Abstract
Background
Functional mitral regurgitation (FMR), a well-recognized component of left ventricular remodeling, is associated with increased morbidity and mortality in heart failure patients. Percutaneous mitral annuloplasty has the potential to serve as a therapeutic adjunct to standard medical care.
Methods and Results
Patients with dilated cardiomyopathy, moderate to severe FMR, an ejection fraction <40%, and a 6-minute walk distance between 150 and 450 m were enrolled in the CARILLON Mitral Annuloplasty Device European Union Study (AMADEUS). Percutaneous mitral annuloplasty was achieved through the coronary sinus with the CARILLON Mitral Contour System. Echocardiographic FMR grade, exercise tolerance, New York Heart Association class, and quality of life were assessed at baseline and 1 and 6 months. Of the 48 patients enrolled in the trial, 30 received the CARILLON device. Eighteen patients did not receive a device because of access issues, insufficient acute FMR reduction, or coronary artery compromise. The major adverse event rate was 13% at 30 days. At 6 months, the degree of FMR reduction among 5 different quantitative echocardiographic measures ranged from 22% to 32%. Six-minute walk distance improved from 307±87 m at baseline to 403±137 m at 6 months (P<0.001). Quality of life, measured by the Kansas City Cardiomyopathy Questionnaire, improved from 47±16 points at baseline to 69±15 points at 6 months (P<0.001).
Conclusions
Percutaneous reduction in FMR with a novel coronary sinus–based mitral annuloplasty device is feasible in patients with heart failure, is associated with a low rate of major adverse events, and is associated with improvement in quality of life and exercise tolerance.
Keywords: heart failure, mitral valve, regurgitation
Functional mitral regurgitation (FMR) is common in patients with advanced systolic heart failure.1 In addition to contributing to morbidity, increasing degrees of FMR have been shown to increase mortality.2 Medical therapy continues to be the standard of care for heart failure patients with FMR.3,4 For patients with FMR undergoing coronary artery bypass surgery, studies have demonstrated clinical benefit to surgical mitral valve repair.5 Ongoing debate surrounds the risk-to-benefit ratio of this approach in terms of its influence on morbidity and mortality.6,7 Although single-arm surgical studies have been conducted to characterize the effects of treating FMR in advanced heart failure patients,8,9 no prospective randomized studies exist to show the benefit of surgically treating FMR.
Increasing interest has been directed toward the nonsurgical management of valvular heart disease. Cardiac Dimensions Inc (Kirkland, Wash) developed a percutaneous, coronary sinus (CS)–based mitral annuloplasty device to treat FMR.10 The feasibility of using this device (the CARILLON Mitral Contour System) to treat FMR was evaluated in a prospective, multicenter, single-arm study, the CARILLON Mitral Annuloplasty Device European Union Study (AMADEUS).
Methods
The primary objective of AMADEUS was to evaluate the safety of deploying the CARILLON implant (Figure 1) in patients with FMR. Secondary objectives included an evaluation of long-term safety and an assessment of FMR reduction and hemodynamic and clinical changes. Hemodynamic changes and MR grade were characterized by echocardiography; clinical efficacy was assessed by changes in New York Heart Association (NYHA) classification, exercise tolerance, and quality of life.
Figure 1.
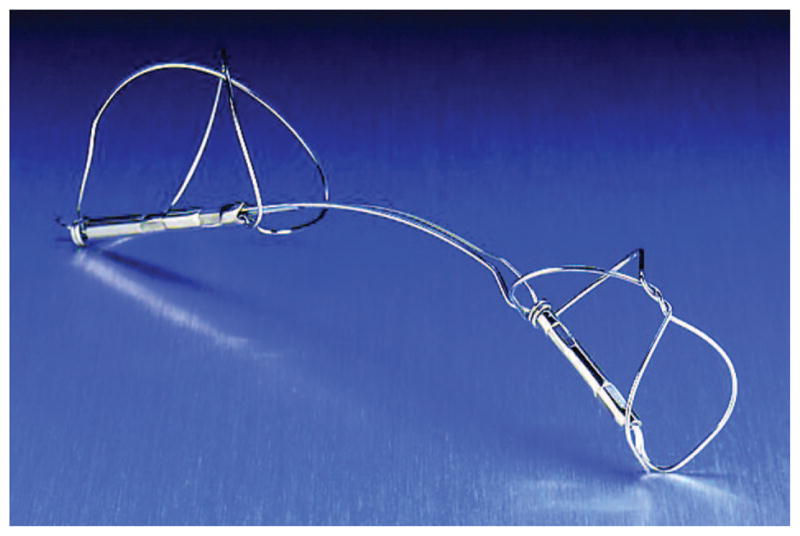
The CARILLON Mitral Contour System is a fixed-length, double-anchor, nitinol device designed to be positioned within the CS/GCV to reduce FMR. The arc of the nitinol ribbon, which connects the 2 anchors, serves to orient the device automatically during deployment.
Study Population
Patients with moderate heart failure were enrolled in this study. Key inclusion criteria included dilated ischemic or nonischemic cardiomyopathy, moderate to severe FMR, NYHA class II to IV, 6-minute walk distance between 150 and 450 m, left ventricular ejection fraction <40%, left ventricular end-diastolic diameter >55 mm, and age >18 years. Additional inclusion criteria stipulated that patients be on a stable heart failure medication regimen (ie, angiotensin-converting enzyme inhibitor [or angiotensin receptor blocker] plus a β-blocker for 3 months, with a stable dose for 1 month and an adjustable diuretic dose for 3 months) unless they had a documented intolerance to the medication. The trial investigators or their cardiology colleagues optimized the patients’ medication regimen.
Key exclusion criteria included hospitalization in the past 3 months for myocardial infarction, history of coronary artery bypass graft surgery, or unstable angina; percutaneous coronary intervention in the past 30 days; both moderate FMR (2+) and NYHA class II; requirement for cardiac surgery within 1 year; pacing lead in the CS; significant organic mitral valve pathology (eg, moderate or severe myxomatous degeneration or rheumatic disease); severe mitral annular calcification; compromised renal function as reflected by a serum creatinine >2.2 mg/dL; severe tricuspid regurgitation; and chronic atrial fibrillation.
Study Design
Prescreening for AMADEUS included an echocardiogram to rule out significant organic mitral valve pathology and to quantify FMR, ventricular size, and left ventricular ejection fraction. An independent echocardiography core laboratory reviewed all screening echocardiograms to qualify patients. Although a multidetector computed tomography scan initially served as a prescreening tool, the protocol was amended after the first 17 patients were enrolled to collect the data for information purposes only because no correlation was seen between anatomy and safety or efficacy.
Baseline data included an echocardiogram with quantitative and semiquantitative measures of FMR (vena contracta, effective regurgitant orifice area, regurgitant volume, and FMR jet area to left atrial area) and measures of cardiac dimensions (eg, left ventricular end-diastolic volume) and function (eg, ejection fraction).11–13 Functional assessments included NYHA class and 6-minute walk distance. Quality of life was assessed by both the Kansas City Cardiomyopathy Questionnaire and the patient component of the global assessment. Additional information included chest x-ray, ECG, cardiac enzymes, and concurrent medications. Baseline variables were reassessed at both 1 and 6 months.
Safety was evaluated by the 30-day rate of major adverse events. Major adverse events were defined as the composite end point of death, myocardial infarction, cardiac perforation necessitating catheter-based or surgical intervention, device embolization, or the occurrence of surgery or percutaneous coronary intervention related to device failure. Myocardial infarction was defined as a rise in creatine kinase-MB ≥3 times the upper limit of normal measured in 8-hour intervals for 24 hours (in all patients) after the procedure. No ECG changes or symptoms were required to make the diagnosis.
Implant Procedure
The implant procedure was done with general anesthesia to facilitate intraprocedural transesophageal echocardiography. A coronary arteriogram was performed to assess for underlying coronary arterial disease. The venous phase of the arteriogram served as a roadmap for CS cannulation.
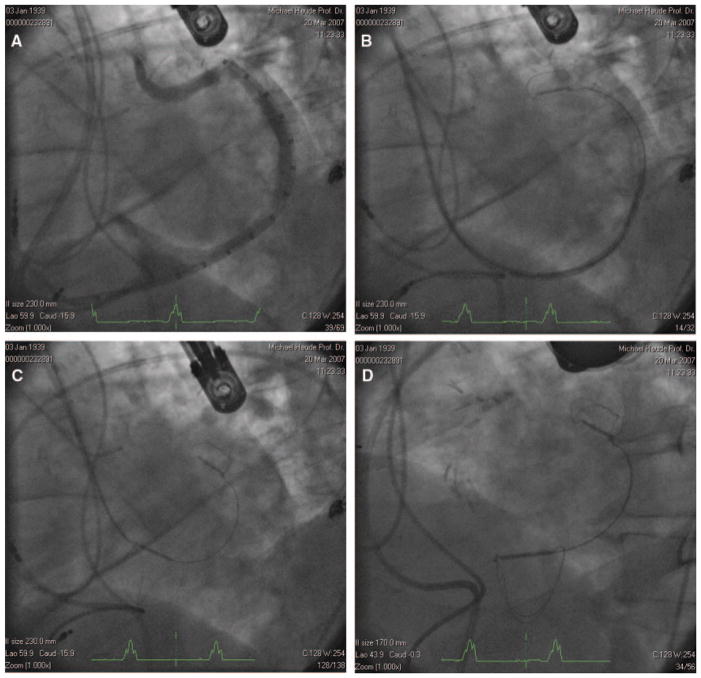
The CS was cannulated with standard techniques, and a 9F delivery catheter was positioned distal in the great cardiac vein (GCV); near the anterior commissure of the mitral valve. A venogram was performed to characterize the length of the CS/GCV and the diameter of the vein in the target location of the GCV and CS anchors (Figure 2A). Once the arteriovenous anatomy was characterized, an appropriately sized implant (CARILLON Mitral Contour System, Cardiac Dimensions) was selected. Deployment of the helical distal anchor involved 2 steps: retraction of the delivery catheter to allow passive expansion of the nitinol wire forms and advancement of the delivery catheter to expand the anchors to their maximum diameter. The anchors were oversized relative to the venous dimensions to apply a circumferential pressure and thereby ensure stable anchoring. After the distal anchor of the CARILLON device was deployed (Figure 2B), manual traction was placed on the delivery system to plicate the periannular tissue (Figure 2C). A combination of echocardiography and fluoroscopy was used to determine the final position of the proximal anchor. Because of the close proximity of the circumflex artery to the GCV, arteriograms were performed throughout the deployment sequence.
Figure 2.
A, Venogram demonstrating vein length and dimensions. B, Distal anchor deployed near the terminal aspect of the GCV. C, Traction applied to the delivery system pulls the proximal anchor toward the CS ostium, plicating the periannular tissue and reducing annular dimensions. D, Device deployed.
Before the implant was decoupled (Figure 2D), coronary arteriography was performed to confirm that coronary flow was not significantly compromised, and echocardiography was performed to confirm that a quantitative reduction in mitral regurgitation was achieved. In the event of coronary artery compromise or insufficient FMR reduction, the implant was recaptured by advancing the delivery catheter forward to collapse first the proximal anchor and then the distal anchor.
Before discharge, an echocardiogram and chest x-ray were performed, and blood samples were collected to evaluate complete blood count, creatine kinase, creatine kinase-MB, and creatinine. Patients who did not receive the CARILLON device were followed up through discharge and assessed for major adverse events at 30 days.
Echocardiographic Methodology
All screening, baseline, and follow-up echocardiograms were evaluated by level III cardiologists at the Echocardiographic Imaging Core Laboratory at Brigham and Women’s Hospital in Boston using comprehensive standard American Society of Echocardiography criteria.14 Regarding measures of FMR, the regurgitant jet area/left atrial area was determined from apical 2- and 4-chamber windows, vena contracta from optimized parasternal long-axis windows, and effective regurgitant orifice area and regurgitant volume from apical windows using a Nyquist limit of 30 to 40 cm/s. Left ventricular ejection fraction was determined from the modified Simpson formula, tracing endocardial borders for end-diastolic and end-systolic volumetric calculations. Mitral annular diameter was measured in the parasternal long-axis view at end diastole.
Independent Oversight
An independent data and safety monitoring board reviewed the data. An independent clinical events committee adjudicated all events contributing to the primary end point, and data coordination was performed by Harvard Clinical Research Institute (Boston, Mass). AMADEUS conformed to Good Clinical Practice guidelines and was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the competent authority and the respective ethics committees. Written informed consent was obtained from all subjects.
Statistical Analysis
For continuous variables, paired t test or signed-rank test was applied to assess change in time within 1 patient. One-way repeated-measures ANOVA and the Friedman test were applied for comparison between >2 points in time. For categorical variables, the Fisher exact test, χ2 test, Cochran-Mantel-Haenszel row mean score, or general association was used for comparison between 2 groups. All statistical testing was based on a 2-sided α=0.05 significance level. There was no imputation for missing data. The Seattle Heart Failure Model was used to estimate annual mortality at baseline before device implantation and at 6 months. Total cholesterol and percent lymphocytes were not collected. They were imputed to allow estimation of survival for all patients.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Patient Demographics
Of the 48 patients enrolled in AMADEUS, 30 received the CARILLON device (Figure 3). Of the remaining 18 patients, no implant was attempted in 5 patients for varied clinical reasons (ie, CS access–related dissection/perforation [n=3] and screen failure [n=2]), and the implant was recaptured in 13 patients. In 3 of these 13 patients, slipping of the distal anchor precluded device delivery. This early experience resulted in a modification to the distal anchor whereby the anchor wire forms were twisted at the apex to increase structural rigidity, thus improving anchoring. Only 1 patient was implanted with the original design. For the 10 patients in whom the CARILLON device was successfully deployed but subsequently recaptured, there were 2 major reasons for the recapture: insufficient FMR reduction and coronary artery compromise.
Figure 3.
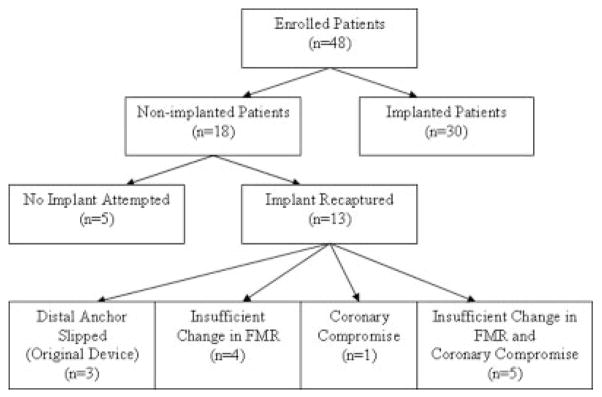
Enrollment tree for the intention-to-treat patient population.
The patient demographics for both the implanted and nonimplanted patients are delineated in Table 1. Most patients were in NYHA class III with a 6-minute walk distance consistent with moderate heart failure.15,16
Table 1.
Demographic Characteristics
| Patient Characteristic | Implanted Patients (n=30) | Nonimplanted Patients (n=18) | P |
|---|---|---|---|
| Age, mean (SD), y | 64 (9) | 65 (15) | 0.75 |
| Male, n (%) | 26 (87) | 14 (78) | 0.45 |
| NYHA class, n (%) | 0.86 | ||
| I | 0/30 (0) | 0/18 (0) | |
| II | 6/30 (20) | 3/18 (17) | |
| III | 22/30 (73) | 15/18 (83) | |
| IV | 2/30 (7) | 0/18 (0) | |
| 6 MWD, mean (SD), m | 307 (87) | 284 (104) | 0.42 |
| Medical history, n/N (%) | |||
| COPD | 6/28 (21) | 5/17 (29) | 0.72 |
| CAD | 25/30 (83) | 10/18 (56) | 0.05 |
| CABG | 11/30 (85) | 6/18 (56) | 0.55 |
| HF admissions in past 12 mo, mean (SD), n | 1.2 (1.5) | 1.5 (1.7) | 0.57 |
| Medications, n (%) | |||
| Diuretics | 25/30 (83) | 17/18 (94) | 0.39 |
| ACE inhibitor/ARB | 27/30 (90) | 18/18 (100) | 0.28 |
| β-Blocker | 29/30 (97) | 17/18 (94) | 1.00 |
| Echocardiographic parameters, mean (SD) | |||
| LVEDD, cm | 6.7 (0.75) | 6.6 (0.94) | 0.83 |
| Mitral annular diameter, cm | 4.2 (0.4) | 3.96 (0.45) | 0.06 |
| LVEF, mean (SD), % | 30 (8) | 29 (8) | 0.59 |
6 MWD indicates 6-minute walk distance; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; CABG, coronary artery bypass graft; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; LVEDD, left ventricular end-diastolic diameter; and LVEF, left ventricular ejection fraction.
Although there were no substantive differences between the implanted and nonimplanted patients from a demographic or echocardiographic standpoint, there were 2 salient differences from a procedural standpoint. Specifically, the device was positioned on average more distal in the CS/GCV in the 30 implanted patients (distal anchor positioned 96% of the distance into the CS/GCV) compared with the 10 patients in whom the device was recaptured (distal anchor positioned 78% of distance into the CS/GCV). Similarly, during the tensioning process to create tissue plication, the CS anchor was pulled closer toward the CS ostium in the implanted patients (4.8±1 cm of displacement) compared with the 10 nonimplanted (device recaptured) patients (3.5±2 cm of displacement) (P=0.097).
Safety
Two patients withdrew from the study before the 30-day follow-up. Six patients of a total of 46 (13%) in the intention-to-treat patient population experienced a total of 7 major adverse events (Table 2). One death occurred in a 56-year-old man with a history of 3-vessel coronary artery disease, chronic renal insufficiency, and chronic obstructive pulmonary disease who had a repeat coronary angiogram the day after the implant procedure to evaluate a rise in his creatine kinase-MB level from 6 to 92 U/L. No significant change in the coronary anatomy was identified; however, the patient developed acute renal failure presumed to be due to contrast-induced nephropathy. This patient died of multisystem organ failure 22 days after the index procedure.
Table 2.
Major Adverse Event Rate (Primary Safety End Point at 30 Days)
| Event | Intention to Treat (n=48*), % (n/N) |
|---|---|
| Death | 2.2 (1/46) |
| Myocardial infarction | 6.5 (3/46) |
| CS dissection/perforation | 6.5 (3/46) |
| Device embolization | 0.0 (0/46) |
| Surgery or PCI related to device | 0.0 (0/46) |
PCI indicates percutaneous coronary intervention.
Two patients withdrew from study before the 30-day follow-up.
Two other patients had a rise in creatine kinase-MB (ie, >3 times the upper limit of normal) after the implant procedure. There were no accompanying ECG changes or clinical symptoms in either patient, and their postprocedure clinical course was uncomplicated. In 1 patient, the proximal anchor of the CARILLON device was noted to cross a small side branch (<1 mm) of the right coronary artery that ran in the atrioventricular groove.
There was no evidence of late coronary compromise in any of the patients who received a permanent implant. Specifically, there were no hospitalizations for new myocardial infarction, and there were no ECG changes suggestive of chronic device-related coronary compromise.
Three patients in AMADEUS suffered either a CS perforation or dissection that qualified as a major adverse event. One patient had a dissection of the CS that resolved without any specific therapy. In 1 patient in whom the CS was perforated with a stiff guidewire, no therapy was needed, and there were no clinical sequelae with observation. The remaining patient in whom the CS was perforated after advancement of a diagnostic catheter required pericardial drainage. The 2 perforations occurred early in the study (first and fourth patients), and the resulting procedural insights were conveyed to all sites before subsequent cases.
Follow-up radiographs were performed to evaluate for device position and integrity. No indication of device movement (ie, migration or embolization) or fracture was observed.
Impact on Mitral Regurgitation
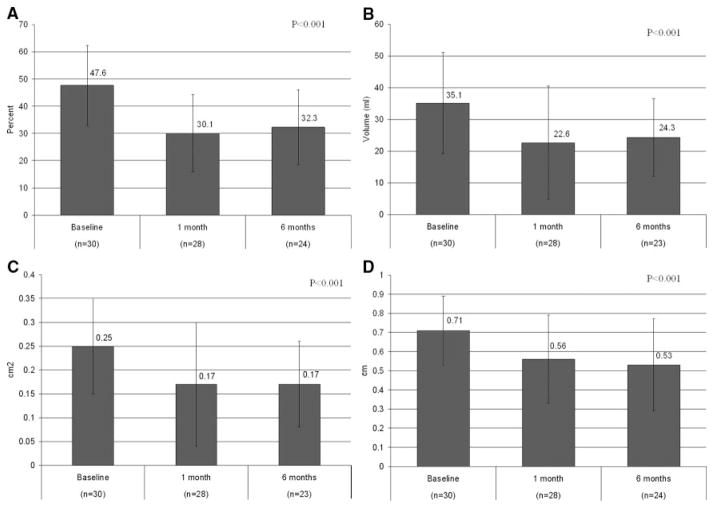
Degree of mitral regurgitation was assessed comprehensively according to strict American Society of Echocardiography standards. There was a statistically significant reduction (P<0.001) in each of the quantitative echocardiographic measures of mitral regurgitation, as illustrated in Figure 4. Across the different indexes, percent reduction between baseline and 6 months ranged from 22% to 32%, thus providing corroboration of similar degrees of reduction by independent methodologies. Of note, 6 data points were missing at the 6-month time point: 2 patients died; 1 patient (listed for transplant before implant procedure) received a transplant; and 3 patients declined to return for the 6-month follow-up visit. A subset analysis of FMR reduction limited to just those patients who had 6-month data (ie, 24 patients with baseline and 6-month data) revealed a 23% average MR reduction across the 5 quantitative echocardiographic measures.
Figure 4.
Comparison of mitral regurgitant jet area and left atrial area (A), regurgitant volumes (B), effective regurgitant orifice area (C), and vena contracta (D).
Although statistically significant reverse remodeling was not seen in the treated patient cohort, there was a trend toward reduced left ventricular end-diastolic volume and a statistically significant reduction in mitral annular diameter (Table 3). Importantly, there was no evidence of a decrease in left ventricular ejection fraction with a reduction in FMR.
Table 3.
Hemodynamic Changes for 30 Implanted Patients
| Parameter | Baseline (n=30) | At 1 mo (n=28) | At 6 mo (n=24) | P |
|---|---|---|---|---|
| LVEDD, cm | 6.7±0.8 | 6.7±0.8 | 6.6±0.7 | 0.92 |
| LVEDV, mL | 217±63 | 204±57 | 192±46 | 0.20 |
| LVEF, % | 29.8±8 | 30.7±8 | 30.8±10 | 0.54 |
| Mitral annular diameter, cm | 4.20±0.4 | 3.81±0.4 | 3.78±0.5 | <0.001 |
LVEDD indicates left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; and LVEF, left ventricular ejection fraction.
Clinical Efficacy
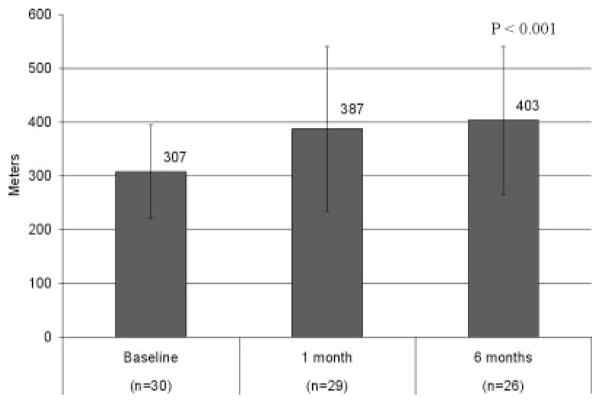
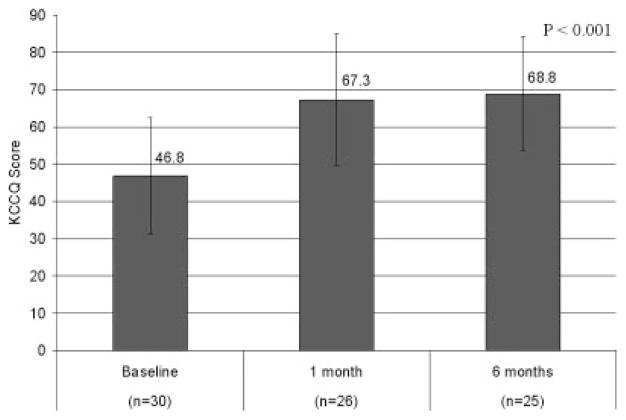
Functional improvement was assessed by a combination of NYHA class, exercise tests, and quality-of-life tools. The NYHA classification was reduced from an average of 2.9 at baseline to 1.8 at 6 months (P<0.001). For this categorical variable, 80% of patients were in NYHA class III or IV at baseline, whereas 88% were in NYHA class I or II at the 6-month follow-up. The class-specific reductions are tabulated in Table 4. The 6-minute walk improved from a baseline of 307±87 to 403±137 m (P<0.001; Figure 5). The results of the Kansas City Cardiomyopathy Questionnaire Overall Summary Score improved from 47 to 69 between baseline and 6 months and are tabulated in Figure 6. The patient component of the global assessment is summarized in Table 5. Of note, 84% of patients reported some degree of improvement between baseline and 6 months, ranging from slight (24%) to marked (36%).
Table 4.
Changes in NYHA Classification
| Parameter | Baseline (n=30), % (n/N) | At 1 mo (n=29), % (n/N) | At 6 mo (n=26), % (n/N) | P |
|---|---|---|---|---|
| NYHA class | <0.001 | |||
| I | 0 (0/30) | 17 (5/29) | 36 (9/25) | |
| II | 20 (6/30) | 62 (18/29) | 52 (13/25) | |
| III | 73 (22/30) | 17 (5/29) | 12 (3/25) | |
| IV | 7 (2/30) | 3 (1/29) | 0 (0/25) |
Figure 5.
Results of 6-minute walk test.
Figure 6.
Changes in Kansas City Cardiomyopathy Questionnaire (KCCQ) before and after CARILLON implantation.
Table 5.
Patient Component of the Global Assessment
| Patient Portion of the Global Assessment Score | At 1 mo (n=26), % (n/N) | At 6 mo (n=26), % (n/N) |
|---|---|---|
| Markedly improved | 26.9 (7/26) | 36.0 (9/25) |
| Moderately improved | 30.8 (8/26) | 24.0 (6/25) |
| Slightly improved | 19.2 (5/26) | 24.0 (6/25) |
| No change | 11.5 (3/26) | 8.0 (2/25) |
| Slightly worse | 7.7 (2/26) | 8.0 (2/25) |
| Moderately worse | 0.0 (0/26) | 0.0 (0/25) |
| Markedly worse | 3.8 (1/26) | 0.0 (0/25) |
Mortality Prediction
Because patient management is facilitated by predictions of improved survival based on therapeutic interventions, heart failure models have been developed to predict the risk of death in specific populations. The Seattle Heart Failure Model was derived recently from a cohort of 1125 heart failure patients and was prospectively validated in 5 additional cohorts totaling 9942 heart failure patients and 17 307 person-years of follow-up.17 The multivariate risk model is designed to allow estimation of the benefit of adding medications or devices to an individual patient’s therapeutic regimen. An analysis of the baseline characteristics of the AMADEUS patients by the Seattle Heart Failure Model predicts an annual mortality of 10%, consistent with moderate heart failure patients who received a cardiac resynchronization therapy device in the Cardiac Resynchronization in Heart Failure (CARE-HF) study.18 An analysis of the 6-month data characteristics predicts an estimated annual mortality of 7% (31% relative reduction compared with baseline; P=0.004).
Discussion
The main findings of the present study are that CS-based annuloplasty for FMR is feasible, is associated with a low rate of major adverse events, and results in improved exercise tolerance and quality of life.
This study is the first to address the potential role of percutaneous intervention designed specifically to reduce FMR in patients with heart failure. Although no prospective surgical trials have been conducted to evaluate the effects of mitral annuloplasty in isolation on patients with FMR, several complementary pieces of data support the use of interventions that may reduce FMR. For example, Badhwar and Bolling8 showed that surgical reduction in FMR with an undersized flexible annuloplasty ring was associated with an improvement in NYHA (3.2 to 1.8) and an improvement in ejection fraction, cardiac output, and end-diastolic volumes. A recent analysis of echocardiographic data from the Valsartan in Acute Myocardial Infarction (VALIANT) trial showed that a 1% increase in FMR jet area/left atrial area increased the odds of death or hospitalization for heart failure.19
Despite the superior and variable position of the CS relative to the mitral valve annulus, FMR reduction was achievable in the majority of patients. Using a series of echocardiographic assessment tools to quantify the influence of device placement on the severity of mitral regurgitation, we showed that the degree of FMR reduction ranged from 22% to 32% at 6 months. These assessments, which were performed at rest, included multiple semiquantitative and quantitative measurements and were facilitated by an independent core laboratory. Given the dynamic nature of FMR,20–22 the changes in resting FMR may underestimate the magnitude of the change that might be seen under conditions of exercise and stress, possibly correlating with the marked improvement in the 6-minute walk seen in our study. Other approaches such as cardiac resynchronization therapy are known to improve FMR and have been shown to attenuate the effect of exertion on MR severity.23
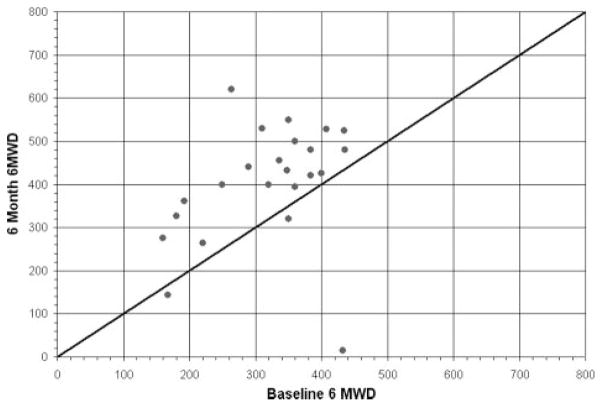
Our study showed that device placement was accompanied by a marked improvement in 6-minute walk distance. Although several potential limitations are recognized with the 6-minute walk test, it continues to be widely used in evaluating the impact of interventions on heart failure.24–26 In the present study, we observed an average improvement in the 6-minute walk of 96 m, in contrast to the 39-m improvement seen in the intervention group of the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) trial for cardiac resynchronization. The present study is limited by the fact that a control group was not included, limiting our ability to account for any potential placebo effect. Of note, however, in the MIRACLE trial, the placebo effect on 6-minute walk was an improvement of 10 m (95% confidence interval, 0 to 25 m).25 In contrast, in our study, 19 of the 23 patients with 6-month follow-up had ≥25-m improvement in their 6-minute walk (Figure 7), greater than that which could reasonably be attributed to a placebo effect. In support of these observations, we noted similar quality-of-life trends in the 7-point Likert score of the patient component of the global assessment in which 84% of patients showed various degrees of symptom improvement. Furthermore, the Kansas City Cardiomyopathy Questionnaire27 documented a 22-point change in overall qualify-of-life improvement. The clinical significance of a change of this magnitude is underscored by a recent study that showed that a 5-point change in patients with heart failure after acute myocardial infarction correlated with both all-cause mortality and cardiovascular mortality or hospitalization.28
Figure 7.
Plot of 6-minute walk distance (6 MWD) at 6 months follow-up compared to baseline, with line of identity depicting no change. As shown, most patients had a significant improvement in 6 MWD at 6 months.
The existence of a subset of nonresponders in our study is not surprising, given reports that surgical annuloplasty has an 80% responder rate29 and cardiac resynchronization therapy has a comparable responder rate.18,25,26 Interestingly, neither demographic nor echocardiographic parameters were clearly predictive of procedural success. Rather, the procedural steps of placing the device further distal in the CS/GCV and applying more traction to plicate more tissue were associated with procedural success. With additional clinical experience, benefits such as reverse remodeling may be found to occur in selected patients treated with percutaneous mitral annuloplasty. Reports from Braun et al7 have shown that reverse remodeling after surgical annuloplasty and surgical revascularization was associated with an left ventricular end-diastolic diameter <65 mm.
In the present study, the observed major adverse events confirm that there is a learning curve to access the CS (similar to that seen in the cardiac resynchronization therapy literature25,26), but once the procedural skills are acquired, the risks for this therapy are low. Careful management of this high-risk patient population during the procedure (eg, judicious contrast use, modest fluid administration) is important. A separate area of potential concern has been the possibility of coronary arterial impingement by a device placed under tension in the CS.10 Careful assessment of coronary arterial flow is required in the event that device recapture and repositioning are required. Coronary arteries were crossed in 36 of the 43 implant attempts. For the 17% of implants in which a significant compromise of a coronary artery was observed, the device was recaptured without sequelae. Chronic device-related adverse events, including clinical ischemic events or myocardial infarction, were not identified in any patients.
The present study was a first-in-human feasibility and safety trial. Limitations include the lack of a randomized, blinded control group with whom to compare safety and efficacy results. Future studies may evaluate not only the long-term durability of this type of therapy but also the merits of improved patient selection and optimal timing of intervention.
Conclusions
These data show for the first time that percutaneous placement of a mitral annuloplasty device based in the CS (CARILLON Mitral Contour System) has the potential to safely and significantly treat FMR in patients with heart failure with resultant symptomatic benefit. Further studies are required to define the long-term efficacy of the therapy, optimal timing for intervention, and effects on survival.
CLINICAL PERSPECTIVE.
Functional mitral regurgitation is common in patients with heart failure and is associated with increased morbidity and mortality. However, its specific contribution to the heart failure syndrome and its progression is not completely understood. Despite the frequent occurrence of moderate to severe functional mitral regurgitation, surgery is rarely performed because of risk per se and the presence of major comorbidities. The impact of medical therapy on functional mitral regurgitation is variable. The CARILLON Mitral Annuloplasty Device European Union Study (AMADEUS) is a single-arm study designed to evaluate the safety and efficacy of a novel percutaneous mitral annuloplasty device. Thirty patients implanted with the device experienced significant improvements in functional capacity and quality of life with reduced mitral regurgitation over a 6-month follow-up period. This study supports the rationale for intervening on functional mitral regurgitation in heart failure patients with further evaluation in appropriate randomized studies.
Acknowledgments
Source of Funding
This work was funded by Cardiac Dimensions Inc, Kirkland, Wash.
Footnotes
Clinical trial registration information: trial received regulatory approval January 14, 2005.
Disclosures
Drs Wu and Kwong worked in the echocardiography and computed tomography core laboratories, respectively. Drs Levy and Kaye are consultants with stock options in Cardiac Dimensions. Drs Van Bibber, Goldberg, and Reuter are employees of Cardiac Dimensions and hold stock options. The remaining authors report no conflicts.
References
- 1.Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10:285–291. doi: 10.1016/j.cardfail.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538–543. doi: 10.1016/s0002-9149(02)03301-5. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Valvular Heart Disease) Circulation. 2006;114:450–527. [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: summary article: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:1116–1143. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Bax JJ, Braun J, Somer ST, Klautz R, Holman ER, Versteegh MIM, Boersma E, Schalij MJ, van der Wall EE, Dion RA. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation. 2004;110(suppl):II-103–II-108. doi: 10.1161/01.CIR.0000138196.06772.4e. [DOI] [PubMed] [Google Scholar]
- 6.Gangemi JJ, Tribble CG, Ross SD, McPherson JA, Kern JA, Kron IL. Does the additive risk of mitral valve repair in patients with ischemic cardiomyopathy prohibit surgical intervention? Ann Surg. 2000;231:710–714. doi: 10.1097/00000658-200005000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun J, Bax JJ, Versteegh MIM, Voigt PG, Holman ER, Klautz RJM, Boersma E, Dion RAE. Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg. 2005;27:847–853. doi: 10.1016/j.ejcts.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Badhwar V, Bolling SF. Mitral valve surgery in the patient with left ventricular dysfunction. Semin Thorac Cardiovasc Surg. 2002;14:133–136. doi: 10.1053/stcs.2002.32314. [DOI] [PubMed] [Google Scholar]
- 9.Shah AS, Hannish SA, Milano CA, Glower DD. Isolated mitral valve repair in patients with depressed left ventricular function. Ann Thorac Surg. 2005;80:1309–1314. doi: 10.1016/j.athoracsur.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Maniu CV, Patel JB, Reuter DG, Meyer DM, Edwards WD, Rihal CS, Redfield MM. Acute and chronic reduction of functional mitral regurgitation in experimental heart failure by percutaneous mitral annuloplasty. J Am Coll Cardiol. 2004;44:1652–1661. doi: 10.1016/j.jacc.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 11.Enriquez-Sarano M, Seward JB, Bailey KR, Tajik AJ. Effective regurgitant orifice area: a noninvasive Doppler development of an old hemodynamic concept. J Am Coll Cardiol. 1994;23:443–451. doi: 10.1016/0735-1097(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 12.Enriquez-Sarano M, Miller FA, Jr, Hayes SN, Bailey KR, Tajik AJ, Seward JB. Effective mitral regurgitant orifice area: clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol. 1995;25:703–709. doi: 10.1016/0735-1097(94)00434-R. [DOI] [PubMed] [Google Scholar]
- 13.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 14.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 15.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M. Prediction of mortality and morbidity with a six minute walk test in patients with left ventricular dysfunction: SOLVD Investigators. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 16.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 17.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 18.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 19.Amigoni M, Meris A, Thune JJ, Mangalat D, Skali H, Bourgoun M, Warnica J, Barvik S, Arnold M, Velazquez E, Werf F, Ghali J, McMurray J, Kober L, Pfeffer M, Solomon SD. Mitral regurgitation in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: prognostic significance and relation to ventricular size and function. Eur Heart J. 2007;28:326–333. doi: 10.1093/eurheartj/ehl464. [DOI] [PubMed] [Google Scholar]
- 20.Pierard LA, Lancellotti P. The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. N Engl J Med. 2004;351:1627–1634. doi: 10.1056/NEJMoa040532. [DOI] [PubMed] [Google Scholar]
- 21.Lebrun F, Lancellotti P, Pierard LA. Quantitation of functional mitral regurgitation during bicycle exercise in patients with heart failure. J Am Coll Cardiol. 2001;38:1685–1692. doi: 10.1016/s0735-1097(01)01605-9. [DOI] [PubMed] [Google Scholar]
- 22.Keren G, Katz S, Strom J, Sonnenblick EH, LeJemtel TH. Dynamic mitral regurgitation: an important determinant of hemodynamic response to load alterations and inotropic therapy in severe heart failure. Circulation. 1989;80:306–313. doi: 10.1161/01.cir.80.2.306. [DOI] [PubMed] [Google Scholar]
- 23.Lancellotti P, Melon P, Sakalihasan N, Waleffe A, Dubois C, Bertholet M, Pierard L. Effect of cardiac resynchronization therapy on functional mitral regurgitation in heart failure. Am J Cardiol. 2004;94:1462–1465. doi: 10.1016/j.amjcard.2004.07.154. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 26.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 27.Green PC, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 28.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, Spertus JA. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115:1975–1981. doi: 10.1161/CIRCULATIONAHA.106.670901. [DOI] [PubMed] [Google Scholar]
- 29.Zhu F, Otsuji Y, Yotsumoto G, Yuasa T, Ueno T, Yu B, Koriyama C, Hamasaki S, Biro S, Kisanuki A, Minagoe S, Levine R, Sakata R, Tei C. Mechanism of persistent ischemic mitral regurgitation after annuloplasty: importance of augmented posterior mitral leaflet tethering. Circulation. 2005;112(suppl):I-396–I-401. doi: 10.1161/CIRCULATIONAHA.104.524561. [DOI] [PubMed] [Google Scholar]