Abstract
Diffuse Noxious Inhibitory Controls (DNIC) involves application of a noxious stimulus outside the testing site to produce analgesia. In human subjects with a variety of chronic pain conditions, DNIC is less effective; however, in animal studies, DNIC is more effective after tissue injury. While opioids are involved in DNIC analgesia, the pathways involved in this opioid-induced analgesia are not clear. The aim of the present study was to test the effectiveness of DNIC in inflammatory muscle pain, and to study which brainstem sites mediate DNIC- analgesia. Rats were injected with 3% carrageenan into their gastrocnemius muscle and responses to cutaneous and muscle stimuli were assessed before and after inflammation, and before and after DNIC induced by noxious heat applied to the tail (45°C and 47°C). Naloxone was administered systemically, into rostral ventromedial medulla (RVM), or bilaterally into the medullary reticularis nucleus dorsalis (MdD) prior to the DNIC-conditioning stimuli. DNIC produced a similar analgesic effect in both acute and the chronic phases of inflammation reducing both cutaneous and muscle sensitivity in a dose-dependent manner. Naloxone systemically or microinjected into the MdD prevented DNIC-analgesia, while naloxone into the RVM had no effect on DNIC analgesia. Thus, DNIC analgesia involves activation of opioid receptors in the MdD.
Keywords: Pain, muscle, inhibition, DNIC, opioid
Diffuse noxious inhibitory control (DNIC) is a mechanism of pain inhibition that occurs when a noxious stimulus is applied outside the test area.1,33 Prior studies show that lesioning either the rostral ventromedial medulla (RVM) or periaqueductal grey (PAG) has no effect on DNIC-induced analgesia.6,9 However, DNIC-conditioning stimuli decreases activity of RVM facilitatory ON-cells.24 Neurons in the medullary reticularis nucleus dorsalis (MdD) respond to high intensity noxious stimuli and may mediate DNIC analgesia.8 DNIC reduces activity of convergent dorsal horn neurons through activation of opioids receptors.5,6,8,32,44,63 Both the RVM and the MdD are rich in μ-opioid receptors39,40 and thus could play a role in DNIC analgesia.
Although the majority of animal studies characterized DNIC in animals without tissue injury, 3 studies show DNIC is more effective after injury when recording from nociceptive dorsal horn or trigeminal neurons in rats16-18; this enhanced effect has yet to be confirmed behaviorally. In human subjects, people who do not produce analgesia to DNIC-conditioning stimuli are more likely to develop chronic pain.64 It is generally thought there is a balance between inhibition and excitation in supraspinal sites so that under normal conditions inhibition is greater than excitation.41,42,55 After tissue injury, alterations in this balance result in a loss of inhibition and/or an increase in excitation.11,53,57 These data suggest the balance between inhibition and facilitation in chronic pain is altered, and DNIC pathways could be a key component in development of chronic pain.
Previous studies use different nociceptive conditioning stimuli to induce DNIC in animals (electrical stimulation, hot water, acupuncture, or pinch30,32,36) and in humans (heat pain, cold pain, and ischemic pain1,29,31,63) In human subjects, intensities of stimulation (45–47°C) are typically lower when compared to animals (48–50°C); both human and animal studies show intensity is important in producing DNIC analgesia.7,33,58 Further, DNIC analgesia is generally tested during the stimulus, and the duration of effect is thought to be short-lasting. Thus, this animal study will investigate: 1) the effectiveness of DNIC using different intensities of conditioning stimuli; 2) the effect of DNIC on both acute and chronic hyperalgesia; 3) if DNIC analgesia outlasts the conditioning stimulus; and 4) participation of endogenous opioids in supraspinal sites in DNIC-induced analgesia.
Materials
Animals
All experiments were approved by the University of Iowa Animal Care and Use Committee and were carried out according to the guidelines of the National Institute of Health. Male Sprague-Dawley rats, weighing between 250 and 350 g (Harlan, St. Louis) were used in this study. The temperature of the behavioral testing room was controlled (23–25°C), and animals were maintained in a 12 hour dark-light cycle with free access to water and food. Behavioral tests were performed between 9 a.m. and 5 p.m.
Induction of Muscle Inflammation
Muscle inflammation was induced in rats by injecting 3% carrageenan (Cg; Type IV carrageenan Lambda; Sigma-Aldrich, St. Louis, MO), dissolved in sterile saline, into the gastrocnemius muscle while the animal was anesthetized with 4% isoflurane.
Diffuse Noxious Inhibitory Control (DNIC)
All rats were acclimated 2× per day for 2 days before the DNIC test to minimize stress during the test. Rats were placed in the restrainer and their tail held in warm (36°C) water for 2 minutes. The DNIC-conditioning stimulus used was immersion of the tail into a hot water bath at 2 different temperatures: 45 and 47°C. The distal 7.0 cm of the tail was immersed into hot water for 2 minutes while the animal was awake. It should be noted that the duration and surface area of the conditioning stimuli were kept constant in this study since modification of these variables can affect DNIC analgesia. The application of the heat stimulus at these temperatures is similar to that used in awake human subjects, and thus is directly transferable. It was noted that the animal tried to withdraw its tail during this stimulus but the experimenter continued to hold the tail in the temperature. Preliminary studies with higher temperatures (49°C) produced significant distress in awake animals, and thus we were unable to separate stress-induced analgesia from DNIC-induced analgesia. Therefore, we used 2 lower temperatures of 45 and 47°C to induce and test DNIC analgesia.
Behavioral Testing
All animals were tested for cutaneous and muscle mechanical sensitivity. Prior to testing, rats were acclimated to the von Frey filaments and tweezer testing procedures for 2 days and 2 times per day to minimize stress during testing and reduce variability of the outcome measures. For acclimation to von Frey filaments, rats were placed on the elevated table in Lucite cubicles for 10 minutes. For the tweezers, mice were placed in the testing glove for 5 minutes.
Test Stimulus 1: Cutaneous Mechanical Sensitivity
Rats were tested for cutaneous sensitivity with von Frey filaments applied to the paw immediately after the DNIC-conditioning stimulus (within 2 minutes) as previously described.27 A series of filaments with various bending forces (501, 224, 140, 88, 74, 57, 34, and 10 mN) was applied to the plantar surface of the hindpaw. The lowest force at which the withdrawal response was obtained was the cutaneous withdrawal threshold of the paw. A decrease in withdrawal threshold was interpreted as secondary cutaneous hyperalgesia and an increase was interpreted as analgesia.
Test Stimulus 2: Muscle Mechanical Sensitivity
Rats were tested for muscle sensitivity with a pair of tweezers applied to the gastrocnemius muscle as previously described.48 To measure the withdrawal threshold, rats were placed in a restraining glove, and the gastrocnemius muscle compressed with a pair of tweezers with calibrated force plates while the hindlimb was extended. Compression was continued until the animal withdrew the leg or vocalized. The maximum force applied at withdrawal was recorded as the compression threshold. A decrease in withdrawal threshold was interpreted as muscle hyperalgesia and an increase was interpreted as analgesia.
Placement of Guide Cannula and Drug Administration
Intracerebral guide cannulae were stereotaxically implanted in the RVM or MdD 3 to 5 days before the first intramuscular injection of carrageenan. The rats were anesthetized with an injection of sodium pentobarbital (Nembutal, 60mg/kg, ip) and secured in a stereotaxic head holder to implant the guide cannula (17.5 mm in length, 26 gauge; Plastics One, Roanoke, VA). After a midline incision, the skull was exposed, and a small hole drilled for placement of the guide cannula. Cannulae were placed 3 mm dorsal to the RVM (interaural: −2.0 mm; mediolateral: .0 mm; and dorsoventral: −7.5 mm27) or the MdD bilaterally (interaural: −5.08 mm; mediolateral: ± 61.5 mm; and dorsoventral: −5.8 mm).37 Site specificity was tested by placing the cannulae 3 mm above the MdD into the nucleus cuneatus and either naloxone or vehicle injected. Cannulae were secured to the skull by stainless steel screws and dental acrylic. A dummy cannula (33 gauge, Plastics One) was inserted into the guide cannula to maintain its patency. All rats were allowed 3 to 5 days recovery before testing began.
The rats were microinjected with naloxone hydrochlo-ride, dissolved in sterile saline (20 μg/.5 μl; Sigma-Aldrich, St. Louis, MO) or vehicle (.5μl/.9% sterile saline) into the RVM or MdD through the guide cannula. For microinjection, a 33-gauge injection cannula was connected to a 10-μl Hamilton syringe through polyethylene-10 tubing backfilled with sterile saline. The microinjection was performed over a 2-minute period and the travel of the air bubble in the tubing was carefully observed to ensure that the drug solution entered the injection cannula. The injection volume of .5 μl was chosen as we previously showed that this volume is sufficient to cover the majority the RVM (nucleus raphe magnus and partial lateral paragigantcellularis) but does not diffuse into the nucleus gigantocellularis 1 mm above the RVM.19 We also showed the dose of naloxone 20μg blocks μ-opioid receptors in the RVM and prevents TENS-induced antihyperalgesia.27
To examine placement of the cannula into RVM and MdD, an equivalent volume of methylene blue dye was injected at the end of the experiment. Rats were then euthanized and transcardially perfused with 4% paraformaldehyde. After this, the brain was removed and stored in 30% sucrose solution. The brain was cross-sectioned at 40 μm on a cryostat and examined under a light microscope for placement of the cannula. Injection sites, mapped from histological sections, are shown in Figs 3 and 4.
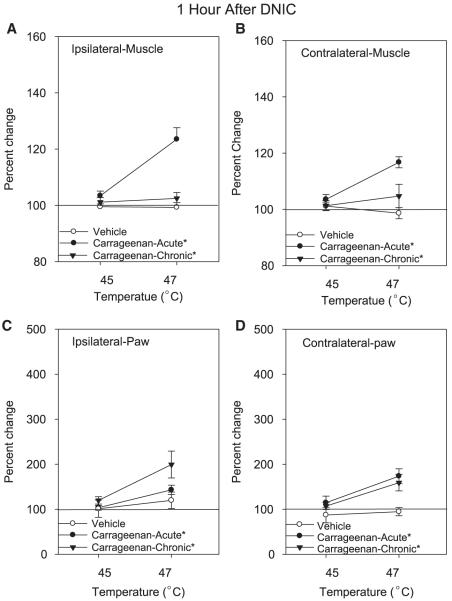
Figure 3.
Graphs representing DNIC-induced analgesia, 1 hour after the conditioning stimulus, on the muscle (A, B) and paw (C, D) withdrawal thresholds in saline-injected controls, acute inflammation, and chronic muscle inflammation. The DNIC-conditioning stimulus was given at 2 intensities, 45°C (n = 6) and 47°C (n = 10). DNIC = diffuse noxious inhibitory controls. Each point represents mean ± S.E.M. *, significantly different from control.
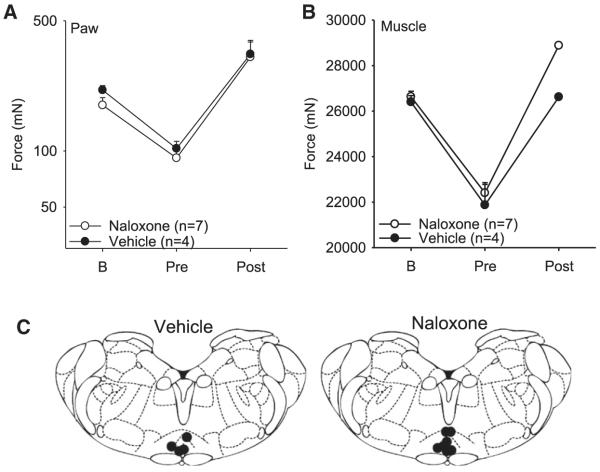
Figure 4.
Paw (A) and muscle (B) withdrawal thresholds before and 24 hours after muscle inflammation, and after the 47°C conditioning stimulus. Naloxone or vehicle was microinjected into the RVM prior to the conditioning stimuli. Each point represents mean ± S.E.M. B = baseline testing before inflammation; RVM = rostral-ventromedial medulla; DNIC = diffuse noxious inhibitory controls. (C) Coronal sections of the medulla showing the microinjection sites for the group injected with naloxone or vehicle.
Experimental Protocol
Experiment 1 tested animals with and without muscle inflammation to compare the effects of the DNIC-conditioning stimuli at 2 different temperatures, 45 and 47°C, on behavioral tests. Behavioral tests were performed before and 24 hours after induction of inflammation (pre-DNIC), considered the acute phase, and 1 week after induction of inflammation, considered the chronic phase. The DNIC-conditioning stimulus was performed at 24 hours and 1 week after induction of inflammation. Behavioral tests were performed immediately after the DNIC-conditioning stimulus and 1 hour after the DNIC-conditioning stimulus. Both 45 and 47°C DNIC-conditioning stimuli were tested and the testing was done in separate groups of animals. A control group was used to compare the DNIC effect in uninflamed animals that were injected with pH 7.2 saline instead of carrageenan. Each animal was tested individually at a separate time. This was done so that cutaneous and muscle testing could both be done in a short time frame after DNIC (within 5 to 10 minutes) since the analgesia produced by DNIC is thought to be short-lasting.
Experiment 2 tested the effect of naloxone (3 mg/kg, sc, 30 minutes prior to DNIC) administered systemically on the behavioral response to the 47°C conditioning stimulus 24 hours after inflammation. The dose of naloxone was based on previous studies showing effectiveness when administered systemically.45 In a separate control group (n = 4), the effects of 3 mg/kg naloxone was tested on the mechanical withdrawal thresholds of the paw and muscle at 24 hours after carrageenan and 8 days after carrageenan.
Experiment 3 tested the effect of naloxone microinjected into the RVM (20 μg/.5 μl) or the MdD (20 μg/.5μl/side) on the behavioral response to 47°C conditioning stimulus 24 hours after muscle inflammation. Naloxone was given 10 minutes prior to the DNIC-conditioning stimulus. Testing was done with the experimenter blinded to the injection of vehicle or naloxone. The dose of naloxone were based on previous studies showing effectiveness when microinjected into the RVM.27 The controls groups were injected with the same volume of saline into the RVM or SRD.
Statistical Analysis
The degree of hyperalgesia induced by carrageenan was presented as the mean ± S.E.M. Percent changes are calculated for the change in DNIC as the withdrawal threshold after DNIC to the withdrawal threshold prior to DNIC but after carrageenan; 0% was no change. For Experiment 1, the effect of DNIC was expressed as a change from the pre-DNIC value either immediately or 1 hour after the DNIC-conditioning stimuli. A repeated measures ANOVA was used for the statistical comparison followed by a Tukey’s post hoc test to compare among groups and temperatures. For Experiment 2, a paired t-test compared withdrawal thresholds after naloxone to that prior to naloxone. For Experiment 3, a repeated measures ANOVA compared withdrawal thresholds across time and between groups. A Tukey’s test compared differences among groups at individual time periods. To verify the hyperalgesia induced by carrageenan the students t-test compared those injected with saline into the muscle gastrocnemius to those injected with carrageenan. Statistical significance was defined as P < .05. Statistical analyses were conducted with SPSS v.17.0 (SPSS Inc, Chicago, IL).
Results
Development of Hyperalgesia After Muscle Inflammation
Carrageenan injected into the gastrocnemius muscle (n = 14) reduced the withdrawal threshold of the paw (cutaneous secondary hyperalgesia) and muscle (primary hyperalgesia) 24 hours after induction of inflammation (acute phase; Fig 1). After 8 days of the inflammation the withdrawal threshold remained decreased for the paw, but not consistently for the muscle (decreases only observed in the group that received 47°C conditioning stimulus). Moreover, this reduced paw withdrawal threshold occurred for both the ipsilateral and contralateral paws when compared with those that received saline into the gastrocnemius muscle. This bilateral hyperalgesia has been found more frequently in musculoskeletal pain models.14,15,43,49,50,60,61
Figure 1.
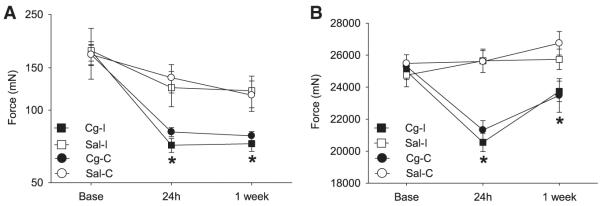
Withdrawal thresholds of the paw (A) and muscle (B) decreased 24 hours after induction of muscle inflammation with 3% carrageenan (Cg) (n = 16). Control animals received intramuscular injection of saline (S) (n = 16). The ipsilateral (I) side is represented by squares and the contralateral (C) side is represented by circles. Each point represents mean ± S.E.M. *, significantly different from saline-injected controls.
Effect of DNIC on Muscle Withdrawal Threshold
Overall Effect
For the muscle withdrawal thresholds there was: 1) a temperature-dependent effect with 47°C producing greater analgesia than 45°C (F1,42 = 14.2; P = .0001); 2) greater analgesia on the inflamed side when compared to the contralateral side (F1,42 = 15.3, P = .0001); and 3) a significant difference between groups (F2,42 = 3.1, P = .05) with the control group significantly different from the acute inflammation group (P = .015), but not from the chronic inflammation group (P = .09 [Fig 2]). There was also a significant effect for changes in the muscle withdrawal threshold 1 hour after DNIC that was dependent on temperature of stimulation and group (inflammation × group: F2,42 = 14.2, P = .0001). In the animals with acute muscle inflammation there was a significant increase in withdrawal thresholds 1 hour after the 47°C conditioning stimulus when compared to controls (P = .0001) and those with chronic inflammation (P = .0001 [Fig 3]).
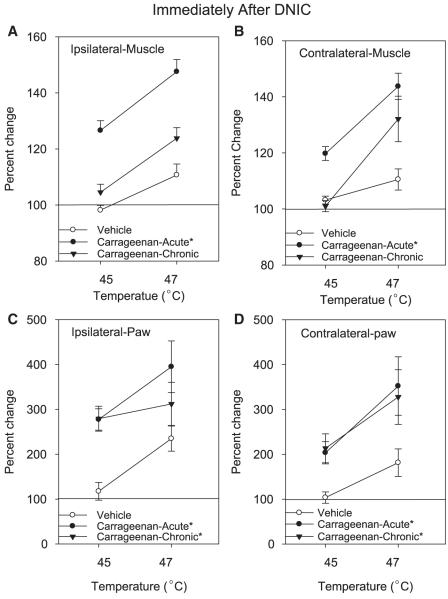
Figure 2.
Graphs representing DNIC-induced analgesia, immediately after the conditioning stimulus, on the muscle (A, B) and paw (C, D) withdrawal thresholds in saline-injected controls (n = 10), acute inflammation (n = 6), and chronic (n = 10) muscle inflammation. The DNIC-conditioning stimulus was given at 2 intensities, 45°C and 47°C. DNIC = diffuse noxious inhibitory controls. Each point represents mean ± S.E.M. *, significantly different from controls.
Normal Animals
In animals without inflammation, the 47°C (n = 8), but not the 45°C (n = 6), conditioning stimulus significantly increased (10.0 ± 4%) the muscle withdrawal threshold (Fig 2). All values retuned to baseline 1 hour after the DNIC-conditioning stimuli.
Acute Inflammation
In animals with acute inflammation, the 45°C (n = 6) and 47°C (n = 8) conditioning stimulus increased the muscle withdrawal threshold (Fig 2). The 45°C conditioning stimulus increased the ipsilateral paw withdrawal threshold (IPL) by 26 ± 3% and the contralateral paw withdrawal threshold (CTL) by 20 ± 2%. Moreover, DNIC delivered at an intensity of 47°C was more robust than 45°C and increased the withdrawal thresholds ipsilaterally by 48 ± 4% and contralaterally by 44 ± 5%. One hour post-DNIC hyperalgesia returned similar to that observed pre-DNIC for the 45°C conditioning stimulus but there was still a significant increase for the group treated with the 47°C conditioning stimulus (Fig 3).
Chronic Inflammation
In animals with chronic inflammation, the 45°C (n = 6) conditioning stimulus was ineffective while the 47°C (n = 8) conditioning stimulus increased the muscle withdrawal thresholds both ipsilaterally (24 ± 3.8%) and contralaterally (32 ± 8.1% [Fig 2]). The hyperalgesia returned to baseline 1 hour after DNIC (Fig 3).
Effect of DNIC on Paw Withdrawal Threshold
Overall effect
For paw withdrawal thresholds there was: 1) a temperature-dependent effect with 47°C producing greater-analgesia than 45°C (F1,42 = 7.4; P = .01); 2) greater analgesia on the inflamed side when compared to the contralateral side (F1,42 = 8.6, P = .005); and 3) a significant difference between groups (F2,42 = 6.0, P = .005) with the control group significantly different from the acute inflammation group (P = .004) and from the chronic inflammation group (P = .025 [Fig 2]). There was also a significant effect for changes in the paw withdrawal threshold 1 hour after DNIC that was dependent on: 1) temperature of stimulation (F1,42 = 16.9, P = .0001); and 2) differences between groups (F2,42 = 6.1, P = .005) with the control group significantly lower than the acute inflammation (P = .02) and the chronic inflammation (P = .001) group (Fig 3).
Normal Animals
DNIC at 45°C (n = 6) had no effect on the paw withdrawal thresholds in animals without inflammation. DNIC at 47°C (n = 8), however, significantly increased with paw withdrawal thresholds in animals without inflammation by 135 ± 29% ipsilaterally, and 181 ± 31% contralaterally (Fig 2).
Acute Inflammation
In animals with acute inflammation, however, the DNIC-conditioning stimulus at either 45°C (n = 6) or 47°C (n = 8) significantly increased the paw withdrawal threshold bilaterally. The 45°C conditioning stimulus increased the paw withdrawal threshold ipsilaterally by 178 ± 24% and contralaterally by 103 ± 25% (Fig 2). One hour after the 45°C DNIC-conditioning stimulus, the withdrawal thresholds returned to baseline values. There was a larger increase in the paw withdrawal thresholds (ipsilateral 295 ± 57%; contralateral 152 ± 65%) after the 47°C conditioning stimulus when compared to 45°C (Fig 3). This increase persisted 1 hour after application of the 47°C DNIC conditioning stimulus (ipsilateral 43 ± 10%; contralateral 74 ± 16%).
Chronic Inflammation
In animals with chronic inflammation both the 45°C (n = 6) and the 47°C (n = 8) conditioning stimulus significantly increased the paw withdrawal threshold bilaterally (45°C: ipsilateral 178 ± 24%; contralateral 103 ± 25%; 47°C: ipsilateral 295 ± 58%; contralateral 252 ± 65% [Fig 2]). There was a significant increase in withdrawal thresholds of the paw 1 hour after the 47°C conditioning stimulus (ipsilateral 99 ± 29%; contralateral 59 ± 18%) when compared to baseline (Fig 3), but not for the 45°C conditioning stimulus.
Effect of Naloxone on DNIC Analgesia
Systemic Naloxone
To verify the participation of endogenous opioids in the mechanism of DNIC as previously described,4,32 naloxone, an opioid antagonist, was injected subcutaneously 30 minutes before the 47°C conditioning stimulus (n = 6). The muscle withdrawal threshold did not increase (ipsilateral 11 ± 6%; contralateral 9 ± 5%) when compared to values in those that did not receive naloxone (ipsilateral 48 ± 4%; contralateral 44 ± 5%), confirming a role for opioids in DNIC-induced analgesia. However, the paw withdrawal threshold still increased significantly after systemic naloxone (ipsilateral 246 ± 45%; contralateral 247 ± 66%). As a control, we tested if systemic naloxone (3 mg/kg, sc) alone had any effect on the muscle or paw withdrawal thresholds (n = 4); there was no significant change 30 minutes after naloxone (Table 1).
Table 1.
Muscle and Paw Withdrawal Thresholds Before and After Treatment With 3 mg/kg Naloxone. Data Are Mean ± S.E.M. and Given in mN of Force
| Muscle Withdrawal Threshold (mN) |
Paw Withdrawal Threshold (mN) |
|||
|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipislateral | Contralateral | |
| Baseline | 2890 ± 261 | 2516 ± 325 | 253 ± 121 | 120 ± 33 |
| 24 h post-Cg | 1365 ± 141 | 2591 ± 187 | 33 ± 13 | 68 ± 32 |
| 30 min post-naloxone | 1432 ± 441 | 2108 ± 390 | 38 ± 22 | 68 ± 32 |
| 8 days post-Cg | 2362 ± 482 | 2765 ± 121 | 86 ± 51 | 116 ± 36 |
| 30 minute post-naloxone | 2000 ± 553 | 2464 ± 362 | 85 ± 49 | 207 ± 103 |
RVM Naloxone
Since the RVM is implicated in opioid induced analgesia,8 we tested if naloxone (n = 7) microinjected into the RVM prevents the analgesic effect of DNIC delivered with the 47°C conditioning stimulus. As seen in Fig 4A and 4B, naloxone had no effect on the analgesia produced by DNIC on either muscle or paw withdrawal thresholds when compared to microinjection of vehicle as a control (n = 4). Thus, opioid receptors in the RVM do not mediate the effects of DNIC.
MdD Naloxone
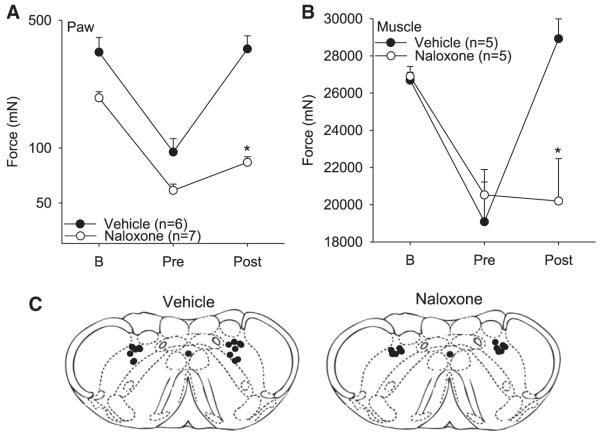
Since prior literature suggests the MdD mediates the analgesic effects of DNIC,6 we tested if microinjection of naloxone administered into the MdD before the 47°C conditioning stimulus prevented DNIC analgesia. Microinjection of naloxone (n = 7) into the MdD bilaterally prevented DNIC-induced analgesia for both the muscle (n = 6) and paw (n = 7) withdrawal thresholds when compared to vehicle controls (P < .05: n = 5 muscle; n = 6 paw [Fig 5]). In animals with misplaced cannula there was still an increase of 221 ± 39% for the paw withdrawal threshold in those injected with naloxone (n = 5) that was significantly greater than those injected with naloxone in the MdD (100 ± 54%). In 4 animals, the cannulae were placed in the cuneate nucleus; in 1 animal, the cannuale was placed lateral reticular nucleus. Thus, opioid receptors in the MdD mediate the analgesic effects of DNIC.
Figure 5.
Paw (A) and muscle (B) withdrawal thresholds before and 24 hours after muscle inflammation, and before and after the 47°C conditioning stimulus. Naloxone or vehicle was microinjected into the MdD prior to the conditioning stimuli. Each point represents mean ± S.E.M. B = baseline testing before inflammation. (C) Coronal sections of the medulla showing the microinjection sites for the group injected with naloxone and vehicle. MdD = medullary reticularis nucleus dorsalis; DNIC = diffuse noxious inhibitory controls.
Discussion
DNIC Increases Pain Thresholds in Uninjured Animals and Humans
Numerous studies have shown that application of a noxious conditioning stimulus produces analgesia in both animals and humans, and a concomitant reduction in dorsal horn activity.34,63 Different studies describe recruitment of DNIC in uninjured animals34,59 and humans20,47 using different conditioning stimuli applied to the skin with the majority of these studies showing analgesia during the test stimulus. We extend these previous findings by showing DNIC analgesia: 1) immediately after the conditioning stimulation; 2) for secondary hyperalgesia; 3) for muscle hyperalgesia; and 4) can last up to 1 hour in animals with muscle inflammation. Our data are in agreement with prior studies recording effects of DNIC on inhibiting dorsal horn neuron activity in rats which show persistent inhibition after removal of the conditioning stimulus.33 For example, DNIC induced by intraperitoneal injection of acetic acid results in analgesia to the tail flick test immediately, to the hot plate test for 15 minutes, and to the vocalization threshold to electrical stimulation for up to 30 minutes.12 On the other hand, in lightly anesthetized rats, 49 to 51°C conditioning stimuli (but not 45 to 48°C) increased the tail flick latency to heat but had no effect on the paw withdrawal latency to heat. Thus, effectiveness and length of action of DNIC differs depending on the test stimulus, intensity of stimulus, and outcome measured.
The current study also shows that DNIC analgesia is dependent on the intensity of the conditioning stimulus, with a greater intensity producing a greater analgesia and is in agreement with prior work in animals.7,33,59 A recent study in human subjects agrees with this concept, showing greater pain intensity and greater DNIC analgesia with the cold-pressor test when compared to intramuscular infusion of hypertonic saline.1 On the other hand, Granot et al23 show no correlation between pain scores and endogenous analgesia in human subjects; however, DNIC-conditioning stimuli resulting in pain below 20/100 do not produce analgesia. Thus, intensity of the DNIC conditioning stimulation appears critical to produce analgesia in both animals and healthy subjects.
DNIC Is Altered in Pain Conditions
The current study shows that DNIC is more effective in animals with muscle inflammation when compared to those without inflammation; the 45°C conditioning stimulus produces analgesia in animals after muscle injury but not in those without muscle injury. These data agree with prior animal studies showing that DNIC is more effective in animals with hind paw inflammation or neuropathic pain when recording central nociceptive neurons in the dorsal horn or trigeminal nucleus.17,18 Interestingly, in humans, cold-pressor-induced DNIC responses applied concomitantly with a hypertonic saline-induced muscle pain resulted in less analgesia.1 This may be comparable to that observed in the current study when the DNIC-conditioning stimulus was given with the muscle withdrawal threshold stimulus which resulted in no analgesia in animals without tissue injury.
We propose that the injury produces changes in spinal and supraspinal sites that alter the effectiveness of DNIC. Our results show that DNIC analgesia is greater on the inflamed side when compared to the contralateral side. This finding was surprising since the analgesia produced by DNIC is systemic and affects all limbs. It may be that in areas of neuron sensitization after tissue injury, effects of DNIC are greater. Indeed opioid agonists delivered spinally or supraspinally are more effective in reducing dorsal horn neuron activity or hyperalgesia after inflammation,26,51,52 suggesting enhanced activity in the opioid system.
The current study showed that DNIC analgesia was stronger in animals with inflammation when compared to uninjured animals. In contrast, human subjects with chronic pain show reduced analgesia to DNIC-conditioning stimuli.10,28,29,31,35,38,46,62 One possibility for the differences between human subjects with chronic pain and animals with tissue injury is the time from initial injury. In human subjects with rheumatoid arthritis the loss of DNIC analgesia occurs in those with long-standing disease (>5 years) but not those with newly diagnosed disease (<1 year).35 In animals with cutaneous inflammation, there is enhanced descending facilitation at early time periods that is then masked at later time periods by enhanced descending inhibition.54 In agreement, in animals with inflammation, DNIC analgesia was greater in the acute stage at a time when there is enhanced inhibition, but not in a more chronic stage.17,18 Thus, these data support the conclusion that there is a shift in the balance between uninjured and injured animals that depends on the time after injury.
Effect of Naloxone on DNIC
The current study shows that the effects of DNIC are not mediated by opioid receptors located in the RVM. This is in agreement with prior studies that show lesions of the RVM do not prevent the effects of DNIC.5,6 On the other hand, the current study shows, for the first time, that blockade of opioid receptors in the MdD prevents DNIC analgesia. Our data agree with prior studies that systemic activation of opioid receptors reduces C-fiber-evoked activity induced in MdD neurons,4 and lesions of the MdD prevent analgesia produced by low doses of naloxone.56 Recent work shows expression of mu-opioid receptors in the MdD in equivalent levels to that in the RVM, and nearly 100% of spinally projecting neurons express the mu-opioid receptors.39,40 Thus, we conclude nociceptive stimuli activate opioid receptors on MdD neurons that project to the spinal cord to reduce dorsal horn neuron activity and result in analgesia.
The current study shows that blockade of opioid receptors systemically reduces DNIC analgesia after inflammation. Surprisingly, however, the current study shows that only the DNIC-induced reduction in muscle hyperalgesia, but not cutaneous hyperalgesia, was prevented by systemic blockade of opioid receptors. One explanation is that different outcome measures have different sensitivity to systemic naloxone. Prior animal studies show a naloxone-reversible blockade of DNIC analgesia when recording activity of dorsal horn neurons in response to noxious electrical stimulation in uninjured animals.32 In healthy human subjects, naloxone blocked the increase in the spinal nociceptive reflex threshold produced by DNIC,63 but had no effect on the increase in cutaneous heat pain produced by DNIC.21
Alternatively, it is possible that systemic naloxone enhanced the response to cutaneous stimuli, thus masking the effects of naloxone on DNIC. Early studies show a bidirectional effect of naloxone on nociception such that low doses produce hyperalgesia and higher doses produce analgesia.2,3,13 The dose of systemic naloxone used in the current study was the same dose that increased cutaneous hyperaglesia in arthritic rats.13 However, in our hands, this dose of naloxone had no effect on either muscle or cutaneous hyperalgesia. Opposite excitatory and inhibitory signals sent from different pools of neurons located in the spinal cord and the MdD could result in a lack of effect of naloxone on DNIC analgesia.
Limitations
One limitation is the application of the DNIC-conditioning stimuli in the awake animal which could produce stress in the animal and thus result in stress-induced analgesia. To minimize this as a concern, we: 1) acclimated animals to the test stimuli; 2) used heat intensities lower than that previously used in animals; and 3) used heat intensities similar to that used in human subjects. Furthermore, stress-induced analgesia is regulated by opioids and involves the RVM.22,25 Our results show that naloxone microinjected into the RVM does not block the DNIC analgesia; thus, we do not believe that there is a stress-induced analgesia in the current study.
Another limitation of the behavioral study was our inability to test during the DNIC-conditioning stimuli. This was not possible as we had to restrain the animals during the DNIC in a separate holder to that used for the behavioral tests. However, we did test immediately after completion of the conditioning stimuli and were able to show changes at this time that likely reflect DNIC-analgesia.
Conclusion
In conclusion, this study showed that a greater intensity of noxious conditioning stimuli produces greater analgesia, and DNIC reduces both primary muscle and secondary cutaneous hyperalgesia during muscle inflammation. We further show that DNIC utilizes opioid receptors in the MdD, but not the RVM, to produce analgesia.
Acknowledgments
We wish to thank Lynn Rasmussen for excellent technical assistance.
Supported by National Institutes of Health AR052316.
Footnotes
Perspective: The current study shows that DNIC activates opioid receptors in the MdD, but not the RVM, to produce analgesia. These data are important for understanding clinical studies on DNIC as well as for potential treatment of chronic pain patients.
References
- 1.Arendt-Nielsen L, Sluka KA, Nie HL. Experimental muscle pain impairs descending inhibition. Pain. 2008;140:465–471. doi: 10.1016/j.pain.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attal N, Jazat F, Kayser V, Guilbauld G. Further evidence for “pain-related” behaviours in a model of unilateral peripheral mononeuropathy. Pain. 1990;41:235–251. doi: 10.1016/0304-3959(90)90022-6. [DOI] [PubMed] [Google Scholar]
- 3.Attal N, Kayser V, Jazat F, Guilbaud G. Behavioural evidence for a bidirectional effect of systemic naloxone in a model of experimental neuropathy in the rat. Brain Res. 1989;494:276–284. doi: 10.1016/0006-8993(89)90596-9. [DOI] [PubMed] [Google Scholar]
- 4.Bing Z, Villanueva L, Le Bars D. Effects of systemic morphine upon A delta- and C-fibre evoked activities of subnucleus reticularis dorsalis neurones in the rat medulla. Eur J Pharmacol. 1989;164:85–92. doi: 10.1016/0014-2999(89)90234-3. [DOI] [PubMed] [Google Scholar]
- 5.Bouhassira D, Chitour D, Villanueva L, Le Bars D. Morphine and diffuse noxious inhibitory controls in the rat: Effects of lesions of the rostral ventromedial medulla. Eur J Pharmacol. 1993;232:207–215. doi: 10.1016/0014-2999(93)90775-d. [DOI] [PubMed] [Google Scholar]
- 6.Bouhassira D, Chitour D, Villaneuva L, Le Bars D. The spinal transmission of nociceptive information: Modulation by the caudal medulla. Neuroscience. 1995;69:931–938. doi: 10.1016/0306-4522(95)00269-o. [DOI] [PubMed] [Google Scholar]
- 7.Bouhassira D, Le Bars D, Villanueva L. Heterotopic activation of A delta and C fibres triggers inhibition of trigeminal and spinal convergent neurones in the rat. J Physiol. 1987;389:301–317. doi: 10.1113/jphysiol.1987.sp016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouhassira D, Villanueva L, Bing Z, Le Bars D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain Res. 1992;595:353–357. doi: 10.1016/0006-8993(92)91071-l. [DOI] [PubMed] [Google Scholar]
- 9.Bouhassira D, Villanueva L, Le Bars D. Effects of systemic morphine on diffuse noxious inhibitory controls: Role of the periaqueductal grey. Eur J Pharmacol. 1992;216:149–156. doi: 10.1016/0014-2999(92)90355-8. [DOI] [PubMed] [Google Scholar]
- 10.Bragdon EE, Light KC, Costello NL, Sigurdsson A, Bunting S, Bhalang K, Maixner W. Group differences in pain modulation: Pain-free women compared to pain-free men and to women with TMD. Pain. 2002;96:227–237. doi: 10.1016/S0304-3959(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 11.Burgess SE, Gardell LR, Ossipov MH, Malan TP, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvino B, Villanueva L, Le Bars D. The heterotopic effects of visceral pain: Behavioural and electrophysiological approaches in the rat. Pain. 1984;20:261–271. doi: 10.1016/0304-3959(84)90015-0. [DOI] [PubMed] [Google Scholar]
- 13.Cattaneo I, Kayser V, Guilbaud G. Differential effects of specific delta and kappa opioid receptor antagonists on the bidirectional dose-dependent effect of systemic naloxone in arthritic rats, an experimental model of persistent pain. Brain Res. 1993;623:201–207. doi: 10.1016/0006-8993(93)91428-u. [DOI] [PubMed] [Google Scholar]
- 14.Coderre TJ, Melzack R. Cutaneous hyperalgesia: Contributions of the peripheral and central nervous systems to the increase in pain sensitivity after injury. Brain Res. 1987;404:95–106. doi: 10.1016/0006-8993(87)91359-x. [DOI] [PubMed] [Google Scholar]
- 15.Coderre TJ, Melzack R. Increased pain sensitivity following heat injury involves a central mechanism. Behav Brain Res. 1985;15:259–262. doi: 10.1016/0166-4328(85)90181-0. [DOI] [PubMed] [Google Scholar]
- 16.Danziger N, Gautron M, Le BD, Bouhassira D. Activation of diffuse noxious inhibitory controls (DNIC) in rats with an experimental peripheral mononeuropathy. Pain. 2001;91:287–296. doi: 10.1016/S0304-3959(00)00451-6. [DOI] [PubMed] [Google Scholar]
- 17.Danziger N, Weil-Fugazza J, Le Bars D, Bouhassira D. Alteration of descending modulation of nociception during the course of monoarthritis in the Rat. J Neurosci. 1999;19:2394–2400. doi: 10.1523/JNEUROSCI.19-06-02394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danziger N, Weil-Fugazza J, Le Bars D, Bouhassira D. Stage-dependent changes in the modulation of spinal nociceptive neuronal activity during the course of inflammation. Eur J Neurosci. 2001;13:230–240. doi: 10.1046/j.0953-816x.2000.01375.x. [DOI] [PubMed] [Google Scholar]
- 19.daSilva LFS, DeSantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, RVM and Gi mediate hyperalgesia produced by repeated intramuscular injections of acidic saline. J Pain. 2010;11:378–387. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards RR, Fillingim RB, Yamauchi S, Sigurdsson A, Bunting S, Mohorn SG, Maixner W. Effects of gender and acute dental pain on thermal pain responses. Clin J Pain. 1999;15:233–237. doi: 10.1097/00002508-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Edwards RR, Ness TJ, Fillingim RB. Endogenous opioids, blood pressure, and diffuse noxious inhibitory controls: A preliminary study. Percept Mot Skills. 2004;99:679–687. doi: 10.2466/pms.99.2.679-687. [DOI] [PubMed] [Google Scholar]
- 22.Foo H, Helmstetter FJ. Expression of antinociception in response to a signal for shock is blocked after selective downregulation of mu-opioid receptors in the rostral ventromedial medulla. Brain Res Mol Brain Res. 2000;76:282–288. doi: 10.1016/s0169-328x(00)00009-7. [DOI] [PubMed] [Google Scholar]
- 23.Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136:142–149. doi: 10.1016/j.pain.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez N, Dmitrieva N, Vanegas H. Medullary on-cell activity during tail-flick inhibition produced by heterotopic noxious stimulation. Pain. 1994;58:393–401. doi: 10.1016/0304-3959(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins E, Spinella M, Pavlovic ZW, Bodnar RJ. Alterations in swim stress-induced analgesia and hypothermia following serotonergic or NMDA antagonists in the rostral ventromedial medulla of rats. Physiol Behav. 1998;64:219–225. doi: 10.1016/s0031-9384(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 26.Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 28.Kosek E, Hansson P. Modulatory influence on somato-sensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 29.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 30.Kraus E, Le BD, Besson JM. Behavioral confirmation of “diffuse noxious inhibitory controls” (DNIC) and evidence for a role of endogenous opiates. Brain Res. 1981;206:495–499. doi: 10.1016/0006-8993(81)90554-0. [DOI] [PubMed] [Google Scholar]
- 31.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Le Bars D, Chitour D, Kraus E, Dickenson AH, Besson JM. Effect of naloxone upon diffuse noxious inhibitory controls (DNIC) in the rat. Brain Res. 1981;204:387–402. doi: 10.1016/0006-8993(81)90597-7. [DOI] [PubMed] [Google Scholar]
- 33.Le Bars D, Villanueva L. Electrophysiological evidence for the activation of descending inhibitory controls by nociceptive afferent pathways. Prog Brain Res. 1988;77:275–299. doi: 10.1016/s0079-6123(08)62795-8. [DOI] [PubMed] [Google Scholar]
- 34.Le Bars D, Dickenson AH, Besson J-M. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979;6:305–327. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- 35.Leffler AS, Hansson P, Kosek E. Somatosensory perception in a remote pain-free area and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from long-term trapezius myalgia. Eur J Pain. 2002;6:149–159. doi: 10.1053/eujp.2001.0312. [DOI] [PubMed] [Google Scholar]
- 36.Murase K, Kawakita K. Diffuse noxious inhibitory controls in anti-nociception produced by acupuncture and moxibustion on trigeminal caudalis neurons in rats. Jpn J Physiol. 2000;50:133–140. doi: 10.2170/jjphysiol.50.133. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edition Academic Press; 2008. [Google Scholar]
- 38.Peters ML, Schmidt AJ, Van den Hout MA, Koopmans R, Sluijter ME. Chronic back pain, acute postoperative pain and the activation of diffuse noxious inhibitory controls (DNIC) Pain. 1992;50:177–187. doi: 10.1016/0304-3959(92)90159-9. [DOI] [PubMed] [Google Scholar]
- 39.Pinto M, Castro AR, Tshudy F, Wilson SP, Lima D, Tavares I. Opioids modulate pain facilitation from the dorsal reticular nucleus. Mol Cell Neurosci. 2008;39:508–518. doi: 10.1016/j.mcn.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Pinto M, Sousa M, Lima D, Tavares I. Participation of muopioid, GABA(B), and NK1 receptors of major pain control medullary areas in pathways targeting the rat spinal cord: Implications for descending modulation of nociceptive transmission. J Comp Neurol. 2008;510:175–187. doi: 10.1002/cne.21793. [DOI] [PubMed] [Google Scholar]
- 41.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 43.Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104:567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain. 2008;139:431–438. doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Resende MA, Sabino GG, Candido CRM, Pereira LSM, Francischi JN. Transcutaneous electrical stimulation (TENS) effects in experimental inflammatory edema and pain. Eur J Pharmacol. 2004;504:217–222. doi: 10.1016/j.ejphar.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 46.Sandrini G, Rossi P, Milanov I, Serrao M, Cecchini AP, Nappi G. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia. 2006;26:782–789. doi: 10.1111/j.1468-2982.2006.01130.x. [DOI] [PubMed] [Google Scholar]
- 47.Serrao M, Rossi P, Sandrini G, Parisi L, Amabile GA, Nappi G, Pierelli F. Effects of diffuse noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain. 2004;112:353–360. doi: 10.1016/j.pain.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J Pain. 2005;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Sluka KA. Stimulation of deep somatic tissue with capsaicin produces long-lasting mechanical allodynia and heat hypoalgesia that depends on early activation of the cAMP pathway. J Neurosci. 2002;22:5687–5693. doi: 10.1523/JNEUROSCI.22-13-05687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Stanfa LC, Dickenson A. Spinal opioid systems in inflammation. Inflammation Research. 1995;44:231–241. doi: 10.1007/BF01782974. [DOI] [PubMed] [Google Scholar]
- 52.Stanfa LC, Sullivan AF, Dickenson AH. Alterations in neuronal excitability and the potency of spinal-mu, delta and kappa opioids after carrageenan-induced inflammation. Pain. 1992;50:345–354. doi: 10.1016/0304-3959(92)90040-I. [DOI] [PubMed] [Google Scholar]
- 53.Terayama R, Dubner R, Ren K. The roles of NMDA receptor activation and nucleus reticularis gigantocellularis in the time-dependent changes in descending inhibition after inflammation. Pain. 2002;97:171–181. doi: 10.1016/s0304-3959(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 54.Terayama R, Guan Y, Dubner R, Ren K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. Neuroreport. 2000;11:1915–1919. doi: 10.1097/00001756-200006260-00022. [DOI] [PubMed] [Google Scholar]
- 55.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136:331–339. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuruoka M, Hiruma Y, Willis WD. The subnucleus reticularis dorsalis is involved in antinociception produced by a low dose of naloxone during carrageenan-induced inflammation. Brain Res. 1997;762:264–268. doi: 10.1016/s0006-8993(97)00509-x. [DOI] [PubMed] [Google Scholar]
- 57.Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 58.Villanueva L, Le Bars D. The encoding of thermal stimuli applied to the tail of the rat by lowering the excitability of trigeminal convergent neurones. Brain Res. 1985;330:245–251. doi: 10.1016/0006-8993(85)90683-3. [DOI] [PubMed] [Google Scholar]
- 59.Villanueva L, Le Bars D. The activation of bulbo-spinal controls by peripheral nociceptive inputs: Diffuse noxious inhibitory controls. Biol Res. 1995;28:113–125. [PubMed] [Google Scholar]
- 60.Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willer JC, Le Bars D, De BT. Diffuse noxious inhibitory controls in man: Involvement of an opioidergic link. Eur J Pharmacol. 1990;182:347–355. doi: 10.1016/0014-2999(90)90293-f. [DOI] [PubMed] [Google Scholar]
- 64.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]