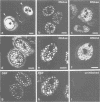
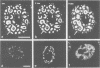
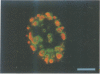
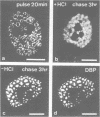
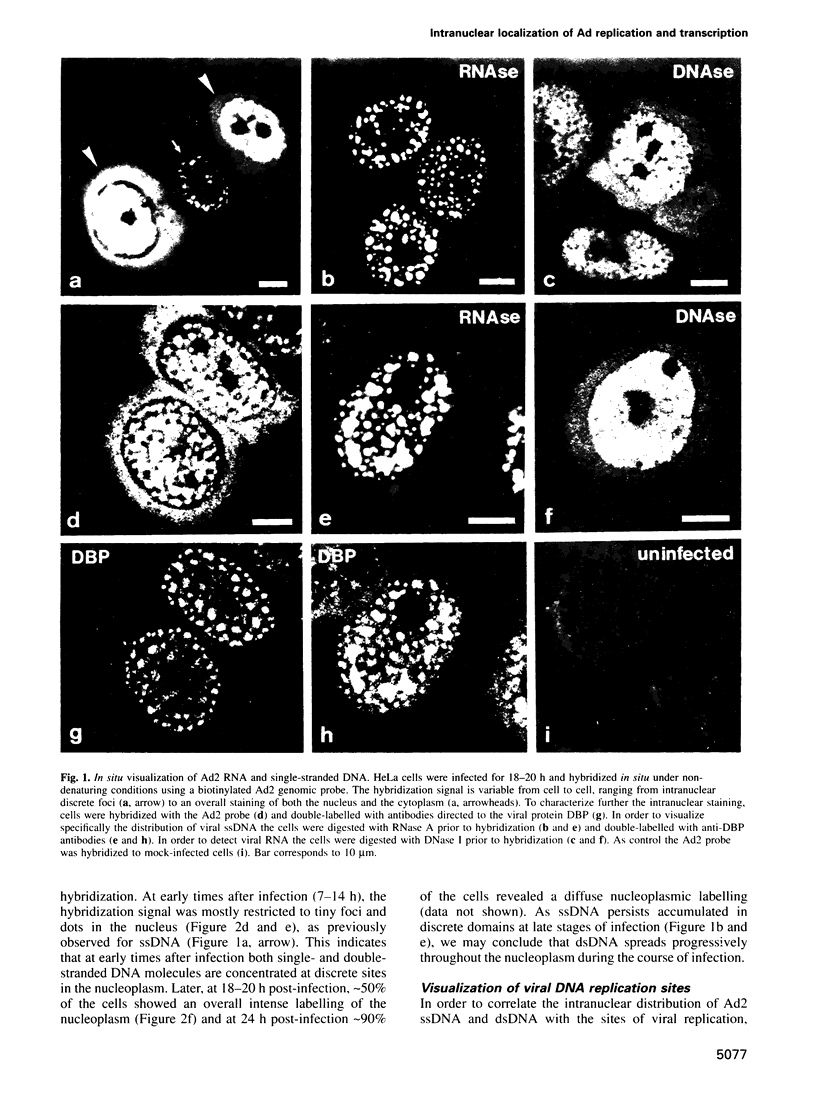
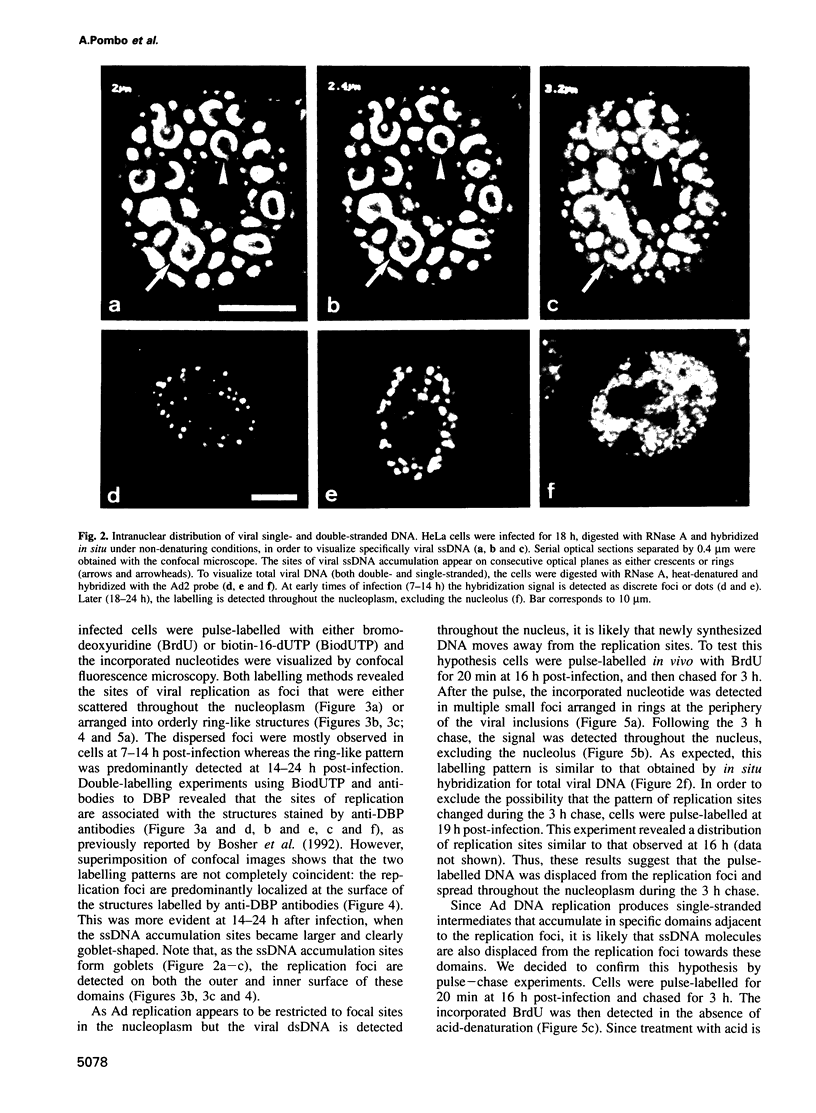
Abstract
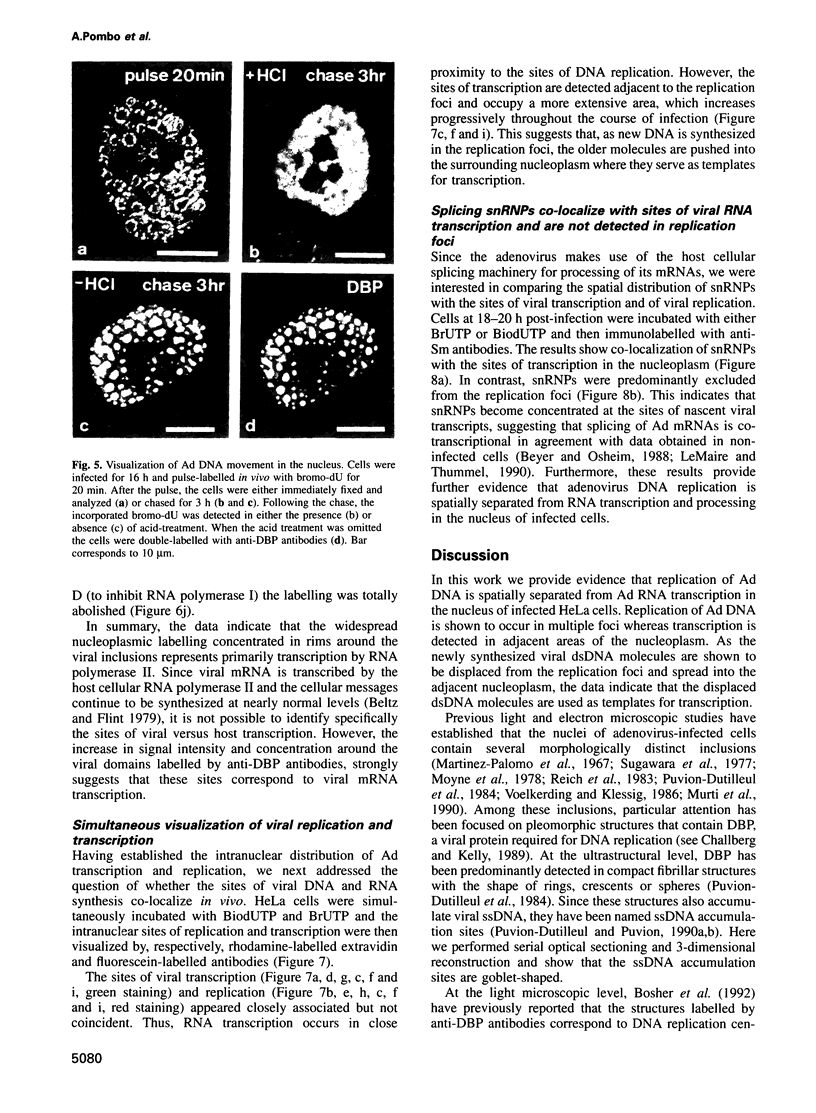
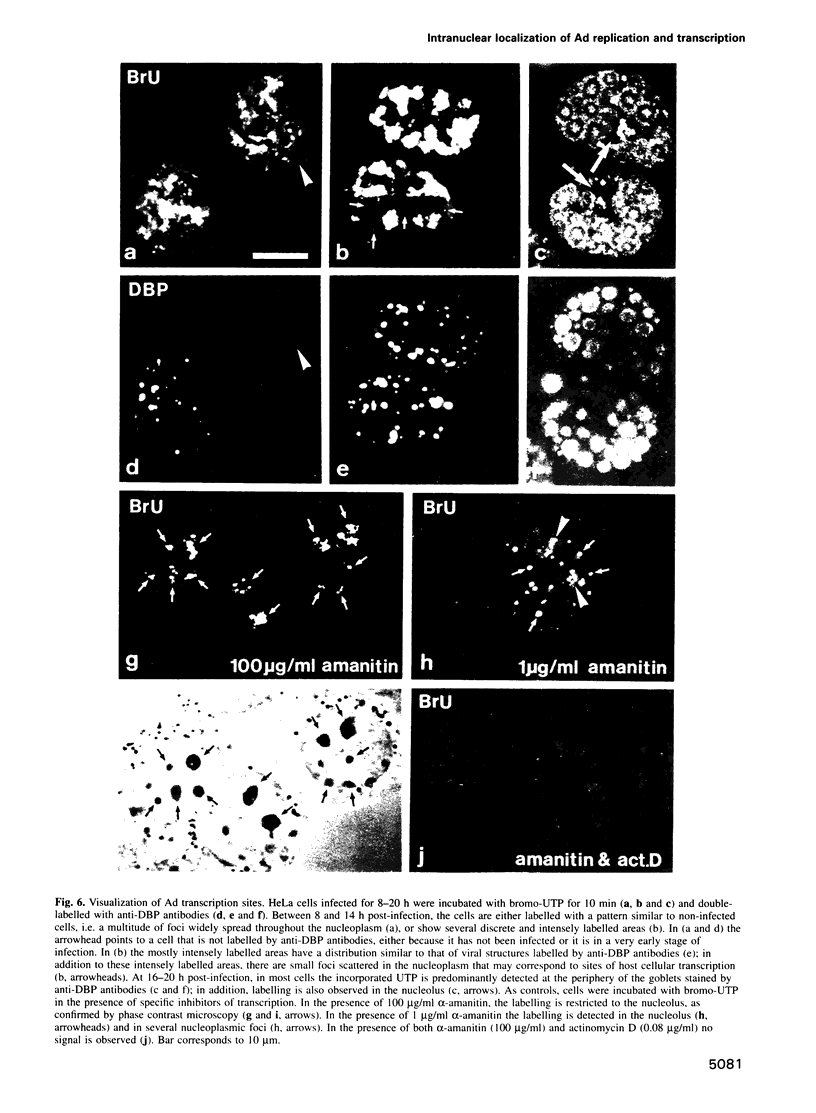
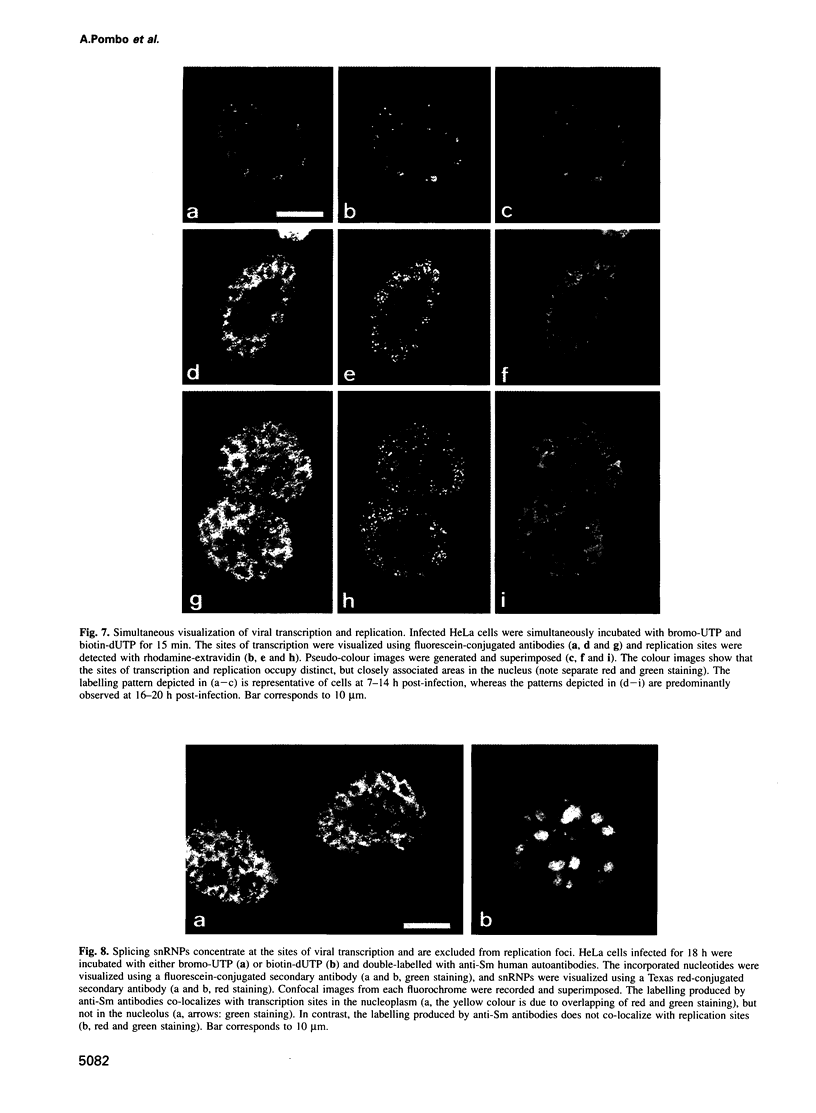
We have visualized the intranuclear topography of adenovirus replication and transcription in infected HeLa cells. The results show that viral DNA replication occurs in multiple foci that are highly organized in the nucleoplasm. Pulse-chase experiments indicate that newly synthesized viral double-stranded DNA molecules are displaced from the replication foci and spread throughout the nucleoplasm, while the single-stranded DNA replication intermediates accumulate in adjacent sites. Double-labelling experiments and confocal microscopy show that replication occurs in foci localized at the periphery of the sites where single-stranded DNA accumulates. The simultaneous visualization of viral replication and transcription reveals that the sites of transcription are predominantly separated from the sites of replication. Transcription is detected adjacent to the replication foci and extends around the sites of single-stranded DNA accumulation. These data indicate that newly synthesized double-stranded DNA molecules are displaced from the replication foci and spread in the surrounding nucleoplasm, where they are used as templates for transcription. Splicing snRNPs are shown to co-localize with the sites of transcription and to be excluded from the sites of replication. This provides evidence that splicing of viral RNAs occurs co-transcriptionally and that the sites of viral DNA replication are spatially distinct from the sites of RNA transcription and processing.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Berezney R. The nuclear matrix: a heuristic model for investigating genomic organization and function in the cell nucleus. J Cell Biochem. 1991 Oct;47(2):109–123. doi: 10.1002/jcb.240470204. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Bouton A. H., Hodge L. D., Miller O. L., Jr Visualization of the major late R strand transcription unit of adenovirus serotype 2. J Mol Biol. 1981 Apr 5;147(2):269–295. doi: 10.1016/0022-2836(81)90441-1. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Osheim Y. N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988 Jun;2(6):754–765. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- Bosher J., Dawson A., Hay R. T. Nuclear factor I is specifically targeted to discrete subnuclear sites in adenovirus type 2-infected cells. J Virol. 1992 May;66(5):3140–3150. doi: 10.1128/jvi.66.5.3140-3150.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge E., Carmo-Fonseca M., Lamond A., Pettersson U. Nuclear organization of splicing small nuclear ribonucleoproteins in adenovirus-infected cells. J Virol. 1993 Oct;67(10):5792–5802. doi: 10.1128/jvi.67.10.5792-5802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison O., Kédinger C., Chambon P. Adenovirus DNA template for late transcription is not a replicative intermediate. J Virol. 1979 Oct;32(1):91–97. doi: 10.1128/jvi.32.1.91-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R. The nucleoskeleton and the topology of replication. Cell. 1991 Aug 23;66(4):627–635. doi: 10.1016/0092-8674(91)90109-c. [DOI] [PubMed] [Google Scholar]
- Cremer T., Kurz A., Zirbel R., Dietzel S., Rinke B., Schröck E., Speicher M. R., Mathieu U., Jauch A., Emmerich P. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb Symp Quant Biol. 1993;58:777–792. doi: 10.1101/sqb.1993.058.01.085. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J. Regulation of adenovirus mRNA formation. Adv Virus Res. 1986;31:169–228. doi: 10.1016/s0065-3527(08)60264-x. [DOI] [PubMed] [Google Scholar]
- Fredman J. N., Engler J. A. Adenovirus precursor to terminal protein interacts with the nuclear matrix in vivo and in vitro. J Virol. 1993 Jun;67(6):3384–3395. doi: 10.1128/jvi.67.6.3384-3395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U. F., Willetts M., Webster P., Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993 Nov 5;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Hassan A. B., Errington R. J., White N. S., Jackson D. A., Cook P. R. Replication and transcription sites are colocalized in human cells. J Cell Sci. 1994 Feb;107(Pt 2):425–434. doi: 10.1242/jcs.107.2.425. [DOI] [PubMed] [Google Scholar]
- Hozák P., Hassan A. B., Jackson D. A., Cook P. R. Visualization of replication factories attached to nucleoskeleton. Cell. 1993 Apr 23;73(2):361–373. doi: 10.1016/0092-8674(93)90235-i. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Hassan A. B., Errington R. J., Cook P. R. Visualization of focal sites of transcription within human nuclei. EMBO J. 1993 Mar;12(3):1059–1065. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-García L. F., Spector D. L. In vivo evidence that transcription and splicing are coordinated by a recruiting mechanism. Cell. 1993 Apr 9;73(1):47–59. doi: 10.1016/0092-8674(93)90159-n. [DOI] [PubMed] [Google Scholar]
- Johnson C. V., Singer R. H., Lawrence J. B. Fluorescent detection of nuclear RNA and DNA: implications for genome organization. Methods Cell Biol. 1991;35:73–99. [PubMed] [Google Scholar]
- Kwant M. M., van der Vliet P. C. Differential effect of aphidicolin on adenovirus DNA synthesis and cellular DNA synthesis. Nucleic Acids Res. 1980 Sep 11;8(17):3993–4007. doi: 10.1093/nar/8.17.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Fairman M. P., Blow J. J. S phase of the cell cycle. Science. 1989 Nov 3;246(4930):609–614. doi: 10.1126/science.2683076. [DOI] [PubMed] [Google Scholar]
- LeMaire M. F., Thummel C. S. Splicing precedes polyadenylation during Drosophila E74A transcription. Mol Cell Biol. 1990 Nov;10(11):6059–6063. doi: 10.1128/mcb.10.11.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. E., Barghusen S. C., Leser G. P., Spear P. G. Redistribution of nuclear ribonucleoprotein antigens during herpes simplex virus infection. J Cell Biol. 1987 Nov;105(5):2069–2082. doi: 10.1083/jcb.105.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A., Le Buis J., Bernhard W. Electron microscopy of adenovirus 12 replication. 1. Fine structural changes in the nucleus of infected KB cells. J Virol. 1967 Aug;1(4):817–829. doi: 10.1128/jvi.1.4.817-829.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Shenk T. Adenovirus virus-associated RNA and translation control. J Virol. 1991 Nov;65(11):5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E. Interaction of adenoviral proteins with pRB and p53. FASEB J. 1993 Jul;7(10):880–885. doi: 10.1096/fasebj.7.10.8344487. [DOI] [PubMed] [Google Scholar]
- Moyne G., Pichard E., Bernhard W. Localization of simian adenovirus 7 (SA 7) transcription and replication in lytic infection. An ultracytochemical and autoradiographical study. J Gen Virol. 1978 Jul;40(1):77–92. doi: 10.1099/0022-1317-40-1-77. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Murti K. G., Davis D. S., Kitchingman G. R. Localization of adenovirus-encoded DNA replication proteins in the nucleus by immunogold electron microscopy. J Gen Virol. 1990 Dec;71(Pt 12):2847–2857. doi: 10.1099/0022-1317-71-12-2847. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Morita T., Sato C. Structural organizations of replicon domains during DNA synthetic phase in the mammalian nucleus. Exp Cell Res. 1986 Aug;165(2):291–297. doi: 10.1016/0014-4827(86)90583-5. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L. Adenovirus assay by the fluorescent cell-counting procedure. Virology. 1961 Nov;15:263–268. doi: 10.1016/0042-6822(61)90357-9. [DOI] [PubMed] [Google Scholar]
- Pettersson I., Hinterberger M., Mimori T., Gottlieb E., Steitz J. A. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984 May 10;259(9):5907–5914. [PubMed] [Google Scholar]
- Phelan A., Carmo-Fonseca M., McLaughlan J., Lamond A. I., Clements J. B. A herpes simplex virus type 1 immediate-early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9056–9060. doi: 10.1073/pnas.90.19.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U. Molecular biology of adenoviruses. Virol Monogr. 1975;14:1–115. doi: 10.1007/978-3-7091-8391-5_1. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Christensen M. E. Alterations of fibrillarin distribution and nucleolar ultrastructure induced by adenovirus infection. Eur J Cell Biol. 1993 Jun;61(1):168–176. [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Puvion E. Analysis by in situ hybridization and autoradiography of sites of replication and storage of single- and double-stranded adenovirus type 5 DNA in lytically infected HeLa cells. J Struct Biol. 1990 May;103(3):280–289. doi: 10.1016/1047-8477(90)90046-f. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Puvion E. Sites of transcription of adenovirus type 5 genomes in relation to early viral DNA replication in infected HeLa cells. A high resolution in situ hybridization and autoradiographical study. Biol Cell. 1991;71(1-2):135–147. doi: 10.1016/0248-4900(91)90060-z. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Pédron J., Cajean-Feroldi C. Identification of intranuclear structures containing the 72K DNA-binding protein of human adenovirus type 5. Eur J Cell Biol. 1984 Jul;34(2):313–322. [PubMed] [Google Scholar]
- Reich N. C., Sarnow P., Duprey E., Levine A. J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983 Jul 30;128(2):480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Singer R. H. RNA travel: tracks from DNA to cytoplasm. Cell. 1993 Nov 5;75(3):399–401. doi: 10.1016/0092-8674(93)90373-x. [DOI] [PubMed] [Google Scholar]
- Schaack J., Ho W. Y., Freimuth P., Shenk T. Adenovirus terminal protein mediates both nuclear matrix association and efficient transcription of adenovirus DNA. Genes Dev. 1990 Jul;4(7):1197–1208. doi: 10.1101/gad.4.7.1197. [DOI] [PubMed] [Google Scholar]
- Spector D. L. Macromolecular domains within the cell nucleus. Annu Rev Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Gilead Z., Wold W. S., Green M. Immunofluorescence study of the adenovirus type 2 single-stranded DNA binding protein in infected and transformed cells. J Virol. 1977 May;22(2):527–539. doi: 10.1128/jvi.22.2.527-539.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkerding K., Klessig D. F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J Virol. 1986 Nov;60(2):353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton T. H., Moen P. T., Jr, Fox E., Bodnar J. W. Interactions of minute virus of mice and adenovirus with host nucleoli. J Virol. 1989 Sep;63(9):3651–3660. doi: 10.1128/jvi.63.9.3651-3660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansink D. G., Manders E. E., van der Kraan I., Aten J. A., van Driel R., de Jong L. RNA polymerase II transcription is concentrated outside replication domains throughout S-phase. J Cell Sci. 1994 Jun;107(Pt 6):1449–1456. doi: 10.1242/jcs.107.6.1449. [DOI] [PubMed] [Google Scholar]
- Wansink D. G., Schul W., van der Kraan I., van Steensel B., van Driel R., de Jong L. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J Cell Biol. 1993 Jul;122(2):283–293. doi: 10.1083/jcb.122.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth D. J., Hsu M. T. Visualization of nascent RNA transcripts and simultaneous transcription and replication in viral nucleoprotein complexes from adenovirus 2-infected HeLa cells. J Mol Biol. 1981 Apr 5;147(2):247–268. doi: 10.1016/0022-2836(81)90440-x. [DOI] [PubMed] [Google Scholar]
- Xing Y., Johnson C. V., Dobner P. R., Lawrence J. B. Higher level organization of individual gene transcription and RNA splicing. Science. 1993 Feb 26;259(5099):1326–1330. doi: 10.1126/science.8446901. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]
- van Driel R., Humbel B., de Jong L. The nucleus: a black box being opened. J Cell Biochem. 1991 Dec;47(4):311–316. doi: 10.1002/jcb.240470405. [DOI] [PubMed] [Google Scholar]