Abstract
OBJECTIVES
The major aim of this study is to test the hypothesis that stress cardiac magnetic resonance (CMR) imaging can provide robust prognostic value in women presenting with suspected ischemia, to the same extent as in men.
BACKGROUND
Compelling evidence indicates that women with coronary artery disease (CAD) experience worse outcomes than men owing to a lack of early diagnosis and management. Numerous clinical studies have shown that stress CMR detects evidence of myocardial ischemia and infarction at high accuracy. Compared to nuclear scintigraphy, CMR is free of ionizing radiation, has high spatial resolution for imaging small hearts, and overcomes breast attenuation artifacts, which are substantial advantages when imaging women for CAD.
METHODS
We performed stress CMR in 405 patients (168 women, mean age 58 ± 14 years) referred for ischemia assessment. CMR techniques included cine cardiac function, perfusion imaging during vasodilating stress, and late gadolinium enhancement imaging. All patients were followed for major adverse cardiac events (MACE).
RESULTS
At a median follow-up of 30 months, MACE occurred in 36 patients (9%) including 21 cardiac deaths and 15 acute myocardial infarctions. In women, CMR evidence of ischemia (ISCHEMIA) demonstrated strong association with MACE (unadjusted hazard ratio: 49.9, p < 0.0001). While women with ISCHEMIA(+) had an annual MACE rate of 15%, women with ISCHEMIA(−) had very low annual MACE rate (0.3%), which was not statistically different from the low annual MACE rate in men with ISCHEMIA(−) (1.1%). CMR myocardial ischemia score was the strongest multivariable predictor of MACE in this cohort, for both women and men, indicating robust cardiac prognostication regardless of sex.
CONCLUSIONS
In addition to avoiding exposure to ionizing radiation, stress CMR myocardial perfusion imaging is an effective and robust risk-stratifying tool for patients of either sex presenting with possible ischemia.
Keywords: cardiac magnetic resonance, infarction, major cardiac adverse events, mortality, myocardial ischemia, women
Despite the advent of medical therapy and improvement in patient prognosis in the past decades, women with coronary artery disease (CAD) continue to experience higher morbidity and mortality than men independent of age (1). Current noninvasive methods have reduced diagnostic accuracy in women leading to delayed CAD recognition and management, which have been associated with the observed adverse outcomes. Noninvasive detection of CAD in women is challenged by atypical symptoms, breast attenuation artifacts, small-sized heart, and age-related comorbidities that limit exercise tolerance (2,3). Cardiac magnetic resonance (CMR) provides a radiation-free assessment of cardiac function, myocardial ischemia, and infarction at high spatial resolution and tissue contrast. Recent studies have shown that stress CMR provides effective cardiac prognostication in patients with chest pain (4,5), but it is unclear whether this robust association with clinical events can be extended to a similar degree to patients of either sex. Our study aimed to test the hypothesis that stress CMR myocardial perfusion imaging for ischemia provides robust cardiac prognostication in women in a consecutive patient cohort referred for ischemia assessment.
METHODS
Patient population
We prospectively studied 424 patients (177 women) who were referred to undergo vasodilator stress CMR for assessment of myocardial ischemia. Detailed medical history was taken immediately before CMR study. A history of dyslipidemia, family history of premature CAD, chronic hypertension, and diabetes were as defined by recent guidelines (6–8). Significant tobacco use was defined as a >10 pack-years of cigarette smoking or any current use of tobacco. Patients were excluded from undergoing CMR in any of the following conditions: 1) acute myocardial infarction (MI) or unstable angina; 2) decompensated heart failure; 3) known infiltrative or hypertrophic cardiomyopathy or myocarditis; 4) hemodynamic instability; 5) absolute contraindication to vasodilator use; 6) severe claustrophobia despite use of sedatives; and 7) presence of metallic hazards. Since June 2008, patients with an estimated glomerular filtration rate of ≤30 ml/min/1.73 m2 within 30 days before CMR were also excluded. The CMR results from the initial 254 patients of the current study cohort were analyzed and reported in another publication (9). All patients provided informed consent at the time of the CMR, and our institutional ethics committee approved the study for clinical follow-up.
Vasodilating stress CMR imaging protocol
All patients were studied while in the supine position either in a 1.5-T scanner (Signa CV/i, GE Health-care, Milwaukee, Wisconsin) with an 8- or 12-element cardiac phased-array coil (n = 381) or in a 3.0-T system (Magnetom TIM TRIO, Siemens, Erlangen, Germany) (n = 43) with a 16-element phased-array coil. Patients were asked to refrain from caffeine for 24 h and maintain fasting for 4 h before CMR. CMR myocardial perfusion imaging was acquired using a saturation-prepared T1-weighted fast gradient-echo sequence (typical parameters included repetition time ≈6 ms, echo time ≈2.3 ms, field of view 34 to 38 cm, matrix size ≈160 × 128 yielding in-plane spatial resolution ≈2.1 × 2.7 mm to 2.4 × 3.0 mm, 8-mm slice thickness, parallel imaging with an acceleration factor of 2, temporal resolution ≈100 to 120 ms with 4 to 5 slices acquired every R-R interval, and time after saturation pulse of 123 ms) during a first-pass bolus of gadolinium–diethylenetriamine pentaacetic acid (Magnevist, Bayer, Wayne, New Jersey) infusion (0.075 to 0.1 mmol/kg at 4 to 5 ml/s) at peak vasodilation. A notched-saturation prepared pulse sequence with an echoplanar read out (echo train length 4) was used in the early studies. Adenosine was infused intravenously at 140 μg/kg/min over 4 to 5 min and CMR myocardial perfusion was acquired during the last minute. Adenosine (Adenocard, Astellas Pharma US, Deerfield, Illinois) was used as the stress agent in the majority of cases (n = 394, 92%), but dipyridamole (Per-santin, Boehringer Ingelheim, Ingelheim am Rhein, Germany) was used in 33 cases (8%) as requested by the referring physicians. Dipyridamole was injected at 0.56 mg/kg over 4 min with a possible addition of 0.28 mg/kg within a 3-min interval after the first injection to achieve a 10% heart rate increase from baseline. When dipyridamole was used, stress CMR myocardial perfusion imaging was acquired when the target heart rate was achieved or at 3 min after dipyridamole injection. Typically 4 to 5 short-axis locations were imaged every heartbeat for 50 to 60 heartbeats to capture the gadolinium bolus transit. Left ventricular (LV) size and function were acquired using cine steady-state free precession (typical repetition time 3.4 ms, echo time 1.2 ms, temporal resolution 40 to 50 ms, in-plane spatial resolution 1.5 to 1.8 mm and 1.8 to 2.1 mm, depending on the field of view) in a stack of parallel short-axis planes (8 mm thick without spacing) and 3 radial long-axis planes. At 10 to 20 min after injection of a cumulative 0.15 to 0.2 mmol/kg of gadolinium, we performed late gadolinium enhancement (LGE) imaging as previously described (10) in short-axis and radial long-axis locations matching cine imaging. All patients were monitored by electrocardiography (ECG), sphygmomanometry, and pulse oximetry during the CMR study, and 12-lead ECG was performed before and after CMR.
CMR image analysis
All images were analyzed using specialized software (QMass MR version 7.1, Medis Medical Imaging Systems, Leiden, the Netherlands) blinded to patient outcome. We manually traced epicardial and endocardial borders of short-axis cine locations at end-systole and end-diastole to determine the LV ejection fraction (LVEF), LV end-diastolic volume index (LVEDVi), LV end-systolic volume index (LVESVi), and LV myocardial mass (11,12). LVEF was measured by the standard Simpson rule using summation of short-axis locations. Segmental wall motion abnormality was graded as present only if it was concordant on both the short-axis and the radial long-axis views. CMR myocardial perfusion images were analyzed by a consensus of 2 experienced cardiologists with qualitative interpretation of stress CMR myocardial perfusion and LGE images (side-by-side display) blinded to clinical information, patient outcome, or cine LV function. Specifically, a stress perfusion defect was considered to be abnormal only if it fulfilled the following criteria, as reported previously by Gerber et al. (13): 1) persistence of defect beyond peak myocardial contrast enhancement; 2) defect did not exist before arrival of first-pass contrast; and 3) segmental locations that conformed to 1 or more coronary territories according to the American Heart Association/American College of Cardiology nomenclature. Peak myocardial enhancement was defined as the last frame of progressive signal enhancement during first-pass transit of gadolinium–diethylenetriamine pentaacetic acid. Following the American Heart Association/American College of Cardiology 17-segment nomenclature (14), any segmental perfusion was interpreted as normal or abnormal. Each segmental perfusion was scored based on the subendocardial extent of any perfusion defect (0, no defect; 1, ≤50%; 2, >50%). Because we were unable to assess the apical cap (segment number 17) due to the short-axis acquisition, perfusion score of this segment was treated as missing. A perfusion defect was considered significant only if it persisted beyond peak myocardial enhancement. Evidence of ischemia (ISCHEMIA) was defined by any segmental stress perfusion defect without any matching segmental LGE. Myocardial extent of ischemia (ISCH-SCORE) (maximal score of 32) was calculated by summing up the segmental perfusion score from stress myocardial perfusion imaging in segments without LGE. Any segment with a stress perfusion defect with LGE present in the matching segment was excluded from the calculation of ISCH-SCORE. Intraobserver and interobserver variability for identifying ISCHEMIA were assessed in 38 randomly selected studies. Intraob-server and interobserver kappa values for ISCHEMIA present (ISCHEMIA [+]) were 0.82 and 0.87, respectively (95% confidence interval: 0.61 to 1.0 and 0.69 to 1.0, respectively). Intraobserver and interobserver concordance correlation coefficients of ISCH-SCORE were 0.85 and 0.88, respectively. Infarct mass was quantified using a semiautomated algorithm that defined LGE as any region with a signal intensity >2 SDs above the mean signal intensity of a remote myocardial region (10).
Invasive coronary angiography
Invasive coronary angiography after CMR was performed at the discretion of the attending physician. Coronary angiography was interpreted by the consensus of 2 experienced invasive cardiologists who reported any severe epicardial coronary stenosis, defined as ≥70% narrowing of luminal diameter of any of the 3 major epicardial arteries or ≥50% narrowing of the left main coronary artery from 2 orthogonal views (15).
Patient follow-up for clinical events
Patient follow-up was performed at least 6 months after the CMR. With institutional approval, we first contacted the patients by mailed questionnaire and telephone calls. If contact was unsuccessful, we then checked our electronic medical records, called each patient’s primary care physician, and checked vital status using the Social Security Death Index (16). We defined major adverse cardiac events (MACE) during follow-up as cardiac death or new acute MI. Cardiac death refers to any death preceded by acute MI, acute or exacerbation of cardiac failure, or documented fatal arrhythmia. Any unexpected death without a noncardiac cause was also considered cardiac. New MI was defined by symptoms consistent with acute MI and elevation of peak serum troponins to twice normal or higher in a temporal pattern consistent with myocardial injury. When a patient experienced >1 event, the first event was chosen. Patients who died of noncardiac causes were censored on the day of death. Patients who underwent early coronary revascularization (either percutaneous or surgical revascularization within the first 60 days after CMR) were not censored. Rather, early coronary revascularization was coded as a binary variable and used in all univariable and multivariable analyses. CMR results were provided to the referring physicians on the day that CMR was performed.
Statistical analyses
Baseline patient characteristics stratified by sex are displayed in Table 1. In Table 1, comparisons were made using the Fisher exact test for binary variables and the student t test or Wilcoxon rank-sum test for continuous variables. Kaplan-Meier curves in either sex were generated for MACE, stratified by ISCHEMIA and compared by log-rank tests. We used the strongest set of clinical variables and their respective regression coefficients reported by Hachamovitch et al. (17) to calculate a weighted clinical risk profile score (SCOREClinical). This SCOREClinical was calculated as reported by Hachamovitch et al. (17) but modified by excluding variables of nuclear scintigraphy. The SCOREClinical was therefore calculated as follows: (patient age in decades · 5.19) + (diabetes mellitus · 3.88) + (coronary revascularization within 60 days after CMR · 4.51) + (dyspnea as a presenting symptom · 5.47) + (resting heart rate per 10 beats · 2.88) − (peak heart rate per 10 beats · 1.42) + (ECG score · 1.95), whereas ECG score was derived as follows: (any heart block · 0.628) + (LV hypertrophy voltage with repolarization abnormality · 0.724) + (presence of premature ventricular beat · 0.832) + (nonspecific ST-T abnormality · 0.331).
Table 1.
Demographic Characteristics of the Study Cohort Stratified by Sex
| Clinical Variables | All Patients (N = 405) | Women (n = 168) | Men (n = 237) | p Value |
|---|---|---|---|---|
| Age, yrs | 57 ± 14 | 58 ± 14 | 57 ± 14 | 0.56 |
| Body mass index, kg/m2 | 28 ± 6 | 28 ± 7 | 28 ± 5 | 0.86 |
| Hypertension | 226 (56) | 103 (61) | 123 (52) | 0.07 |
| Diabetes | 87 (22) | 31 (19) | 56 (24) | 0.22 |
| Hyperlipidemia | 228 (57) | 83 (50) | 145 (62) | <0.05 |
| Tobacco use | 60 (15) | 19 (11) | 41 (17) | 0.12 |
| Family Hx of CAD | 104 (26) | 48 (29) | 56 (24) | 0.35 |
| SCOREClinical | 39 ± 9 | 39 ± 10 | 40 ± 9 | 0.77 |
| Hx of MI | 82 (20) | 24 (14) | 58 (25) | <0.05 |
| Hx of PCI | 66 (16) | 13 (8) | 53 (22) | <0.0001 |
| Hx of CABG | 32 (8) | 9 (5) | 23 (10) | 0.14 |
| Early coronary revascularization | 43 (11) | 11 (6.6) | 32 (14) | <0.05 |
| Medications | ||||
| Aspirin | 219 (55) | 81 (49) | 138 (60) | <0.05 |
| Beta-blocker | 214 (53) | 92 (55) | 122 (52) | 0.54 |
| ACE inhibitor | 166 (41) | 60 (36) | 106 (45) | 0.08 |
| Calcium channel blocker | 68 (17) | 35 (21) | 33 (14) | 0.08 |
| Statins | 221 (55) | 82 (50) | 139 (59) | 0.07 |
| Electrocardiography | ||||
| Resting heart rate, beats/min | 69 ± 14 | 71 ± 15 | 68 ± 14 | <0.01 |
| Sinus rhythm | 374 (94) | 155 (93) | 219 (94) | 0.54 |
| QRS >120 ms | 33 (8) | 11 (7) | 22 (10) | 0.36 |
| QTc >400 ms | 9 (2.3) | 6 (3.6) | 3 (1.3) | 0.17 |
| Left bundle branch block | 21(5.3) | 11 (6.6) | 10 (4.3) | 0.37 |
| Right bundle branch block | 20(5.0) | 6 (3.6) | 14 (6.0) | 0.35 |
| LV hypertrophy | 32 (8.0) | 13 (7.7) | 19 (8.2) | 0.99 |
| Significant Q waves | 56 (14) | 14 (8.3) | 42 (18) | <0.01 |
| Resting ST changes | 51 (12.9) | 20 (12.1) | 31 (13.5) | 0.76 |
| Resting T-wave inversions | 81 (20.5) | 27 (16.4) | 54 (23.5) | 0.1 |
| CMR variables | ||||
| LVEF, % | 57 ± 13 | 60 ± 12 | 56 ± 13 | <0.001 |
| LV mass index, g/m2 | 62 ± 17 | 56 ± 15 | 66 ± 18 | <0.0001 |
| LVEDVi, ml/m2 | 86 ± 26 | 79 ± 21 | 92 ± 27 | <0.0001 |
| LVESVi, ml/m2 | 39 ± 24 | 33 ± 19 | 43 ± 27 | <0.0001 |
| Resting RWMA | 101 (25.1) | 25 (15) | 76 (32.5) | <0.0001 |
| LGE presence | 119 (30) | 22 (13) | 97 (41) | <0.0001 |
| LGE mass, per gram of tissue | 4.4 ± 11 | 1.8 ± 7 | 6.2 ± 13 | <0.0001 |
| % of LGE, per 10% of LV mass | 3.3 ± 8.3 | 1.8 ± 6.3 | 4.4 ± 9.3 | <0.0001 |
| Rest perfusion defect | 53 (13) | 16 (10) | 37 (16) | 0.09 |
| Stress perfusion defect | 126 (31) | 39 (23) | 87 (37) | <0.005 |
| ISCHEMIA(+) | 109 (27) | 36 (21) | 73 (31) | <0.05 |
| ISCH-SCORE | 1.3 ± 3.0 | 0.9 ± 2.2 | 1.6 ± 3.5 | <0.05 |
| ISCH-SCORE, ISCHEMIA(+) | 5.0 ± 4.0 | 4.3 ± 2.9 | 5.3 ± 4.4 | 0.15 |
Values shown are mean ± SD or n (%).
ACE = angiotensin-converting enzyme; CABG = coronary artery bypass graft; CAD = coronary artery disease; CMR = cardiac magnetic resonance; Hx = history; ISCHEMIA(+) = evidence of ischemia present; ISCH-SCORE = myocardial extent of ischemia; LGE = late gadolinium enhancement; LV = left ventricular; LVEDVi = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESVi = left ventricular end-systolic volume index; MI = myocardial infarction; RWMA = regional wall motion abnormality; PCI = percutaneous coronary intervention; SCOREClinical = clinical risk profile score.
We used Cox regression in univariable association of clinical, ECG, and CMR variables with MACE. Considering all variables in Table 1, we sought the best overall multivariable models for MACE, separately in women and in men, by stepwise forward selection using p < 0.01 for model entry or stay. We also specifically tested for any effect modification by sex to the prognostic association of ISCHEMIA with MACE by adding both sex and an interaction term of sex and ISCHEMIA into a Cox model that associated ISCHEMIA with MACE. In building any of the multivariable models, care was taken to follow the “10 MACE for every variable” rule as much as possible to avoid overfitting of any of the multivariable models. The proportional hazard assumption was tested valid in each multivariable model by adding a time-dependent interaction variable for every variable included in each best overall model. Because SCOREClinical contained data collected during the study follow-up period, it was treated as a time-varying covariate in any multivariable model. We therefore created a binary variable by dichotomizing SCOREClinical at its median value from the whole study cohort (median value = 39.7). For any multivariable model that included SCOREClinical, we repeated the model with stratification to the binary variable (obtained from dichotomizing SCOREClinical). We determined the diagnostic utility of ISCHEMIA in detecting coronary stenosis in patients who were referred to undergo coronary angiography within 2 years after CMR. All statistical analyses were performed with SAS version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
Patient characteristics
We enrolled 424 consecutive subjects referred to have vasodilator stress CMR for assessment of ischemia. Nineteen enrolled patients (4%, 8 of whom were women) were excluded from study analysis due to the following reasons: scanner failure (n = 2), patient claustrophobia (n = 8), patient intolerance of pharmacological stress (n = 7), and nondiagnostic image quality (n = 2). Of these 19 excluded patients, 2 patients (11%) experienced cardiac death. The remaining 405 patients (96%) constituted the study cohort. Table 1 provides the demographic characteristics of the study cohort stratified by sex. Compared with men, women had a lower prevalence of CAD based on fewer histories of MI and coronary interventions, fewer electrocardiographic Q waves, higher LVEF, lower LVEDVi and LVESVi, less wall motion abnormality, and fewer previous MIs by LGE.
Univariable analyses for MACE
Clinical follow-up by either mailed questionnaire or telephone contact was successful in all patients (median, 30 months; range, 6 months to 6.9 years). No patient was followed based only on Social Security Death Index. During study follow-up, 36 patients (9%) experienced MACE (21 cardiac deaths and 15 acute MIs). Among them, 14 were women (7 cardiac deaths and 7 acute MIs) and 22 were men (14 cardiac deaths and 8 acute MIs). Another 19 patients (8 women, 11 men) died of noncardiac causes and were censored on the day of death. ISCHEMIA(+) was observed in 36 women (21%) and in 73 men (31%). Univariable associations of clinical, electrocardiographic, and CMR variables with MACE for the entire study cohort and stratified by sex are presented in Table 2. Patient age, history of MI, early coronary revascularization after CMR, SCOREClinical, and resting ST changes on an electrocardiogram were the strongest clinical predictors of MACE in the entire study cohort and in either sex. Although LV end-systolic and end-diastolic indexes, regional wall motion abnormality, LGE presence, and abnormal CMR stress perfusion by CMR all demonstrated strong univariable association with MACE, ISCH-SCORE was the strongest predictor by univariable analyses in the entire cohort and in patients of either sex (likelihood ratio [LR] chi-square test 62.12, 37.15, and 25.95; all p values < 0.0001, in whole cohort, women, and men, respectively). On average, the hazard for MACE increased by 43% and 16% for each unadjusted unit increase in ISCH-SCORE in women and in men, respectively. ISCH-SCORE was right skewed, and we performed a log-transformation of ISCH-SCORE. The resultant log-transformed ISCH-SCORE maintained robust prognostic association with MACE (LR chi-square test = 63.84, p < 0.0001). ISCHEMIA(+) portended a 17-, 50-, and 11-fold unadjusted hazard increase in MACE in the entire cohort, in women, and in men, respectively (LR chi-square test 34.58, 14.18, and 14.98; all p values < 0.0001, respectively). Among the 36 women who had ISCHEMIA(+), ISCH-SCORE was significantly higher in those who experienced MACE (n = 13) than those who did not (n = 23) (median ISCH-SCORE 5 vs. 3, p = 0.04 by nonparametric Wilcoxon rank-sum test). LGE presence was a strong predictor of MACE, but its association with MACE appeared to be stronger in men than in women (LR chi-square test = 6.63 vs. 3.88, p = 0.01 vs. p < 0.05, respectively).
Table 2.
Univariable Prognostic Association With MACE Stratified by Sex
| Clinical Characteristics | All Patients (N = 405)
|
Women (n = 168)
|
Men (n = 237)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| LR Chi-Square Test | HR (95% CI) | p Value | LR Chi-Square Test | HR (95% CI) | p Value | LR Chi-Square Test | HR (95% CI) | p Value | |
| Age, yrs | 17.31 | 1.07 (1.03–1.1) | <0.0001 | 7.95 | 1.07 (1.02–1.11) | <0.005 | 9.16 | 1.06 (1.02–1.11) | <0.005 |
| Female | 0.01 | 0.98 (0.49–1.93) | 0.95 | * | * | * | * | * | * |
| Hypertension | 12.13 | 5.4 (2.09–13.96) | 0.0005 | 2.09 | 2.56 (0.71–9.19) | 0.15 | 9.96 | 10.11 (2.35–43.51) | <0.005 |
| Diabetes | 4.23 | 2.05 (1.04–4.08) | <0.05 | 0.95 | 1.78 (0.56–5.63) | 0.33 | 4.43 | 3.21 (0.93–5.32) | 0.07 |
| Hyperlipidemia | 3.38 | 2.04 (0.95–4.4) | 0.07 | 1.18 | 1.84 (0.62–5.48) | 0.28 | 2.36 | 2.35 (0.79–6.99) | 0.12 |
| Hx of MI | 13.94 | 3.56 (1.82–6.93) | <0.0005 | 13.18 | 6.98 (2.45–19.91) | <0.0005 | 4.05 | 2.44 (1.02–5.8) | <0.05 |
| Hx of PCI | 17.91 | 4.21 (2.16–8.18) | <0.0001 | 3.34 | 3.29 (0.92–11.79) | 0.07 | 14.59 | 5.56 (2.31–13.42) | <0.0005 |
| Hx of CABG | 1.02 | 1.63 (0.63–4.21) | 0.32 | 0.02 | 1.16 (0.15–8.89) | 0.89 | 1.23 | 1.85 (0.62–5.17) | 0.27 |
| Early revascularization | 19.05 | 4.74 (2.36–9.56) | <0.0001 | 10.46 | 6.79 (2.13–21.66) | <0.005 | 11.23 | 4.54 (1.87–11.01) | <0.001 |
| SCOREClinical | 26.51 | 1.11 (1.07–1.15) | <0.0001 | 10.63 | 1.10 (1.04–1.16) | <0.005 | 15.69 | 1.12 (1.06–1.19) | <0.0001 |
| Electrocardiographic findings | |||||||||
| Left bundle branch block | 6.31 | 3.37 (1.31–8.73) | 0.01 | 0.06 | 1.28 (0.17–9.8) | 0.81 | 10.38 | 6.12 (2.03–18.44) | <0.005 |
| Significant Q waves | 6.93 | 2.69 (1.29–5.61) | <0.05 | 7.31 | 4.95 (1.55–15.8) | <0.01 | 2.2 | 2.05 (0.79–5.31) | 0.14 |
| Resting ST changes | 22.66 | 5.19 (2.63–10.22) | <0.0001 | 7.52 | 4.62 (1.57–13.79) | <0.01 | 14.41 | 5.38 (2.56–12.83) | 0.0001 |
| CMR results | |||||||||
| LVEF, per 10% | 22.58 | 0.95 (0.93–0.97) | <0.0001 | 3.29 | 0.97 (0.9–1.01) | 0.07 | 21.41 | 0.94 (0.91–0.96) | <0.0001 |
| LV mass index, g/m2 | 12.72 | 1.03 (1.01–1.04) | <0.0005 | 2.67 | 1.02 (0.99–1.05) | 0.11 | 10.92 | 1.03 (1.01–1.05) | <0.0005 |
| LVEDVi, per 10 ml/m2 | 20.84 | 1.02 (1.01–1.03) | <0.0001 | 4.35 | 1.03 (1.01–1.06) | <0.05 | 16.39 | 1.02 (1.01–1.03) | <0.0001 |
| LVESVi, per 10 ml/m2 | 28.25 | 1.02 (1.01–1.02) | <0.0001 | 6.47 | 1.03 (1.01–1.05) | 0.01 | 21.56 | 1.02 (1.01–1.03) | <0.0001 |
| Resting RWMA | 28.31 | 7.06 (3.44–14.5) | <0.0001 | 13.26 | 7.61 (2.55–22.67) | <0.0005 | 16.06 | 7.94 (2.89–21.86) | <0.0001 |
| LGE presence | 10.29 | 3.08 (1.55–6.11) | 0.001 | 3.88 | 3.34 (1.01–11.1) | <0.05 | 6.63 | 3.31 (1.33–8.24) | 0.01 |
| LGE mass, per gram of tissue | 4.73 | 1.02 (1.01–1.04) | <0.05 | 0.9 | 1.03(0.97–1.08) | 0.34 | 3.46 | 1.02 (0.99–1.04) | 0.06 |
| LGE presence without Hx of MI | 9.62 | 4.3 (1.7–10.79) | <0.005 | 0.88 | 2.76 (0.33–22.97) | 0.34 | 8.41 | 5.74 (1.76–18.69) | <0.005 |
| ISCHEMIA(+) | 34.58 | 17.16 (6.65–44.25) | <0.0001 | 14.18 | 49.9 (6.52–381.67) | <0.0001 | 14.98 | 11.19 (3.29–38.03) | <0.0001 |
| ISCH-SCORE | 62.12 | 1.19 (1.14–1.24) | <0.0001 | 37.15 | 1.36 (1.23–1.5) | <0.0001 | 25.95 | 1.14 (1.1–1.2) | <0.0001 |
Could not be determined due to low event.
CI = confidence interval; HR = hazard ratio; LR = likelihood ratio; MACE = major adverse cardiac events; other abbreviations as in Table 1.
Survival by Kaplan-Meier and event rates analysis
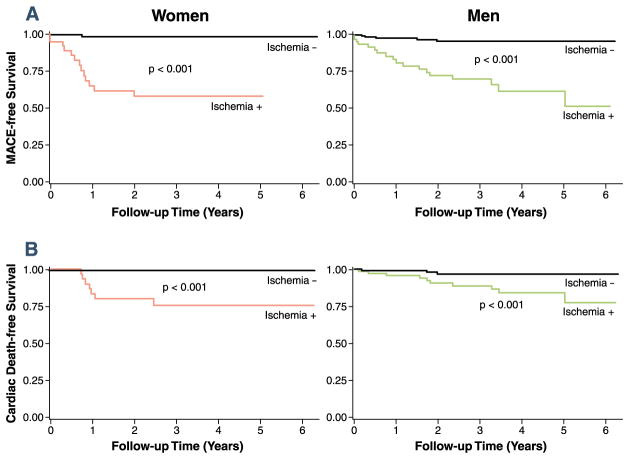
Figure 1 shows the Kaplan-Meier curves stratified by ISCHEMIA(+) in each sex. Figure 1 demonstrated that ISCHEMIA(+) was associated with reduced MACE-free survival and cardiac survival for women and for men (all p values < 0.001). Figure 2 shows the annual crude event rates of MACE and cardiac death stratified by ISCHEMIA in either sex. Annual event rates of MACE and cardiac death were significantly higher in patients with ISCHEMIA(+) in either sex. ISCHEMIA(−) was associated with very low annual MACE in women (0.3%) and no women with ISCHEMIA(−) experienced cardiac death during follow-up (0%). On the contrary, ISCHEMIA(+) in women was associated with high annual rates of MACE and cardiac death (15.1% and 8.2%, respectively). These annual MACE and cardiac death rates in women were not different from the annual event rates in the groups of men stratified by ISCHEMIA. Figure 3 demonstrates a stress CMR case in a female patient.
Figure 1. Kaplan-Meier Curves for MACE and Cardiac Death.
Kaplan-Meier curves for major adverse cardiac events (MACE) (A) and cardiac death (B) stratified by evidence of ischemia in each sex.
Figure 2. Patient Annual Event Rates of MACE and of Cardiac Death.
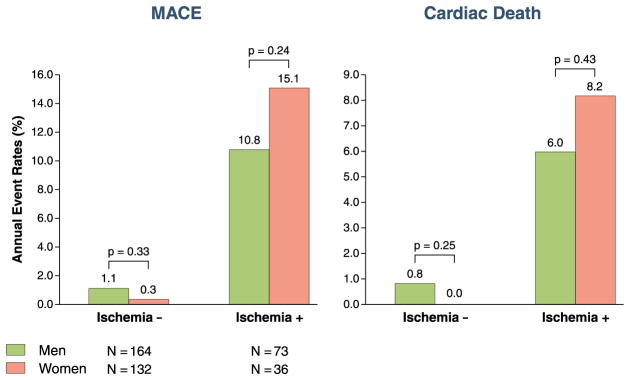
Patient annual event rates of major adverse cardiac events (MACE) and cardiac death, stratified by sex and evidence of ischemia.
Ischemia − = evidence of ischemia absent; ischemia + = evidence of ischemia present.
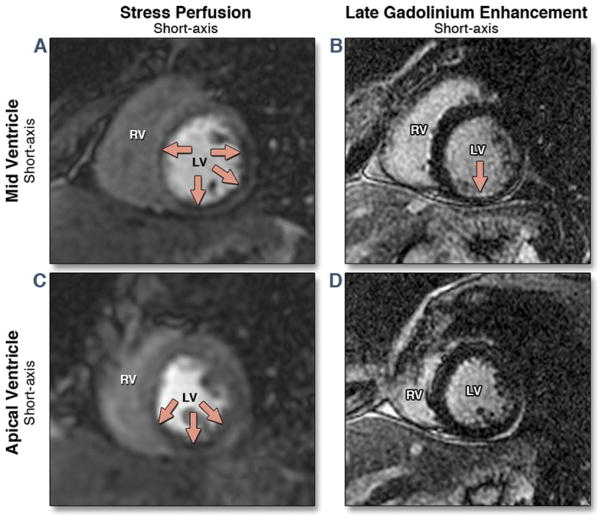
Figure 3. Stress CMR Study From a 47-Year-Old Woman With a Previous MI Referred for Assessment of Myocardial Ischemia.
Mid and apical short-axis views of the stress perfusion images show an extensive perfusion defect within the mid and apical inferior, inferoseptal, and inferolateral walls (red arrows) (A,C). Matching late gadolinium enhancement (B,D) demonstrates a small subendocardial myocardial infarction (MI) within the mid-inferior wall. CMR = cardiac magnetic resonance; LV = left ventricle; RV = right ventricle.
Multivariable analyses for MACE
When sex, ISCHEMIA, and an interaction term that described any effect modification of ISCHEMIA by sex were entered into a multivariable model for MACE, the interaction term did not demonstrate any prognostic significance, suggesting against any evidence that patient sex modified the strong association of ISCHEMIA with MACE.
The best overall multivariable models for MACE are summarized in Table 3. For the entire study cohort, the strongest multivariable predictors of MACE included ISCH-SCORE, ISCHEMIA, and SCOREClinical. ISCH-SCORE was consistently the strongest multivariable predictor for MACE in each of the best overall multivariable models for MACE in all patients, women, and men, respectively (Table 3, model LR chi-square test = 68.67, 37.71, and 30.81, all p < 0.0001, respectively). Along with early revascularization, ISCH-SCORE formed the best overall model for MACE in women (LR chi-square test = 12.14 and 32.52, p = 0.0005 and p < 0.0001, respectively). Along with SCOREClinical, ISCH-SCORE formed the best overall model for MACE in men (LR chi-square test = 14.95 and 17.52, p = 0.0001 and p < 0.0001, respectively). Additional analyses were performed to assess the time-varying effects of SCOREClinical being selected in the best overall multivariable models for MACE of the whole cohort and of men (Table 3), respectively. Although stratified to the binary variable obtained from dichotomizing SCOREClinical at its median value, the best overall multivariable models of the whole cohort and of men both maintained strong association with MACE, respectively (model LR chi-square test = 56.72 and 23.59, both p < 0.0001, respectively).
Table 3.
Best Overall Multivariable Models by Stepwise Selection in All Patients, Women, and Men
| Variables | Adjusted LR Chi-Square of the Variable | HR (95% CI), p Value of the Variable | Model LR Chi-Square,p Value |
|---|---|---|---|
| All patients (N = 405) | |||
| ISCH-SCORE | 7.13 | 1.11 (1.03–1.19), p = 0.008 | 68.67, p < 0.0001 |
| ISCHEMIA | 10.61 | 6.18 (2.07–18.51), p = 0.001 | |
| SCOREClinical | 10.84 | 1.08 (1.03–1.12), p = 0.001 | |
| Women (n = 168) | |||
| ISCH-SCORE | 32.52 | 1.49 (1.31–1.69), p = 0.0001 | 37.71, p < 0.0001 |
| Early revascularization | 12.14 | 9.09 (2.63–31.45), p = 0.0005 | |
| Men (n = 237) | |||
| ISCH-SCORE | 17.52 | 1.17 (1.09–1.25), p = 0.0001 | 30.81, p < 0.0001 |
| SCOREClinical | 14.95 | 1.11 (1.05–1.17), p = 0.0001 | |
ISCHEMIA = evidence of ischemia; other abbreviations as in Table 1.
In the 323 patients (80%) without a history of MI, ISCHEMIA(+) was observed in 60 patients (19%) and only ISCHEMIA was selected to form the best overall model for MACE. However, LGE presence, LGE mass, and percentage of LGE were also strong univariable predictors of MACE (LR chi-square test = 9.58, 7.14, and 8.17; hazard ratio [HR]: 4.28, 1.05, and 1.72; p < 0.005, p < 0.01, and p < 0.005, respectively). These strong associations of LGE variables with MACE appeared to complement ISCHEMIA in patients without a history of MI. Adjusted to ISCHEMIA, infarct size measured by percentage of LGE (per 10% increase of LV mass) portended to an adjusted hazard increase in MACE of 67% (adjusted LR chi-square test = 5.46, adjusted HR: 1.67; 95% confidence interval: 1.09–2.57, p = 0.02). In women without a history of MI, ISCH-SCORE and percentage of LGE were the strongest variables selected to form the best overall model for MACE. Adjusting for known CAD variables (number of angiographic significant coronary arteries known before CMR, history of percutaneous coronary intervention, and history of MI), ISCHEMIA and ISCH-SCORE both maintained a strong association with MACE (adjusted HR: 2.52 and 1.18; adjusted LR chi-square test = 24.81 and 31.71; both p < 0.0001, respectively). In addition, in 285 patients without any history of CAD (defined as any history of coronary intervention, previous MI, and significant Q waves on ECG), both ISCHEMIA and ISCH-SCORE were significant predictors of MACE during the follow-up (HR: 17.60 and 1.32; LR chi-square test = 18.34 and 26.46; both p < 0.0001, respectively).
Diagnosis of significant coronary stenosis by ISCHEMIA
At the discretion of the referring physician, 77 patients (19%) underwent coronary angiography within 24 months after CMR; of them, 48 patients (61%, 12 were women) demonstrated significant stenosis. Table 4 shows the patient numbers in groups stratified by ISCHEMIA, presence of significant stenosis, and single- or multiple-vessel stenosis. ISCHEMIA detected significant stenosis at sensitivities of 88%, 92%, and 86% for both sexes combined, women, and men, respectively. Although all cases of multiple-vessel stenosis were detected by ISCHEMIA (100% sensitivity), single-vessel stenosis was detected at sensitivities of 80%, 89%, and 76% for both sexes combined, women, and men, respectively. ISCHEMIA detected significant stenosis at specificities of 76%, 80%, and 74% in these groups, respectively.
Table 4.
Sensitivity and Specificity of ISCHEMIA to Detect Significant (≥70%) Angiographic Coronary Stenosis in Both Sexes, Women, and Men
| CA (−) | CA (+) | Single-Vessel CAD | Multiple-Vessel CAD | |
|---|---|---|---|---|
| Both sexes (N = 77) | ||||
| ISCHEMIA(−) | 22 | 6 | 6 | 0 |
| ISCHEMIA(+) | 7 | 42 | 24 | 18 |
| Correct, % | 76 | 88 | 80 | 100 |
| Women (n = 22) | ||||
| ISCHEMIA(−) | 8 | 1 | 1 | 0 |
| ISCHEMIA(+) | 2 | 11 | 8 | 3 |
| Correct, % | 80 | 92 | 89 | 100 |
| Men (n = 55) | ||||
| ISCHEMIA(−) | 14 | 5 | 5 | 0 |
| ISCHEMIA(+) | 5 | 31 | 16 | 15 |
| Correct, % | 74 | 86 | 76 | 100 |
CA (−) = coronary angiography without significant stenosis; CA (+) = coronary angiography with significant stenosis; CAD = coronary artery disease; ISCHEMIA(−) = evidence of ischemia absent; ISCHEMIA(+) = evidence of ischemia present.
DISCUSSION
Accurate noninvasive risk stratification may be the first of many steps that can improve the outlook of women with CAD. Stress CMR may be ideally suited for this purpose. We examined the prognostic implications of CMR stress myocardial perfusion imaging in a large single-center consecutive cohort. We observed a strong association of ISCHEMIA(+) detected by CMR with hard cardiac events (cardiac death or acute MI) regardless of patient sex. CMR is thus a highly promising alternative noninvasive prognosticating tool that overcomes the limitations of other techniques in imaging women with suspected myocardial ischemia. Specifically, ISCHEMIA(−) indicated very low annual rates of MACE and of cardiac death in women (0.3% and 0%, respectively), which were similar to the observed low annual rates in men. In contrast, ISCHEMIA(+) was associated with a markedly elevated HR of 56 for MACE in women. In addition, we found that the segmental extent of myocardial ischemia, quantified by ISCH-SCORE, was the strongest multivariable predictor for MACE in the whole cohort, in women, and in men. These findings support consistent and robust prognostication by CMR myocardial perfusion imaging regardless of patient sex.
Several factors challenge both noninvasive CAD diagnosis and cardiac prognostication by noninvasive imaging in women. Compared with men, women more often present with atypical symptoms, have a higher burden of microvascular dysfunction, are less likely to perform an adequate exercise test due to comorbidities, and are more difficult to image due to smaller heart size and breast attenuation. It has also been reported that varying estrogen levels may cause digitalis-like false positivity on stress ECG testing (18). These factors, either alone or in combination, may make exercise ECG, stress nuclear scintigraphy, or echocardiography less accurate in assessing women with chest pain. These reasons and possible underuse of stress imaging in women may have resulted in underdetection of CAD in women and delivery of treatment only at a later stage of disease (19–21). CMR is appealing to image women at risk because it provides noninvasive characterization of myocardial ischemia and infarction at high spatial resolution, is relatively free of attenuation artifacts, and does not use ionizing radiation. The results of the current study extend from a previous study showing high accuracy of detecting CAD in women by CMR. Klem et al. (22) reported a diagnostic accuracy of 87% by stress CMR myocardial perfusion imaging in 147 women who underwent coronary angiography.
Prognostic evidence using CMR myocardial perfusion imaging in CAD is growing and promising, with several recent single-center studies all reporting excellent negative event-free survival rates by CMR at up to 3 years of follow-up (4,5,23). However, these studies did not specifically assess the prognostic value of CMR myocardial perfusion imaging in women. A relatively small number of studies focus on sex differences in prognosis based on findings of stress imaging modalities. Our results are in accordance with other reports using nuclear imaging, stress echocardiography, and exercise treadmill testing. In a study of 6,173 consecutive patients, Berman et al. (24) found an incremental prognostic value of adenosine myocardial perfusion single-photon emission computed tomography results over baseline data for the prediction of cardiac death in both sexes. America et al. (25) studied a homogeneous cohort of 453 consecutive female patients using quantitative gated technetium-99m tetrofosmin single-photon emission computed tomography. In that study, patients with a summed stress score ≥14 were at risk of any cardiac event, including death, MI, and need for revascularization. Cortigiani et al. (26) reported additive prognostic information from stress echocardiography over clinical findings and resting wall motion score index in 8,737 patients in men and women with known and suspected CAD. Alexander et al. (27) studied 976 women and 2,249 men undergoing exercise treadmill testing and cardiac catheterization. The Duke Treadmill Score provided information beyond clinical predictors of CAD and survival and performed equally well in stratifying both sexes in prognostic categories.
The consecutive cohort of women in our study has a risk profile comparable to that of large population cohorts (8% MACE during study follow-up) (19,28). The results of our study suggest that the prognostic value of stress CMR for MACE including cardiac death is excellent regardless of patient sex. Given the limitations often observed with stress ECG, nuclear scintigraphy, and stress echocardiography, the evidence put forth by the current study indicates that stress CMR myocardial perfusion imaging may be an appropriate method for studying female patients with possible ischemia.
Study limitations
This study has several limitations. Most important of all is the limited study power from a small number of MACE from a relatively small single-center study. However, the finding that ISCH-SCORE was one of the strongest multivariable predictors consistent in each of the 3 best overall models supports that its prognostic robustness is independent of patient sex. Given the small number of MACE, the current experience cannot determine the prognostic association of ISCH-SCORE adjusted to many of the known prognostic markers in this clinical setting.
Our results are based on observation of a consecutive clinical cohort of 405 patients. We could not plan an equivalence test because the magnitude of the difference in observed event rates between men and women was unknown. As part of the clinical workup of this patient group, only a subset of patients was referred for invasive angiography, and therefore the prevalence of significant coronary stenosis could not be determined. The lack angiographic data in all patients in the cohort prohibits accurate determination of the negative and positive predictive values of CMR myocardial perfusion imaging in the detection of significant angiographic stenosis. This limitation was patient care related and reflects common clinical practice. Because the main goal of the current study was the evaluation of the prognostic value of CMR myocardial perfusion imaging in women and men, not detection of significant CAD, we believe that these limitations do not nullify the observed prognostic significance. In 15% of our patients, stress CMR was ordered due to inconclusive findings on another noninvasive test or presence of intermediate coronary stenosis on angiography performed within 30 days before the CMR study. The current pattern of CMR use may result in a higher pretest likelihood of CAD than if CMR had been chosen as the first-line noninvasive test in all patients. Nevertheless, the high negative event rates seen in the whole cohort and in each sex attest to the high prognostic value of stress CMR. Whether information from CMR can alter the currently adverse outlook of women with CAD will need to be addressed in a future study.
CONCLUSIONS
CMR myocardial perfusion imaging provides robust prognostication for cardiac events in both women and men. A CMR study without evidence of myocardial ischemia indicates a very low risk of MACE and cardiac death in women. The prognostic association of CMR myocardial perfusion imaging with MACE appeared to be as strong in women as in men, and CMR offers the advantage of freedom from using ionizing radiation and high image resolution.
Acknowledgments
Dr. Kwong is in part supported by the National Institutes of Health R01HL091157.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance
- ECG
electrocardiography
- ISCHEMIA
evidence of ischemia
- ISCHEMIA(+)
evidence of ischemia present
- ISCHEMIA(−)
evidence of ischemia absent
- ISCH-SCORE
myocardial extent of ischemia
- LGE
late gadolinium enhancement
- LR
likelihood ratio
- LV
left ventricular
- LVEDVi
left ventricular end-diastolic volume index
- LVEF
left ventricular ejection fraction
- LVESVi
left ventricular end-systolic volume index
- MACE
major adverse cardiac event(s)
- MI
myocardial infarction
- SCOREClinical
clinical risk profile score
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Stramba-Badiale M, Fox KM, Priori SG, et al. Cardiovascular diseases in women: a statement from the policy conference of the European Society of Cardiology. Eur Heart J. 2006;27:994–1005. doi: 10.1093/eurheartj/ehi819. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. Heart Disease and Stroke Statistics—2005 Update. Dallas, Texas: 2005. [Google Scholar]
- 3.Benjamin EJ, Smith SC, Jr, Cooper RS, Hill MN, Luepker RV. Task force #1—magnitude of the prevention problem: opportunities and challenges. 33rd Bethesda Conference. J Am Coll Cardiol. 2002;40:588–603. doi: 10.1016/s0735-1097(02)02082-x. [DOI] [PubMed] [Google Scholar]
- 4.Ingkanisorn WP, Kwong RY, Bohme NS, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427–32. doi: 10.1016/j.jacc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–76. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 9.Steel K, Broderick R, Gandla V, et al. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120:1390–400. doi: 10.1161/CIRCULATIONAHA.108.812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 11.Salton CJ, Chuang ML, O’Donnell CJ, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39:1055–60. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 12.Alfakih K, Reid S, Jones T, Sivananthan M. Assessment of ventricular function and mass by cardiac magnetic resonance imaging. Eur Radiol. 2004;14:1813–22. doi: 10.1007/s00330-004-2387-0. [DOI] [PubMed] [Google Scholar]
- 13.Gerber BL, Raman SV, Nayak K, et al. Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. J Cardiovasc Magn Reson. 2008;10:18. doi: 10.1186/1532-429X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539– 42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–68. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 16.Davis KB, Fisher L, Gillespie MJ, Pettinger M. A test of the National Death Index using the Coronary Artery Surgery Study (CASS) Control Clin Trials. 1985;6:179–91. doi: 10.1016/0197-2456(85)90001-7. [DOI] [PubMed] [Google Scholar]
- 17.Hachamovitch R, Hayes SW, Fried-man JD, Cohen I, Berman DS. A prognostic score for prediction of cardiac mortality risk after adenosine stress myocardial perfusion scintigraphy. J Am Coll Cardiol. 2005;45:722–9. doi: 10.1016/j.jacc.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 18.Clark PI, Glasser SP, Lyman GH, Krug-Fite J, Root A. Relation of results of exercise stress tests in young women to phases of the menstrual cycle. Am J Cardiol. 1988;61:197–9. doi: 10.1016/0002-9149(88)91334-3. [DOI] [PubMed] [Google Scholar]
- 19.State-specific mortality from sudden cardiac death—United States, 1999. MMWR Morb Mortal Wkly Rep. 2002;51:123–6. [PubMed] [Google Scholar]
- 20.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–5. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 21.Daly C, Clemens F, Lopez Sendon JL, et al. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–8. doi: 10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]
- 22.Klem I, Greulich S, Heitner JF, et al. Value of cardiovascular magnetic resonance stress perfusion testing for the detection of coronary artery disease in women. J Am Coll Cardiol Img. 2008;1:436–45. doi: 10.1016/j.jcmg.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Pilz G, Jeske A, Klos M, et al. Prognostic value of normal adenosine-stress cardiac magnetic resonance imaging. Am J Cardiol. 2008;101:1408–12. doi: 10.1016/j.amjcard.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Berman DS, Kang X, Hayes SW, et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men. Impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol. 2003;41:1125–33. doi: 10.1016/s0735-1097(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 25.America YG, Bax JJ, Boersma E, Stokkel M, van der Wall EE. The additive prognostic value of perfusion and functional data assessed by quantitative gated SPECT in women. J Nucl Cardiol. 2009;16:10–9. doi: 10.1007/s12350-008-9012-6. [DOI] [PubMed] [Google Scholar]
- 26.Cortigiani L, Sicari R, Bigi R, Landi P, Bovenzi F, Picano E. Impact of gender on risk stratification by stress echocardiography. Am J Med. 2009;122:301–9. doi: 10.1016/j.amjmed.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Alexander KP, Shaw LJ, Shaw LK, Delong ER, Mark DB, Peterson ED. Value of exercise treadmill testing in women. J Am Coll Cardiol. 1998;32:1657–64. doi: 10.1016/s0735-1097(98)00451-3. [DOI] [PubMed] [Google Scholar]
- 28.Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]