Abstract
Purpose of review
This review is meant as a balanced summary of the current state of cardiac magnetic resonance (CMR) perfusion imaging in assessing alterations in myocardial blood flow due to coronary artery disease (CAD). We aim to provide first an accessible technical overview of first-pass CMR perfusion imaging and contrast it with other conventional perfusion imaging modalities, and then address the potential advantages of CMR for a qualitative assessment of perfusion defects, as well as quantitative blood flow measurements. Most recent results from clinical trials on the utility of CMR perfusion and novel directions will be explored.
Recent findings
Recent results of the first multicenter multivendor CMR perfusion study demonstrated superior diagnostic utility in detecting CAD by CMR compared with conventional nuclear single-photon emission computed tomography. Several large clinical trials provide additional evidence indicating the strong prognostic implications when CMR perfusion was performed in a clinical setting in patients with an intermediate clinical likelihood of CAD. A negative adenosine stress CMR perfusion study conferred a favorable 3-year prognosis towards nonfatal myocardial infarction or cardiac death.
Summary
CMR perfusion imaging during the first pass of gadolinium-based contrast agents has undergone many technical improvements and levels of clinical validation. Rapidly increasing clinical use worldwide over the last years in diagnosing chest pain syndromes supports the role of CMR in a comprehensive and efficient noninvasive assessment of altered myocardial physiology in CAD.
Keywords: first-pass myocardial perfusion, gadolinium, ischemia, mortality, myocardial blood flow, myocardial infarction
Introduction
Healthcare practitioners looking after patients with suspected or known cardiac diseases are often challenged by, first of all, the need to diagnose the presence and severity of coronary artery disease (CAD) in patients with suspected symptoms, and second, patient risk stratification, both of which are needed to decide on next-level invasive procedures. With the advent over the past 3 decades of various imaging technologies, extensive experience had been gained in understanding the physiology and pathophysiology of CAD by assessing myocardial blood flow (MBF) at different hemodynamic states. In many single-center studies, and recently a multicenter study, cardiac magnetic resonance (CMR) perfusion imaging has demonstrated high diagnostic utility for detecting angiographic significant CAD, and also demonstrated strong noninvasive patient prognostic implications. This article will serve to provide an up-to-date review of the technical aspects of CMR perfusion and its current clinical values.
Imaging strategies and technical challenges
The in-vivo visualization of the coronary tree from the major proximal epicardial branches down to the capillary level is beyond the reach of clinically applicable imaging modalities, as it covers several orders of magnitude in vessel diameter [1]. Instead, the spatial resolution limits of clinical imaging modalities such as CMR or echocardiography require strategies that are either tailored to the direct evaluation of the coronary wall [2,3] and measurement of flow in the epicardial vessels [4], or the indirect detection of flow in the intramural coronary microcirculation by perfusion imaging. In the latter case, one uses injected tracers or indicators whose distribution in tissue is flow-dependent and allows external detection [5]. The principles of indicator-dilution theory, originally introduced by Stewart in 1893 for estimating cardiac output from the circulation time of an injected indicator, were extended to estimate blood flow in tissue, and they form an essential underpinning of myocardial perfusion imaging.
There are several reasons for assessing coronary flow at different levels in the coronary tree. Luminal narrowing, in particular in the presence of diffuse arterial narrowing [6], is not a good predictor of the functional severity of epicardial lesions. Collateral vessels can effectively help in preserving tissue blood flow at baseline. Conversely, microvascular dysfunction can precede the appearance of flow-limiting epicardial lesions, for example, in hypertensive patients [7,8]. Although complementary tests may be useful for risk stratification, performing multiple imaging tests can be cumbersome on the patient and may entail an increased burden of radiation exposure. CMR is one of the most versatile modalities allowing a combined assessment of myocardial perfusion, coronary flow, and ventricular function, with proven benefits of combining some of these complementary tests for the diagnosing coronary heart disease [9••], without any exposure to ionizing radiation. We discuss here which specific imaging strategies based on CMR are increasingly being viewed as optimal for the assessment coronary blood flow in patients with CAD.
Cardiac magnetic resonance perfusion imaging techniques
The assessment of contrast enhancement during the first pass of a contrast bolus constitutes one of the most widely used approaches to detect regional MBF deficits with MRI [10–12]. It involves a bolus injection of contrast agent during rapid imaging of the heart to allow a visual or quantitative assessment of the transit of an extracellular contrast agent through the chambers of the heart and myocardial tissue. A conceptual illustration of an MRI ‘first-pass’ perfusion study is shown in Fig. 1. Areas with reduced signal enhancement during the first pass of the blood-borne contrast agent reveal hypoperfusion, at least with respect to other territories. Comparison of the myocardial contrast enhancement between baseline and stress states is used to evaluate the myocardial perfusion reserve [13–15]. The feasibility of evaluating the myocardial perfusion reserve by MRI has by now been firmly established, including through validation by comparison with invasive measures of the coronary flow reserve [16]. Epicardial, flow-limiting lesions cause the most pronounced reduction of MBF in the endocardial layer of the heart muscle. The ability to detect hemodynamically relevant coronary lesions is therefore determined by the spatial resolution of the imaging technique to resolve flow reductions in the endocardial layer. The imaging techniques used for magnetic resonance perfusion imaging yield an in-plane spatial resolution on the order of 2–3 mm, which makes it feasible to evaluate tissue blood flow in the endocardial layer. Novel fast imaging techniques can afford an in-plane spatial resolution of approximately 1mm [17], while preserving adequate temporal resolution.
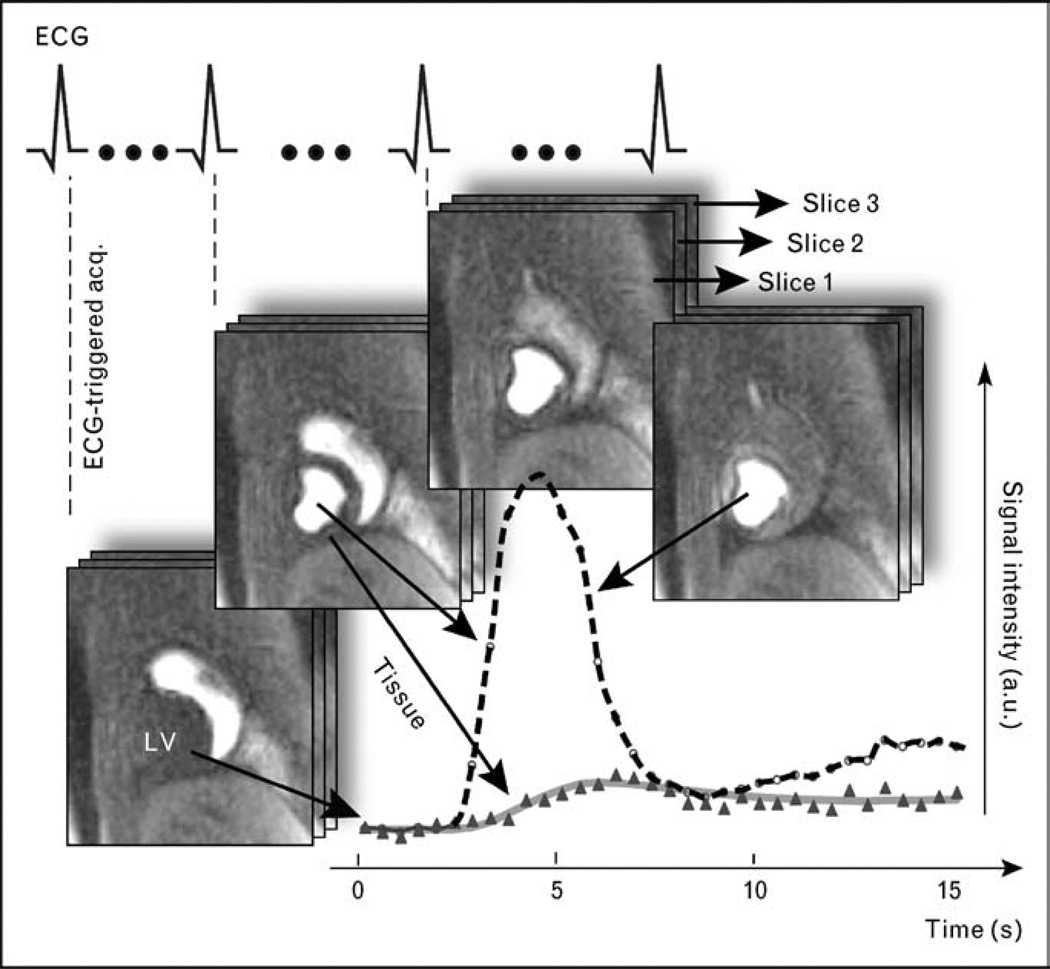
Figure 1. Schematic illustration of cardiac magnetic resonance data acquisition in myocardial perfusion imaging during the first-pass transit of a tight intravenous bolus injection of gadolinium-based contrast agent.
Images are acquired with an electrocardiogram-triggered, multislice imaging protocol, for example, in the short-axis orientation as in this example, and at a rate equal to the heart rate, to achieve adequate temporal resolution. A visual or quantitative analysis of the signal intensity changes in the myocardium allows the identification of hypoperfused areas. The signal intensity changes shown in the graph of this figure were determined for a myocardial region and a region in the center of the left ventricle (LV), with the latter serving as arterial reference or input function. For a quantitative analysis, this arterial input is used as ‘reference’ for analysis of the myocardial enhancement.
Cardiac and respiratory motion rank high among the technical challenges for the assessment of coronary flow and myocardial perfusion. Solutions, common to most tomographic approaches for perfusion imaging, are the use of gating to synchronize the image acquisition with the cardiac cycle, and shortening the image acquisition time. In the field of MRI, so-called parallel imaging and novel data sampling schemes have been developed to allow image acquisitions times on the order of 100 ms or shorter [18]. Parallel imaging involves the use of receiver coil arrays, which reduce the number of spatial encoding steps because each receiver array element has a localized and well characterized spatial sensitivity profile with only partial overlap with neighboring coil profiles [19]. Combining information about the spatial sensitivity profiles of the coil array elements with the conventional spatial encoding with magnetic field gradients allows an overall reduction of the image acquisition time. For myocardial perfusion imaging, this creates the opportunity to image more slices during each heart cycle while maintaining the in-plane spatial resolution, thereby overcoming limits in anatomical coverage that initially stymied myocardial perfusion studies.
Parallel imaging involves a reduction of the spatial encoding steps for acquisition of each image, but further gains in acquisition speed can be achieved in a perfusion study by extending sparse sampling from the spatial to the spatial-temporal (‘k-t’) domain [17,20–22]. In other words, the same spatial encoding steps need not to be repeated for each image acquisition, because contrast enhancement is limited to certain regions of the image, implying some redundancy if complete image acquisitions are performed during each heart beat. With sparse sampling in ‘k-t’ space, missing spatial encoding steps are estimated from equivalent encoding steps in other images using a reconstruction filter that is based on the contrast changes measured in lower-resolution ‘training’ data sets. A recent study by Jahnke et al. [23] with such a temporal and spatial acceleration technique (‘BLAST’ – k-space and time broad-use linear acquisition speed-up technique) reported sensitivity, specificity, and diagnostic accuracy of 86, 78, and 83%, using only visual analysis, which compares favorably with studies using nonaccelerated techniques [24,25••], but requiring longer image acquisition times.
Similarly to developments in the catheterization laboratory, such as measurement of the fractional coronary flow reserve [26], there is increasing interest in the field of myocardial perfusion imaging to obtain quantitative, largely observer-independent measures of coronary function in the microcirculation. Quantitative approaches for the estimation of the perfusion reserve [15], and absolute myocardial flow were validated successfully in animal models [27–30] and are now increasingly being used in CAD patients [31,32••]. Using the initial rate of myocardial contrast enhancement (‘up-slope’ parameter), it was shown by noninvasive magnetic resonance perfusion imaging in patients with single-vessel disease that the perfusion reserve in ischemic segments improved significantly after successful angioplasty but did not normalize in comparison with remote segments [32••].
The basic concept behind quantitative contrast-enhanced MRI perfusion techniques lies in the analysis of the myocardial contrast enhancement kinetics, using the arterial contrast enhancement (e.g. the enhancement in the left ventricular cavity or the ascending aorta) as reference. For example, the rate at which the signal intensity increases during the initial stage of contrast transit through the myocardium is an indirect measure of blood flow in a given myocardial region. The rate of contrast enhancement during the first pass, often referred to as the ‘up-slope parameter’, normalized by the rate of enhancement in the blood pool, was shown to be a sensitive index to detect coronary disease and assess the benefit of coronary interventions [14,33].
For quantitative studies, it was until recently necessary to use low-contrast dosages to avoid signal saturation in the arterial input, which is used to estimate the MBF. Low-contrast dosages render a visual evaluation of myocardial perfusion studies more difficult. Two technical developments have helped to overcome this divergence of requirements for quantitative and qualitative studies: In the dual bolus technique, a smaller contrast dosage [29] is used akin to a ‘test’ bolus to characterize the arterial input of contrast, whereas a larger dosage bolus is injected shortly afterwards to measure myocardial enhancement with optimal contrast-to-noise settings. The larger bolus injection is also well suited for a qualitative, visual evaluation. As an alternative, a recently developed image acquisition technique combines a low-resolution arterial input scout image, taking approximately 30–50 ms during every heart beat, with high-resolution myocardial imaging with a dual contrast weighting that largely overcomes saturation effects [34]. With these developments, it can be foreseen that quantitative perfusion imaging will no longer remain the realm of research studies that impose special conditions for the image acquisition and contrast injection.
The contrast-based perfusion MRI techniques are well suited for detecting microvascular obstruction [35,36]. The extent of microvascular obstruction observed with MRI was recently found to correlate well with invasive Doppler coronary flow measurements [37]. In addition, late gadolinium enhancement (LGE) can be used to image myocardial infarcts. Viability imaging with MRI, although based on techniques quite similar to those used for imaging perfusion during the first pass, can be carried out at considerably higher spatial resolution as the contrast distribution has reached steady-state equilibrium [38]. A time window starting as early as 5 min after the contrast injection extending to approximately 20 min can be used to map out nonviable myocardium with a spatial resolution that allows detection of small or microinfarcts [39]. The relation between a disturbance of microcirculatory function and distal myonecrosis was recently elucidated by a combination of magnetic resonance perfusion and viability imaging [31]. The combined assessment of myocardial perfusion, microvascular obstruction, and LGE in a single CMR examination of a patient with CAD is illustrated in Fig. 2.
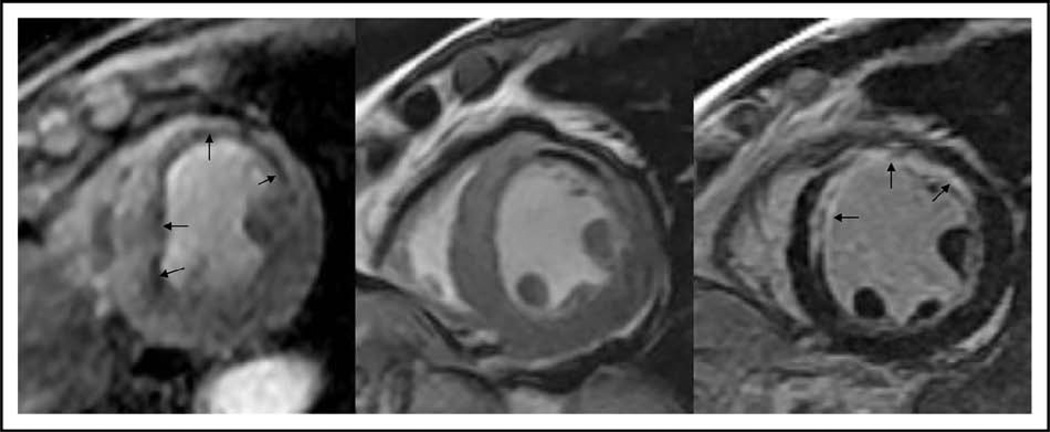
Figure 2. Depiction of a clinical case of a 55-year-old man who experienced recurrent chest pain several years after suffering a ST-elevation myocardial infarction.
Only matching short-axis location of adenosine stress CMR perfusion (left), diastolic frame of cine function (middle), and late enhancement for infarction (right) were shown. The patient had evidence of a full-thickness anterior myocardial infarction as demonstrated by late enhancement (arrows in the right image) with thinned anterior wall (arrows in middle image), during first-pass perfusion, there was an extensive subendocardial perfusion defect matching the location of the infarction but also extended into surrounding segments with preserved myocardial thickness and noninfarcted segments (arrows in the left image). This patient had returned as a result of the attempt to stent the proximal LAD had failed and this was confirmed on subsequent X-ray angiography. Matching locations of the different components of CMR and the high spatial resolution of CMR perfusion contributed to this study. CMR, cardiac magnetic resonance; LAD, left anterior descending artery.
Other cardiac magnetic resonance techniques in quantifying myocardial blood flow: phase-contrast imaging
A robust and well validated technique for measuring blood flow velocities relies on the measurement of the phase of the MRI signal in combination with flow-sensitive spatial encoding to quantify for each pixel location the velocity component(s) perpendicular or within the image plane. This so-called phase-contrast technique can be used not only for measuring flow in the great vessels, but also for determining the coronary flow reserve [40,41] and thereby allow detection of restenosis after percutaneous coronary revascularization [42], and also to check patency in coronary bypass grafts [43]. Both high-spatial resolution and the absence of artifacts from respiratory motion remained significant challenges, until the introduction of navigator techniques [44,45]. The navigator techniques, first devised to compensate for breathing motion in coronary magnetic resonance angiography [46], consist of rapidly and periodically measuring (~20 ms) signal intensity profiles along thin cylindrical regions perpendicular to the diaphragm to monitor respiratory motion. Image data are acquired continuously, but only those data acquired during periods of respiratory motion quiescence are retrospectively selected for the image reconstruction. The image acquisition is also cardiac gated to obtain velocity data for a defined number of cardiac phases. This navigator-guided, retrospectively gated phase-contrast technique can be used to measure coronary blood flow velocities in the proximal portions of the left or right coronary artery. Although not suitable for localizing a coronary lesion, this technique nevertheless allows detection of flow reserve reductions. It is in a sense the noninvasive equivalent to coronary blood velocity as measured by a Doppler flow catheter during cardiac catheterization.
Cardiac magnetic resonance perfusion in diagnosing clinical coronary artery disease and patient risk prognostication
Compared with conventional nuclear scintigraphy, the reduced burden of image artifacts and substantially higher spatial resolution contributed to the ability of acquiring highly reproducible quantitative MBF measurements. It is therefore not surprising that over the years many clinical and preclinical studies had utilized CMR perfusion as surrogate in studying pathophysiology and potential beneficial effects of novel treatments, in which large-scale clinical outcome studies are not feasible. In our opinion, though nuclear scintigraphy has played a dominating role of clinical patient care in the last decades, CMR perfusion imaging can provide a more precise and complete characterization of alteration of cardiac physiology by qualitative and quantitative assessment of MBF [32••], ventricular function and reserve, and the extent of myocardial scar from CAD (Fig. 3). The Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial (MR-IMPACT) study is the first multicenter multivendor clinical trial that direct compared the diagnostic performance of CMR perfusion imaging with nuclear single-photon emission computed tomography (SPECT) imaging with X-ray angiography as a reference [25••]. A dose-finding study was prospectively performed before the beginning of the multicenter study to conclude the use of 0.1 mmol/kg of first-pass contrast. With 234 patients from 18 centers studied, overall CMR perfusion performed favorably compared to SPECT by receiver operating characteristic analysis (area under the curve 86±6 vs. 67±5%). This study was limited by suboptimal SPECT results, which was in part due to a lack of gated SPECT data and as a result, led to poor specificity of SPECT in this comparison. Nevertheless, this is the first study, which demonstrated that CMR perfusion is a technology that maintains its high diagnostic utility in a multicenter multivendor setting, which provides incremental information to the numerous single-centered studies in the past decade showing excellent sensitivity for detection of significant CAD.
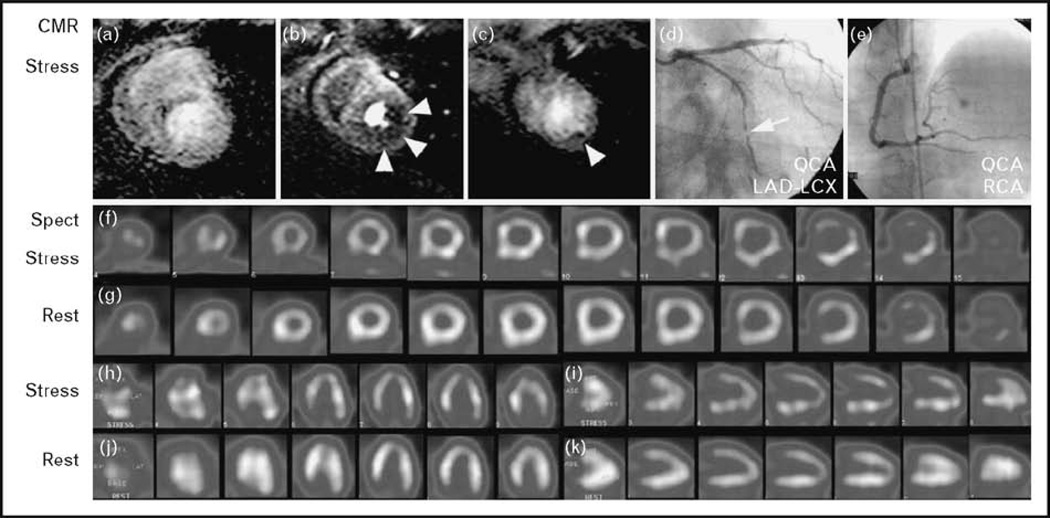
Figure 3. Case example from the MR-IMPACT study.
A 47-year-old patient is shown 2 months after successful stenting of the LAD and experienced mild angina. The perfusion CMR study during hyperemia (at 0.1 mmol/kg gadolinium – DTPA) demonstrates a perfusion deficit in the subendocardium of the lateral wall (b and c; arrow head) appreciated by all three readers. SPECT in this patient was positive for the presence of CAD for one reader only. Coronary X-ray angiography demonstrated a significant stenosis in the circumflex coronary artery. Perfusion in the anterior wall was assessed correctly by both techniques (normal perfusion) despite a stent in the LAD. CAD, coronary artery disease; DTPA, diethylenetriaminepentaacetic acid; LDA, left anterior descending artery; MR-IMPACT, Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial; SPECT, single-photon emission computed tomography. Reproduced with permission from [13].
There is increasing evidence that CMR perfusion provides strong patient prognostic implication at a clinical setting. Jahnke et al. [9••] assessed the prognostic implication of CMR perfusion with adenosine stress and dobutamine stress CMR performed in a concurrent imaging session, in 513 patients who were suspected to have significant CAD (Fig. 4). With an intermediate prevalence of CAD in this cohort, the authors demonstrated strong differentiating characteristics by CMR perfusion in patients who subsequently suffered a nonfatal myocardial infarction (MI) or cardiac death (cumulative event rate in the first 12 months after CMR was 0.7% with normal CMR stress perfusion vs. 9% with abnormal stress perfusion). The authors found that prognostic potential was similar between adenosine stress CMR perfusion and dobutamine stress CMR function. This study is the first large single-center study that established the excellent negative predictive value of CMR perfusion in risk stratifying patients with an intermediate likelihood of CAD and provided strong adjunctive evidence for the role of CMR perfusion in aiding clinical decision making.
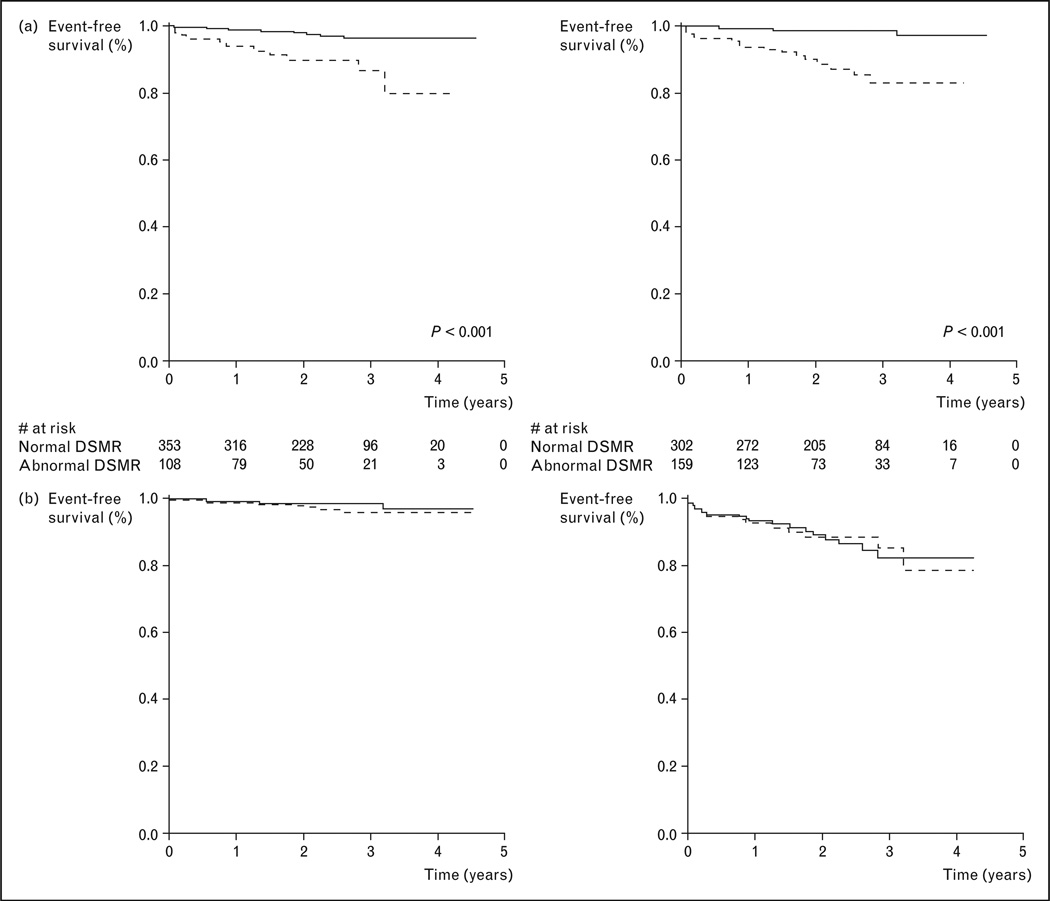
Figure 4. Kaplan–Meier survival curves illustrating the strong prognostic value of CMR perfusion imaging in a study of 513 patients who were followed for a median of 2.3 years.
Dobutamine stress CMR function and adenosine stress perfusion achieved similar prognostic values. (a) ——, Normal DSMR; -----, abnormal DSMR; ——, normal magnetic resonance perfusion; -----, abnormal magnetic resonance perfusion. (b) -----, Normal DSMR; ——, normal magnetic resonance perfusion; -----, abnormal DSMR; ——, abnormal magnetic resonance perfusion. CMR, cardiac magnetic resonance; DSMR, dobutamine stress magnetic resonance imaging. Reproduced with permission from [23].
In addition, the growing interests in high field CMR such as 3T imaging can potentially strengthen the clinical application of CMR perfusion. With a higher reserve for signal noise ratio at 3T compared with 1.5 T, accelerated data acquisition by parallel imaging can improve perfusion application by reducing breatholding time, increasing cardiac coverage, or improved contrast noise ratio. Cheng et al. [47] compared the diagnostic utility of 3T CMR with 1.5T in 61 patients who had a clinical indication for X-ray angiography and found improvement in both sensitivity and specificity by 3T CMR imaging (sensitivity 90 vs. 82% and specificity 98 vs. 90%). Recently, Lanza et al. [48] demonstrated CMR evidence of myocardial hypoperfusion in patients and reduced coronary flow reserve in patients a clinical diagnosis of syndrome X. This provides additional evidence to prior literature that owing to a high-spatial resolution, CMR perfusion can characterize the abnormal endo-MBF due to microvascular dysfunction.
Conclusion
In our opinion, CMR is the technique that provides the most comprehensive quantitation of myocardial physiology including MBF, infarction, and ventricular function. Strong diagnostic utility is supported by excellent spatial resolution and resultant sensitivity of the technique in detecting subtle reduction of endo-MBF. Growing results from outcome studies demonstrated that these findings are concordant with the correspondingly strong prognostic power of this novel technique.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Wischgoll T, Meyer J, Kaimovitz B, et al. A novel method for visualization of entire coronary arterial tree. Ann Biomed Eng. 2007;35:694–710. doi: 10.1007/s10439-007-9278-x. [DOI] [PubMed] [Google Scholar]
- 2.Fayad ZA, Fuster V, Fallon JT, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102:506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- 3.Worthley SG, Helft G, Fayad ZA, et al. Cardiac gated breath-hold black blood MRI of the coronary artery wall: an in vivo and ex vivo comparison. Int J Cardiovasc Imaging. 2001;17:195–201. doi: 10.1023/a:1010688122184. [DOI] [PubMed] [Google Scholar]
- 4.Maintz D, Ozgun M, Hoffmeier A, et al. Selective coronary artery plaque visualization and differentiation by contrast-enhanced inversion prepared MRI. Eur Heart J. 2006;27:1732–1736. doi: 10.1093/eurheartj/ehl102. [DOI] [PubMed] [Google Scholar]
- 5.Bassingthwaigthe JB, Raymond GR, Chan JIS. Principles of tracer kinetics. In: Zaret BL, Beller GA, editors. Nuclear cardiology: state of the art and future directions. St. Louis: Mosby-Year Book; 1993. pp. 3–23. [Google Scholar]
- 6.Wilson RF, Marcus ML, White CW. Prediction of the physiologic significance of coronary arterial lesions by quantitative lesion geometry in patients with limited coronary artery disease. Circulation. 1987;75:723–732. doi: 10.1161/01.cir.75.4.723. [DOI] [PubMed] [Google Scholar]
- 7.Erdogan D, Yildirim I, Ciftci O, et al. Effects of normal blood pressure, prehypertension, and hypertension on coronary microvascular function. Circulation. 2007;115:593–599. doi: 10.1161/CIRCULATIONAHA.106.650747. [DOI] [PubMed] [Google Scholar]
- 8.Laine H, Raitakari OT, Niinikoski H, et al. Early impairment of coronary flow reserve in young men with borderline hypertension. J Am Coll Cardiol. 1998;32:147–153. doi: 10.1016/s0735-1097(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 9. Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. This study is the first single-center experience in assessing the prognostic implication of 513 patients without prior infarction in long-term hard outcomes including cardiac death and nonfatal MI.
- 10.Atkinson DJ, Burstein D, Edelman RR. First-pass cardiac perfusion: evaluation with ultrafast MR imaging. Radiology. 1990;174(3 Pt 1):757–762. doi: 10.1148/radiology.174.3.2305058. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer S, Tyen RV, Saloner O. Evaluation of myocardial perfusion abnormalities with gadolinium-enhanced snapshot MR imaging in humans. Radiology. 1992;185:795–801. doi: 10.1148/radiology.185.3.1438765. [DOI] [PubMed] [Google Scholar]
- 12.Wilke N, Simm C, Zhang J, et al. Contrast-enhanced first pass myocardial perfusion imaging: correlation between myocardial blood flow in dogs at rest and during hyperemia. Magn Reson Med. 1993;29:485–497. doi: 10.1002/mrm.1910290410. [DOI] [PubMed] [Google Scholar]
- 13.Schwitter J, Nanz D, Kneifel S, et al. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–2235. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 14.Al-Saadi N, Nagel E, Gross M, et al. Improvement of myocardial perfusion reserve early after coronary intervention: assessment with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2000;36:1557–1564. doi: 10.1016/s0735-1097(00)00914-1. [DOI] [PubMed] [Google Scholar]
- 15.Jerosch-Herold M, Wilke N, Stillman AE. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys. 1998;25:73–84. doi: 10.1118/1.598163. [DOI] [PubMed] [Google Scholar]
- 16.Wilke N, Jerosch-Herold M, Wang Y, et al. Myocardial perfusion reserve: assessment with multisection, quantitative, first-pass MR imaging. Radiology. 1997;204:373–384. doi: 10.1148/radiology.204.2.9240523. [DOI] [PubMed] [Google Scholar]
- 17.Plein S, Ryf S, Schwitter J, et al. Dynamic contrast-enhanced myocardial perfusion MRI accelerated with k-t sense. Magn Reson Med. 2007;58:777–785. doi: 10.1002/mrm.21381. [DOI] [PubMed] [Google Scholar]
- 18.Niendorf T, Sodickson DK. Parallel imaging in cardiovascular MRI: methods and applications. NMR Biomed. 2006;19:325–341. doi: 10.1002/nbm.1051. [DOI] [PubMed] [Google Scholar]
- 19.Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med. 1997;38:591–603. doi: 10.1002/mrm.1910380414. [DOI] [PubMed] [Google Scholar]
- 20.Madore B. Using UNFOLD to remove artifacts in parallel imaging and in partial-Fourier imaging. Magn Reson Med. 2002;48:493–501. doi: 10.1002/mrm.10229. [DOI] [PubMed] [Google Scholar]
- 21.Tsao J, Kozerke S, Boesiger P, Pruessmann KP. Optimizing spatiotemporal sampling for k-t BLAST and k-t SENSE: application to high-resolution real-time cardiac steady-state free precession. Magn Reson Med. 2005;53:1372–1382. doi: 10.1002/mrm.20483. [DOI] [PubMed] [Google Scholar]
- 22.Di Bella EV, Wu YJ, Alexander AL, et al. Comparison of temporal filtering methods for dynamic contrast MRI myocardial perfusion studies. Magn Reson Med. 2003;49:895–902. doi: 10.1002/mrm.10439. [DOI] [PubMed] [Google Scholar]
- 23.Jahnke C, Paetsch I, Gebker R, et al. Accelerated 4D dobutamine stress MR imaging with k-t BLAST: feasibility and diagnostic performance. Radiology. 2006;241:718–728. doi: 10.1148/radiol.2413051522. [DOI] [PubMed] [Google Scholar]
- 24.Nagel E, Klein C, Paetsch I, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432–437. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 25. Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. This multicenter study in 241 patients found that the diagnostic performance of perfusion imaging by MRI was superior in comparison with SPECT, confirming similar findings from previous smaller single-center studies.
- 26.Wilson RF. Assessment of the human coronary circulation using a Doppler catheter. Am J Cardioly. 1991;67:44D–56D. doi: 10.1016/s0002-9149(05)80007-4. [DOI] [PubMed] [Google Scholar]
- 27.Jerosch-Herold M, Wilke N, Manning WJ, Pennell DJ. Myocardial Perfusion-Theory. Cardiovascular Magnetic Resonance. Churchill Livingstone. 2002:31–40. [Google Scholar]
- 28.Jerosch-Herold M, Hu X, Murthy NS, et al. Magnetic resonance imaging of myocardial contrast enhancement with MS-325 and its relation to myocardial blood flow and the perfusion reserve. J Magn Reson Imaging. 2003;18:544–554. doi: 10.1002/jmri.10384. [DOI] [PubMed] [Google Scholar]
- 29.Christian TF, Aletras AH, Arai AE. Estimation of absolute myocardial blood flow during first-pass MR perfusion imaging using a dual-bolus injection technique: comparison to single-bolus injection method. J Magn Reson Imaging. 2008;27:1271–1277. doi: 10.1002/jmri.21383. [DOI] [PubMed] [Google Scholar]
- 30.Christian TF, Rettmann DW, Aletras AH, et al. Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging. Radiology. 2004;232:677–684. doi: 10.1148/radiol.2323030573. [DOI] [PubMed] [Google Scholar]
- 31.Selvanayagam JB, Cheng AS, Jerosch-Herold M, et al. Effect of distal embolization on myocardial perfusion reserve after percutaneous coronary intervention: a quantitative magnetic resonance perfusion study. Circulation. 2007;116:1458–1464. doi: 10.1161/CIRCULATIONAHA.106.671909. [DOI] [PubMed] [Google Scholar]
- 32. Selvanayagam JB, Jerosch-Herold M, Porto I, et al. Resting myocardial blood flow is impaired in hibernating myocardium: a magnetic resonance study of quantitative perfusion assessment. Circulation. 2005;112:3289–3296. doi: 10.1161/CIRCULATIONAHA.105.549170. The study provided convincing evidence for the hibernation paradigm with quantitative magnetic resonance perfusion imaging after almost three decades of debate on whether blood flow under resting conditions is significantly reduced in viable nonischemic myocardium in patients with CAD.
- 33.Al-Saadi N, Nagel E, Gross M, et al. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101:1379–1383. doi: 10.1161/01.cir.101.12.1379. [DOI] [PubMed] [Google Scholar]
- 34.Gatehouse PD, Elkington AG, Ablitt NA, et al. Accurate assessment of the arterial input function during high-dose myocardial perfusion cardiovascular magnetic resonance. J Magn Reson Imaging. 2004;20:39–45. doi: 10.1002/jmri.20054. [DOI] [PubMed] [Google Scholar]
- 35.Wu KC, Kim RJ, Bluemke DA, et al. Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol. 1998;32:1756–1764. doi: 10.1016/s0735-1097(98)00429-x. [DOI] [PubMed] [Google Scholar]
- 36.Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 37.Hirsch A, Nijveldt R, Haeck JD, et al. Relation between the assessment of microvascular injury by cardiovascular magnetic resonance and coronary Doppler flow velocity measurements in patients with acute anterior wall myocardial infarction. J Am Coll Cardiol. 2008;51:2230–2238. doi: 10.1016/j.jacc.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 38.Simonetti O, Kim RJ, Fieno DS, et al. An improved MRI technique for the visualization of myocardial infarction. Radiology. 2000;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 39.Ricciardi MJ, Wu E, Davidson CJ, et al. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–2783. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 40.Hofman MB, van Rossum AC, Sprenger M, Westerhof N. Assessment of flow in the right human coronary artery by magnetic resonance phase contrast velocity measurement: effects of cardiac and respiratory motion. Magn Reson Med. 1996;35:521–531. doi: 10.1002/mrm.1910350411. [DOI] [PubMed] [Google Scholar]
- 41.Nagel E, Bornstedt A, Hug J, et al. Noninvasive determination of coronary blood flow velocity with magnetic resonance imaging: comparison of breath-hold and navigator techniques with intravascular ultrasound. Magn Reson Med. 1999;41:544–549. doi: 10.1002/(sici)1522-2594(199903)41:3<544::aid-mrm17>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Hundley WG, Hillis LD, Hamilton CA, et al. Assessment of coronary arterial restenosis with phase-contrast magnetic resonance imaging measurements of coronary flow reserve. Circulation. 2000;101:2375–2381. doi: 10.1161/01.cir.101.20.2375. [DOI] [PubMed] [Google Scholar]
- 43.van Rossum AC, Galjee MA, Post JC, Visser CA. A practical approach to MRI of coronary artery bypass graft patency and flow. Int J Card Imaging. 1997;13:199–204. doi: 10.1023/a:1005859101088. [DOI] [PubMed] [Google Scholar]
- 44.Nagel E, Underwood R, Pennell D, et al. New developments in noninvasive cardiac imaging: critical assessment of the clinical role of cardiac magnetic resonance imaging. Eur Heart J. 1998;19:1286–1293. [PubMed] [Google Scholar]
- 45.Johnson K, Sharma P, Oshinski J. Coronary artery flow measurement using navigator echo gated phase contrast magnetic resonance velocity mapping at 3.0T. J Biomech. 2008;41:595–602. doi: 10.1016/j.jbiomech.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Rossman PJ, Grimm RC, et al. Navigator-echo-based real-time respiratory gating and triggering for reduction of respiration effects in three-dimensional coronary MR angiography. Radiology. 1996;198:55–60. doi: 10.1148/radiology.198.1.8539406. [DOI] [PubMed] [Google Scholar]
- 47.Cheng AS, Pegg TJ, Karamitsos TD, et al. Cardiovascular magnetic resonance perfusion imaging at 3-tesla for the detection of coronary artery disease: a comparison with 1.5-tesla. J Am Coll Cardiol. 2007;49:2440–2449. doi: 10.1016/j.jacc.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 48.Lanza GA, Buffon A, Sestito A, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466–472. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]