Abstract
Objective
To determine if bacterial colonization of drains can be reduced by local antiseptic interventions.
Summary Background
Drains are a potential source of bacterial entry into surgical wounds and may contribute to surgical site infection (SSI) after breast surgery.
Methods
Following IRB approval, patients undergoing total mastectomy and/or axillary lymph node dissection were randomized to standard drain care (control) or drain antisepsis (treated). Standard drain care comprised twice daily cleansing with alcohol swabs. Antisepsis drain care included 1) a chlorhexidine disc at the drain exit site and 2) irrigation of the drain bulb twice daily with dilute sodium hypochlorite (Dakin’s) solution. Cultures results of drain fluid and tubing were compared between control and antisepsis groups.
Results
Overall, 100 patients with 125 drains completed the study with 48 patients (58 drains) in the control group and 52 patients (67 drains) in the antisepsis group. Cultures of drain bulb fluid at one week were positive (1+ or greater growth) in 66% (38/58) of control drains compared to 21% of antisepsis drains (14/67), (p=0.0001). Drain tubing cultures demonstrated >50 CFU in 19% (8/43) of control drains versus 0% (0/53) of treated drains (p=0.004). SSI was diagnosed in 6 patients (6%) - 5 patients in the control group and 1 patient in the antisepsis group (p=0.06).
Conclusions
Simple and inexpensive local antiseptic interventions with a chlorhexidine disc and hypochlorite solution reduce bacterial colonization of drains. Based on these data, further study of drain antisepsis and its potential impact on SSI rate is warranted.
Introduction
Surgical site infection (SSI) rates after mastectomy are higher than expected for clean procedures, with reported rates of up to 26%.1–6 In addition to patient risk factors for infection such as obesity and smoking,7, 8 the presence of a surgical drain and its prolonged use are also associated with increased infection risk.5, 9, 10
Some surgeons recommend postoperative antibiotic prophylaxis until drains are removed, but this practice may select for resistant organisms without impacting the infection rate.11–13 In addition, prolonged antibiotic use carries other consequences including allergic reactions, gastrointestinal intolerance, yeast infections, and Clostridium difficile infection.14 We hypothesize that bacterial colonization of surgical drains contributes to SSI after breast surgery and that measures of local antisepsis are likely to be well-tolerated and effective at reducing drain colonization and SSI rates. Therefore, we undertook a proof of principle, prospective, surgeon-blinded, randomized controlled trial to determine if simple local antisepsis measures can effectively reduce bacterial colonization of drains after breast surgery.
Methods
Study population
Following approval by the Mayo Clinic Institutional Review Board, eligible subjects were recruited prospectively from the breast surgical practice at Mayo Clinic in Rochester, MN between January 2009 and May 2011. Individuals were included if undergoing total mastectomy (TM) and/or axillary lymph node dissection (ALND) for benign or malignant disease in which surgical drains were placed. Subjects were ineligible if they were pregnant, had received antibiotics within 14 days prior to surgery, had a known allergy to chlorhexidine, or were undergoing immediate reconstruction (because of the common use of postoperative antibiotics in this subgroup).
Randomization
Following informed consent, subjects were randomized to either the standard drain care regimen or the drain antisepsis regimen by a computerized randomization program, using dynamic allocation and stratifying by surgical procedure (mastectomy or lumpectomy with axillary dissection), surgeon, and body mass index (BMI<30 or ≥30). In the event of bilateral procedures, patients with a unilateral cancer had sample collection and analysis from the side affected with cancer. Subjects who had bilateral cancer or bilateral prophylactic mastectomies underwent computerized randomization to select the side to be evaluated on study. The operating surgeon remained blinded to the assigned treatment arm.
Perioperative standardization
All subjects received a single dose of intravenous preoperative cefazolin (dosed per weight) within 30 minutes prior to skin incision. In the case of cefazolin allergy, vancomycin or levofloxacin were alternates, and antibiotics were redosed intraoperatively when appropriate. A chlorhexidine/ alcohol skin prep (ChloraPrep®, CareFusion Corporation, San Diego, CA) or iodine/alcohol (DuraPrep™, 3M™, St. Paul, MN) was used per surgeon preference. The surgical drainage tube used was a 15Fr round channel hubless drain secured with nonabsorbable monofilament suture. Antibiotics were not permitted beyond 24 hours after surgery. Patients could shower 48 hours after surgery, but immersion bathing was not permitted.
Drain care regimens
Study subjects and family members received personal instruction on drain care on the first postoperative day by the nurse study coordinator, and they were advised not to divulge the drain care regimen to their surgeon. All subjects in both treatment arms were instructed to strip the drain tubing, empty the bulb, and record fluid volume at least twice daily. Individuals assigned to standard drain care were also advised to cleanse the drain exit site with prepackaged 70% isopropyl alcohol wipes twice daily and to cover the drain site with a sterile gauze dressing. Subjects in the drain antisepsis arm were shown how to cleanse the drain exit site with alcohol, then apply a chlorhexidine gluconate disc dressing (Biopatch®, Johnson & Johnson Medical) at the drain site and secure it with an adherent sterile transparent occlusive dressing (Figure 1). The chlorhexidine disc and occlusive dressing were changed every three days until drain removal. In addition, subjects in the drain antisepsis arm were instructed to perform antiseptic irrigation of the drainage bulb twice daily as follows: instill 10 milliliters of dilute Dakin’s solution (0.0125% buffered sodium hypochlorite) into the drainage bulb via the exit valve (prepped with alcohol), then swish occasionally over 10 minutes, then empty and return the bulb to suction. At time of showering the chlorhexidine disc/occlusive dressing was left intact.
FIGURE 1.
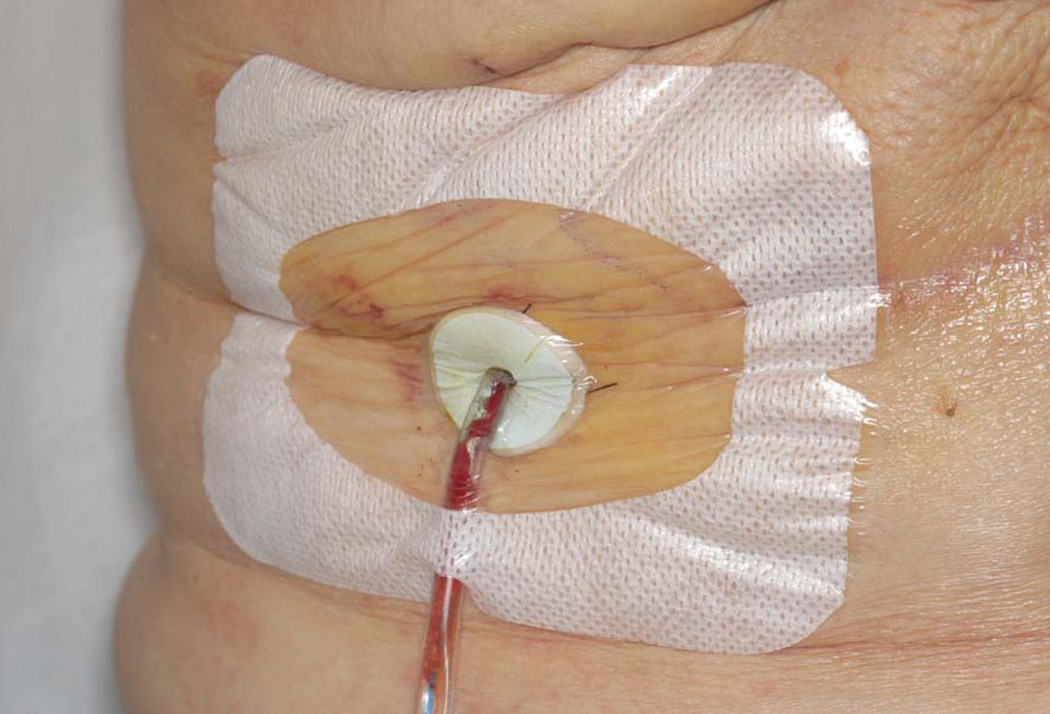
Chlorhexidine disc dressing with occlusive adherent dressing.
Management of multiple drains
If more than one drain was placed per surgical site (i.e. two drains in the setting of a modified radical mastectomy), then both drains associated with that surgical site were treated according to the assigned treatment arm. Each drain associated with the surgical site was evaluated separately for bacterial colonization endpoints.
Follow-up visits and cultures
For 30 days after operation, a standardized data collection form was completed at every follow-up visit, with details including drainage volume, erythema or skin changes at the incision and drain sites, and evidence of seroma or infection. In addition, the medical record was reviewed to screen for late infections. Blinding of the surgeon was maintained at postoperative visits by the study coordinator removing all dressing materials before surgeon evaluation. A mandatory follow-up visit occurred at one week [on postoperative day (POD) 7 +/− one day] for study cultures and for clinical evaluation for signs of infection or adverse reactions to drain antisepsis regimen. If drains were ready for removal prior to this, all cultures were obtained on the day of drain removal. At each visit, compliance with the antiseptic interventions was assessed by the study coordinator, who asked the subject if they had any difficulties that prevented completing the prescribed regimen. For patients with clinical evidence of infection, diagnostic cultures and antibiotic therapy were performed per routine clinical care. Guidelines for drain removal specified output ≤30 ml/24 hours for two consecutive days or at POD19, whichever came first. If drain removal did not occur at the one week visit, then a repeat culture of drain fluid was obtained on the day of drain removal; therefore some subjects had drain fluid cultures at two timepoints. Drain tubing cultures at removal were added to the protocol after the study was underway and are available in 76/100 patients.
Microbiology
At the one week visit, a 2 mL sample of drain fluid from the bulb was obtained aseptically for semiquantitative aerobic and anaerobic cultures. On the day of drain removal, cultures were obtained of both drain bulb fluid and drain tubing. Drains were removed in a sterile fashion after chlorhexidine preparation and sterile draping of the drain exit site. A 5 cm portion of the subcutaneous drain tubing was harvested, starting approximately 1–2 cm internal to the skin exit site.
For drain fluid culture, 1–2 drops of fluid was inoculated onto sheep blood, eosin methylene blue, and colistin-nalidixic acid agar plates, and anaerobic sheep blood agar plates, and 1 ml of drain fluid was inoculated into thioglycollate broth. The aerobic agar plates were incubated at 35°C in 5–7% CO2 for 4 days, or until positive. The anaerobic agar plate was incubated anaerobically for 7 days, or until positive. The thioglycollate broth was incubated anaerobically for 14 days, or until positive. Growth was reported as negative, growth from broth only, or if there was growth on a plate it was quantitated as 1+, 2+, 3+ or 4+ according to standardized laboratory protocol. All isolates were speciated.
For drain tubing culture, the tube was rolled over the surface of a sheep blood agar plate four times in different directions,15 and the plate incubated aerobically at 35°C in 5–7% CO2 for 4 days, or until positive. Growth was identified and reported semiquantitatively as <10 colony forming units (CFU), 10–19 CFU, 20–50 CFU, 51–100 CFU or >100 CFU. Laboratory personnel were blinded to patients’ drain care regimens, and results of study cultures were not reported or included in the participants’ medical records.
Endpoints and statistical power
The primary endpoint of the study was bacterial growth in the fluid of the drainage bulb at the one week follow-up visit. Estimating a bacterial colonization rate of 33% in drainage fluid at one week, a sample size of 100 was projected to provide 80% power to detect a 70% reduction in colonization with antisepsis measures. A drain fluid culture with bacterial growth of 1+ or greater was defined as positive based on the assumption that growth from broth only should not be clinically meaningful. A positive drain tubing culture was defined as growth of greater than 50 CFU, based upon prior published data demonstrating catheter site inflammation in a majority of subjects with >50 CFU.15 Given the limitations in selecting these cutoffs, we examined endpoints not dependent on the chosen cutoff for positivity; the ordinal quantification of degree of colonization was also analyzed for both drain fluid and drain tubing cultures. In samples colonized with multiple organisms, the highest degree of quantification across all organisms was used to classify the sample for analysis. SSIs included any of the following within 30 days after operation: purulent drainage, positive aseptically collected culture from the wound, signs of inflammation with opening of incision and absence of a negative culture, or physician diagnosis of infection (which could include cellulitis). Cases of equivocal SSI were reviewed in detail by the research team without knowledge of the assigned treatment arm and were decided by consensus.
Statistical Analysis
Two-sample comparisons at the per patient level were performed using two-sample t-tests or Wilcoxon rank-sum tests for continuous and ordinal variables and likelihood ratio chi-square tests for nominal variables. Drain duration and volume were compared using linear mixed effects models to account for multiple drains within patient. Colonization rates were analyzed on a per drain level with generalized linear mixed models (random intercept logistic regression) to account for the non-independence of multiple drains from the same patient. Ordinal colonization quantification levels were compared between treatment arms using a generalized estimating equations approach to fit ordinal logistic regression. A per patient analysis of drain colonization was also performed, as was a comparison of SSI rates, using chi-square tests. The sign test for paired proportions was used to compare positivity rates between mastectomy and axillary drains in patients who had both. P-values < 0.05 were considered statistically significant. Analysis was performed using SAS (Version 9.2, SAS Institute Inc., Cary, NC).
Results
One hundred thirteen patients were enrolled with 13 of them excluded prior to study completion for various reasons including: difficulties with returning for drain removal (travel distance/bad weather (6), patient consented but changed their mind on POD1 (3), patient prescribed antibiotics between time of consent and surgery (2), patient had immediate reconstruction (1), and significant language barrier (1).
The remaining100 patients with 125 drains completed the study. Forty-eight women (58 drains) were randomized to the control group and 52 women (67 drains) to the antisepsis group. The control and antisepsis groups were similar with respect to age, BMI, ASA class, prior chemotherapy or radiation, smoking, preoperative skin prep, operative time, day of drain removal and drain volume (see Table 1). Total mastectomy +/− sentinel node biopsy, axillary node dissection, and modified radical mastectomy were performed in 66, 6, and 28 patients respectively. Median duration of drain use was 7 days (range 4–19 days), with a median output of 23 ml (range 3–136 ml) for the preceding 24 hours at the one week visit and a median of 19 ml (range 3–57 ml) for the 24 hours prior to drain removal.
Table 1.
Characteristics of the study cohort.
| Control | Antisepsis | p-value* | |
|---|---|---|---|
| Subjects (n) | 48 | 52 | |
| Number of drains | 58 | 67 | |
| Number of subjects with two drains (%) | 10 (21%) | 15 (29%) | 0.36 |
| Number of subjects with axillary drain (%) | 13 (27%) | 18 (35%) | 0.42 |
| Age, years, mean (range) | 61.6 (39–84) | 61.5 (32–82) | 0.96 |
| BMI, mean (range) | 27.8 (16–45) | 27.3 (15–42) | 0.65 |
| ASA Class, n (%) | 0.24 | ||
| I | 5 (10%) | 10 (19%) | |
| II | 37 (77%) | 37 (71%) | |
| III | 5 (10%) | 5 (10%) | |
| IV | 1 (2%) | 0 | |
| Neoadjuvant chemotherapy, n (%) | 9 (19%) | 7 (13%) | 0.47 |
| Prior radiation treatment, n (%) | 4 (8%) | 3 (6%) | 0.62 |
| Smoking within 4 weeks preop, n (%) | 4 (8%) | 4 (8%) | 0.91 |
| Operation, n (%) | 0.28 | ||
| Lumpectomy / axillary dissection | 3 (6%) | 1 (2%) | |
| Mastectomy | 5 (10%) | 5 (10%) | |
| Mastectomy + sentinel node | 29 (60%) | 27 (52%) | |
| Modified radical mastectomy | 11 (23%) | 17 (33%) | |
| Axillary dissection | 0 | 2 (4%) | |
| Preoperative skin prep, n (%) | 0.97 | ||
| Alcohol/ chlorhexidine | 38 (79%) | 41 (79%) | |
| Alcohol/ iodine | 10 (21%) | 11 (21%) | |
| Operative time, hours:minutes | 0.56 | ||
| Mean | 2:28 | 2:34 | |
| Median | 2:17 | 2:27 | |
| Range | 1:12–5:13 | 1:14–4:45 | |
| Drain removal day (POD)† | 0.15 | ||
| Mean | 8.2 | 9.1 | |
| Median | 7 | 7 | |
| Range | 5–16 | 4–19 | |
| 24-hour drain volume at one week†ml | 0.44 | ||
| Mean | 26.6 | 29.6 | |
| Median | 23 | 24 | |
| Range | 5–65 | 3–136 | |
| 24-hour drain volume prior to drain removal†ml |
0.81 | ||
| Mean | 20.5 | 20.1 | |
| Median | 19 | 19 | |
| Range | 5–57 | 3–45 |
P-values were calculated using either two-sample t-tests (per patient comparisons) or linear mixed models (per drain comparisons) for continuous variables, the Wilcoxon rank-sum test for ordinal ASA class, and likelihood-ratio chi-square tests for nominal variables.
Including all drains (n = 58 Control, n = 67 Antisepsis).
Colonization of drain fluid at one week
Cultures of drain bulb fluid at one week in the treated group showed significantly less bacterial growth compared to the control group. At the cutoff of 1+ or greater growth for drain fluid cultures, 21% (14/67) of treated drains were positive compared to 66% (38/58) of control drains (p=0.0001). Analysis performed on a per patient basis showed a similar result with 13/52 (25%) of patients on the antisepsis arm experiencing 1+ or greater colonization on any drain compared to 31/48 (65%) on the control arm (p<0.0001). To examine an analysis not dependent on the choice of cutoff, the ordinal quantification result was also compared between the two groups (Table 2) and again demonstrated strong statistical significance (p<0.0001). In drains removed after the one week visit, a second culture was obtained at drain removal. All drains positive (≥1+) at one week were also positive at drain removal, with at least one organism in common between the two cultures for 11/14 (79%).
Table 2.
Concentration of bacterial colonization for drain fluid at one week and drain tubing at the time of drain removal, analyzed per drain. Samples with multiple organisms were classified according to the highest level of colonization across all organisms. (CFU= colony forming units)
| Drain Fluid POD6–8 | Drain Tubing | ||||
|---|---|---|---|---|---|
| Growth | Control (n=58) |
Antisepsis (n=67) |
CFU | Control (n=43) |
Antisepsis (n=53) |
| 0 | 14 | 49 | 0 | 30 | 47 |
| Broth | 6 | 4 | <10 | 3 | 4 |
| 1+ | 16 | 10 | 10–19 | 0 | 1 |
| 2+ | 8 | 1 | 20–50 | 2 | 1 |
| 3+ | 5 | 1 | 51–100 | 3 | 0 |
| 4+ | 9 | 2 | >100 | 5 | 0 |
Colonization of drain tubing
Drain tubing was cultured at time of drain removal from 76 subjects (96 drains- 43 control and 53 antisepsis). Using a cutoff value of 50 CFU, drain tubing cultures were positive in 0% (0/53) of treated drains compared to 19% (8/43) of control drains (p=0.004). In a per patient analysis, 0/40 patients in the antisepsis group demonstrated >50 CFU colonization for any drain compared to 7/36 (19%) of patients in the control arm (p=0.0008). Treating degree of colonization as an ordinal variable (see Table 2) also resulted in a statistically significant difference between the treatment arms (p=0.04). Subjects in the drain antisepsis group were much less likely to have high levels of bacterial colonization in the drain fluid, and none of them had >50 CFU from drain tubing. Among drains with positive bulb fluid cultures (≥1+) at the time of drain removal, the drain tubing was also positive (>50 CFU) in 28% of control drains versus 0% of antisepsis drains. Conversely, among the 8 drains (7 patients) with positive tubing cultures (all in the control group), all 8 had ≥1+ growth in drain fluid, and 7 of 8 had the same organism in both fluid and tubing.
Multiple drains
Twenty-five patients (10 control,15 antisepsis) had two ipsilateral drains. Regarding fluid cultures, the two bulb fluid cultures at one week were concordant for 20 drain pairs (12 negative, 8 positive) and discordant for 5; 4/5 discordant drain pairs were positive (≥1+) for the mastectomy drain fluid but not the axillary drain fluid. The difference in positivity rate was not significant (p=0.38), and the kappa agreement statistic was 0.60. Similar results were observed for tubing cultures among the 20 cases with both a mastectomy drain and an axillary drain. The tubing culture results were concordant for 19 pairs (18 with both drain tubing cultures negative, 1 pair with both positive) and discordant for one patient who had a positive axillary drain at >100 CFU but a mastectomy drain with growth of only 20–50 CFU (below the 50 CFU of positivity and therefore negative).
Bacterial colonization and drain duration
In the control group, bacterial colonization was a time-dependent phenomenon and increased in frequency with longer duration of drain presence, both for bulb fluid and drain tubing (see Figure 2). In the antisepsis group, positive fluid cultures also increased in frequency over time but were less frequent compared to the control group at all time intervals. Tubing cultures in the antisepsis group remained negative at all time points.
FIGURE 2.
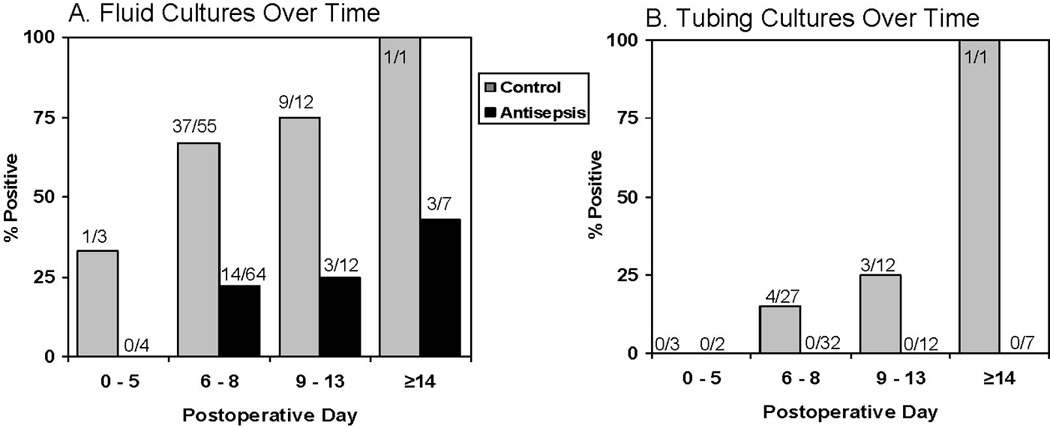
Frequency of bacterial growth in surgical drain fluid and tubing as a function of time. A, Drain fluid cultures. B, Drain tubing cultures. Positive culture was defined as 1+ or greater growth from fluid and greater than 50 CFU from tubing.
Microbiology
A wide variety of microorganisms were identified in bulb fluid, with 35% of cultures demonstrating multiple organisms (Table 3). Staphylococci were the most common recovered (71%); predominantly coagulase-negative staphylococcus with some Staphylococcus aureus. Gram-negative rods and anaerobes were identified with lower frequency. Drain tubing showed less variation in types of microorganisms, with Staphylococcus species the most common.
Table 3.
Prevalence of microbial isolates in cultures of drain fluid or tubing with colonization. The 82 drain fluid samples include those taken per protocol at one week and those taken at drain removal if it occurred after one week. All drain tubing samples occurred at drain removal.
| Drain Fluid (82 samples) |
Drain Tubing (19 samples) |
|||
|---|---|---|---|---|
| Organism | N | %* | N | %* |
| Aerobic Bacteria | ||||
| Gram positive bacteria | ||||
| Coagulase-negative staphylococci | 50 | 61 | 15 | 79 |
| Staphylococcus aureus | 8 | 10 | 2 | 11 |
| Corynebacterium species | 14 | 17† | 3 | 16‡ |
| Enterococcus species | 10 | 12 | ||
| Viridans group streptococci | 8 | 10 | ||
| Abiotrophia/Granulicatella species | 3 | 4 | ||
| Beta-hemolytic Streptococcus, group B | 2 | 2 | 1 | 5 |
| Beta-hemolytic Streptococcus, group G | 2 | 2 | ||
| Micrococcus species | 1 | 1 | ||
| Gram negative bacteria | ||||
| Proteus mirabilis | 2 | 2 | 1 | 5 |
| Pseudomonas aeruginosa | 2 | 2 | 1 | 5 |
| Acinetobacter species | 2 | 2 | ||
| Neisseria elongata | 1 | 1 | ||
| Serratia marcescens | 1 | 1 | ||
| Anaerobic Bacteria | ||||
| Propionibacterium acnes | 5 | 6 | ||
| Bacteroides diastasonis | 1 | 1 | ||
| Bacteroides vulgatus | 1 | 1 | ||
| Clostridium innocuum | 1 | 1 | ||
| Clostridium ramosum | 1 | 1 | ||
Percentages collectively are >100% because some samples had more than one isolate. Among 82 drain fluid samples with microbial growth, 29 (35%) had more than one organism; among 19 drain tubing samples with microbial growth, 3 (16%) grew more than one organism.
Corynebacterium species includes organisms identified as nonsporeforming Gram positive bacillus resembling Corynebacterium (n=5), gram positive bacillus (n=2), and bacillus (n=4).
Corynebacterium species includes organisms identified as nonsporeforming gram positive bacillus resembling Corynebacterium (n=2), Gram positive bacillus (n=1).
Drain site erythema and colonization
The extent of erythema in the skin around the drain exit site as a radial measurement was significantly less among subjects in the antisepsis group compared to the control group (mean 1.1 mm versus 2.6 mm, p=0.001) at one week. Although drains with positive (>50 CFU) tubing cultures on average had greater drain site erythema (mean 4.4 mm versus 1.1 mm, p=0.86), as did patients with SSI (mean 3.0 mm versus 2.1 mm, p=0.41), neither of these comparisons reached statistical significance.
Surgical site infections
SSI was diagnosed in 6 patients; 5 in the control group and 1 in the antisepsis group (Table 4). Of the 5 SSIs in the control group, 2 were abscesses that required incision and drainage, and a third demonstrated cellulitis with a positive culture. The remaining two SSIs were cases of cellulitis without cultures, but at the time of treatment they were judged by a physician blinded to the study group to represent infection, were treated with antibiotics, and improved on antibiotic therapy, thus on final review these were deemed to be SSIs.
Table 4.
Clinical features of cases with surgical site infection.
| Subject | Group | BMI | ASA | Operation | POD Drain Removal | Study Cultures | Infection Date & Culture Results |
Notes | |
|---|---|---|---|---|---|---|---|---|---|
| Ax | Mastx | ||||||||
| 1 | Control | 33 | 2 | MRM | 7 | 9 |
Fluid POD7: 4+ GpB Strep in both mastx and ax drains Tubing POD7: 51–100 CFU ax drain (mastx drain not cultured; was removed at time of abscess drainage) |
POD9: 1+GpB strep (36 hours after oral antibiotics begun) |
POD8: fever and erythema treated with cephalexin and sulfa/trimethoprim; POD9: progressive erythema, I&D abscess in OR with intraoperative culture |
| 2 | Control | 28 | 3 | TM | - | 5 |
Fluid POD5: No growth Tubing POD5: No growth |
POD19: 3+S. aureus |
POD19: tender erythema, I&D abscess in OR with intraoperative culture |
| 3 | Control | 31` | 2 | MRM | 7 | 11 |
Fluid POD7: 4+ S. aureus in both mastx and ax drains Tubing POD7: >100 CFU S. aureus ax drain (mastx tubing not cultured due to removal on POD11 after SSI treatment) |
POD9: 4+ S. aureus, 1+ Pseudomonas sp. |
POD7: ax drain removed, cellulitis POD9, culture+, treated with cephalexin and resolved |
| 4 | Control | 34 | 2 | TM+SN | - | 5 |
Fluid POD5: No growth Tubing POD5: No growth |
POD5: no culture | Cellulitis POD5; drain removed and cephalexin started; cellulitis resolved |
| 5 | Control | 38 | 3 | TM+SN | - | 7 |
Fluid POD7: No growth Tubing POD7: N/A (before tubing cultures were added to study) |
POD7: no culture | Cellulitis POD7; drain removed and levofloxacin/minocycline started; cellulitis resolved |
| 6 | Antisepsis | 33 | 2 | MRM | 8 | 6 |
Fluid POD6: no growth either drain Tubing POD6 mastx drain: no growth Fluid POD8 ax drain: no growth Tubing POD8 ax drain: no growth |
POD38: 4+ S. aureus |
Both drains out by POD8; chemo started POD21; fever unknown origin POD31; axillary erythema noted POD35; surgical drainage POD38 |
Abbreviations: MRM – modified radical mastectomy, TM – total mastectomy, TM+SN – total mastectomy with sentinel lymph node biopsy, mastx – mastectomy, ax – axillary, POD – postoperative day
There was only one case of SSI that occurred in the drain antisepsis group, presenting with symptoms on POD31. That patient started chemotherapy on POD21 and developed fever of unknown origin on POD31, with localized signs of axillary infection developing over the next week leading to incision and drainage of an axillary abscess. Since the patient’s symptoms began just outside the standard 30-day timeframe of the CDC definition, it is debatable whether this SSI should be included or not, but we included it for a conservative assessment of the differences between the control and antisepsis groups. Therefore, with 5 SSIs among 48 women in the control group and 1 SSI among 52 subjects in the antisepsis group, the difference in the SSI rate between the two groups (10.4% versus 1.9%) was not statistically significant but showed a strong trend (p=0.06). If this case of SSI in the antisepsis group is excluded due to its occurrence after 30 days, then the SSI rate in the control group (10.4%) is significantly higher than in the antisepsis group (0%), p=0.01.
Correlation of SSI and degree of drain colonization
Although the analysis was limited by a small number of SSIs, the trend was that SSI occurred more frequently among subjects with greater bacterial colonization, in either drain fluid or drain tubing, as compared to those with less or no bacterial colonization. Among patients with heavy (4+) bacterial growth in drain fluid from any drain at one week, the SSI rate was 2/9 (22%) compared to those with less heavily colonized fluid or no growth (4/91=4%, p=0.08). Similarly, the SSI rate was 2/7 (29%) for subjects with tubing growth >50 CFU in any drain compared to 3/69 (4%) for those with fewer CFU or no growth (p=0.05).
Intervention toxicity and compliance
There were no allergic reactions to the chlorhexidine disc. Compliance with the antiseptic interventions was excellent based upon subjects’ reports; at the one week follow-up visit and beyond there were no subjects who reported any compliance failures with the interventions. Two subjects felt unsure about their ability to perform the chlorhexidine disc dressing change. Both of these subjects returned to the clinic for study coordinator assistance with the first dressing change; one performed dressing changes independently after that, and the other subject elected to return for study coordinator assistance with the remaining dressing changes.
Discussion
In this study, we demonstrated that local measures of antisepsis significantly reduce bacterial colonization of surgical drains after breast and axillary surgery, both in the fluid of the drainage bulb and in the subcutaneous portion of the drain tubing. This work provides proof of principle that simple and inexpensive local care measures deserve further study in clinical trials as a means to reduce SSI. SSIs result in increased cost and morbidity and therefore have gained national attention with programs established to minimize these events.16, 17
The frequency of SSI after breast and axillary surgery in many studies is higher than would be expected for “clean” cases.1–6 In two recent multicenter prospective studies, the SSI rates after axillary dissection (including cases of cellulitis) were 8% and 14%.18, 19 Reasons for these higher than expected rates remain undefined, but are likely multifactorial. In addition to true differences in the SSI rate, apparent differences may also exist due to SSI definition (e.g. whether cellulitis cases are included or excluded),20 and differences in surveillance methods to ascertain SSI cases.9, 21
The presence of a surgical drain and its prolonged presence have been associated with increased risk of infection,5, 9, 10 which is logical because surgical drains provide a conduit for bacterial entry into the wound environment. A recent study investigated rates of bacterial colonization in surgical drains after mastectomy and found that drain fluid is colonized with bacteria in 33% of drains at one week after mastectomy and in 81% by two weeks.22 Furthermore, among patients who developed a surgical site infection, the microorganism identified was the same as that previously identified in the drain fluid cultures in 85% of cases. These findings strongly implicate the surgical drain as a source of bacteria that contributes to SSI.
Many risk factors associated with SSI after breast and axillary surgery are host-related (BMI, diabetes, smoking etc),6–9 but few of these can be modified within the preoperative timeframe of a few weeks that is common after a diagnosis of early stage breast cancer. However, the presence and duration of a surgical drain are clinical factors that may be modified to reduce infection risk. Surgical drains can be removed earlier in the postoperative course or omitted altogether, but the theoretical benefit of reduced infection risk without drains would result in an increased frequency of seroma formation,23–25 which in turn has also been associated with increased risk of SSI .26 Therefore, working under the assumptions that drains after mastectomy and axillary dissection are necessary and provide a source of bacterial entry causally related to SSI,22 we hypothesized that mitigating bacterial colonization of drains may help to reduce SSI.
Although some surgeons prescribe systemic antibiotics in the postoperative period after mastectomy with intent to reduce infection risk associated with indwelling drains,27, 28 the efficacy of this strategy is unproven and carries other risks.29, 30 Therefore, we selected an approach of local antiseptic measures to minimize the bacterial burden of the drain and subcutaneous wound.
Local antisepsis for indwelling drains is logical and extrapolated based upon an extensive experience with its use to prevent intravascular catheter-related infections (CRI). Research on CRIs demonstrates that the majority of infections with short-term intravascular devices are related to extraluminal migration of bacteria along the catheter, whereas intraluminal contamination is responsible for infections in most permanent intravascular devices.31 Reducing CRIs is successfully approached with a bundled approach of interventions that target both possible routes of bacterial access.32 In the case of surgical drains, bacteria may also access the wound environment via both extraluminal and intraluminal routes; the relative contributions of these routes to SSI risk is unknown. For this reason, antiseptic approaches to target both extraluminal and intraluminal colonization in this proof-of-principle trial were used. The chlorhexidine disc dressing was included based upon existing evidence of its efficacy in reducing CRIs.33, 34 In addition to the chlorhexidine disc dressing, we added hypochlorite rinses of the drain bulb, a reservoir that is intermittently opened to the external environment for emptying. This intervention was included based upon evidence of reduced bacteriuria after acetic acid irrigation of urinary drainage reservoirs in patients with longterm indwelling urethral catheters.35 Dilute Dakin’s solution for drain bulb irrigation was selected for use because of its broad antimicrobial spectrum, its tradition of use in clinical practice for infected wounds, and its low toxicity profile.36–38
Our data demonstrate that bacterial colonization of the drain bulb and subcutaneous drain tubing can be reduced with local, nontoxic antiseptic measures. Our findings also suggest that this might translate into reduced SSI rates. However, the findings must be interpreted with appropriate caution because the study was not powered to a primary endpoint of SSI, and our findings on SSI did not reach statistical significance. Therefore, though the findings are encouraging, we cannot assume that these interventions will reduce SSI. Furthermore, although the antiseptic interventions dramatically reduced bacterial colonization of the surgical drains, culture results varied in the small number of SSIs that occurred, with some individuals developing infection in the presence of negative drain fluid and tubing cultures, as well as the converse situation- a small number of individuals with “heavier” bacterial growth who did not develop infection. It is tempting to speculate that these findings suggest host factors likely play an important role in the development of SSI. Individuals with host factors conferring increased risk (increased BMI, smoking, immunosuppression) may have lower thresholds for the burden of bacteria that can be tolerated before manifesting as a clinical infection. Our study was not powered to separately analyze these subsets. Another limitation of this study is the combination of two interventions to address possible bacterial entry into the mastectomy/axillary dissection wound environment, so we are unable to determine the relative contribution of each route of contamination to SSI, and we could not assess the independent effects of each intervention.
These interventions are simple to perform, easy to learn, have low toxicity, and are relatively inexpensive. These methods could also be applied to other surgical procedures in which drains are required and in which the consequences of infection are high, e.g. groin dissection, ventral hernia repair with mesh, breast reconstruction with implants. With proof of principle that drain antisepsis may reduce SSI by reducing bacterial colonization of drains, we intend to pursue a larger randomized trial powered to an endpoint of SSI.
Acknowledgements
Funds for this study were provided by the Mayo Clinic Rochester Department of Surgery, Division of Gastroenterologic and General Surgery. ACD is supported by the Paul Calabresi Program in Clinical/ Translational Research in the Mayo Clinic Cancer Center. This project was also supported by NIH/NCRR CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Sincere thanks to Jill Randolph R.Ph. and Sue McCluskey R.Ph. for preparation of dilute Dakin’s solution, Lisa M Nyre and David T Lynch in Microbiology, and to Marilyn Churchward for assistance with manuscript preparation. Most importantly, this study was made possible by the individuals who elected to participate in this research study.
References
- 1.Prospero E, Cavicchi A, Bacelli S, et al. Surveillance for surgical site infection after hospital discharge: a surgical procedure-specific perspective. Infect Control Hosp Epidemiol. 2006;27(12):1313–1317. doi: 10.1086/509838. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37(10):783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Nahabedian MY, Tsangaris T, Momen B, et al. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112(2):467–476. doi: 10.1097/01.PRS.0000070727.02992.54. [DOI] [PubMed] [Google Scholar]
- 4.Ruvalcaba-Limon E, Robles-Vidal C, Poitevin-Chacon A, et al. Complications after breast cancer surgery in patients treated with concomitant preoperative chemoradiation: A case-control analysis. Breast Cancer Res Treat. 2006;95(2):147–152. doi: 10.1007/s10549-005-9058-y. [DOI] [PubMed] [Google Scholar]
- 5.Vilar-Compte D, Jacquemin B, Robles-Vidal C, et al. Surgical site infections in breast surgery: case-control study. World J Surg. 2004;28(3):242–246. doi: 10.1007/s00268-003-7193-3. [DOI] [PubMed] [Google Scholar]
- 6.El-Tamer MB, Ward BM, Schifftner T, et al. Morbidity and mortality following breast cancer surgery in women: national benchmarks for standards of care. Ann Surg. 2007;245(5):665–671. doi: 10.1097/01.sla.0000245833.48399.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen MA, Lefta M, Dietz JR, et al. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207(3):326–335. doi: 10.1016/j.jamcollsurg.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Blacam C, Ogunleye AA, Momoh AO, et al. High body mass index and smoking predict morbidity in breast cancer surgery: a multivariate analysis of 26,988 patients from the national surgical quality improvement program database. Ann Surg. 2012;255(3):551–555. doi: 10.1097/SLA.0b013e318246c294. [DOI] [PubMed] [Google Scholar]
- 9.Rey JE, Gardner SM, Cushing RD. Determinants of surgical site infection after breast biopsy. Am J Infect Control. 2005;33(2):126–129. doi: 10.1016/j.ajic.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Rotstein C, Ferguson R, Cummings KM, et al. Determinants of clean surgical wound infections for breast procedures at an oncology center. Infect Control Hosp Epidemiol. 1992;13(4):207–214. doi: 10.1086/646511. [DOI] [PubMed] [Google Scholar]
- 11.Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc. 2011;86(11):1113–1123. doi: 10.4065/mcp.2011.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barie PS. Multidrug-resistant organisms and antibiotic management. Surg Clin North Am. 2012;92(2):345–391. doi: 10.1016/j.suc.2012.01.015. ix-x. [DOI] [PubMed] [Google Scholar]
- 13.Throckmorton AD, Boughey JC, Boostrom SY, et al. Postoperative prophylactic antibiotics and surgical site infection rates in breast surgery patients. Ann Surg Oncol. 2009;16(9):2464–2469. doi: 10.1245/s10434-009-0542-1. [DOI] [PubMed] [Google Scholar]
- 14.Throckmorton AD, Boostrom SY, Hoskin TL, et al. Surgical site infection (SSI) after breast surgery: How good is the CDC definition? Ann Surg Oncol. 2008;15(Supplement 2):38. [Google Scholar]
- 15.Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296(23):1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 16.Preventing Healthcare Associated Infections. [Accessed May 20, 2012, 2012]; Available at: http://www.cdc.gov/HAI/prevent/prevention.html.
- 17.Surgical Care Improvement Project. [Accessed May 20, 2012, 2012]; Available at: http://www.jointcommission.org/surgical_care_improvement_project.
- 18.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 19.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 20.Degnim AC, Throckmorton AD, Boostrom SY, et al. Surgical site infection (SSI) after breast surgery: Impact of 2010 CDC reporting guidelines. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2448-6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss CA, 3rd, Statz CL, Dahms RA, et al. Six years of surgical wound infection surveillance at a tertiary care center: review of the microbiologic and epidemiological aspects of 20,007 wounds. Arch Surg. 1999;134(10):1041–1048. doi: 10.1001/archsurg.134.10.1041. [DOI] [PubMed] [Google Scholar]
- 22.Felippe WA, Werneck GL, Santoro-Lopes G. Surgical site infection among women discharged with a drain in situ after breast cancer surgery. World J Surg. 2007;31(12):2293–2299. doi: 10.1007/s00268-007-9248-3. [DOI] [PubMed] [Google Scholar]
- 23.Tadych K, Donegan WL. Postmastectomy seromas and wound drainage. Surg Gynecol Obstet. 1987;165(6):483–487. [PubMed] [Google Scholar]
- 24.Gupta R, Pate K, Varshney S, et al. A comparison of 5-day and 8-day drainage following mastectomy and axillary clearance. Eur J Surg Oncol. 2001;27(1):26–30. doi: 10.1053/ejso.2000.1054. [DOI] [PubMed] [Google Scholar]
- 25.Somers RG, Jablon LK, Kaplan MJ, et al. The use of closed suction drainage after lumpectomy and axillary node dissection for breast cancer. A prospective randomized trial. Ann Surg. 1992;215(2):146–149. doi: 10.1097/00000658-199202000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boostrom SY, Throckmorton AD, Boughey JC, et al. Incidence of clinically significant seroma after breast and axillary surgery. J Am Coll Surg. 2009;208(1):148–150. doi: 10.1016/j.jamcollsurg.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Brahmbhatt RD, Huebner M, Scow JS, et al. National practice patterns in preoperative and postoperative antibiotic prophylaxis in breast procedures requiring drains: Survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2477-1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips BT, Wang ED, Mirrer J, et al. Current practice among plastic surgeons of antibiotic prophylaxis and closed-suction drains in breast reconstruction: experience, evidence, and implications for postoperative care. Ann Plast Surg. 2011;66(5):460–465. doi: 10.1097/SAP.0b013e31820c0593. [DOI] [PubMed] [Google Scholar]
- 29.Landes G, Harris PG, Lemaine V, et al. Prevention of surgical site infection and appropriateness of antibiotic prescribing habits in plastic surgery. J Plast Reconstr Aesthet Surg: JPRAS. 2008;61(11):1347–1356. doi: 10.1016/j.bjps.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Throckmorton AD, Hoskin T, Boostrom SY, et al. Complications associated with postoperative antibiotic prophylaxis after breast surgery. Am J Surg. 2009;198(4):553–556. doi: 10.1016/j.amjsurg.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device-related bloodstream infection. I. Pathogenesis and short-term devices. Clin Infect Dis. 2002;34(9):1232–1242. doi: 10.1086/339863. [DOI] [PubMed] [Google Scholar]
- 32.O'Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087–1099. doi: 10.1093/cid/cir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231–1241. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 34.Levy I, Katz J, Solter E, et al. Chlorhexidine-impregnated dressing for prevention of colonization of central venous catheters in infants and children: a randomized controlled study. Pediatr Infect Dis J. 2005;24(8):676–679. doi: 10.1097/01.inf.0000172934.98865.14. [DOI] [PubMed] [Google Scholar]
- 35.Washington EA. Instillation of 3% hydrogen peroxide or distilled vinegar in urethral catheter drainage bag to decrease catheter-associated bacteriuria. Biol Res Nurs. 2001;3(2):78–87. doi: 10.1177/109980040200300203. [DOI] [PubMed] [Google Scholar]
- 36.Lineaweaver W, Howard R, Soucy D, et al. Topical antimicrobial toxicity. Arch Surg. 1985;120(3):267–270. doi: 10.1001/archsurg.1985.01390270007001. [DOI] [PubMed] [Google Scholar]
- 37.McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27(3):265–268. doi: 10.1097/00000637-199109000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Doughty D. A rational approach to the use of topical antiseptics. J Wound Ostomy Continence Nurs. 1994;21(6):224–231. doi: 10.1097/00152192-199411000-00008. [DOI] [PubMed] [Google Scholar]


