Abstract
A functional polymorphism in the promoter region of the serotonin transporter (5-HTTLPR) gene has been associated with variation in anxiety and hypothalamus–pituitary–adrenal (HPA) axis function in humans and rhesus macaques. Individuals carrying the short allele are at a higher risk for developmental psychopathology, and this risk is magnified in short allele carriers who have experienced early life stress. This study investigated the relationship between 5-HTTLPR allelic variation, infant abuse, and behavioral and hormonal responses to stress in rhesus macaques. Subjects were 10 abusive mothers and their infants, and 10 nonabusive mother–infant pairs. Mothers and infants were genotyped for the rh5-HTTLPR, and studied in the first 6 months of infant life. For mothers and infants, we measured social group behavior, behavioral responses to handling procedures, and plasma concentrations of ACTH and cortisol under basal conditions and in response to stress tests. The proportion of individuals carrying the short rh5-HTTLPR allele was significantly higher among abusive mothers than controls. Among mothers and infants, the short allele was associated with higher basal cortisol levels and greater hormonal stress responses in the infants. In addition, infants who carried the short rh5-HTTLPR allele had higher anxiety scores than infants homozygous for the long allele. The rh5-HTTLPR genotype also interacted with early adverse experience to impact HPA axis function in the infants. These results are consistent with those of previous studies which demonstrate associations between serotonergic activity and anxiety and stress reactivity, and add additional evidence suggesting that genetic variation in serotonergic function may contribute to the occurrence of abusive parenting in rhesus macaques and modulate emotional behavior and HPA axis function.
Keywords: 5-HTTLPR, rh5-HTTLPR, Serotonin transporter, Macaca mulatta, Child maltreatment, Maternal behavior, Developmental psychopathology, Stress reactivity, Genetic effects, Early experience
Introduction
The human serotonin transporter gene (5-HTT) contains a length polymorphism in the 5′regulatory region (5-HTTLPR) that alters in vitro levels of transcriptional activity and density of the transporter protein (Lesch et al., 1996). Of the two common alleles (long (l) and short (s)), the short (low 5-HTT activity) allele has been associated with higher neuroticism and anxiety (Greenberg et al., 2000; Lesch et al., 1996; Mazzanti et al., 1998; Osher et al., 2000), suicide attempts (Baca-Garcia et al., 2002), and alcoholism (Lichtermann et al., 2000). Individuals carrying one or two copies of the short allele have also been reported to exhibit greater amygdala activation in response to fearful stimuli compared to individuals homozygous for the long allele (Hariri et al., 2002). These results suggest that 5-HTTLPR polymorphisms regulate emotional behaviors and individual reactivity to stressful situations.
5-HTTLPR polymorphisms have also been found to moderate the impact of early life stress on the development of depression. Caspi et al. (2003) found that individuals who experienced early life stress were more vulnerable to developing depression later in life if they carried one or more copies of the short allele. Although a recent study failed to replicate these findings (Surtees et al., 2006), other groups have found similar gene by environment effects, such as Kaufman et al. (2004), who not only found that abused children carrying at least one short allele were more vulnerable to developing depression, but that this was moderated by levels of social support. These findings suggest that there are complex interactions between 5-HTTLPR genotypes and life stress events that modulate vulnerability to the development of psychopathology.
Rhesus monkeys (Macaca mulatta) possess a 21-base pair insertion/deletion polymorphism that is orthologous to the human 5-HTTLPR length variant (rh5-HTTLPR) with two predominant alleles (short and long) of similar effects on transcriptional activity in vitro (Bennett et al., 2002). As in humans, rhesus monkeys with one or more copies of the short allele exhibit greater levels of anxiety and are more behaviorally reactive on temperament tests than individuals homozygous for the long allele (Bethea et al., 2004; Champoux et al., 2002). There is also evidence that rh5-HTTLPR allelic variation affects individual vulnerability to early adverse experiences in rhesus monkeys. Among monkeys that were separated from their mothers at birth and reared in adult absent peer-only groups, individuals carrying at least one of the short allele variants have been found to be more aggressive, sensitive to alcohol, and to have lower orientation scores on temperament tests than mother-reared animals with either genotype and peer-reared animals with the l/l genotype (Barr et al., 2003a,b; Champoux et al., 2002). Peer-reared animals with a short allele also had lower CSF levels of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA), lower levels of basal plasma cortisol, and higher levels of plasma ACTH in response to social separation (Barr et al., 2004a,b; Bennett et al., 2002). This association between the short 5-HTTLPR allele and HPA axis reactivity is of importance, as serotonin is directly involved in the activation and negative feedback of the HPA axis (Cassan and D'mello, 2001; Lowry, 2002). This evidence indicates that rh5-HTTLPR polymorphisms in rhesus macaques are associated with similar behavioral and physiological effects as found in humans, and may interact with early life stress to impact development.
Child maltreatment is a form of early life stress that occurs in both humans and rhesus monkeys. In large captive populations of rhesus monkeys, between 2 and 10% of all infants are physically abused by their mothers (Maestripieri, 1999; Maestripieri and Carroll, 1998; Maestripieri et al.,1997a,b). Infant abuse is defined as violent behavior including infant dragging, crushing, throwing, stepping or sitting on by the mother. These behaviors are extreme, and are never exhibited, not even in milder forms, by non-abusive mothers. Abusive behavior is generally exhibited in the first 3 months of an infant's life, and commonly repeated with successive infants. Infant abuse has a higher prevalence in some matrilines and among related females, suggesting an intergenerational transmission along the maternal line. Previous research found an association between abusive behavior and increased anxiety in these mothers (Maestripieri, 1994; McCormack et al., 2006; Troisi and D'Amato, 1991), and, interestingly, a recent study suggests that altered serotonergic function may be one of the mechanisms underlying the intergenerational transmission of infant abuse in rhesus monkeys (Maestripieri et al., 2006). In that study, approximately half of the female infants that were physically abused by their biological or foster mothers in their first month of life exhibited abusive parenting with their own offspring (Maestripieri, 2005), and the group of abused females that later became abusive mothers had lower CSF levels of 5-HIAA than those who did not (Maestripieri et al., 2006). Although we reported that rh5-HTTLPR genotypic differences among infants were not associated with significant differences in CSF 5-HIAA, we did not examine potential interactions between rh5-HTTLPR genotype and early experience in the behavioral and neuroendocrine developmental outcomes of abused and nonabused infants. Furthermore, the rh5-HTTLPR genotype of abusive mothers already present in our subject population has not been previously investigated. Given the aforementioned associations between low serotonergic activity and abusive behavior, the associations between the short rh5-HTTLPR allele and anxiety, and because previous work with nonhuman primates has detected an association between abusive behavior and increased anxiety in abusive mothers, we were interested in further exploring whether there is an association between abusive behavior and the 5-HTTLPR short allele.
The purpose of the present study was to evaluate the effects of rh5-HTTLPR polymorphisms on behavior and HPA axis function of rhesus monkey mother–infant pairs living in social groups, and to examine potential gene by environment interactive effects of this locus on the developmental impact of infant abuse. We have previously characterized the behavioral, neuroendocrine and neurochemical correlates of abusive mothering, and the developmental consequences of infant abuse in the offspring (Maestripieri et al., 2006; McCormack et al., 2006; McCormack et al., submitted for publication; Sanchez et al., 2007). In the present study we genotyped abusive and nonabusive mothers for the rh5-HTTLPR gene and investigated whether these polymorphisms accounted for variation in behavioral and neuroendocrine responsiveness to stress in mothers and infants. Specifically, we tested the following hypotheses: 1) abusive mothers would be more likely to carry the rh5-HTTLPR short allele than nonabusive mothers; 2) mothers and infants with the short allele would show greater behavioral reactivity to challenges and greater HPA axis activity under basal condition and in response to stress than individuals with the long allele; and 3) carrying the short allele would increase the vulnerability to the effects of abuse on infant behavioral and neuroendocrine development. The findings reported here provide a broader understanding of the genetic factors associated with infant abuse and how they moderate its developmental consequences in a non-human primate model.
Methods
Subjects and housing
This study was conducted at the Field Station of the Yerkes National Primate Research Center (YNPRC), Emory University, in Lawrenceville, GA. Subjects were rhesus macaque mother–infant pairs living in four large social groups. Each group consisted of two or three adult males and 18–49 adult females with their sub-adult and juvenile offspring. The groups were housed in 38×38 m outdoor compounds with indoor housing areas. Animals were fed in the morning and evening (with excess chow typically remaining from the previous feeding in order to avoid potential conflicts due to food availability), and water was freely available. The studies described in this section were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Emory University Institutional Animal Care and Use Committee.
Ten multiparous adult females with a previous history of abusive parenting and their newborn infants (6 female, 4 male) were included in the study after substantiation of maternal physical abuse (Maltreated group; see definition below). For this, prior to the birth season, fifteen adult females with a history of abusive parenting of most of their prior offspring were assigned to the studies as “potentially abusive mothers”. Of those fifteen, only 10 mothers whose frequency and severity of abuse was substantiated and did not jeopardize their infant's health were used in these studies (three of the infants were removed from the studies due to severity of abuse and three previously abusive mothers did not abuse their infants during our observations, leaving nine abusive mother–infant pairs; a tenth previously abusive mother was added to the study after she was observed to abuse her infant during the first week of life).Ten nonabusive mothers and their infants served as controls. Although the selection of potentially “abusive” and “control” mothers was done based on prior maternal history, final assignment of infants to either the “abused” or “nonabused” groups was done based on direct observation of mother–infant interactions during the first 4 weeks of infant's life. Controls were matched to maltreated subjects by infant sex (6 female, 4 male), infant age and, whenever possible, social group of origin and the mother's dominance rank so that the two experimental groups did not differ significantly on any of these variables. Abusive mothers had an average of 6 prior offspring (range: 3–12), and the control mothers had an average of 6.2 prior offspring (range: 2–12). In order to avoid confounding effects of heritability on our HPA axis measures, the adult females were selected from different matrilines (i.e., they were unrelated individuals).
Behavioral data collection: mother and infant behavior in the social group
Behavioral observations were made from an observation tower situated at the corner of each social group. Behavioral data were collected using binoculars and a handheld computer (Palm Pilot IIIXE) programmed to record the frequency, duration and sequence of behaviors. Three experienced observers collected the data. For reliability purposes, prior to the beginning of data collection, observers watched and recorded behavior until percent agreement exceeded 90% and Cohen's Kappa exceeded 0.8.
Focal observations began on the second day of infant life. Each mother–infant pair was observed for a 30-minute period, five times per week during the first month of life, two times per week during the second month of life, and one time per week from the third month through the sixth month of life. This observation schedule was chosen in order to best document the occurrence of infant abuse, as the frequency of abuse is highest in the first month and decreases steadily thereafter (Maestripieri, 1998; McCormack et al., 2006). Observations were done between 7:00 and 11:00 AM, when the animals were most active. Data collection included infant abuse, other mother–infant interactions (time spent in contact and frequencies of: contacts made and broken, rejections, and restrains), as well as the social and nonsocial behavior of the infants (frequencies of vocalizations, anxiety behavior, agonistic interactions with others, and social and solitary play), following previous behavioral definitions described in a well-established rhesus ethogram (Altman, 1962) and other publications (Maestripieri, 1998; McCormack et al., 2006). The following maternal behavior patterns were included in the infant physical abuse category following previously published criteria for this species (Maestripieri, 1998; Troisi and D'Amato, 1983): infant dragging, crushing, throwing, stepping/sitting on, rough grooming or abusive carrying. Infant abuse was scored independently of all the other mother–infant interactions (e.g. infant rejection). Abuse events did not last more than a few seconds and therefore only frequencies were recorded, as is the standard for this type of research (Maestripieri, 1998; Troisi and D'Amato, 1983). Infant abuse was recorded as two separate events if there was a transition in the pattern of behavior or if there was a pause of at least 10 s during the behavior.
Experimental procedures
Training and awake blood sampling procedures
Prior to the studies of HPA axis function, animals were trained and habituated to the experimental procedures in order to facilitate quick blood drawing without anesthesia and to minimize arousal. Animals are commonly trained to move on command from the outdoor corral into an indoor capture unit, from this to a transfer box, and from the box to a squeeze cage for awake blood sampling. Training is done using positive reinforcement and following guidelines and protocols approved by the YNPRC and Emory University IACUC. Because individual training can be done only with animals 12 months or older, the infants in the present study were carried by their mothers, who had been previously trained and habituated to the procedures.
Once in the squeeze cage, the infant was quickly removed from the mother, and a basal blood sample was collected from the mother and the infant. Blood was collected in the awake state within <10 min from the time the monkeys saw the experimenters approach their outdoor compound. Research at the YNPRC has shown that, when blood is collected with these training/habituation procedures, rhesus monkeys exhibit minimal arousal and cortisol elevation (e.g. Blank et al., 1983) and that infants who have experienced these procedures exhibit no alterations in normal development (Wilson et al., 1986). All blood samples were collected via femoral (infant) or saphenous (mother) venipuncture in pre-chilled polypropylene tubes containing EDTA and immediately placed on ice. Plasma was separated by centrifugation at 1000 ×g for 10 min at 4 °C, then aliquoted and stored at −80 °C until assayed.
Basal blood sample collection for cortisol measurements
Mother–infant pairs were captured at 1, 2, 3, 4, and 6 months of age for the collection of blood samples to assess basal cortisol concentrations. Mother–infant pairs were captured and released twice at each age, on different days, to obtain an early morning sample (at sunrise) and an evening sample (at sunset) for analysis of diurnal cortisol secretion (Sanchez et al., 2005; Zeitzer et al., 2003). Because the animals live under natural lighting conditions, we selected our time points from sunrise and set times published by the U.S. Naval Observatory in order to use the daylight, and not the clock time, as a reference for our diurnal samples. This is important due to the acute effect of light on plasma cortisol concentrations in the early morning, in addition to the light's ability to shift the endogenous circadian rhythm (Scheer and Buijs, 1999).
When infants were <4 months old, basal blood samples were drawn only at the early morning time point, to avoid repetitive disruption of the mother–infant pairs very early in life. When collecting the diurnal samples, only one blood sample per week was collected per mother–infant pair following a counterbalanced design for order of diurnal time point collection. Blood samples were collected during the same season for all experimental groups, to control for circannual differences in HPA axis activity. Although the animals were trained and habituated to the procedures, and the mother's presence buffers the infants' physiological activations at the ages of study (see findings below; and Gunnar and Vazquez, 2006 for review), ACTH elevations could potentially be detected within the few minutes that these procedures take. Thus, we focused on cortisol (and not ACTH) for these basal measures.
ACTH and cortisol responses to stress
(1) Novel environment stress test in the presence of the mother (2 months of age). Mother–infant pairs were quickly captured from the social group and transported together to a novel testing room, where they were placed (together) in a novel cage for 30 min. One blood sample was collected from both infant and mother at the end of the test (post-test: 30 min). The infant was then quickly returned to its mother and the mother–infant pair immediately returned to the social group. The basal measurement was taken on a different day in order to avoid stress activations prior to the test due to separation. The presence of a social companion (e.g., the mother) has been previously demonstrated to buffer physiological and behavioral activations caused by stressful stimuli such as novel environments and social separations in primates, including rhesus monkeys (Gunnar and Vazquez, 2006; Heinrichs et al., 2003; McCormack et al., submitted for publication).
(2) Separation and novel environment stress test, without the mother (3 and 6 months of age). Mother–infant pairs were quickly captured from the social group, separated from each other, and placed in separate cages (alone) in separate unfamiliar rooms for 30 min. A basalblood sample was collected from both infant and mother at the beginning (pre-test: 0 min) and another at the end (post-test: 30 min) of the stress test. The infant was then quickly returned to its mother, and the mother–infant pair was immediately returned to the social group. Mother–infant separations at this age cause known behavioral and HPA axis stress responses in rhesus monkey mothers and infants (Bowlby, 1968; Hinde and McGinnis, 1977; for review see Sanchez et al., 2001).
Assessment of mother and infant behavioral responses to experimental procedures
Every time mother–infant pairs were captured for blood sample collection and experimental testing (monthly), both infants and mothers were rated by an observer on two behavioral dimensions: 1) behavioral reactivity to capture and handling, and 2) mother–infant interactions during the process. The scales used to assess the infants reactivity were adapted from two sources: 1) the Behavioral Temperament Scale (Ruppenthal and Sackett, 1992), and 2) the state control portion of the Neonatal Neurobehavioral Assessment (Schneider and Suomi, 1992) that provides measures of temperament, in addition to orientation and neuromotor functioning in infant rhesus monkeys. The items and rating scales used are listed in Tables 1 and 2. Ratings were only collected until infants were 4 months old because after that age their behavioral responses became homogeneous, consisting of intense struggle.
Table 1.
Maternal assessment scale (adapted from Ruppenthal and Sackett, 1992; Schneider and Suomi, 1992)
| 1. Predominant state in capture unit |
| 0 = relaxed |
| 1 = somewhat agitated |
| 2 = extremely agitated (body jerks and screams) |
| 3 = frozen, hanging from mesh; difficult to transfer to box |
| 2. Predominant state in squeeze cage |
| 0 = holding infant tightly |
| 1 = infant on and off of mother |
| 2 = does not hold infant |
| 3. Initial reaction of mother to testers attempt to get infant (in cage) |
| 0 = not distressed at infant removal |
| 1 = moderately distressed, mild attempts to prevent removal |
| 2 = very distressed, bites/grabs at tester, holds infant tightly |
| 4. Mother's response during blood draw |
| 0 = no resistance (passive or compliant) |
| 1 = moderate resistance |
| 2 = constantly resists tester's hold |
| 5. Occurrence of distress calls to infant |
| 0 = no obvious distress vocalizations |
| 1 = one vocalization (screams, grunts) |
| 2 = two to four vocalizations |
| 3 = many vocalizations |
| 6. Response of mother to return of infant |
| 0 = takes infant immediately, holds it tightly |
| 1 = takes infant immediately, but does not hold it tightly |
| 2 = does not take infant immediately |
| 3 = rejects, hits infant |
Table 2.
Infant assessment scale (adapted from Ruppenthal and Sackett, 1992; Schneider and Suomi, 1992)
| 1. Predominant state in capture unit |
| 0 = alert, awake, and aware |
| 1 = alert, but somewhat agitated |
| 2 = extremely agitated (body jerks and screams) |
| 3 = freezing behavior, hanging from mesh |
| 2. Infant contact with the mother in capture unit |
| 0 = on mother |
| 1 = 50% of time on mother |
| 2 = off mother |
| 3. Degree to which infant clings to tester before and after blood draw |
| 0 = passive/no cling |
| 1 = moderate cling |
| 2 = moderate cling with slight grasps and releases |
| 3 = tight cling or frantic intermittent grasps and releases |
| 4. Infant's response during blood draw: |
| 0 = no resistance (passive or compliant) |
| 1 = moderate resistance |
| 2 = constantly resists tester's hold |
| 5. Ease with which infant was consoled or calmed: |
| 0 = not necessary to console |
| 1 = easy to console |
| 2 = consoles with difficulty |
| 3 = cannot be consoled |
| 6. Occurrence of distress coos or screams |
| 0 = no obvious distress vocalization |
| 1 = one coo or one scream |
| 2 = 2 coos, 3 or fewer screams |
| 3 = many coos and/or screams |
| 7. Occurrence of body jerks or tantrums |
| 0 = no obvious jerks or tantrums |
| 1 = one body jerk or tantrum |
| 2 = two to four body jerks or tantrums |
| 3 = many body jerks or tantrums |
Hormonal assays
Plasma concentrations of cortisol were assayed in duplicate 10 µl aliquots by RIA using commercially available kits (Diagnostic Systems Lab, Webster, TX). The sensitivity of this assay was 1.25 µg/dl and intra- and inter-assay coefficients of variation in each assay were <10%. ACTH plasma levels were assayed in duplicate 200 µl aliquots by a two-site IRMA method using commercial kits (Nichols Institute Diagnostics, CA). The sensitivity of the assay was 1 pg/ml and inter- and intra-assay coefficients of variation were <6%.
rh-5HTTLPR genotyping
At the end of the study period, 4 ml of blood were drawn from mothers and infants for the purpose of rh-5HTTLPR genotyping. DNA was isolated from whole blood collected in EDTA-containing polypropylene tubes using a commercial extraction kit (Gentra Systems, Inc). The short (s: 398 bp) and long allele (l: 410 bp) alleles of the rhesus 5HTT gene promoter region were amplified using DNA primers (stpr5, 5′ GGCGTTGCCGCTCTGAATGC; intl, 5′CAGGGGAGATCCTGGGAGGG) and polymerase chain reaction conditions modified from that of Lesch and colleagues (1996). Amplicons were separated by electrophoresis and the s and l alleles identified by direct visualization by ethidium bromide staining.
Data analysis
The distributions of the rh5-HTTLPR genotype in mothers and infants in each group were analyzed with chi square tests. Data on maternal and infant behavior in the social groups were analyzed using mixed-design ANOVAs in which the fixed factors were group (abuse, non-abuse) and genotype (l/l, l/s; see distribution of genotypes in “Results” for the rationale for excluding the s/s genotype), and the repeated measure was age (monthly averages of behavioral scores). Behaviors were analyzed as either rates per 30 min (the observational period), or as proportions of observational time. Because the main effects of group for these behaviors in the social group have been reported extensively elsewhere (McCormack et al., 2006), the focus of the behavioral analyses in this study was on the genotype and the genotype-by-group effects.
Our analysis of the infant and mother responses during experimental testing procedures was modeled after Schneider and Suomi (1992). The behavioral responses of the mothers during testing procedures was analyzed using mixed design ANOVA's with group and genotype as fixed factors, and age as the repeated measure (month 1, 2, 3, 4). However, because the infants displayed high behavioral variability at each of the monthly time points, due to species-typical socioemotional development (Hinde, 1974; Hinde and Spencer-Booth, 1967), we chose to analyze each time point of the infant behavioral responses separately using two-way between subjects ANOVA's, with group and genotype as the fixed factors.
The cortisol and ACTH data were also analyzed using mixed design ANOVA's, with group and genotype as fixed factors, and time as the repeated measures factor (age, time of day — for diurnal cortisol, or 0 min vs.30 min — for stress tests). Because the main effects of group for the infant HPA axis activity have been reported extensively elsewhere (McCormack et al., submitted for publication), the focus of the infant HPA axis analyses in this study was on the genotype and the genotype-by-group effects. Regarding our findings for maternal HPA axis activity, we report all main effects and interactions with group (as these have not been previously reported).
When the assumption of sphericity for ANOVAs was violated, the corrected Huhn–Feldt statistic was used. Post-hoc analyses of significant interactions were performed with Bonferroni- corrected t-tests. Main effects for the time variable are not specifically reported here because some have been previously reported (McCormack et al., 2006; McCormack et al., submitted for publication) and the main focus of this research was on the effects and interactions of group and genotype. All data were analyzed using SPSS, version 13. Statistically significant results were set at the p ≤ 0.05 level across each family of analyses.
Results
Genotype and abuse
One abusive mother, 1 abused infant, and 1 control mother could not be genotyped for rh5-HTTLPR (animals had died by the end of the study period, and no tissue was available for genotyping). Of the remaining 9 abusive mothers, 1 individual had the l/l genotype and 8 had the l/s genotype. Of the 9 control mothers, 5 had the l/l genotype, 2 had the l/s genotype, and 2 had the s/s genotype. There was a significant difference in the distribution of the rh5-HTTLPR genotype among the abusive and non-abusive mothers (χ2(2, N = 18) = 8.27, p = .02), with the short allele being more common among the abusive mothers compared to the control mothers.
Of the 9 abused infants that could be genotyped, 1 had the l/l genotype, 5 had the l/s genotype, and 3 had the s/s genotype. Of the 10 non-abused infants, 3 had the l/l genotype and 7 had the l/s genotype. There was not a significant difference in the distribution of the rh5-HTTLPR genotype among the abused and non-abused infants (χ2(2, N = 19) = 4.23, p = .12).
Given the low incidence of the s/s genotype in our subjects, subsequent data analyses were limited to the comparison between the l/l and l/s genotypes, similarly to analyses previously conducted in this species (Barr et al., 2003a; Barr et al., 2004b; Champoux et al., 2002). We have also chosen this approach, as opposed to evaluating the l/s and s/s animals together in one group, based on recent evidence in the literature suggesting that the short allele may not always be additive in its effects, a phenomenon known as negative heterosis (Middeldorp et al., 2007; Uher and McGuffin, 2008), and in order to be consistent with other primate studies which compared the l/l versus the l/s genotypes. The rh5-HTTLPR genotype distribution in our colony is approximately 50% l/l and 50% “short” (40% l/s, 10% s/s), which is comparable to other primate centers (Barr et al., 2004a,b; Bennett et al., 2002; Bethea et al., 2004).
Mother and infant behavior in the social group
We have previously reported main effects for group (McCormack et al., 2006), such that (1) abusive mothers rejected their infants more, spent less time in ventral contact and broke contact more often with their infants during the first 3 months of life; and (2) the abused infants exhibited more tantrums and screams during the same developmental period, and broke contact with their mothers less often than controls. Our current analyses of the social group data (which focused on the genotype and genotype-by-group effects) detected a significant rh5-HTTLPR genotype-by-group interaction for infant anxiety rates (average frequency of yawns) (F(1,12) = 4.80, p = .049). Abused infants with the l/s genotype had the highest anxiety rates, although post-hoc analyses revealed significant differences only with control l/s infants (abuse: 0.03 ± 0.008; control: .004 ± .008; t(10)=3.51, p = 0.006). There were no other significant genotype or genotype-by-group effects on any of the other infant behaviors, nor were there any for the maternal behaviors.
Assessment of mother and infant behavioral responses to experimental procedures
Mothers
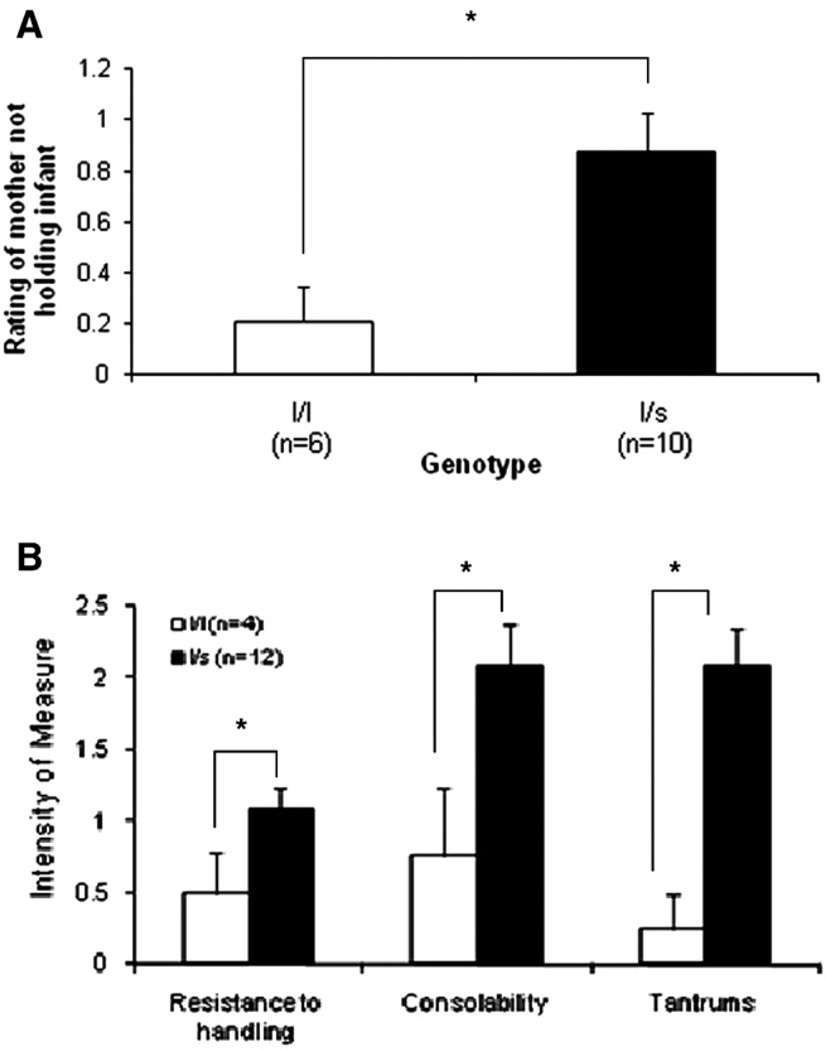
There was a significant main effect of genotype on the mother's response to capture and handling during procedures (Table 1, Question 2), (F(1,12) = 5.96, p = .03; Fig. 1A). Mothers with the l/s genotype (.88 ± .15) were less likely to hold their infants while in the cage, compared to mothers with the l/l genotype (.21 ± .14). There were no other significant effects (group, genotype, or group-by-genotype) for the maternal assessment questions.
Fig. 1.
(A) Maternal behavioral reactivity during testing. Mothers with the l/s genotype were more likely to not hold their infants while in the squeeze cage, compared to l/l mothers (F(1,12) = 5.96, p = .03; *p<.05, l/s mothers versus l/s mothers). (B) Infant behavioral reactivity during testing. Animals with the l/s genotype were more distressed during the testing procedures. (Resistance: F(1,12) = 4.98, p = .045; Consolability: F(1,12) = 5.77, p = .04; Tantrums: F(1,12) = 11.33, p = .01; *p<.05, l/s versus l/l).
Infants
There were several significant findings among the assessments of infant behavioral responses, all at 3 months of age (Fig.1B). Infants with the l/s genotype were rated as more resistant to handling and experimental procedures (Table 2, Question 4), (1.08 ± .15) than l/l infants (.5 ± .29; F(1,12) = 4.98, p = .045). Infants with the l/s genotype were also rated as more difficult to console (Table 2, Question 5), and exhibited more tantrums and body jerks (Table 2, Question 7) than l/l infants in response to handling and experimental procedures (console: l/s = 2.09 ± .28, l/l = .75 ± .48, F(1,12) = 5.77, p = .04; tantrums/body jerks: l/s = 2.08 ± .26, l/l = .25 ± .25), F(1,12) = 11.33, p = .01). There were no other significant effects (genotype, group, or genotype-by-group) for any of the remaining questions at any of the other time points.
Basal plasma cortisol
Mothers
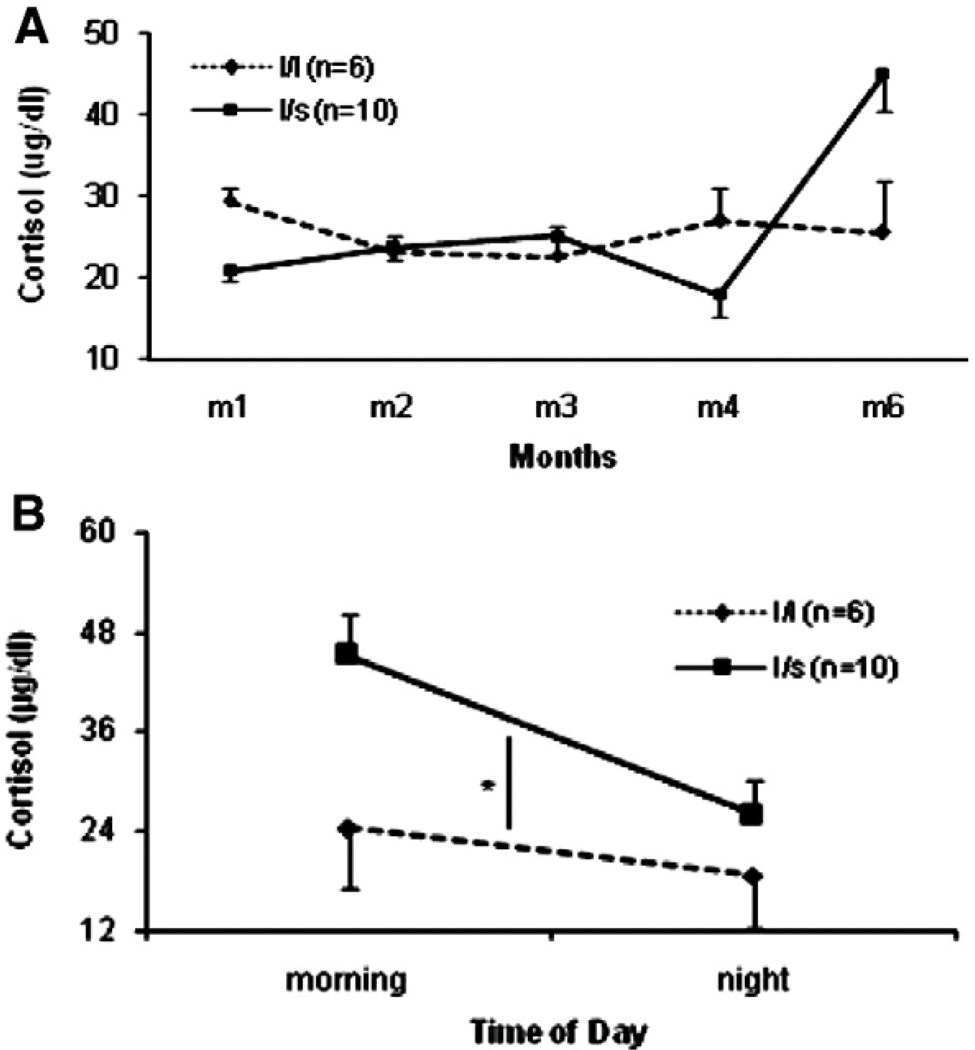
Across the first 6 months postpartum, there was a significant month-by-genotype interaction for early morning basal cortisol (F (2.38, 28.56) = 6.77, p = .003). Fig. 2A illustrates that mothers with the l/l genotype had consistently stable baseline cortisol levels across all 6 months, in contrast to the l/s mothers who in month 6, exhibited significantly higher variability in basal cortisol levels. To further examine this variability, difference scores were calculated for each animal by subtracting their month 1 cortisol values from their month 6 cortisol values. Mothers with the l/s genotype had greater mean difference scores (19.16 µg/dl ± 4.43) than did mothers with the l/l genotype (2.37 µg/dl ± 3.99; t(14) = 2.57, p = .02), indicating a significantly higher increase from month 1 to month 6 for the l/s than l/l mothers. This spike in cortisol at month 6 coincides with the beginning of the mating season, which often results in increased mother–infant conflict and group competition. There were no other significant main effects or interactions for basal HPA axis activity.
Fig. 2.
(A) Mothers basal levels of cortisol during the first 6 months postpartum. Compared to l/l mothers, l/s mothers demonstrated higher variability in their basal cortisol levels across the 6 months postpartum (F(2.38, 28.56) = 6.77, p = .003). (B) Mothers basal levels of cortisol across the day (month 6 postpartum). Mothers with the l/s genotype secreted higher levels of basal cortisol across the day than l/l mothers (F(1,10) = 5.48, p = .04; *p<.05, l/l mothers versus l/s mothers).
When the daytime cortisol rhythm of the mothers was examined (at months 4 and 6), a significant main effect of genotype was detected at month 6, (F(1,10) = 5.48, p = .04; Fig. 2B). Thus, mothers with the l/s genotype secreted higher levels of cortisol across the day, but particularly in the early morning, (AM = 45.07 µg/dl ± 4.94; night = 25.84 µg/dl ± 4.21) than did mothers with the l/l genotype (AM = 24.25 ± 7.23; night = 18.62 ± 6.16). There were no other significant main effects or interactions for diurnal HPA axis activity.
Infants
We have previously reported a month-by-group interaction on basal cortisol secretion, so that at 1 month of age (when abuse rates are the highest) the abused infants have higher basal cortisol levels than controls (McCormack et al., submitted for publication). This pattern is inverted after month 2, so that the abused infants tend to have lower cortisol than controls. In our current analyses we detected significant effects of rh5-HTTLPR genotype on infants HPA axis basal function. A month-by-genotype interaction was detected for infant basal cortisol levels (F(4,48) = 2.88, p = .03). Although post-hoc tests were not statistically significant, data presented in Fig. 3A suggest that l/s infants had higher cortisol levels than l/l infants when they were 1 month old, and that basal cortisol concentrations were similar thereafter. There was also a significant month-by-genotype-by-group interaction for basal cortisol levels (F(4,48) = 3.28, p = .02, Fig. 3B). Although the findings have to be interpreted with caution due to the small sample size, during the first month, when abuse rates were the highest, abused l/s infants had higher levels of cortisol compared to all the other groups (control l/l: t(6) = 3.091, p = .02; control l/s: t(10) = 2.23,p = .05; abusedl/l: t(4) = 4.21,p = .01), however, this pattern switched in month 2, such that control l/l infants had higher cortisol levels than all the other groups (control l/s: t(8) = 4.11, p = .003; abused l/s: t (6) = 3.62, p = .01; abused l/l: t(2) = 4.08, p = .05). After month 2, no significant differences were detected in relation to group or genotype.
Fig. 3.
(A) Resting levels of infant cortisol. Infants with the l/s genotype had higher levels of basal cortisol at month 1 compared to the l/l infants (F(4,48) = 2.88, p = .03). (B) Resting levels of infant cortisol. There was a significant month by genotype by group effect (F(4,48) = 3.28, p = .02). During the first month, abused l/s infants had the highest levels of resting cortisol (*p<.05, abused l/s infants versus all others). During the second month, control l/l infants had the highest levels of resting cortisol (*p<.05, control l/l infants versus all others).
No significant main or interaction effects of genotype were detected on the infants' month 4 and 6 diurnal pattern of cortisol secretion.
ACTH and cortisol responses to stress
Mothers
There were no significant differences between the cortisol or ACTH responses of l/l and l/s mothers (or interactions with group) during the Novel Environment Stress Tests in either month 2 or 3. However, during the month 6 test, a significant genotype-by-time (0 min versus 30 min) interaction was detected in the mothers' plasma cortisol levels (F(1,12) = 6.93, p = .02). Thus, whereas the l/l mothers exhibited significant increases in cortisol levels from baseline (0 min: 25.62 ± 6.57 µg/dl) to the end of the separation period (30 min: 46.27 ± 6.72 µg/dl) (t(5) = 2.93, p = .03), this stress-induced increase was not shown by the l/s mothers (0 min: 45.08 ± 4.74; 30 min: 48.55 ± 4.84 t(9) = 2.00, p>.05), which seemed to be due to the higher baseline cortisol levels in the latter (see above, under basal cortisol analyses). There were no other significant main effects or interactions for maternal HPA axis activity for the stress tests.
Infants
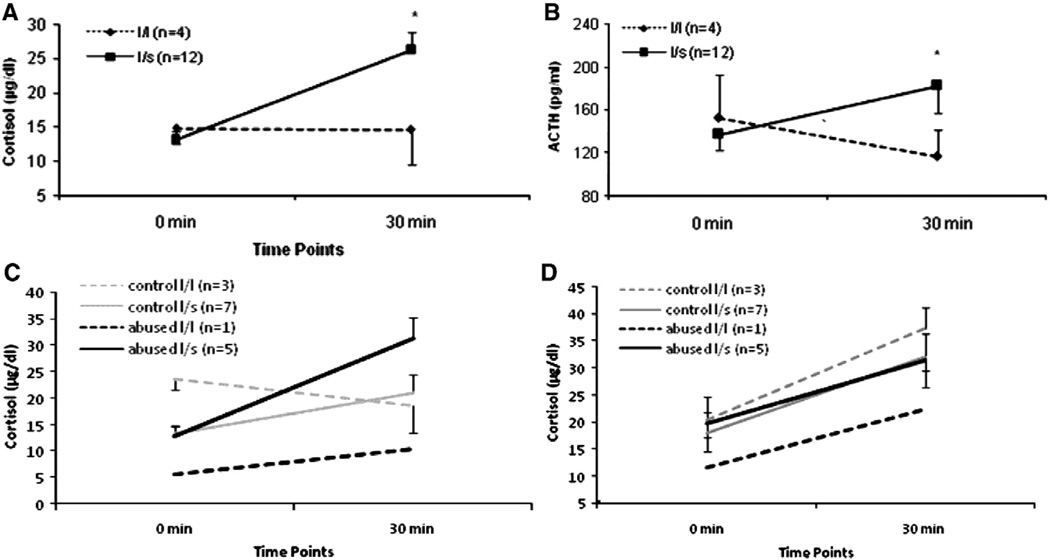
We have previously reported that abused infants exhibit higher cortisol reactivity to stress, even when the mother is present (McCormack et al., submitted for publication). When we examined the effects of rh5-HTTLPR genotype on cortisol reactivity during the Novel Environment Stress Test (mother and infant together) at month 2, we found a significant genotype-by-time effect (F(1,12) = 6.47, p = .03). As shown in Fig. 4A, infants with the l/l genotype showed no cortisol elevations during the stress test when the mother was present (“buffering” effect: t(3) = 1.02, n.s.).
Fig. 4.
(A) Infant cortisol response to the Novel Environment Stress Test at month 2. Infants with the l/l genotype were buffered from an increase in cortisol during the test, while l/s infants demonstrated an increase in cortisol to the test (F(1,12) = 6.47, p = .03; *p<.01, l/s infants, 0 min versus 30 min). (B) Infant ACTH response to the Novel Environment Stress Test at month 2. Infants with the l/l genotype were buffered from an increase in ACTH during the test, while l/s infants demonstrated an increase in ACTH to the test (F(1,12) = 7.65, p = .02; *p<.05, l/s infants, 0 min versus 30 min). (C) Infant cortisol response to the Novel Environment Stress Test at month 2 (F(1,12 = 6.30, p = .03). Abused l/s infants had higher levels of cortisol across the paradigm compared to the other groups. (D) Infant cortisol response to the Separation and Novel Environment Stress Test at month 6. Abused l/s infants demonstrated higher levels of cortisol compared to abused l/l infants (F(1,12) = 4.98, p = .04; *p<.05, abused l/s versus abused l/l).
In contrast, l/s infants showed significant cortisol elevations in response to the novelty stress, despite their mother's presence (t(11) = 4.19, p<.01). Differences in the infants' ACTH responses were similar to those observed for cortisol, (F(1,12) = 7.65, p = .02; see Fig. 4B), with l/l infants showing no ACTH elevations in response to the stress test when the mother was present (t(3) = 1.58, n.s.), whereas l/s infants lacked such a maternal buffering effect, as demonstrated by their significant ACTH elevations during the novelty stress (t(11) = 2.71, p = .02).
A significant genotype-by-group interaction was also detected in the cortisol responses during the Novel Environment Stress Test at month 2,(F(1,12) = 6.30, p = .03). Although post-hoc tests were not significant, and findings have to be interpreted with caution due to the small sample sizes, Fig. 4C suggests that average cortisol levels were higher for the abused l/s infants compared to the abused l/l infant, and higher for the control l/l infants compared to the control l/s infants.
Although no significant differences were detected in the responses to the mother–infant Separation and Novel Environment Stress test at month 3, a significant genotype-by-group effect was detected at month 6 (F(1,12) = 4.98, p = .04; see Fig. 4D). The pattern of effects is similar to that detected in the month-2 stress test. Across the test, abused l/s animals demonstrated higher levels of cortisol than the abused l/l animal, (t(4) = 4.54, p = .01). There were no other significant findings for either cortisol or ACTH in response to the month 6 mother–infant separations.
Discussion
The findings of this study provide evidence that the short allelic variant in the rh5-HTTLPR locus accounts for an increased occurrence of abusive parenting and low infant protectiveness among adult rhesus macaque females, as well as increased behavioral reactivity and HPA axis activity for both mothers and offspring. Moreover, many of these genotypic effects are environmentally limited, indicating that while the genotype may confer risk, phenotypic outcomes of such risk are dependent on the early environment and context in which they are measured. Consistent with previous evidence, psychopathological outcomes, in this case infant abuse and its toll on the developing offspring, seems to be a product of a gene by environment interaction.
The genotype analyses revealed that abusive mothers were more likely to carry the short rh5-HTTLPR allele. In addition, across both abusive and control mothers, animals with the short allele were less likely to hold their infants during experimental procedures. Taken together, these findings suggest that the short 5-HTTLPR allele is associated with poor maternal care in this species, or at least with physical abuse and low protectiveness of the infant during threatening situations. These findings are consistent with previous reports of altered brain 5HT function in macaque females exhibiting poor maternal care. This includes evidence that abused rhesus monkey females that become abusive mothers themselves have lower CSF levels of the serotonin metabolite 5-HIAA than abused females that did not perpetuate abuse with their own offspring (Maestripieri et al., 2006) and with evidence that high rates of infant rejection is also associated with low CSF 5-HIAA levels in the mothers (Maestripieri et al., 2007). Taken together, these findings suggest that infant abuse (and other forms of poor caregiving, such as infant rejection or low infant protectiveness) by adult macaque females could be associated with lower brain serotonergic function. Although previous findings point to the role of maternal care experienced as an important determinant of both altered serotonergic function and perpetuation of abuse later in life (Maestripieri, 2005; Maestripieri et al., 2006), the results of the present study suggest that genetic factors may also play a role. In particular, genetic predispositions for low serotonergic function paradoxically associated with the short rh5-HTTLPR allele seem to impact important maternal behaviors, supporting previous evidence of gene by environment interactions modulating the consequences of early adverse experiences (see, for example, Bennett et al., 2002 and Caspi et al., 2003).
Previous studies in nonhuman primates have also characterized abusive mothers as having higher levels of anxiety and being more rejecting of their infants (McCormack et al., 2006; Maestripieri, 1998; Troisi and D'Amato, 1984). Given that the human literature links the serotonin transporter gene to anxiety-related traits in humans (Lesch et al., 1996; Mazzanti et al., 1998; Osher et al., 2000) one possible explanation for our findings is that the poor maternal care exhibited by mothers with the rh5-HTTLPR short allele (abusive behavior, lower infant protectiveness) could be related to higher levels of anxiety, supporting a potential link between the rh5-HTTLPR short allele, increased anxiety and alterations in proper maternal care. This possibility needs to be tested in further studies with bigger sample sizes.
We also found that adult females with the short allele exhibited higher basal cortisol secretion across the day, and greater variability in morning cortisol across the 6 months postpartum. Associations between the rh5-HTTLPR polymorphisms and HPA axis activity have been previously reported (Barr et al., 2004a,b), and our study adds additional evidence that the presence of the short rh5-HTTLPR allele may have behavioral and stress reactivity implications (see below for similar effects in the infants). In conclusion, our data suggest that the presence of the short rh5-HTTLPR allele is associated with alterations in the neuroendocrine mechanisms regulating anxiety and reactivity to stress, and may also be associated with alterations in maternal care. Although not possible in this study, due to our small sample size, it would be important to evaluate whether mothers with the s/s genotype exhibited even more severe phenotypes (i.e. greater instances of abuse, exaggerated HPA axis responses, etc.) than those with the l/s genotype. This would suggest that the short allele has an additive effect, and confers an even greater risk to the development of alterations in maternal behavior and HPA axis function.
Although our findings suggest a potential genetic influence on maternal behavior, this does not negate the fundamental effect of experience and environment in understanding maternal care. Previous research indicates that infant abuse is concentrated along specific matrilines in both rhesus and pigtail macaques, such that daughters of abusive mothers are more likely to abuse their own offspring than those of non-abusive mothers (Maestripieri and Carroll, 1998; Maestripieri et al.,1997b). In an important cross-fostering study, Maestripieri (2005) reported that abusive parenting may be explained more by early life experiences than genetic inheritance. However, it may be the case that the poor maternal care demonstrated by the mothers in this study resulted from the interaction between early life stress (being abused as an infant) and some genetic factors (such as their rh5-HTTLPR genotype). Caspi et al. (2002) found a similar interaction between the MAOA polymorphism and child abuse, such that maltreated males were more likely to be antisocial and violent if they had the low-activity MAOA genotype. As with the 5-HTTLPR, the rhesus MAOA gene also contains a polymorphism that affects transcriptional activity, and interacts with rearing experience to influence aggressive behavior in adult rhesus males (Newman et al., 2005). Clearly, given our small sample size, our results should be interpreted with caution, and more research is needed to examine the genetic and environmental influences on anxious behavior and maternal care.
In this study, we also found effects for the rh5-HTTLPR genotype on infant behavior. Infants with the short allele were more behaviorally reactive during experimental procedures at month 3 (they exhibited more tantrums, resistance to handling and were more difficult to console), compared to l/l infants. These results are consistent with other reports of behavioral reactivity of rhesus infants, and also suggest that the short allele may be associated with increased emotional reactivity and levels of anxiety and fear as exhibited in the infants' social groups. Champoux et al. (2002) found that infants with the l/s genotype were more difficult to console and demonstrated higher levels of emotional distress, and Bethea et al. (2004) found that infants with the s/s genotype had higher levels of anxiety when exposed to various behavioral tests. It is also of interest that all the behavioral reactivity differences detected in our study occurred during month 3. This may be due to the fact that this is a critical developmental period for rhesus macaques, with fearful behavior becoming more prevalent and regulated (Kalin et al., 1991), in parallel to an increase in exploratory behavior and an increase in responsibility of keeping in contact with the mother (Hinde and Spencer-Booth, 1967). Thus, the genotype effects detected on the infants' behavioral reactivity, not only support the findings in the human literature that the short 5-HTTLPR allele may be tied to anxiety-related disorders (Lesch et al., 1996; Mazzanti et al., 1998; Osher et al., 2000), but that the influence of the 5-HTTLPR genotype on emotional behavior may emerge at a critical developmental juncture, shortly after birth.
This study also revealed an association between the rh5-HTTLPR genotype and infant HPA axis activity. Infants with the l/s genotype exhibited higher basal morning levels of cortisol at month one, and cortisol and ACTH responses to stress at month 2, compared to l/l infants. In addition, the rh5-HTTLPR genotype interacted with rearing history to influence HPA activity. In a previous study we reported that during the first month (when abuse rates were the highest), abused infants had elevated basal cortisol, suggesting that this was a stressful experience (McCormack et al., submitted for publication). We now report that abused l/s infants showed the highest levels of resting cortisol at this age compared to all other groups. This suggests that during the first month, l/s infants may have been more vulnerable to the effects of abuse on basal cortisol levels than the l/l infants. The decline in cortisol after month 1, suggests a potential downregulation in the HPA system in response/adaptation to the heightened levels of cortisol during the first month (McCormack et al., submitted for publication). This pattern of higher followed by lower basal cortisol levels detected in the abused infants after the first month is consistent with similar effects observed in monkeys exposed to other early adverse experiences (Capitanio et al., 2005; Clarke et al., 1998; Dettling et al., 2002; Sanchez et al., 2005; Shannon et al.,1998) and in human children and adults with early life stress (Cicchetti and Rogosch, 2001; Gunnar and Vazquez, 2001; Heim et al., 2001; Tarullo and Gunnar, 2006). Abused l/s infants also showed higher cortisol responses to stress (both at months 2 and 6) compared to abused l/l infants. Although these interaction effects have to be interpreted with caution due to the small sample size, these results suggest that the HPA axis of abused l/s infants may be sensitized to early life stress, thus explaining the heightened cortisol levels during stressful experiences.
A wide variety of studies have shown that humans with major depressive disorder exhibit altered HPA axis functioning (Heim et al., 2008; Plotsky et al., 1998). Previous studies of rhesus macaques also reported a heightened HPA axis response to separation in l/s infants, especially among peer-reared individuals (Barr et al., 2004a; Bennett et al., 2002). In addition, targeted disruption of the serotonin transporter gene in rodents also results in increased HPA axis responses to stress (Lanfumey et al., 2000). Altogether, these findings are consistent with the important role of serotonin regulating not only HPA stress reactivity, but its negative feedback (Lanfumey et al., 2008; Porter et al., 2004). In humans, individuals with the short allele who experienced early abuse or neglect were also more likely to develop depression later in life than individuals with the long allele (Caspi et al., 2003; Kendler et al., 2005; see also Collier et al., 1996; Joiner et al., 2003, for a link between the short 5-HTTLPR allele and risk to develop depression). In the current study we found that abused infants with the l/s genotype had higher rates of anxiety and increased HPA axis stress reactivity, which is compelling given the consistency with previous human (Caspi et al., 2003; Kendler et al., 2005) and nonhuman primate literature (Barr et al., 2003a,b; Champoux et al., 2002) which report that the short allele confers vulnerability to behavioral and HPA axis alterations in individuals with early adverse experiences. Altogether, previous results, and the findings from our study, suggest an important role of 5-HTT polymorphisms in humans and nonhuman primates modulating the impact of early life stress on anxiety-like behavior, basal HPA axis activity, and HPA axis reactivity to stress. Clearly, further research with larger samples sizes is needed to clarify the role that the serotonin transporter (and these allelic variants) has in mediating development, and how early life stress may impact this relationship.
The continued longitudinal investigation of abused and nonabused monkeys will provide us with a broader picture of the effects of early life stress and rh5-HTTLPR genotype on behavioral and neuroendocrine development in nonhuman primates. Although the findings of this study are intriguing and generally congruent with those of human studies they also need to be interpreted with caution due to our small sample size and replicated in future studies. Further work with this nonhuman primate model of child maltreatment can continue to inform human developmental psychopathology research and have potential implications for intervention as well.
Acknowledgments
This work was supported by NIH grants MH065046 and MH58922 (MMS), MH62577 and MH63097 (DM), MD000215 (KM), and RR-00165 to the Yerkes National Primate Research Center, as well as by a FIRST fellowship (NIH K-12 grant GM00680 to KM), a NARSAD Young Investigator Award (MMS), and NIAAA Intramural funds. We thank Richelle Fulks and Anne Graff for their technical assistance, Paul Plotsky for his support of this project, and the members of the NIMH “Early Experience, Stress and Prevention Science Network” (MH65046) for stimulating discussion of this research. We also thank Dr. James Ritchie's laboratory at Emory University for their assistance with ACTH and cortisol assays.
References
- Altman SA. A field study of the sociobiology of rhesus monkeys. Ann. N.Y. Acad. Sci. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Baca-Garcia E, Vaquero C, Diaz-Sastre C, Saiz-Ruiz J, Fernandez-Piqueras J, deLeon J. A gender-specific association between the serotonin transporter gene and suicide attempts. Neuropsychopharmacology. 2002;26:692–695. doi: 10.1016/S0893-133X(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol. Clin. Exp. Res. 2003a;27:812–817. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2003b;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi S, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc. Natl. Acad. Sci. 2004a;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi S, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic–hypothalamic–pituitary–adrenal axis response to stress in infant macaques. Biol. Psychiatry. 2004b;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol. Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FKY, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5-HTTLPR) Behav. Genet. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta Endocrinol. (Copenh.) 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Effects on behaviour of disruption of an affectional bond. Eugen. Soc. Symp. 1968;4:94–108. [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic–pituitary–adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev. Psychobiol. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cassan WJ, D'mello A. Acute stress-induced facilitation of the hypothalamic–pituitary–adrenal axis: evidence for the roles of stressor duration and serotonin. Neuroendocrinology. 2001;74:167–177. doi: 10.1159/000054683. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus neonates. Mol. Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev. Psychopathol. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Clarke SA, Kraemer GW, Kupfer DJ. Effects of rearing condition on HPA axis response to fluoxetine and desipramine treatment over repeated social separations in young rhesus monkeys. Psychiatry Res. 1998;79:91–104. doi: 10.1016/s0165-1781(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Collier DA, Stöber G, Li T, Heils A, Catalano M, DiBella D, Arranz MJ, Murray RM, Vallada HP, Bengel D, Müller CR, Roberts GW, Smeraldi E, Kirov G, Sham P, Lesch KP. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol. Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacol. Biochem. Behav. 2002;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, Lesch KP, Hamer D, Murphy DL. Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. Am. J. Med. Genet. 2000;96:202–216. doi: 10.1002/(sici)1096-8628(20000403)96:2<202::aid-ajmg16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Volume 2, Developmental Neuroscience, 2nd ed. New York: Wiley Press; 2006. pp. 533–577. [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bosnall R, Miller AH, Nemeroff CB. Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Mother–infant relations in rhesus monkeys. In: White NF, editor. Ethology and Psychiatry. Toronto: University of Toronto Press; 1974. pp. 29–46. [Google Scholar]
- Hinde RA, Spencer-Booth Y. The behavior of socially living rhesus monkeys in their first two and a half years. Anim. Behav. 1967;15:169–196. doi: 10.1016/s0003-3472(67)80029-0. [DOI] [PubMed] [Google Scholar]
- Hinde RA, McGinnis L. Some factors influencing the effects of temporary mother–infant separation: some experiments with rhesus monkeys. Psychol. Med. 1977;7:197–212. doi: 10.1017/s0033291700029275. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Johnson F, Soderstrom K, Brown JS. Is there an association between serotonin transporter gene polymorphismand family history of depression? J. Affect. Disord. 2003;77:273–275. doi: 10.1016/s0165-0327(02)00171-4. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social support and serotonin transporter gene moderate depression in maltreated children. Proc. Natl. Acad. Sci. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhm JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression. Arch. Gen. Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Mannoury La Cour C, Froger N, Hamon M. 5-HT-HPA interaction in two models of transgenic mice relevant to major depression. Neurochem. Res. 2000;25:11099–11206. doi: 10.1023/a:1007683810230. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid–serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci. Biobehav. Rev. 2008;32:1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller C, Hamer D, Murphy D. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lichtermann D, Hranilović D, Trixler M, Franke P, Jernej B, Delmo CD, Knapp M, Schwab SG, Maier W, Wildenauer DB. Support for allelic association of a polymorphic site in the promoter region of the serotonin transporter gene with risk for alcohol dependence. Am. J. Psychiatry. 2000;157:2045–2047. doi: 10.1176/appi.ajp.157.12.2045. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurons: implications for control of the hypothalamic–pituitary–adrenal axis. J. Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Infant abuse associated with psychosocial stress in a group-living pigtail macaque (Macaca nemestrina) mother. Am. J. Primatol. 1994;32:41–49. doi: 10.1002/ajp.1350320105. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim. Behav. 1998;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. The biology of human parenting: insights from nonhuman primates. Neurosci. Biobehav. Rev. 1999;23:411–422. doi: 10.1016/s0149-7634(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc. Natl. Acad. Sci. 2005;102:9726–9729. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Risk factors for infant abuse and neglect in group-living rhesus monkeys. Psychol. Sci. 1998;9:143–145. [Google Scholar]
- Maestripieri D, Wallen K, Carroll KA. Genealogical and demographic influences on infant abuse and neglect in group-living sooty mangabeys (Cercocebus atys) Dev. Psychobiol. 1997a;31:175–180. doi: 10.1002/(sici)1098-2302(199711)31:3<175::aid-dev2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Wallen K, Carroll KA. Infant abuse runs in families of group-living pigtail macaques. Child Abuse Negl. 1997b;21:465–471. doi: 10.1016/s0145-2134(97)00006-9. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lindell SG, Higley JD, Newman TK, McCormack KM, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques. Behav. Neurosci. 2006;120:1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lindell SG, Higley JD. Intergenerational transmission of maternal behavior in rhesus macaques and its underlying mechanisms. Dev. Psychobiol. 2007;49:165–171. doi: 10.1002/dev.20200. [DOI] [PubMed] [Google Scholar]
- Mazzanti CM, Lappalainen J, Long JC, Bengel D, Naukkarinen H, Eggert M, Virkkunen M, Linnoila M, Goldman D. Role of serotonin transporter promoter polymorphism in anxiety-related traits. Arch. Gen. Psychiatry. 1998;55:936–940. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- McCormack K, Grand AP, LaPrairie J, Maestripieri D, Sanchez MM. The effects of maternal abuse on HPA axis function in rhesus macaques. submitted for publication. [Google Scholar]
- McCormack K, Sanchez MM, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Dev. Psychobiol. 2006;48:537–550. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, deGeus EJC, Beem AL, Lakenberg N, Hottenga J, Slagboom PE, Boomsma DI. Family based association analyses between the serotonin transporter gene polymorphism (5-HTTLPR) and neuroticism, anxiety and depression. Behav. Genet. 2007;37:294–301. doi: 10.1007/s10519-006-9139-7. [DOI] [PubMed] [Google Scholar]
- Newman TK, Syagailo Y, Barr CS, Wentland J, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. MAOA gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol. Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Osher Y, Hamer D, Benjamin J. Association and linkage of anxiety-related traits with a functional polymorphism of the serotonin transporter gene regulatory region in Israeli sibling pairs. Mol. Psychiatry. 2000;5:216–219. doi: 10.1038/sj.mp.4000660. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic–pituitary–adrenal axis. Psychiatr. Clin. North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Watson S, Young AH. Corticosteroid–serotonin interactions in depression: a review of the human literature. Psychopharmacology. 2004;173:1–17. doi: 10.1007/s00213-004-1774-1. [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC, Sackett GP. Research Protocol and Technicians Manual: A Guide to the Care, Feeding, and Evaluation of Infant Monkeys. Infant Primate Research Laboratory, Seattle, WA. 1992 [Google Scholar]
- Sanchez MM, Alagbe O, Felger J, Zhang J, Graff AE, Grand AP, Maestripieri D, Miller AH. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol. Psychiatry. 2007;12:895–897. doi: 10.1038/sj.mp.4002025. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in non-human primates with adverse rearing. Biol. Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Scheer FAJL, Buijs RM. Light affects morning salivary cortisol in humans. J. Clin. Endocrinol. Metab. 1999;84:3395–3398. doi: 10.1210/jcem.84.9.6102. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability and early experience. Infant Behav. Dev. 1992;15:155–177. [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing conditions and plasma cortisol in rhesus monkey infants. Am. J. Primatol. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NWJ, Willis-Owen SAG, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol. Psychiatry. 2006;59:224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm. Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Troisi A, D'Amato FR. Is monkey maternal abuse of offspring aggressive behavior? Aggress. Behav. 1983;9:167–173. [Google Scholar]
- Troisi A, D'Amato FR. Ambivalence in monkey mothering: infant abuse combined with maternal possessiveness. J. Nerv. Ment. Dis. 1984;172:105–108. doi: 10.1097/00005053-198402000-00007. [DOI] [PubMed] [Google Scholar]
- Troisi A, D'Amato FR. Anxiety in the pathogenesis of primate infant abuse: a pharmacological study. Psychopharmacology. 1991;103:571–572. doi: 10.1007/BF02244261. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol. Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Gordon TP, Collins DC. Ontogeny of luteinizing hormone secretion and first ovulation in seasonal breeding rhesus monkeys. Endocrinology. 1986;118:293–301. doi: 10.1210/endo-118-1-293. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J. Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]