infiltrative cardiovascular diseases: cardiomyopathies that look alike. J Am Coll Cardiol. 2010;55:1769–1779. doi: 10.1016/j.jacc.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 2.De Cobelli F, Esposito A, Belloni E, et al. Delayed-enhanced cardiac MRI for differentiation of Fabry’s disease from symmetric hypertrophic cardiomyopathy. AJR Am J Roentgenol. 2009;192:W97–W102. doi: 10.2214/AJR.08.1201. [DOI] [PubMed] [Google Scholar]
- 3.Arad M, Maron BJ, Gorham JM, et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352:362–372. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- 4.Maceira AM, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 5.Hosch W, Kristen AV, Libicher M, et al. Late enhancement in cardiac amyloidosis: correlation of MRI enhancement pattern with histopathological findings. Amyloid. 2008;15:196–204. doi: 10.1080/13506120802193233. [DOI] [PubMed] [Google Scholar]
- 6.Austin BA, Tang WH, Rodriguez ER, et al. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. J Am Coll Cardiol Img. 2009;2:1369–1377. doi: 10.1016/j.jcmg.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Ruberg FL, Appelbaum E, Davidoff R, et al. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am J Cardiol. 2009;103:544–549. doi: 10.1016/j.amjcard.2008.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. J Am Coll Cardiol Img. 2010;3:155–164. doi: 10.1016/j.jcmg.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51:1022–1030. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Migrino RQ, Christenson R, Szabo A, Bright M, Truran S, Hari P. Prognostic implication of late gadolinium enhancement on cardiac MRI in light chain (AL) amyloidosis on long term follow up. BMC Med Phys. 2009;9:5. doi: 10.1186/1756-6649-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piotrowska-Kownacka D, Kownacki L, Kuch M, et al. Cardiovascular magnetic resonance findings in a case of Danon disease. J Cardiovasc Magn Reson. 2009;11:12. doi: 10.1186/1532-429X-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudolph A, Abdel-Aty H, Bohl S, et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284–291. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 13.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 15.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 16.Thornhill RE, Prato FS, Wisenberg G, Moran GR, Sykes J. Determining the extent to which delayed-enhancement images reflect the partition-coefficient of Gd-DTPA in canine studies of reperfused and unreperfused myocardial infarction. Magn Reson Med. 2004;52:1069–1079. doi: 10.1002/mrm.20236. [DOI] [PubMed] [Google Scholar]
- 17.Thornhill RE, Prato FS, Wisenberg G, White JA, Nowell J, Sauer A. Feasibility of the single-bolus strategy for measuring the partition coefficient of Gd-DTPA in patients with myocardial infarction: independence of image delay time and maturity of scar. Magn Reson Med. 2006;55:780–789. doi: 10.1002/mrm.20830. [DOI] [PubMed] [Google Scholar]
- 18.Arheden H, Saeed M, Higgins CB, et al. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology. 1999;211:698–708. doi: 10.1148/radiology.211.3.r99jn41698. [DOI] [PubMed] [Google Scholar]
- 19.Jerosch-Herold M, Sheridan DC, Kushner JD, et al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;295:H1234–H1242. doi: 10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 22.Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson. 2008;10:54. doi: 10.1186/1532-429X-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49:9–13. doi: 10.1016/0002-9149(82)90270-3. [DOI] [PubMed] [Google Scholar]
- 24.Ha JW, Ommen SR, Tajik AJ, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy using mitral annular velocity by tissue Doppler echocardiography. Am J Cardiol. 2004;94:316–319. doi: 10.1016/j.amjcard.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52:347–361. doi: 10.1016/j.pcad.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218:703–710. doi: 10.1148/radiology.218.3.r01fe18703. [DOI] [PubMed] [Google Scholar]
- 27.Schelbert EB, Testa SM, Meier CG, et al. Myocardial extravascular extra-cellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011;13:16. doi: 10.1186/1532-429X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol. 2009;54:1407–1424. doi: 10.1016/j.jacc.2009.04.094. [DOI] [PubMed] [Google Scholar]