Abstract
Patients with hematologic malignancies undergoing allogeneic stem cell transplantation (HSCT) commonly have an elevated serum ferritin prior to HSCT, which has been associated with increased mortality after transplantation. This has led to the suggestion that iron overload is common and deleterious in this patient population. However, the relationship between serum ferritin and parenchymal iron overload in such patients is unknown. We report a prospective study of 48 patients with acute leukemia (AL) or myelodysplastic syndromes (MDS) under going myeloablative HSCT, using magnetic resonance imaging (MRI) to estimate liver iron content (LIC) and cardiac iron. The median (and range) pre-HSCT value of serum ferritin was 1549 ng/ mL (20–6989); serum hepcidin, 59 ng/mL (10–468); labile plasma iron, 0 LPI units (0.0–0.9). Eighty-five percent of patients had hepatic iron overload (HIO), and 42% had significant HIO (LIC ≥5.0 mg/gdw). Only 1 patient had cardiac iron overload. There was a strong correlation between pre-HSCT serum ferritin and estimated LIC (r =.75), which was mostly dependent on prior transfusion history. Serum hepcidin was appropriately elevated in patients with HIO. Labile plasma iron elevation was rare. A regression calibration analysis supported the hypothesis that elevated pre-HSCT LIC is significantly associated with inferior post-HSCT survival. These results contribute to our understanding of the prevalence, mechanism, and consequences of iron overload in HSCT.
Keywords: Iron overload, Acute myeloid leukemia, Acute lymphoblastic leukemia, Myelodysplastic syndromes, Stem cell transplantation
INTRODUCTION
There is growing evidence that iron overload is common in patients with hematologic malignancies undergoing hematopoietic allogeneic stem cell transplantation (HSCT), and that it may have a deleterious effect on the outcomes of transplantation. This evidence comes mostly from studies using serum ferritin as a surrogate for iron burden, which have repeatedly shown that an increased pre-HSCT serum ferritin is associated with inferior post-HSCT survival [1–11], especially in patients who receive myeloablative conditioning. In most of these studies, the predominant association is between high ferritin and nonrelapse mortality (NRM) rather than with disease relapse. Some reports have suggested an increase in acute graft-versus-host disease (GVHD) [1,7,8] or hepatic veno-occlusive disease [3,12–14], but the strongest evidence seems to be for an increase in the risk of infection [4,6,8,9,15,16]. Unfortunately, serum ferritin is also an acute phase reactant and is therefore an imperfect surrogate for iron burden. This could be particularly problematic in this group of patients, who are frequently receiving chemotherapy or dealing with infectious issues pretransplantation. Therefore, although ferritin clearly has prognostic value, it is not clear that iron overload is itself an important contributor to post-HSCT morbidity and mortality. Moreover, we have little understanding of the mechanisms that may underlie iron overload in this setting or of the distribution of iron in those patients. We therefore conducted a prospective study in patients with myelodysplastic syndromes (MDS) or acute leukemia undergoing myeloablative HSCT, using hepatic and cardiac magnetic resonance imaging (MRI) to better estimate the prevalence of iron overload, and to clarify the relationship between iron burden and serum ferritin. We also measured labile plasma iron (LPI) and hepcidin levels in plasma and urine.
MATERIALS AND METHODS
Patients
We approached consecutive adult patients with acute myelogenous (AML) or lymphoblastic (ALL) leukemia or MDS who were scheduled to undergo allogeneic HSCT with myeloablative conditioning at the Dana-Farber/Brigham and Women’s Hospital (DF/BWH) transplant program beginning in June 2008. Patients who agreed to participate were enrolled in this prospective observational study. Accrual closed in January 2010. Sixteen eligible patients were not enrolled, because of patient refusal, logistic challenges of obtaining the required testing, or claustrophobia precluding MRI. Patients who met criteria for a companion study of pretransplantation chelation (NCT00658411) could enroll in the chelation study instead, and because we obtained the same pretransplantation studies as in the observational study (prior to any chelation), they are included in this report. A total of 48 patients are included in this analysis. Informed consent was obtained from all patients. Institutional review board (IRB) approval was obtained from the Office for the Protection of Research Subjects (OPRS) at Dana-Farber/Harvard Cancer Center to perform this study. This study was performed in accordance with the principles of the Declaration of Helsinki, and registered at ClinicalTrials.gov (NCT00954720).
Transplantation
Patients were transplanted under several treatment and investigational protocols over the period covered by this study. All patients received an ablative conditioning regimen, mostly cyclophosphamide plus total body irradiation (88% of patients). Ninety percent of patients received a peripheral blood stem cell graft; 44% of patients had a matched related donor, 48% a matched unrelated donor, and 8% received a mismatched transplant. GVHD prophylaxis regimens consisted mostly of a combination of calcineurin inhibitor and methotrexate, with or without sirolimus [17].
Laboratory Studies
Serum iron, total iron binding capacity, ferritin, and C-reactive protein were measured using standard commercial assays in the clinical laboratory at Brigham and Women’s Hospital. We defined adjusted serum ferritin (ASF) as serum ferritin (in ng/mL) divided by the log10 of C-reactive protein (CRP) (in mg/L), when CRP is higher than 10.0 mg/L. If CRP is <10.0 mg/L, the ASF is simply the serum ferritin (see Results section on ASF). HFE genotyping was performed using a commercial assay (Mayo Clinic, Rochester, MN). Patients’ plasma and urine were prospectively collected and frozen for measurements of hepcidin. LPI was measured in plasma by Afferix Ltd (Ashkelon, Israel). Transfusion history was obtained from the medical records of DF/BWH blood bank and of all the institutions where the patients reported to have received transfusions.
Hepcidin Assay
The assay was based on the detection and quantification of endogenous hepcidin relative to that of a stable isotope-labeled hepcidin internal standard added to samples at a known concentration [18]. The plasma version of this method has been recently validated (Anderson et al., manuscript in preparation). Plasma or urine samples stored at 80°C were thawed at 25°C and vortexed briefly. Internal standard was mixed with neat plasma or urine, and samples were equilibrated at 25°C in 96-well polypropylene microtiter filter plate wells. Binding buffer was mixed, and samples were then subjected to centrifugation 10 min at 3000 ×g at 25°C in an Eppendorf microcentrifuge. In situ hepcidin enrichment was performed by incubating plasma filtrate (5 μL) on the MALDI spot surface for 20 minutes under controlled environmental conditions (20°C, 50% relative humidity). Following incubation, plasma/urine was removed and several brief (10-second) washes (5 μL) were performed using finishing solution prior to MALDI-TOF MS analysis, performed as previously described [18].
Magnetic Resonance Imaging (MRI)
All patients had an MRI scan for liver and cardiac iron assessment prior to transplantation. All scans were done at Brigham and Women’s Hospital and read under the supervision of 1 of the study investigators (R.Y.K.). All MRI studies were performed on a 1.5-Tesla magnetic resonance system (General Electric HealthCare, Excite HDx, Piscataway, NJ) with an 8-channel phased-array receiving surface coil. MRI included both cardiac and liver imaging with dedicated pulse sequences. Vector ECG signals were used for cardiac gating for all cardiac MRI data acquisition and for patient monitoring. Cardiac imaging included cine imaging of ventricular structure and function and imaging for quantification of myocardial iron content. Cine imaging was performed using standard cine steady-state free precession fast gradient-echo technique with the following parameters (repetition time 3 milliseconds, echo time 1–1.3 milliseconds, flip angle 45–55 degrees, in-plane spatial resolution 1.5 ×2 mm at a slice thickness of 8 mm and 0 mm interslice spacing). Quantification of myocardial iron was performed using a validated fast gradient-echo technique that acquires 8 images from a range of echo times (ranging from 2 milliseconds to 30 milliseconds, echo spacing 4 milliseconds) in a midventricular short-axis location [19]. Hepatic iron was quantified using the same pulse sequence technique but without cardiac gating and acquired in a transaxial location of the liver. For liver iron imaging, this pulse sequence technique typically acquires 16 images corresponding to a range of progressive echo times from 1 millisecond to 32 milliseconds (echo spacing of 2 milliseconds). No intravenous contrast was used during MRI studies. Breath holds were performed during cardiac imaging to minimize respiratory motions. Offline analysis was performed to obtain left ventricular volumes and ejection fraction using commercial available software (QMASS®7.1, Medis, Leiden, The Netherlands) or iron quantification using validated research software (CineTool version 8.0, General Electric Healthcare). Liver iron content was estimated from the hepatic parenchyma T2* value using the following formula [20]: .
Statistics
Patient baseline characteristics were analyzed descriptively. Correlations between various parameters were assessed graphically using a hierarchical clustering tree and estimated using the Spearman correlation coefficient and Hoeffding’s D statistics. To establish a cutoff value for predictive serum ferritin, adjusted serum ferritin, and transfusion history, analysis of the receiver operating characteristic (ROC) curves was performed. Regression calibration was performed using the method of Rosner and colleagues [21,22]. This method corrects bias in estimates of relative risk of covariates obtained from regression models because of measurement error. We reanalyzed from our previous study [3] the survival of 321 patients with AML, ALL, or MDS who underwent myeloablative HSCT, assuming that ferritin was measuring LIC with measurement error. We used the present dataset as the source of external validation for the relationship between LIC and serum ferritin. We corrected the estimates of the hazard ratio for mortality of elevated ferritin in the proportional hazards model using the BLINPLUS8 macro for SAS (provided by Dr. Rosner). All calculations were done using SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
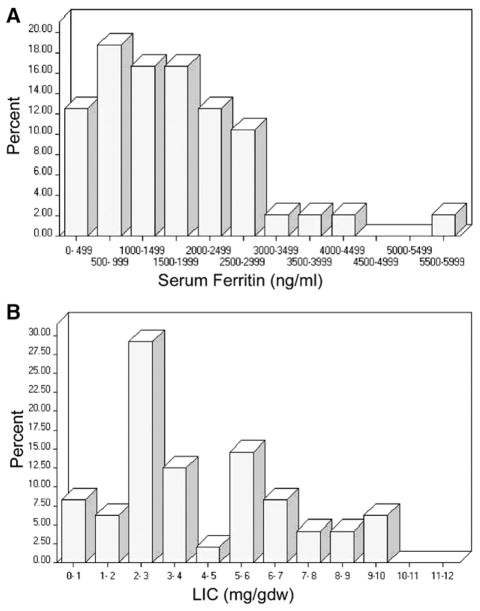
This analysis includes 48 patients who underwent myeloablative HSCT for AML, ALL, or MDS. Their demographic and clinical characteristics are summarized in Table 1. The median age was 47 years (range: 18–63). Sixty percent of patients had AML, 23% had ALL, and 17% had MDS. Most were transplanted with early stage disease, after having received a median of 2 (range: 0–6) prior lines of therapy. Patients had received a median of 20 packed red blood cells units (PRBCs) prior to their pre-HSCT MRI (range: 0–77). Table 2 details the iron-related data for this patient cohort. The median serum ferritin was 1549 ng/mL (range: 20–6989; upper limit of normal (ULN), 300 ng/mL, which changed during the course of this study at our institution to 400 ng/mL). The distribution of serum ferritin values is shown in Figure 1A. Median transferrin saturation was 35% (range: 5%–91%). H63D or C282Y mutations were assayed in all patients; 31% were heterozygous for 1 mutation, but none had a homozygous or a compound heterozygous mutation. LIC was estimated using MRI T2* measurement. Median LIC was 3.1 mg/g dry weight (mg/gdw) (range: 0.6–12.9; ULN, 1.8 mg/ gdw); 85% had hepatic iron overload (HIO), defined as LIC above the upper limit of normal. We defined significant HIO as LIC ≥5.0 mg/gdw (see Discussion). Based on this definition, 42% of patients had significant HIO. The distribution of LIC values is shown in Figure 1B. Only 1 patient had evidence of cardiac iron overload (defined as cardiac T2* <20 milliseconds); this patient was heterozygous for the C282Y mutation, had AML, a serum ferritin of 5523 ng/mL, a serum hepcidin of 139 ng/mL, an LPI of 0.0 units, and an estimated LIC of 5.6 mg/gdw. This patient was also the only one who exhibited significant left ventricular dysfunction (left ventricular ejection fraction [LVEF] 34%); none of the other patients had an LVEF below 40%.
Table 1.
Baseline Characteristics of the Patients
| Variable | No. (%)* |
|---|---|
| Number of patients | 48 |
| Age (years) | |
| Median (range) | 47 (18–63) |
| Disease | |
| MDS | 8 (17) |
| Low risk† | 3 (6) |
| High risk† | 5 (10) |
| AML | 29 (60) |
| ALL | 11 (23) |
| Stage at HSCT | |
| Early | 41 (85) |
| Untreated | 8 (17) |
| CR1 | 25 (52) |
| CR>1 | 8 (17) |
| Advanced | 7 (15) |
| Induction failure/PR | 2 (4) |
| Relapse | 5 (10) |
| Number of prior treatments | |
| Median (range) | 2 (0–6) |
| Cytogenetics | |
| Favorable | 3 (6) |
| Intermediate | 20 (42) |
| Adverse | 25 (52) |
MDS indicates myelodysplastic syndromes; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; HSCT, hematopoietic stem cell transplantation; CR, complete remission; PR, partial remission.
Percentages may not add to 100 because of rounding.
Low-risk MDS includes refractory anemia with or without ringed side-roblasts, and refractory cytopenias with multilineage dysplasia with or without ringed sideroblasts; high-risk MDS includes refractory anemia with excess blasts.
Table 2.
Iron-Related Parameters
| Variable | No. (%)* |
|---|---|
| Number of patients | 48 |
| Serum values | |
| Ferritin (ng/mL) (median, range) | 1549 (20–6989) |
| Patients with ferritin > ULN† | 42 (88%) |
| Patients with ferritin >1000 ng/mL | 33 (69%) |
| Patients with ferritin >2500 ng/mL | 11 (23%) |
| C-reactive protein (median, range)‡ | 4.3 (0.4–209) |
| C-reactive protein > ULN§ | 16 (33%) |
| Adjusted ferritin‡¶ (median, range) | 1297 (20–6989) |
| Transferrin saturationt ⊥ (median, range) | 35% (5%–91%) |
| Labile plasma iron (median, range) (in LPI units)** | 0 (0.0–0.9) |
| Low-positive labile plasma iron†† | 0 (0%) |
| Positive labile plasma iron†† | 3 (7%) |
| Serum hepcidin‡‡ (median, range) (ng/mL) | 59 (10–468) |
| Serum hepcidin > ULN§§ | 29 (74%) |
| Urine hepcidin¶¶ (median, range) (ng/mg creatinine) | 110 (5–955) |
| MRI results | |
| Estimated LIC (mg/g dry weight) ⊥⊥ (median, range) | 3.1 (0.6–12.9) |
| Patients with liver iron overload (LIC > ULN***) | 41 (85%) |
| Patients with significant liver iron overload (LIC ≥5 mg/g dry weight) | 20 (42%) |
| Patients with LIC ≥7 mg/g dry weight | 9 (19%) |
| Cardiac T2* (msec) (median, range) | 57 (13–171) |
| Patients with cardiac iron overload (T2* < LLN†††) | 1 (2%) |
| HFE genotype‡‡‡ | |
| Wild type | 33 (69%) |
| H63D heterozygous | 10 (21%) |
| C282Y heterozygous | 5 (10%) |
| Transfusion history | |
| Prior PRBCs (median, range) | 20 (0–77) |
ULN indicates upper limit of normal; LIC, liver iron content; LLN, lower limit of normal; PRBCs, packed red blood cell units.
Percentages may not add to 100 because of rounding.
ULN for serum ferritin =400 ng/mL.
C-reactive protein not available for 1 patient.
ULN for C-reactive protein =8.0 mg/L.
Ferritin adjusted by dividing by log(CRP) when CRP>10. See text for explanation.
Data missing on 2 patients.
Available on 45 patients.
Low-positive LPI is between 0.4 and 0.6 units; positive is ≥0.6 units.
Measurements were unavailable for 6 patients, and not technically obtainable for 3 patients.
Divided by simultaneously measured urine creatinine. Measurements were unavailable for 13 patients, and not technically obtainable for 2 patients.
Upper limit of normal in this assay 30 ng/mL.
Based on hepatic T2*.
ULN for liver LIC =1.8 mg/g dry weight.
LLN for cardiac T2* =20 msec.
No patients were homozygous or compound homozygous.
Figure 1.
Distribution of pretransplantation serum ferritin and liver iron content (estimated by MRI). (A) Serum ferritin (in ng/mL); (B) Liver iron content (in mg/g dry weight).
Correlations
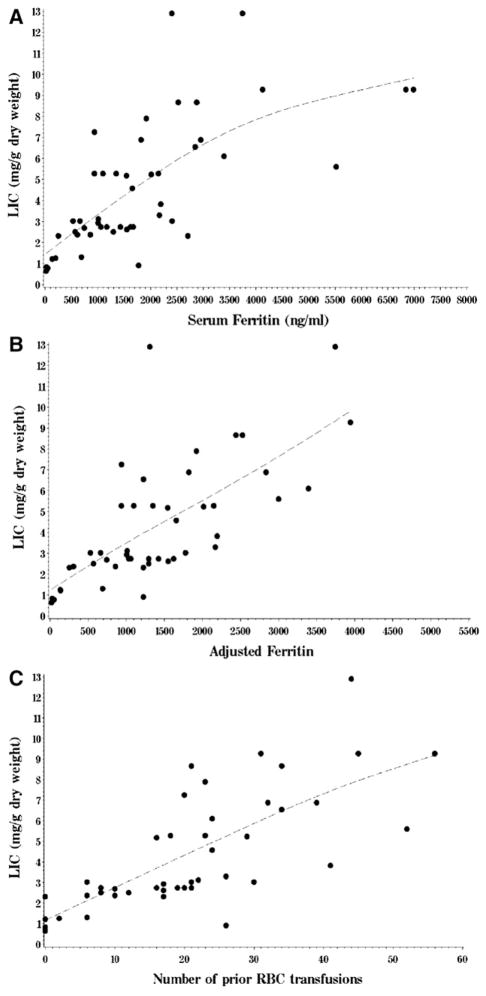
We investigated correlations between LIC and other potentially relevant baseline characteristics (Table 3). The strongest correlations were between LIC, serum ferritin and transfusion history. The Spearman correlation coefficient between LIC and serum ferritin was 0.75, and between LIC and number of prior red cell transfusions was 0.81. Ferritin itself was strongly correlated to transfusion history (r =.85). Of note, LIC had only a weak association with transferrin saturation (r=.36) and cardiac T2* (r=−.33) (cardiac T2* is inversely related to cardiac iron content; because we used a nonparametric correlation coefficient, it would not improve by using the inverse or log of cardiac T2*). LIC was moderately correlated to number of prior therapies (r =.55), presumably because this variable was itself correlated to transfusion history (r =.76). LIC was also significantly associated with disease stage, presumably because disease stage was itself associated with transfusion history (median number of red cell units transfused was 19 for patients with early stage disease versus 34 for those with advanced stage disease, P =.025). Finally, there was no significant association between LIC and HFE genotype. When transfusion history (or ferritin), number of prior chemotherapies, and transferrin saturation were entered in a multivariable regression model for LIC, only transfusion history (or ferritin) was significant (P<.0001). The model fit was better using transfusion history (R2 =.62 versus 0.49 using ferritin), presumably because ferritin is also influenced by acute phase issues. Figure 2 depicts the relationship between LIC and serum ferritin (Figure 2A), and between LIC and transfusion history (Figure 2C).
Table 3.
Association between Liver Iron Content (Estimated by MRI) and Other Baseline Characteristics
| Variable | Value*† |
|---|---|
| Correlations* | |
| Number of red cell units transfused | 0.81 |
| Serum ferritin | 0.75 |
| Adjusted serum ferritin‡ | 0.76 |
| Number of prior therapies | 0.55 |
| Serum hepcidin concentration | 0.53 |
| Transferrin saturation | 0.36 |
| LVEF | −0.34 |
| Cardiac T2* | −0.33 |
| Urine hepcidin concentration | 0.31 |
| Labile plasma iron | 0.18 |
| Age | −0.04 |
| Associations† | |
| Stage of disease (early versus advanced)§ | 3.0 vs. 6.6 (p=0.048) |
| HFE genotype | |
| C282Y heterozygosity versus wild type | 5.2 vs. 3.0 (p=0.7) |
| H63D heterozygosity versus wild type | 2.9 vs. 3.0 (p=1.0) |
LVEF, left ventricular ejection fraction.
Correlation was examined using the Spearman correlation coefficient.
Association tested using Wilcoxon rank-sum test, reported as difference in median LIC and associated P value.
Ferritin adjusted by dividing by log(CRP) when CRP >10. See text for explanation.
Early stage disease includes patients with AML in complete remission or untreated MDS, whereas advanced stage includes all other patients (see Table 1).
Figure 2.
Relationship between liver iron content, ferritin, and transfusion history. (A) Scatterplot of liver iron content (in mg/g dry weight) versus serum ferritin (in ng/mL); (B) Liver iron content versus adjusted serum ferritin (see text for details); (C) Liver iron content versus number of prior red cell units transfused. Dotted lines were fit to data using a cubic spline.
Labile Plasma Iron (LPI)
We measured pre-SCT LPI in 45 of the 48 patients, at the same time as other iron parameters. Thirty-eight out of 45 patients (84%) had no detectable LPI pre-SCT; the remaining had pre-SCT LPI between 0.1 and 0.9 LPI units, among whom 4 (9%) had low-positive LPI (0.4–0.6 units), and 3 patients (7%) had positive LPI (≥0.6 units). There was no meaningful correlation between LPI and LIC, serum ferritin, transferrin saturation, or transfusion history. Also, the patients with positive LPI were not those with the highest ferritin, LIC, or transferrin saturation. For example, none of the patients with transferrin saturation >75% had positive or low-positive LPI.
Hepcidin
We measured serum hepcidin on 39 of the 48 patients (results were technically not obtainable for 3 and specimens were missing for 6 patients). Median serum value was 59 ng/mL (range: 10–468; ULN in this assay 30 ng/mL). Serum hepcidin values were strongly correlated to serum ferritin (r=.70) and to transfusion burden (r =.65). They correlated to a lesser extent to LIC (r =.53), and weakly to C-reactive protein (r =.39). Similar but weaker correlations were obtained using serum hepcidin divided by log10(transferrin saturation), which has been proposed as a better measure of the appropriateness of the hepcidin response to iron loading (eg, r=.44 with LIC and r=.63 with ferritin). In a linear regression model using both LIC and CRP as covariates (which had no correlation with each other), both showed a significant association (P =.009 and P<.0001, respectively). We also measured urine hepcidin (divided by urine creatinine to adjust for urine concentration). Samples were available for 35 patients, of which 2 were technically not obtainable, leaving 33 usable samples. Median adjusted urine hepcidin was 110 ng/mg creatinine (range: 5–955). Urine hepcidin was moderately correlated to serum ferritin (r=.52), and poorly to LIC (r=.31). The correlation between serum and urine hepcidin was modest (r =.41).
Adjusted Serum Ferritin
Because ferritin is an acute phase reactant, we attempted to derive an ASF that would take this into account. We used CRP, another acute phase reactant, to adjust for inflammatory status (see Materials and Methods). An ASF was calculable for 47 of the 48 patients. For those patients, the ASF was slightly better correlated to LIC than the unadjusted ferritin (Spearman r=.76 versus .73). The relationship between LIC and ASF is shown in Figure 2B.
Predicting Elevated LIC
Given the costs and discomfort (mostly claustrophobia) associated with MRI scanning, it would be useful to be able to predict which patients are likely to have significant hepatic iron overload (HIO) using readily available measurements. Based on the above, the best such candidates would be serum ferritin or transfusion history. We performed an analysis of ROC curves for each of those tests, using as outcome significant HIO (LIC ≥5.0 mg/gdw). Both measurements produced similar results (see Table 4 for selected cutoff points). Combining ferritin with transfusion history did not improve the test characteristics.
Table 4.
Test Characteristics of Selected Values of Serum Ferritin and Transfusion History to Predict Significant Hepatic Iron Overload (LIC >5.0 mg/gdw)
| Test | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Serum ferritin >1000 ng/mL | 90% | 46% | 55% | 87% |
| Serum ferritin >1500 ng/mL | 80% | 68% | 64% | 83% |
| Serum ferritin >2000 ng/mL | 65% | 86% | 76% | 77% |
| Serum ferritin >2500 ng/mL | 50% | 96% | 91% | 73% |
| More than 15 red blood cell units transfused | 100% | 50% | 59% | 100% |
| More than 20 red blood cell units transfused | 75% | 71% | 65% | 80% |
| More than 25 red blood cell units transfused | 55% | 86% | 73% | 73% |
| More than 30 red blood cell units transfused | 50% | 96% | 91% | 73% |
PPV indicates positive predictive value; NPV, negative predictive value.
LIC and Posttransplantation Survival
We and others have previously reported a strong association between pre-HSCT hyperferritinemia and decreased overall survival (OS) [1–11]. Although those data argue that iron overload is detrimental in the context of transplantation, the major question is whether ferritin is an adequate surrogate for iron overload, or whether other causes of hyperferritinemia (such as acute phase reaction) may, in fact, explain the association with posttransplantation mortality. The strong correlation between serum ferritin and LIC described in the present study argues that most of the variability in ferritin is explained by variability in LIC. Because our cohort is not of sufficient power to check for a significant effect of HIO on OS, we sought to use the present data on the relationship between LIC and ferritin to reexamine our prior data on ferritin and post-HSCT outcome [3]. Specifically, we considered, within the cohort of patients who underwent myeloablative HSCT between 1997 and 2005 at our institution, and who were described in our previously published study [3], the 321 patients with MDS, AML, or ALL (who should most closely resemble the cohort in the present study, other than the time period of HSCT). Using the method proposed by Rosner et al. [21,22], we performed regression calibration on the multivariable proportional hazards model to account for the error associated with the measurement of LIC, using the present dataset as our external validation study. The uncorrected hazard ratio (HR) for mortality associated with LIC (as a continuous variable) was 1.2 (P =.00001); the corrected HR was 1.2 (P =.005). Thus, assuming that ferritin measures LIC with error, and using the present study to correct for that error, this analysis suggests that increasing pre-HSCT LIC is associated with worse OS after ablative HSCT.
DISCUSSION
In this study, we sought to better define the prevalence of iron overload in patients with AML, ALL, or MDS undergoing myeloablative HSCT, to better understand the distribution of the various forms of iron in those patients and the correlations between their measurements, to begin to investigate what mechanisms may perturb normal iron homeostasis in this population, and to leverage those results to increase our understanding of the impact of iron overload on post-HSCT survival. We used as our “gold standard” measurement of iron overload the LIC estimated from MRI T2* reading. Although this is an indirect measurement, there is a strong correlation between liver T2* values and LIC measured by liver biopsy [20,23]. It would clearly be impossible to subject patients with hematologic malignancies to the risk of liver biopsy for an observational study; furthermore, even liver biopsy is likely not an appropriate gold standard, given the significant intrapatient variability [24].
Some limitations of this work must be noted. First, we cannot rule out a selection bias in this cohort. Second, the derivation of an adjusted serum ferritin is intended as an exploratory analysis that should be validated in other cohorts. Third, we stress that the use of regression calibration is also only intended as an exploratory analysis. It is unlikely that a prospective study of iron overload that is adequately powered for an analysis of survival could be conducted using MRI, given the financial and logistic challenges associated with MRI scanning. We therefore chose the regression calibration method as the best way to leverage the present data in interpreting the many studies showing an adverse impact of hyperferritinemia. We present the results of this analysis to support, and not to prove, the hypothesis that iron overload has a detrimental effect on post-HSCT survival.
The results presented here offer several insights into the issue of iron overload in HSCT. First, they clearly demonstrate that HIO is very common in acute leukemia/MDS patients undergoing HSCT, with a prevalence of 85%. It is also clear that iron overload in this population is at least in large part because of red cell transfusion, as the number of transfused units is the single best predictor of elevated LIC in multivariable analysis. It is also apparent that patients without any identified HFE mutations and without aberrantly low hepcidin levels may have HIO with relatively few (<20) RBC transfusions. Disease type and stage, prior chemotherapy, and HFE genotype did not seem to play a significant role in iron overload in this patient population, except for the association of prior therapy with transfusion burden. It is also apparent that significant HIO is found in a large proportion of those patients (42% in our cohort). In this respect, it must be acknowledged that we do not yet know what constitutes clinically meaningful iron overload in this patient population. In patients with benign hematologic diseases, a threshold of 7 mg/gdw LIC has been used to predict clinical complications [25]. However, patients with acute leukemia or MDS undergoing ablative HSCT are at much higher risk of morbidity and mortality (especially from organ failure or infection) than patients who are chronically transfused for benign disease, and the relevant threshold may be lower. Prior studies using serum ferritin as a surrogate marker for iron overload have suggested that the ferritin threshold that best predicts increased post-HSCT mortality is somewhere between 1000 and 2500 ng/mL. In our own data [3], the optimal threshold was 2500 ng/mL, which, based on the relationship between LIC and ferritin (Figure 2A), corresponds roughly to an LIC of 5 mg/gdw. Thus, we used this value as our putative clinically relevant LIC cutoff. If the relevant ferritin cutoff is closer to 1000 ng/mL, then the relevant LIC cutoff would be lower than 5 mg/gdw, which would imply an even higher prevalence of significant HIO.
Our results further suggest that, although significant HIO is common, cardiac iron overload is very rare (2%), which is in contrast to the situation for patients with thalassemia whose principal iron-related toxicity may be cardiac. This is not entirely unexpected because the kinetics of cardiac iron loading are known to be slower than those of hepatic loading [26]. Patients with AML/ALL/MDS who come to transplantation probably accumulate iron over a much shorter time frame than patients who are chronically transfused for benign hematologic diseases. Moreover, the transfusion threshold to develop cardiac iron overload may be higher than that for HIO, and patients with thalassemia often receive many more transfusions than did the patients in our cohort. At any rate, on the basis of our results, we would not recommend cardiac MRI to screen for iron overload in patients before HSCT, unless they have unexplained cardiac dysfunction.
Another surprising result of our study is the rarity of elevated labile plasma iron, and the lack of a relationship between LPI and LIC, even for patients whose transferrin is highly saturated. On the basis of this, it would seem imprudent to use ferritin or MRI as surrogate markers for LPI, and large prospective studies measuring LPI will need to be done to determine whether this parameter influences HSCT outcomes. It may also be that the previously described increase in LPI after transplantation (in particular, from the conditioning regimen) [27] is more important than pre-HSCT LPI in mediating iron-dependent toxicity in this setting.
In this cohort, serum hepcidin was strongly correlated with serum ferritin as well as with transfusion burden, as has been previously described in healthy volunteers [28]. The good correlation between hepcidin and ferritin suggests that this component of the iron homeostasis system in these patients is relatively well preserved at least prior to HSCT; this argues against the hypothesis that such patients have hepcidin levels that are abnormally low for their degree of iron overload, which would increase their iron loading out of proportion to transfusion burden. It is interesting to note that serum hepcidin seemed to be determined both by iron burden (as reflected by LIC) and inflammatory state (as reflected by CRP), consistent with our understanding of hepcidin biology [29]. We also found that serum hepcidin and urine hepcidin were only weakly correlated, even after adjusting for urine concentration; given our result that serum hepcidin is more strongly correlated to other iron parameters (especially LIC) than urine hepcidin, it may be that serum hepcidin is the preferred measurement.
An important finding from this study is the demonstration of a strong correlation between serum ferritin and LIC. Although this may have been an expected result, and one that has been previously reported in other patient populations [30], it has not previously been demonstrated in patients undergoing HSCT for hematologic malignancies. As those patients are often battling illness or treatment complications around the time of HSCT, the value of ferritin as a marker for iron overload has often been questioned. The importance of our result is that it lends some measure of retrospective validity to the numerous studies that have used an elevated pre-HSCT serum ferritin as a surrogate marker for iron overload and demonstrated a significant impact on post-HSCT treatment-related and overall mortality [2–11,31]. This is further strengthened by our regression calibration analysis. We are also able to propose some thresholds for using serum ferritin as a screening test for significant iron overload. Given that ferritin and transfusion history are imperfect predictors of iron overload, it is probably not appropriate to use these parameters alone to select patients for trials of chelation therapy or other iron manipulations in HSCT. However, the thresholds provided here may be useful to select patients for liver MRI. In particular, it is unlikely that a patient with a serum ferritin below 1000 ng/ mL, or a patient who has received 15 or fewer red cell units, would have significant hepatic iron overload.
In conclusion, we have shown that hepatic iron overload and significant HIO, unlike cardiac iron overload or elevated LPI, are common in patients with acute leukemia or MDS undergoing ablative HSCT, primarily resulting from red cell transfusion burden. Moreover, serum ferritin may be used as a reasonable surrogate marker of LIC, and in particular, to select patients for further testing. We anticipate that longer follow-up on the present patient cohort, as well as several ongoing and planned prospective studies, will further elucidate the mechanisms of this phenomenon, the clinical significance of iron overload in this population, and the best methods to remedy it.
Acknowledgments
We thank Dr. Nancy Andrews and Dr. Andrew Powell for their advice and guidance in the design of this study. We also thank Dr. Bernard Rosner and Weiliang Qiu for providing the software and help for regression calibration.
Footnotes
Financial disclosure: This work was funded in part by grants from Novartis Oncology, the Jock and Bunny Adams Research and Education Endowment, and the National Institutes of Health, CA142106-06. P.A. is a recipient of a Special Fellowship in Clinical Research from the Leukemia and Lymphoma Society.
References
- 1.Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95:476–484. doi: 10.3324/haematol.2009.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altes A, Remacha AF, Sureda A, et al. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:987–989. doi: 10.1038/sj.bmt.1703570. [DOI] [PubMed] [Google Scholar]
- 3.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kataoka K, Nannya Y, Hangaishi A, et al. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:195–204. doi: 10.1016/j.bbmt.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Kang HJ, Kim EK, Kim H, Shin HY, Ahn HS. Effect of iron overload and iron-chelating therapy on/allogeneic hematopoietic SCT in children. Bone Marrow Transplant. 2009;44:793–797. doi: 10.1038/bmt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahindra A, Bolwell B, Sobecks R, et al. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus- host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009;146:310–316. doi: 10.1111/j.1365-2141.2009.07774.x. [DOI] [PubMed] [Google Scholar]
- 7.Platzbecker U, Bornhauser M, Germing U, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS) Biol Blood Marrow Transplant. 2008;14:1217–1225. doi: 10.1016/j.bbmt.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42:799–805. doi: 10.1038/bmt.2008.262. [DOI] [PubMed] [Google Scholar]
- 9.Storey JA, Connor RF, Lewis ZT, et al. The transplant iron score as a predictor of stem cell transplant survival. J Hematol Oncol. 2009;2:44. doi: 10.1186/1756-8722-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YR, Kim JS, Cheong JW, Song JW, Min YH. Transfusion-associated iron overload as an adverse risk factor for transplantation outcome in patients undergoing reduced-intensity stem cell transplantation for myeloid malignancies. Acta Haematol. 2008;120:182–189. doi: 10.1159/000187646. [DOI] [PubMed] [Google Scholar]
- 11.Lim ZY, Fiaccadori V, Gandhi S, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2009;34:723–727. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Morado M, Ojeda E, Garcia-Bustos J, et al. BMT: serum ferritin as risk factor for veno-occlusive disease of the liver. Prospective Cohort Study. Hematology. 2000;4:505–512. [PubMed] [Google Scholar]
- 13.Maradei SC, Maiolino A, de Azevedo AM, Colares M, Bouzas LF, Nucci M. Serum ferritin as risk factor for sinusoidal obstruction syndrome of the liver in patients undergoing hematopoietic stem cell transplantation. Blood. 2009;114:1270–1275. doi: 10.1182/blood-2009-03-212282. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Yoo KH, Sung KW, et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Bone Marrow Transplant. 2010;45:1287–1293. doi: 10.1038/bmt.2009.349. [DOI] [PubMed] [Google Scholar]
- 15.Kontoyiannis DP, Chamilos G, Lewis RE, et al. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer. 2007;110:1303–1306. doi: 10.1002/cncr.22909. [DOI] [PubMed] [Google Scholar]
- 16.Ozyilmaz E, Aydogdu M, Sucak G, et al. Risk factors for fungal pulmonary infections in hematopoietic stem cell transplantation recipients: the role of iron overload. Bone Marrow Transplant. 2010 Oct;45(10):1528–1533. doi: 10.1038/bmt.2009.383. [DOI] [PubMed] [Google Scholar]
- 17.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson DS, Heeney MM, Roth U, Menzel C, Fleming MD, Steen H. High-throughput matrix-assisted laser desorption ionization-time-of-flight mass spectrometry method for quantification of hepcidin in human urine. Anal Chem. 2010;82:1551–1555. doi: 10.1021/ac902479p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westwood M, Anderson LJ, Firmin DN, et al. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18:33–39. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- 20.Wood JC, Enriquez C, Ghugre N, et al. MRIR2 andR2*mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med. 1989;8:1051–1069. doi: 10.1002/sim.4780080905. discussion 1071–1053. [DOI] [PubMed] [Google Scholar]
- 22.Spiegelman D, McDermott A, Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr. 1997;65:1179S–1186S. doi: 10.1093/ajcn/65.4.1179S. [DOI] [PubMed] [Google Scholar]
- 23.Hankins JS, McCarville MB, Loeffler RB, et al. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853–4855. doi: 10.1182/blood-2008-12-191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emond MJ, Bronner MP, Carlson TH, Lin M, Labbe RF, Kowdley KV. Quantitative study of the variability of hepatic iron concentrations. Clin Chem. 1999;45:340–346. [PubMed] [Google Scholar]
- 25.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761. [PubMed] [Google Scholar]
- 26.Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112:2973–2978. doi: 10.1182/blood-2008-04-148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahlstedt L, von Bonsdorff L, Ebeling F, Parkkinen J, Juvonen E, Ruutu T. Non-transferrin-bound iron in haematological patients during chemotherapy and conditioning for autologous stem cell transplantation. Eur J Haematol. 2009;83:455–459. doi: 10.1111/j.1600-0609.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- 28.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 29.Zhang AS, Enns CA. Molecular mechanisms of normal iron homeostasis. Hematology Am Soc Hematol Educ Program. 2009:207–214. doi: 10.1182/asheducation-2009.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexopoulou E, Stripeli F, Baras P, et al. R2 relaxometry with MRI for the quantification of tissue iron overload in beta-thalassemic patients. J Magn Reson Imaging. 2006;23:163–170. doi: 10.1002/jmri.20489. [DOI] [PubMed] [Google Scholar]
- 31.Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Haematologica. 2010 Mar;95(3):476–484. doi: 10.3324/haematol.2009.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]