Abstract
Background
Noninsulinoma pancreatogenous hypoglycemia (NIPH) is a rare cause of hypoglycemia from excessive insulin secretion, especially affecting post-bariatric surgery patients. Partial pancreatectomy may control hypoglycemia; however, multiple patients experienced symptomatic relapse. Our study goal was to assess frequency and severity of recurrent symptoms postoperatively.
Methods
Demographics, preoperative testing, operative and postoperative details were reviewed for all patients who underwent pancreatic resection for NIPH at Mayo Clinic from January 1996 - December 2008. Patient records and mail surveys (including European Quality of Life Survey (EQ-5D) and Fear of Hypoglycemia Scale (FOHS-98)) were used to assess outcome.
Results
75 patients underwent pancreatic resection for NIPH. 48 patients (70%) completed the survey (median follow-up 53 mo.). Median time to recurrent symptoms was 16 months (N=41, 87%). Despite symptom recurrence, 75% of patients reported overall improvement in quality of life, with marked reduction in psychological stress and hypoglycemic symptoms (greater than 50% decrease in FOHS-98 scores (p<0.001). Overall, half of the patients were classified as highly/moderately surgically-successful. Nevertheless, 25% of patients experienced no apparent benefit.
Conclusions
Although nearly 90% of NIPH patients reported recurrent symptoms suggestive of hypoglycemia, a majority reported significant improvements in QOL and marked reduction in other symptoms after pancreatic resection.
Keywords: noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS), adult-onset nesidioblastosis, post-gastric bypass hypoglycemia
BACKGROUND
Hyperinsulinemic hypoglycemia can be of endogenous (over-secretion from an insulin-producing islet cell tumor or abnormal pancreas) or exogenous (self-administration of insulin) origin. Noninsulinoma pancreatogenous hypoglycemia (NIPH) is a cause of endogenous hyperinsulinemic hypoglycemia related to hypersecretion of insulin diffusely from the pancreas, rather than a functional tumor (insulinoma)1,2. This can be related to nesidioblastosis or islet cell hyperplasia, and is most often encountered in patients who have undergone gastric surgery, most-commonly Roux en Y gastric bypass for obesity, but has also been reported after anti-reflux and ulcer surgery, or rarely in patients without prior surgery3–6. These patients can develop severe symptomatic hypoglycemia after food ingestion due to robust over-secretion of insulin. Glucose measurements in these patients may fall below 30 mg/dl, causing neuroglycopenic symptoms such as coma, seizures, confusion, and visual disturbances. These symptoms can be as severe and debilitating as in patients with insulinomas. However, these patients characteristically have a negative 72-hour fast, as the hyperinsulinemia occurs in the postprandial period.
Most patients’ symptoms can be controlled with diet-modifications and medications such as, acarbose, diazoxide, verapamil and somatostatin2, 7, 8. However, a small proportion of patients fail medical management. For these patients, surgical resection of the pancreas, with the intent of reducing islet cell mass, is the recommended treatment7. In 1996, we began performing pancreatic resections for noninsulinoma pancreatogenous hypoglycemia at the Mayo Clinic, with initial promising results3, supported by reports from other centers2. Over the ensuing years, with additional patient experience and follow-up, we have recognized a number of patients with recurrent symptoms, again mirrored by others’ reports of long-term surgical failure8–11.
The goals of our study were to determine the incidence of recurrent symptoms in our patient cohort. Additionally, we sought to quantify the impact of surgical resection on symptom severity and overall quality of life.
METHODS
After receiving IRB approval, we identified all patients who had undergone pancreatic resection for noninsulinoma pancreatogenous hypoglycemia at the Mayo Clinic from January 1996 through December 2008. Patient medical records were reviewed to obtain data on: patient demographics, medical co-morbidities, pre-surgical evaluation, perioperative findings and complications, and postoperative outcomes. In addition to clinical data available through the patient medical records, we also wished to assess quality of life and symptomatology that may not have been reported in their medical record. We therefore provided mail surveys, consisting primarily of 5 point Likert-scale format questions addressing patient’s perceived health/symptoms before surgery and at the present time. The European Quality of Life Survey (E5-QD), and the Fear of Hypoglycemia Scale (FOHS-98) were included in the survey packet.
The E5-QD is a validated quality of life instrument used for an overall assessment of a patient’s health status12. We chose this instrument over the more commonly used SF-36 and SF-12 surveys as it was the shortest validated global assessment tool, and was intended for pairing with disease-specific tools. It consists of 15 questions, subdivided into 5 global health categories (Mobility, Self-care, Usual Activities, Pain/Discomfort, Anxiety/Depression) paired with a 100-point visual analog scale for assessment of perceived overall health status. The global questions are then converted into an overall health index score, which has been validated in the US population and has been used in cost-analysis calculations of Quality-adjusted Life Years (QALY)12. The US index scale ranges from −0.11 (no quality/death) to 1.0 (perfect health)13.
To date, no disease-specific instrument has been used or validated in patients with insulinoma or noninsulinoma pancreatogenous hypoglycemia. However, the Fear of Hypoglycemia Scale (FOHS-98) has been validated in medically-treated diabetes, and measures the psychological impact of hypoglycemia and behavioral modifications made by respondents who experience hypoglycemia14. Since the symptoms of hypoglycemia caused by endogenous or exogenous hyperinsulinemic hypoglycemia are indistinguishable, we felt this instrument was well-suited for use in noninsulinoma pancreatogenous hypoglycemia, as it addresses the symptomatology, psychological impact, and subsequent behavioral responses of patients with symptomatic hypoglycemia. This instrument consists of 27 Likert-scale questions related to the frequency of events precipitating fear of hypoglycemia and related behavioral responses (graded from 0=“Never” to 4=“Always” with overall scale from 0 – 108).
Additionally, we included questions specific to symptoms and outcomes of pancreatic resection. These included questions about gastrointestinal symptoms and postoperative development of diabetes, and questions about symptom recurrence, medications, and diet. We then developed and assigned patients to an assessment of overall surgical outcome, categorized into four groups: high, moderate, and minimal surgical success, and surgical failure. The basis for this categorization was the EQ5D and FOHS-98 scores, clinical symptoms related to the pancreatic resection, possible development and severity of diabetes, and the need for additional surgical intervention for hypoglycemia (Table 1).
Table 1.
Grading criteria and assessment of surgical success in patients undergoing surgery for noninsulinoma pancreatogenous hypoglycemia.
| Category | Criteria | Patients, N (%) |
|---|---|---|
| I – High surgical success (significant reduction in symptoms with no long-term complications) |
|
10 (21%) |
| II – Moderate surgical success (Moderate reduction in symptoms with few long-term complications) |
|
13 (27%) |
| III – Minimal surgical success (Reduction in symptoms with significant long-term complications) |
|
13 (27%) |
| IV – Surgical Failure (No long-term reduction of symptoms, and overall worse quality of life) |
|
12 (25%) |
Upon receipt of the completed surveys, results were compiled and analyzed. Unpaired t-tests were used for continuous data with normal distributions (age, glucose nadirs only) Chi-square tests were used to compare categorical data. Paired data-points (pre- and post-operative survey data) were analyzed with Wilcoxon rank-sum tests. Kaplan-Meier curves were estimated for recurrent symptoms – patients were deemed to have recurrent symptoms if they reported recurrence of symptoms identical to their preoperative symptoms and the reported month of recurrence. Analyses were performed with Stata SE 9.2 (StataCorp, College Station, TX). Validation of symptomatic hypoglycemia by reflex glucose monitor or lab tests was infrequently available. We looked at overall outcomes of the group as well as potential preoperative predictors; specifically, prior gastric bypass surgery (as this has been proposed to have a unique pathologic mechanism of beta cell hyperplasia7) and results of preoperative selective arterial calcium stimulation testing (SACS).
RESULTS
Seventy-five patients were identified that underwent pancreatic resection for treatment of Noninsulinoma pancreatogenous hypoglycemia. Mean age at operation was 46 years, and the majority (64%) had undergone prior bariatric surgery (N=48). Patients typically presented with severe neuroglycopenic symptoms, such as: loss of consciousness (N=27, 36%) seizures (N=15, 20%), or confusion (N=28, 37%). There was no perioperative mortality; however 37% of patients (N=28) suffered one or more postoperative complications. Fourteen patients (20%) developed a pancreatic fistula or pseudocyst, none of whom required operative intervention. One additional patient developed pancreatitis, with multiple readmissions for recurrent pancreatitis. Three patients (4%) had significant postoperative hemorrhage, two of whom required urgent reoperation. Two patients (3%) developed deep surgical site infections, and two patients (3%) developed superficial wound infections, all treated non-operatively. Additional non-pancreatic complications included: Clostridium difficile colitis (N=3), portal vein thrombosis (N=2), malabsorptive diarrhea (N=1), hypoventilation associated hypoxemia (N=1), postoperative atrial fibrillation (N=1), and small bowel obstruction (N=1).
Four patients have undergone additional operations for symptoms: two patients underwent completion pancreatectomy for persistent noninsulinoma pancreatogenous hypoglycemia (one with subsequent pancreas transplantation); one underwent feeding tube placement for treatment of persistent hypoglycemia; and one underwent gastric bypass revision complicated by fistulas precluding oral intake.
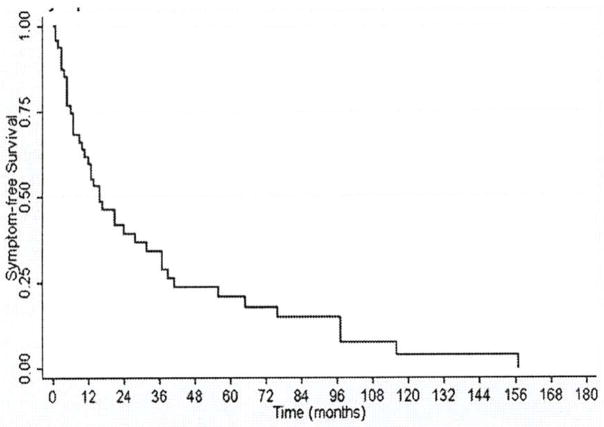
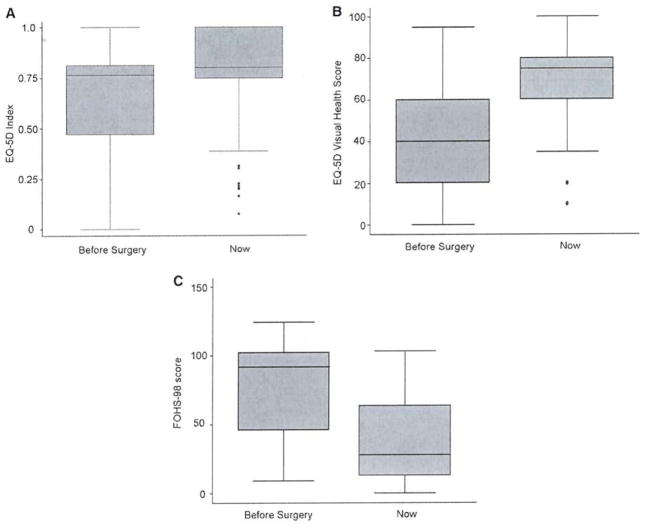
At the time of the survey, 69 of the 75 patients were eligible for participation (5 patients expired, 1 patient incarcerated). Forty-eight patients (70%) completed the survey with mean follow-up of 53 months. Demographics of respondents vs. non-respondents is presented in Table 2. There were no significant differences in demographics, preoperative glucose nadir, or symptoms in respondents vs. non-respondents (Table 2). Survey results are presented in Tables 3–6. Forty-one patients (87%) reported recurrent symptoms, with one patient undergoing total pancreatectomy for severe persistent symptoms. Median time to recurrent symptoms was 16 months (range <1 – 157 mo.; Figure 1). Although most patients reported symptom recurrence, there was a marked reduction in psychological stress and hypoglycemic symptoms postoperatively as measured by the FOHS-98 (Tables 3 and 4). Median FOHS-98 behavioral, worry, and overall scores all significantly decreased by more than 50% postoperatively, with almost all patients reporting overall improvement in their perceived quality of life: median EQ5D visual health scores increased from 40 to 75 out of 100 (p<0.001; Table 5, Figures 2–4). The most significant improvements were seen in patients’ reported time off work and travel limitations, as well as marked reduction in worry about passing out, driving, being alone, and losing control. There was a trend toward improvement in overall functional status as measure by the in EQ-5D index scores, but this did not reach statistical significance.
Table 2.
Patient Demographics: Respondents vs. non-respondents.
| Respondents N=48 |
Non-respondents N=21 |
P value | |
|---|---|---|---|
|
| |||
| Mean age at operation (range) | 45 y (15–78) | 44 y (28– 63) | 0.56 |
|
| |||
| Males:Females | 13:35 | 6:15 | 0.89 |
|
| |||
| Prior surgeries | |||
| Bariatric procedure | 33 (69%) | 15 (70%) | |
| Esophagectomy/ gastrectomy | 4 (8%) | 0 (0%) | |
| Anti-ulcer procedure | 1 (2%) | 2 (10%) | |
| Anti-reflux procedure | 0 (0%) | 2 (10%) | |
| No prior upper GI surgery | 10 (21%) | 2 (10%) | 0.23 |
|
| |||
| Mean preoperative glucose nadir (SD) | 39.8 (1.4) | 39.3 (1.9) | 0.81 |
|
| |||
| Most severe preoperative symptom | |||
| Loss of consciousness, automobile crash | 15 (31%) | 12 (51%) | |
| Seizures | 8 (17%) | 7 (33%) | |
| Confusion/ behavior changes | 17 (35%) | 9 (43%) | |
| Vision changes/ other | 8 (17%) | 0 (0%) | 0.22 |
|
| |||
| Procedures performed | |||
| Distal pancreatectomy with splenectomy | 44 (92%) | 21 (100%) | |
| Spleen-sparing distal pancreatectomy | 2 (4%) | 0 (0%) | |
| Pancreaticoduodenectomy | 1 (2%) | 0 (0%) | |
| Total pancreatectomy | *1 (2%) | (0%) | |
| Laparoscopic distal pancreatectomy | 1 (2%) | 0 (0%) | 0.40 |
|
| |||
| Operative complications | |||
| Superficial surgical site infection | 0 (0%) | 2 (10%) | |
| Deep surgical site infection | 2 (5%) | 0 (0%) | |
| Pancreatic leak/fistula | 9 (19%) | 5 (24%) | |
| Hemorrhage | 1 (2%) | 1 (0%) | |
| Portal vein occlusion | 2 (4%) | 0 (0%) | |
| Bowel obstruction | 1 (2%) | 0 (0%) | |
| C. Difficile colitis | 2 (4%) | 1 (5%) | |
| Atelectasis | 0 (0%) | 1 (5%) | |
| Prolonged abdominal pain | 1(2%) | 0 (5%) | 0.44 |
1 patient underwent total pancreatectomy for severe persistent symptoms after subtotal pancreatectomy
Table 3.
Postoperative outcomes in patients undergoing pancreatic resection for noninsulinoma pancreatogenous hypoglycemia –Fear of Hypoglycemia Scale behavior scores. (Individual questions scaled: 0 – Never; 1 – Rarely; 2 – Sometimes; 3 – Often; 4 – Always; lower overall score is better.)
| To avoid low blood sugar and how it affects me, I… | Median (mean) preop value |
Median (mean) postop value |
P value |
|---|---|---|---|
| Ate large snacks | 2 (1.81) | 1 (1.19) | <0.001 |
| Tried to keep my blood sugar above 150 | 0 (1.23) | 0 (0.81) | <0.001 |
| Changed my medications when my sugar was low | 0 (0.46) | 0 (0.50) | 0.96 |
| Measured my blood six or more times a day | 3 (2.46) | 0 (0.90) | <0.001 |
| Made sure I had someone with me when I go out | 3 (2.48) | 1 (1.10) | <0.001 |
| Limited my out-of-town travel | 3 (2.27) | 0 (0.94) | <0.001 |
| Limited my driving (car, truck, or bicycle) | 3 (2.42) | 0.5(1.04) | <0.001 |
| Avoided visiting friends | 2 (1.88) | 0 (0.69) | <0.001 |
| Stayed at home more than I liked | 3 (2.40) | 0.5 (1.15) | <0.001 |
| Limited my exercise/physical activity | 3 (2.52) | 2 (1.52) | <0.001 |
| Made sure there were other people around | 3 (2.54) | 1 (1.25) | <0.001 |
| Avoided sex | 1 (1.29) | 0 (0.98) | 0.11 |
| Kept my blood sugar higher than usual in social situations | 2 (1.60) | 1 (1.02) | 0.003 |
| Kept my blood sugar higher than usual when doing important tasks | 2 (1.85) | 1 (1.25) | 0.05 |
| Had people check on me several times during the day or night | 3 (2.06) | 0 (0.98) | <0.001 |
| FOHS Behavior Score (0–60) | 34 (29.27) | 13 (15.31) | <0.001 |
Table 6.
Symptomatology of patients pre- and post-resection for noninsulinoma pancreatogenous hypoglycemia (Scale: 0 – No symptoms; 1 – rare; 2 – Monthly; 3 – Weekly; 4 – daily symptoms).
| Median (mean) preop value |
Median (mean) postop value |
P value | |
|---|---|---|---|
| Experience symptoms of low blood sugar | 4 (3.84) | 2 (2.15) | <0.001 |
| Experience fullness after eating | 4 (3.17) | 4 (3.31) | 0.85 |
| Experience symptoms of diarrhea | 1 (1.83) | 1 (1.60) | 0.40 |
| Have greasy, foul-smelling stools | 1 (1.65) | 1 (1.58) | 0.55 |
| Experience nausea or vomiting | 3 (2.19) | 1 (1.65) | 0.003 |
| Check your blood sugars at home | 4 (3.38) | 3 (2.65) | 0.002 |
| Experience high blood sugars (>200mg/dl) | 0 (0.79) | 0.5(1.27) | 0.06 |
| Experience low blood sugars (<50mg/dl) | 4 (3.57) | 2 (2.17) | <0.001 |
| Stay home from work because of symptoms | 3 (2.17) | 0 (1.00) | <0.001 |
| Get evaluated in a doctor’s office | 2 (2.14) | 1 (1.39) | <0.001 |
| Get evaluated in the hospital/Emergency Room | 1 (1.39) | 0 (0.56) | <0.001 |
Figure 1.
Kaplan-Meier estimates of symptom-free survival after pancreatic resection for noninsulinoma pancreatogenous hypoglycemia.
Table 4.
Postoperative outcomes in patients undergoing pancreatic resection for noninsulinoma pancreatogenous hypoglycemia –Fear of Hypoglycemia Scale worry scores. (Individual questions scaled: 0 – Never; 1 – Rarely; 2 – Sometimes; 3 – Often; 4 – Always; lower overall score is better.)
| Because my blood sugar could go low, I worried about… | Median (mean) preop value |
Median (mean) postop value |
P value |
|---|---|---|---|
| Not recognizing/realizing I was having low blood sugar | 3 (2.75) | 2 (1.75) | <0.001 |
| Not having food, fruit, or juice available | 3 (2.60) | 1.5 (1.63) | <0.001 |
| Passing out in public | 3 (2.67) | 1 (0.88) | <0.001 |
| Embarrassing myself or my friends in a social situation | 3 (2.42) | 0.5 (0.88) | <0.001 |
| Having a hypoglycemic episode while alone | 3.5 (3.02) | 1 (1.56) | <0.001 |
| Appearing stupid or drunk | 3 (2.60) | 1 (1.08) | <0.001 |
| Losing control | 3 (2.71) | 1 (1.12) | <0.001 |
| No one being around to help me during a hypoglycemic episode | 3 (2.58) | 1 (1.19) | <0.001 |
| Having a hypoglycemic episode while driving | 3 (2.94) | 1 (1.31) | <0.001 |
| Making a mistake or having and accident | 4 (3.00) | 1 (1.29) | <0.001 |
| Getting a bad evaluation or being criticized | 3 (2.31) | 1 (1.10) | <0.001 |
| Difficulty thinking clearly when responsible for others | 3 (2.54) | 1 (1.33) | <0.001 |
| Feeling light-headed or dizzy | 3 (3.02) | 2 (1.58) | <0.001 |
| Accidentally injuring myself or others | 3 (2.44) | 1 (1.25) | <0.001 |
| Permanent injury or damage to my health or body | 3 (2.56) | 1 (1.23) | <0.001 |
| Low blood sugar interfering with important things I was doing | 4 (3.15) | 2 (1.65) | <0.001 |
| Becoming hypoglycemic during sleep | 3 (2.50) | 1 (1.62) | <0.001 |
| Getting emotionally upset and difficult to deal with | 3 (1.15) | 1 (1.38) | <0.001 |
| FOHS Worry Score (0–72) | 55.5 (48.13) | 18 (23.17) | <0.001 |
Table 5.
Postoperative outcomes in patients undergoing pancreatic resection for noninsulinoma pancreatogenous hypoglycemia – overall quality of life pre- and postoperatively (E5-QD), and overall FOHS-98 scores.
| Median (mean) preop value |
Median (mean) postop value |
P value | |
|---|---|---|---|
| EQ-5D index score (scale −0.11 – 1.00) | 0.81 (0.73) | 0.84 (0.80) | 0.09 |
| Perceived EQ-5D global health state score (visual scale: 0–100) | 40 (44) | 75 (70) | <0.001 |
| Combined FOHS Behavior + Worry Scores (0–132) | 91.5 (77.40) | 27.5 (38.38) | <0.001 |
Figure 2.
A EQ-5D Index scores – before and after pancreatic resection for noninsulinoma pancreatogenous hypoglycemia. B EQ-5D Self reported perceived health (100-point analog scale) - before and after pancreatic resection for noninsulinoma pancreatogenous hypoglycemia. C Figure 4. FOHS-98 Overall scores – before and after pancreatic resection for noninsulinoma pancreatogenous hypoglycemia.
There was no significant increase in gastrointestinal symptoms (nausea, diarrhea, and steatorrhea) post-operatively (Table 6). Nine patients (18%) reported being diagnosed with diabetes since their operations, of whom 4 are insulin-treated. Seventy-five percent of patients reported continued diet modifications for symptom control (such as eating high protein diets, N=15; and/or eating multiple small meals, N=9).
Overall surgical outcomes are presented in Table 1. Twenty-three patients (48%) had highly or moderately successful outcomes, based on good symptom reduction and minimal long-term morbidity. Thirteen patients (27%) had a modestly successful outcome, with only minimal symptom control or significant morbidity such as insulin-dependent diabetes or new daily gastrointestinal symptoms. Twelve patients (25%) were considered to have a poor outcome based on lack of long-term improvement, need for additional surgical intervention for treatment of hypoglycemia, or overall decline in health status since surgery. Although the rate of surgical failure was not different in male vs. female patients (23% vs. 29%, p=0.7), male patients showed a trend towards a moderate or highly successful outcome (69% vs. 40%, p=0.07). There was no statistical difference in the proportion of patients with moderate or highly successful outcome based on prior bariatric vs. non-bariatric gastric surgery (14/33 vs. 4/5, p=0.12) or between post-bariatric vs. patients with no prior operations (14/33 vs. 5/10, p=0.67). Furthermore, there was no difference in the proportion of patients with nesidioblastosis (vs. hyperplasia) on histology between the post-bariatric, other gastric, or no prior surgery groups (80% vs. 80% vs. 79%, p=0.99). There was also no correlation between preoperative SACS findings and outcome – patients who had regionalization by SACS to a single arterial distribution (N=11, 23%) did no better or worse than patients who did not localize (4/7 vs. 19/37 moderately or highly successful, p=0.38).
DISCUSSION
Overall patients reported significant improvement in quality of life after pancreatic resection for noninsulinoma pancreatogenous hypoglycemia. Postoperatively, all patients had initial documented resolution of hypoglycemia and symptoms. However, the vast majority of patients did report symptom recurrence, albeit much less severe and debilitating. Further follow-up will be required to determine if symptoms progress and become debilitating or if they remain mild and manageable.
We had few patients with either venous or capillary verification of hypoglycemia associated with their recurrent symptoms, which limits our ability to conclusively link patients’ symptomatology to recurrent hypoglycemia. Our primary aim, however, was to evaluate patient perceptions of their quality of life and symptomatic improvement. Additional controlled testing of postoperative patients with mixed meal testing and validation of perceived symptom relationship to glucose concentrations wold be useful in understanding this disease and confirming on-going neuroglycopenia. Additionally, our median follow-up was only 43 months, and additional follow-up of this cohort will be necessary to determine how these patients progress over time.
Some studies have tried to differentiate between post-gastric bypass hypoglycemia (PGBPH) and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS) from other causes7. We did not see any difference in outcome in patients with prior bariatric surgery vs. any of our other patients with diffuse islet cell disease. However, the vast majority of our patients had a history of bariatric surgery and our study may not have adequate numbers for statistical power to detect such a difference. Additionally, we were unable to differentiate any specific pathologic entity (e.g., nesidioblastosis or islet cell hypertrophy) or clinical differences that would relate to outcomes in post-bariatric or other adult-onset noninsulinoma pancreatogenous hypoglycemia. One proposed treatment for persistent hypoglycemia in post-bariatric patients with documented loss of gastric restriction is silastic ring implantation6. This is thought to reduce late dumping, and potentially reduce dumping-related hypoglycemia. We have no experience with this approach. In the series by Z’graggen et al.6, 3 of 10 patients still required pancreatic resection for severe recurrent of symptoms during their 2 year follow-up after surgical re-restriction procedures; which appears worrisome that further bypass revision/restriction may not have lasting long-term results in improving hypoglycemia.
We attempted to find a correlation between preoperative SACS testing as a potential predictor of outcome, as it is one of the few preoperative studies that could potentially guide surgical care. Unfortunately this was also not a predictor of ultimate outcome. Other centers have also failed to find a correlation between SACS results and histopathology6. Our initial concept of a “gradient-directed” partial pancreatectomy guided by SACS offers little advantage over the concept that the pancreas diffusely hypersecretes insulin and the resection accomplishes a debulking of the islet cell mass.
One weakness of our study is its retrospective design, which makes our results subject to recollection bias. We recognize that the patients were asked to recall their symptoms and score their quality of life prior to the operation. How this may skew their perceived severity of symptoms or quality of life is unclear. Nor do we use quality of life scores to help determine who might benefit from pancreatic resection. However, our study does demonstrate that patients perceive a significant improvement in symptoms and quality of life after pancreatic resection for medically-refractory NIPH due to diffuse islet cell disease. Our own experience would support this perception. Specifically, we have found that patients with severe debilitating disease can have a dramatic response to surgical treatment with substantial symptomatic improvement which allows return to normal life activities (such as work, driving, travel, and independent self-care). Moreover, half of the patients were substantially better in long-term follow-up and 75% of patients at least somewhat better in overall symptoms and quality of life. However, we are equally convinced that pancreatic resection is rarely curative for noninsulinoma pancreatogenous hypoglycemia, as the majority of our patients report recurrent symptoms in long-term follow-up. Furthermore, we have only offered resection to patients with severe debility from neuroglycopenia and these results would dissuade us from offering surgical intervention to patients with mild or moderate symptoms. In conclusion, partial pancreatectomy for NIPH from diffuse disease does provide improvement in quality of life and symptoms in most patients; however, symptoms are likely to recur, but are typically less severe and debilitating. A better understanding of this disease process and successful non-invasive treatment strategies are therefore greatly needed.
LISTING OF RELEVANT ABBREVIATIONS
- E5-QD
European Quality of Life Survey
- FOHS-98
Fear of Hypoglycemia Scale, 1998 revision
- NIPH
Noninsulinoma pancreatogenous hypoglycemia
- NIPHS
Noninsulinoma pancreatogenous hypoblycemia syndrome
- PGBPH
Post-gastric bypass hypoglycemia
- SACS
Arteriography with selective arterial calcium stimulation
References
- 1.Service FJ, Natt N, Thompson GB, Grant CS, van Heerden JA, Andrews JC, et al. Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kir6. 2 and SUR1 genes. J Clin Endocrinol Metab. 1999;84:1582–9. doi: 10.1210/jcem.84.5.5645. [DOI] [PubMed] [Google Scholar]
- 2.Won JG, Tseng HS, Yang AH, Tang KT, Jap TS, Lee CH, et al. Clinical features and morphological characterization of 10 patients with noninsulinoma pancreatogenous hypoglycaemia syndrome (NIPHS) Clin Endocrinol. 2006;65:566–78. doi: 10.1111/j.1365-2265.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson GB, Service FJ, Andrews JC, Lloyd RV, Natt N, van Heerden JA, Grant CS. Noninsulinoma pancreatogenous hypoglycemia syndrome: an update in 10 surgically treated patients. Surgery. 2000;128:937–44. doi: 10.1067/msy.2000.110243. [DOI] [PubMed] [Google Scholar]
- 4.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–54. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 5.Tsujino M, Sugiyama T, Nishida K, Takada Y, Takanishi K, Ishizawa M, Hirata Y. Noninsulinoma pancreatogenous hypoglycemia syndrome: a rare cause of adult-onset nesidioblastosis. Intern Med. 2005;44:843–7. doi: 10.2169/internalmedicine.44.843. [DOI] [PubMed] [Google Scholar]
- 6.Z’graggen K, Guweidhi A, Steffen R, Potoczna N, Biral R, Walther F, et al. Severe recurrent hypoglycemia after bypass surgery. Obes Surg. 2008;18:981–8. doi: 10.1007/s11695-008-9480-4. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor RR, James C, Hussain K. Advances in the diagnosis and management of hyperinsulinemic hypoglycemia. Nat Clin Pract Endocrinol Metab. 2009;5:101–12. doi: 10.1038/ncpendmet1046. [DOI] [PubMed] [Google Scholar]
- 8.Moriera RO, Moreira RB, Machado NA, Goncalves TB, Coutinho WF. Post-prandial hypoglycemia after bariatric surgery; pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618–21. doi: 10.1007/s11695-008-9569-9. [DOI] [PubMed] [Google Scholar]
- 9.Witteles RM, Straus FH, Sugg SL, Koka MR, Costa EA, Kaplan EL. Adult-onset nesidioblastosis causing hypoglycemia: an important clinical entity and continuing treatment dilemma. Arch Surg. 2001;136:656–63. doi: 10.1001/archsurg.136.6.656. [DOI] [PubMed] [Google Scholar]
- 10.Raffel A, Krausch M, Anlauf M, Wieben D, Braunstein S, Kloppel G, Roher HD. Diffuse nesidioblastosis as a cause of hyperinsulinemic hypoglycemia in adults: a diagnostic and therapeutic challenge. Surgery. 2007;141:179–84. doi: 10.1016/j.surg.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Karawagh AM, Abdullah LS, Gasim AM, Abdelaziz MM. Noninsulinoma pancreatogenous hypoglycemia syndrome in a Saudi male. Saudi Med J. 2008;29:1654–7. [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. Clinical Economics Resource Links: Outcomes Measurement [Internet] Rockville, MD: [accessed 11/19/09]. updated Feb 2005. Available from: http://www.ahrq.gov/ric/ceoutc.htm. [Google Scholar]
- 13.Agency for Healthcare Research and Quality. Calculating the US Population-based EQ-5D Index Score [Internet] Rockville, MD: [accessed 11/19/09]. updated Aug 2005. Available from: http://www.ahrq.gov.rice/EQ5Dscore.htm. [Google Scholar]
- 14.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10:617–22. doi: 10.2337/diacare.10.5.617. [DOI] [PubMed] [Google Scholar]