Abstract
Besides the liver, it has been difficult to identify which organ(s) and/or cellular component(s) contribute significantly to the production of human FVIII:c (FVIII). Thus far, only endothelial cells have been shown to constitute a robust extrahepatic source of FVIII, possibly explaining both the diverse presence of FVIII mRNA in the body, and the observed increase in FVIII levels during liver failure. Here, we investigate whether human mesenchymal stem cells (MSC), ubiquitously present in different organs, could also contribute to FVIII production. MSC isolated from human lung, liver, brain, and bone marrow expressed FVIII message as determined by quantitative-RT-PCR. Using an antibody specific for FVIII, confocal microscopy, and umbilical cord-derived endothelial cells (HUVEC) as a negative control, we demonstrated that, in MSC, FVIII protein was not stored in granules; rather, it localized to the perinuclear region. Furthermore, functional FVIII was detected in MSC supernatants and cell lysates by aPTT and chromogenic assays. These results demonstrate that MSC can contribute at low levels to the functional FVIII pool, and advance the understanding of the physiology of FVIII production and secretion.
Keywords: FVIII, Endothelial Cells, Mesenchymal Stem cells, Pericytes, von Willibrand-Factor
INTRODUCTION
Factor VIII:c (FVIII) is a glycoprotein essential for normal blood clotting. Its role in the coagulation cascade, as a cofactor, is to increase factor IXa-mediated activation of Factor X. Deficiency in factor VIII causes the hereditary bleeding disorder hemophilia A (HemA). The liver is considered to be the primary site of FVIII production, with both hepatocytes and sinusoidal endothelial cells being reported as the main FVIII-producing cells.(Do et al., 1999; Hollestelle et al., 2001; Kelly et al., 1984; Wion et al., 1985) However, secondary sites of FVIII synthesis appear to play important roles in maintaining circulating FVIII levels, since both acute liver failure and chronic liver disease can result in elevated FVIII levels.(Hollestellel et al., 2004; Rapaport et al., 1960) Further evidence of extra-hepatic FVIII production comes from liver transplantation studies. While it is known that transplanting HemA patients with livers derived from normal donors will cure their disease,(Bontempo et al., 1987) studies in HemA dogs, and in one human case study, show that transplantation of livers derived from HemA donors into normal recipients will not cause hemophilia.(Madeira et al., 2009; Webster et al., 1971) Furthermore, transplantation of a normal spleen or lung into HemA canine recipients was also able to resolve the disease.(Veltkamp et al., 1974; Webster et al., 1967) Therefore, these observations strongly support the idea that extra-hepatic sources of FVIII exist, and are able to provide physiological/curative levels of FVIII. Recent reports have implicated endothelial cells as the most probable FVIII source (Follenzi et al., 2008; Jacquemin et al., 2006; Kumaran et al., 2005; Shahani et al., 2010; Shi et al., 2010; Shovlin et al., 2010) and this finding is consistent with current in vivo evidence that FVIII mRNA is present in various tissues throughout the body.(Lenting et al., 1998; Wu et al., 2009) Furthermore, the ability of endothelial cells to co-store FVIII with von Willebrand Factor (VWF), previously known as Factor VIII-related antigen (FVIIIR:Ag), supports a role for these cells as a releasable storage pool for FVIII. However, the possibility exists that other cell types present throughout the body that have not been clearly identified (Doering et al., 2002; Lamont and Ragni, 2005; Xu et al., 2005), can also contribute, even if at smaller levels, to circulating FVIII. Mesenchymal Stem Cells (MSC) have been isolated from a variety of tissues including bone marrow, liver, lung, spleen, skeletal muscle, kidney, brain and thymus (reviewed in (Bianco et al., 2008; Porada and Almeida-Porada, 2010)). A large percentage of MSC within the body is believed to reside in and derive from the perivascular niche,(Crisan et al., 2008; Sacchetti et al., 2007; Shi and Gronthos, 2003; Zannettino et al., 2008) a location that could allow these cells to secrete FVIII into circulation. Nevertheless, to our knowledge, no one has ever reported on the innate ability of these cells to express FVIII. We hypothesized that MSC, ubiquitously present throughout the body and sitting in prime perivascular locations, could constitute another putative source of FVIII production. Several markers have been used to identify and isolate MSC from bone marrow and other tissues. (Caplan and Bruder, 2001) Amongst the many reported, Stro-1(Caplan and Bruder, 2001; Simmons and Torok-Storb, 1991) is able to select populations of human MSC from the bone marrow as well as from several other tissues (reviewed in (Porada and Almeida-Porada, 2010)). Here, we demonstrate for the first time, to our knowledge, that populations of MSC isolated from human lung, liver, brain, and bone marrow based on Stro-1 positivity express RNA and secrete functional FVIII protein, and that this protein is not stored within the cell, but is released into the culture supernatant.
MATERIALS AND METHODS
Isolation and culture of Human MSCs
Heparinized human BM was obtained from healthy donors after informed consent according to guidelines from the Office of Human Research Protection at the University of Nevada at Reno. Human fetal bone, liver, lung, and brain were purchased from Advanced Bioscience Resources (Alameda, CA). Four different donors were used for each tissue. Stro-1+/CD45- MSC were isolated, cultured expanded, and confirmed to meet the criteria of MSC by flow cytometric analysis and differentiation into bone, cartilage, and adipocytes, as previously described (Airey et al., 2004; Chamberlain et al., 2007; Colletti et al., 2009). Briefly, Stro-1+ CD45- MSC isolated from the different tissues were maintained on gelatin-coated flasks using MSC growth media (MSCGM, Lonza, Walkersville, MD) in a humidified 37°C incubator at 5% CO2.
Culture of Human Hepatic Sinusoidal Endothelial Cells (HHSEC) and Umbilical Vein Endothelial Cells (HUVEC)
HUVEC were purchased from Lonza, and grown in EGM-2 culture media (Lonza, Walkersville, MD as per vendor instruction. Hepatic Sinusoidal Endothelial Cells (HHSEC) were purchased from Science Cell Research Laboratories and grown in endothelial cell media (ECM) (Science Cell Research Laboratories , Carlsbad CA).
Activated partial thromboplastin time (aPTT) and Chromogenic assays to measure FVIII
Different MSC populations, at identical passages, were plated at the same density in 6 well plates and cultured in Mesencult-XF (Stem Cell Technologies Inc. Vancouver BC Canada), xeno-free serum culture media. Culture supernatants were collected at 24 and 48 hours in culture. The number of independent experiments performed is given for each cell population, in the results section. At 48h, cultures were terminated to determine the final number of cells in culture and for quantitation of FVIII intracellular levels. The media was centrifuged at 1000g for 10min to remove cell debris and was mixed with 0.1M sodium citrate solution at a ratio of 1 part citrate to 9 parts media. In order to keep the same culture supernatant conditions for the aPTT assays, human hepatic sinusoidal endothelial cell (HHSEC) media was replaced with Mesencult-XF for 24 hours prior to harvesting the supernatants. Biologically active FVIII in cells cultured in xeno-free serum culture media Mesencult-XF (Stem Cell Technologies Inc. Vancouver BC Canada) was determined by performing a modified activated partial thromboplastin time test (Instrumentation Laboratory Company - Lexington, MA) on an IL ACL 10000 Coagulation Analyzer (Instrumentation Laboratory Company - Lexington, MA). Samples were diluted and added to human plasma deficient in factor VIII. Correction of the clotting time of the deficient plasma is proportional to the concentration (% activity) of that factor in the sample, interpolated from a calibration curve constructed using pooled human plasma. In order to measure intracellular FVIII activity, chromogenic assays were performed on cell lysates using the Chromogenix Coatest SP FVIII Kit (Diapharma, West Chester, OH). Modifications were made to the manufacturer's protocol in order to increase the sensitivity of the assay, as previously reported (Shahani et al., 2010) and described in detail in the Supplementary Materials and Methods.
Quantitative RT-PCR
Total RNA was RQ1 DNase (Promega, Madison, WI) treated to remove genomic DNA contamination. 1ug of DNA-free RNA was converted into cDNA using an RT2 First Strand Kit (Qiagen, Valencia, CA). SYBER Green-based qPCR was conducted by using the qRT-PCR Primer Assay (Qiagen) with the following primers: human FVIII: (qRT-F8-FWD: 5’ATGCACAGCATCAATGGCTAT3’ , qRT-F8-REV: 5’GTGAGTGTGTCTTCATAGAC3’); human vWF specific primers Cat. Number PPH02567E (Qiagen). Human GAPDH was used as an internal reference/housekeeping gene with commercially available primers (Cat. Number PPH00150E, Qiagen). The qPCR master mix was loaded into a ABI PRISM 96 well plate and processed in the 7300 Real Time PCR system (ABI Applied Biosystems).
Immunofluorescence Staining of Cell Cultures
MSC and HUVEC were plated at various passages (P5-P10) onto 2-well chamber slides (Nalge Nunc, Rochester, NY). At 50-80% confluency, staining was performed as previously described (Shahani et al., 2010) using sheep anti-human FVIII:c affinity purified polyclonal antibody (cat# ISAF8C-AP, Innovative Research, Novi, MI), mouse Anti-Factor VIII-Related antigen (cat# AM016-5M, Biogenex, Fremont, CA), and/or mouse anti-human CD105 (cat# ab4967, AbCam, Cambridge, MA). Slides were washed, permeabilized, and incubated overnight at 4°C with the primary antibodies at the following concentrations: FVIII:c 5 μg/mL, CD105 1:1000, VWF 1:5. Slides were washed again and stained with secondary antibody for 30’at RT. Slides were washed, counterstained with DAPI (4,6 diamidino-2-phenylindole, Biogenex), and mounted using Cytoseal 60 (Thermo Scientific, Barrington, IL). Slides were imaged using a Fluoview 1000 confocal microscope system (Olympus America, Center Valley, PA). Images were taken as z-stacks with 5-10 slices per image and then projected as 2-D images before being saved and processed using Adobe Photoshop. One well stained with only secondary antibody served as a negative control in all slides tested.
Transduction of MSC with an expression/secretion-optimized porcine FVIII vector
MSC and HUVEC were transduced with a lentivector encoding an expression/secretion optimized B-domain deleted FVIII gene (Gangadharan et al., 2006) kindly supplied by Drs. H.T. Spencer and C. Doering, Department of Pediatrics, Aflac Cancer Center and Blood Disorders Service, Emory University, Atlanta. Upon subsequent passage, some of the cells were plated on chamber slides and stained as described above.
Statistical analysis
Experiments were independently repeated at least three times. Results are presented as mean±SD. Mann–Whitney–Wilcoxon test was used to analyze the statistical significance of the results; p values < 0.05 were considered to be statistically significant.
RESULTS
Human MSC express FVIII at the transcriptional level
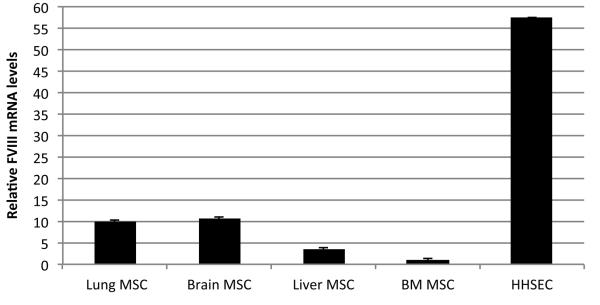
As previously described, bone marrow- (BM), fetal lung-, liver- and brain-derived MSC were isolated based on Stro-1 positivity and CD45 negativity(Airey et al., 2004; Colletti et al., 2008,{Chamberlain, 2007 #136)}. The cells were cultured and expanded, and flow cytometry analysis demonstrated that these cells displayed markers characteristic of mesenchymal progenitors/adventitial reticular cells/pericytes, including CD146, CD105, CXCL12, CD90, CD44, that have been identified by us and others as a perivascular population of cells that exists in several different organs (Bianco, 2011; Crisan et al., 2011). Furthermore, upon culture in appropriate media these cells differentiated into bone, cartilage, and adipocyte (Airey et al., 2004; Chamberlain et al., 2007; Colletti et al., 2009). No hematopoietic, or endothelial cell contamination was found in these cultures as confirmed by the absence of CD45, CD34 and CD31 expression. In addition, no hepatocytic or hepatoblastic markers were found in these cultures. Quantification of FVIII mRNA in the different cell populations was performed by qRT-PCR. Since human hepatic sinusoidal endothelial cells (HHSEC) have been reported as the main FVIII-producing cells (Do et al., 1999) we used these cells as a positive control. Fig. 1 is a representative image of 4 different independent experiments and depicts the levels of FVIII mRNA. FVIII mRNA was present in the MSC derived from all of the different tissues, albeit at lower levels than HHSEC.
Figure 1. Relative FVIII mRNA levels in Human MSC.
qRT-PCR data showing relative FVIII mRNA levels in different MSC populations in comparison to HHSEC. Values represent an average of 4 different independent experiments for each cell population.
Identification of FVIII protein in MSC cell cultures
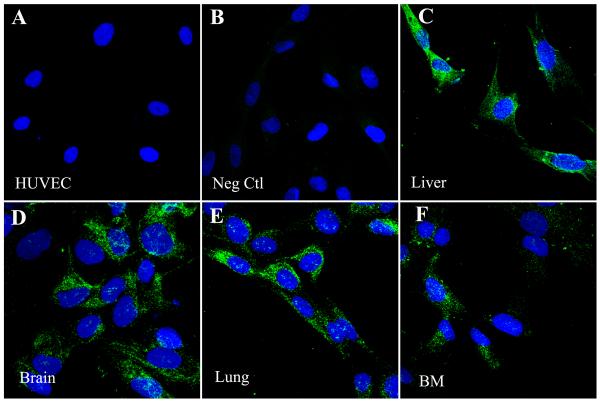
In order to evaluate whether the different MSC populations were producing FVIII protein, immunofluorescence staining was performed. Since several of the anti-human FVIII antibodies cross react with von Willebrand Factor/FVIII-related antigen (vWF), we first confirmed that the antibody that we used was specific for FVIII and did not cross-react with vWF. Because umbilical vein endothelial cells (HUVEC) express vWF but not FVIII protein (Shahani et al., 2010; Shovlin et al., 2010) we used these cells as a control to test the specificity of our antibody. As can be seen in Fig. 2, when HUVEC and MSC were stained in parallel, HUVEC were negative for FVIII (Figure 2A), demonstrating the specificity of the antibody, while all of the different MSC populations were found to be positive for FVIII (Fig. 2B-F). When MSC were stained with an antibody specific to vWF, MSC in contrast to HUVEC were found to be negative for vWF protein (data not shown). In order to confirm these results, we performed qRT-PCR for vWF mRNA using the same cell populations. The results obtained are displayed in (Fig. 3) and show that, while HUVEC produce vWF mRNA at high levels MSC do not.
Figure 2. Human MSC express FVIII protein.
Cells were stained for FVIII (green) using an affinity purified FVIII:c antibody. Nuclei are counterstained with DAPI (blue). Images were taken on an Olympus Fluoview 1000 confocal microscope using a 40x objective. Representative images have an additional 3x digital zoom. HUVEC were used as a specificity control for the anti-FVIII:c antibody, showing that the antibody does not cross-react with VWF (A). Secondary antibody control (B). Panels C-F show different populations: Liver (C), Brain (D), Lung (E), BM (F).
Figure 3. Relative vWF mRNA levels in Human MSC.
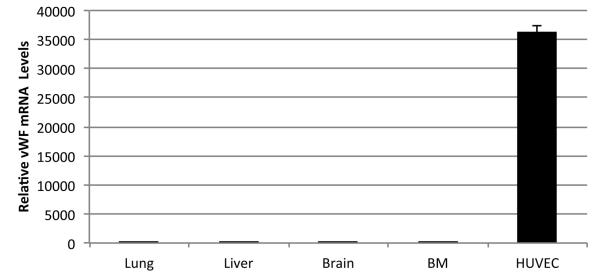
qRT-PCR data showing vWF mRNA levels in different MSC populations in comparison to HUVEC. Values represent an average of 3 different independent experiments for each cell population.
MSC secrete biologically active FVIII at lower levels than Hepatic Sinusoidal Endothelial Cells (HHSEC)
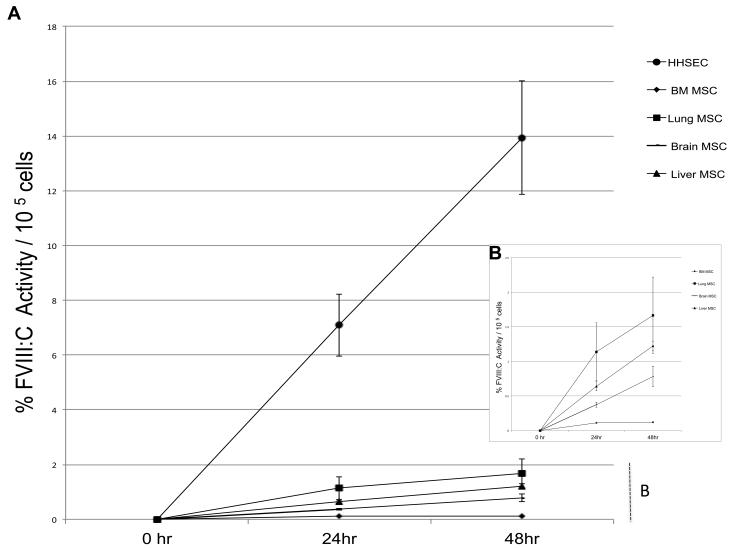
We next investigated whether the different MSC populations were secreting biologically active FVIII, and compared the levels of FVIII released by these cells with those produced by HHSEC. MSC- and HHSEC-conditioned media were harvested at 24 and 48 hours of culture, and FVIII activity determined in each sample by performing a modified activated partial thromboplastin time test (aPTT), as described in the Materials and Methods. Fig. 4 is a representative image of 4 different independent experiments and depicts the percentage of FVIII activity for each cell population (Fig. 4A) expressed as a percentage of that present in normal human plasma. The percentage of activity in normal plasma obtained with aPTT ranged between 62-102%. In agreement with what has been reported for HHSEC (Do et al., 1999), our data shows that HHSEC robustly secrete increasing amounts of active FVIII into the culture medium (Fig 4A) . In similarity, but at much lower level, all of the MSC cell populations secreted active FVIII in a similar temporal pattern. As can be seen in detail in Fig. 4B, at the 24h time point, the percentage of FVIII:C activity /105 cells in the culture media of lung-derived MSC was 1.1±0.4 %; Liver derived-MSC 0.6±0.06%; Brain-derived MSC 0.3±0.03%; and BM-derived MSC 0.1±0.0%. At the 48h time point, the percentage of FVIII:C activity/105 cells in the culture media of Lung-derived MSC was 1.7±0.6%; Liver derived-MSC 1.2±0.07%; Brain-derived MSC 0.7±0.1%; and BM-derived MSC 0.12±0.0%. Despite the slight difference in FVIII production between Lung, Brain and Liver MSC, these differences, regardless of time point were not statistically significant (p>0.05).
Figure 4. Human MSC secrete biologically active FVIII, but at lower levels than Hepatic Sinusoidal Endothelial Cells (HHSEC).
(A) Comparison between the different MSC populations and HHSEC of FVIII activity at 24 and 48h of culture, as determined by aPTT, expressed as a percentage of the activity present in normal human plasma. (B) Detail showing the FVIII activity of the different MSC populations as determined by aPTT, expressed as a percentage of that present in normal human plasma at 24 and 48h of culture.
MSC do not store FVIII in intracellular granules
The increase in percentage of FVIII activity in the culture supernatants with the time in culture suggested that FVIII was being constantly secreted to the media and not stored within the cells. To directly investigate this we measured the levels of intracellular FVIII present in the different MSC populations. To be able to detect the minute amounts of FVIII present inside the cells, we used a modification of the Coatest SP FVIII chromogenic assay, as previously published (Shahani et al., 2010), and the reference curves for these assays can be seen in Supplementary Fig. 1B. The amount of FVIII found inside each of the MSC populations was lower more than 100 fold, than the amount measured in their supernatant counterparts. Specifically, lung-, brain-, liver and BM-derived MSC contained intracellular levels of 0.02±0.001 (n=2), 0.014±0.00 (n=2), and 0.013±0.004 (n=4) and 0.004±0.001 (n=2) mU FVIII/106 cells, respectively. Overall, the very low levels of intracellular FVIII and the increase of FVIII in culture supernatants with time in culture suggest that MSCs do not store FVIII protein, and likely secrete most of the FVIII they produce.
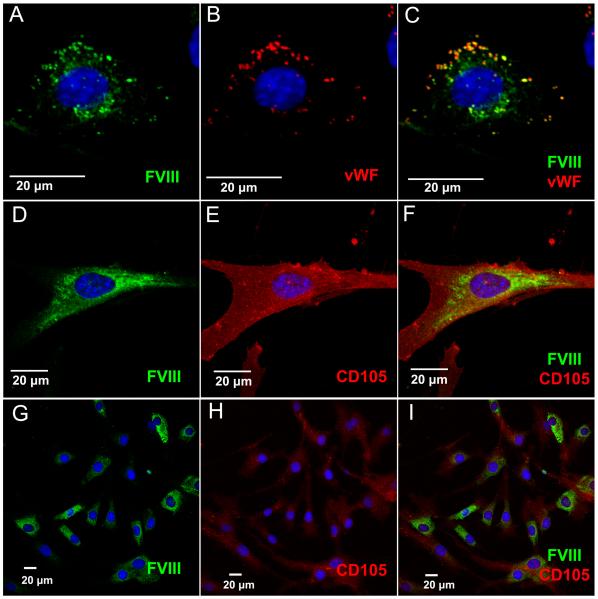
Previous studies showed that MSC could efficiently secrete FVIII at high levels when transduced with FVIII-encoding vectors (Gangadharan et al., 2006; Van Damme et al., 2006). Therefore, in order to ascertain that when high amounts of FVIII are being produced, FVIII is still not stored in granules, MSC populations were transduced with a lentivector encoding an expression/secretion-optimized porcine-FVIII. Because HUVEC store vWF in granules, and vWF binds FVIII, we also transduced HUVEC with the same FVIII-encoding lentivector to provide a reference for the staining pattern of granule-stored FVIII. Immunofluorescence staining was performed on transduced cell populations and analyzed by confocal microscopy. As can be seen in Fig 5., robust homogeneous FVIII staining is detected in the cytoplasm of MSC, but no obvious storage granules are observed (Fig. 5D-I). By contrast, in FVIII-transduced HUVEC cells granules co-storing FVIII with VWF are easily visualized (Fig. 5A-C).
Figure 5. Immunofluorescence Analysis Demonstrates Homogeneous Staining of FVIII in MSC.
MSC and HUVEC were transduced with an expression/secretion optimized porcine FVIII in order to visualize intracellular FVIII localization. FVIII was detected by staining with an antibody against FVIII (green), while CD105 (red) staining reveals the MSC cell surface. All images were taken on a Fluoview 1000 confocal microscope with a 40x objective with various amounts of digital zoom factors as indicated by scale bars. (A-C) FVIII-transduced HUVEC co-store FVIII and VWF (red) in granules, and were used as a control. (D-F) High magnification of human MSC showing homogenous cytoplasmic staining of FVIII. (G-I) Low magnification of MSC labeled with an antibody against FVIII. Nuclei are counterstained with DAPI (blue).
DISCUSSION
The sites and physiology of FVIII production remains an open question to scientists and physicians dealing with patients with liver disease, liver transplant, and Hemophilia A. Until recently, cells expressing endogenous FVIII could not be reliably identified (Hollestelle et al., 2001; Webster et al., 1971), and although FVIII mRNA was shown to be present in different tissues, detection of the FVIII protein at the cellular level has been a more difficult task.(Hollestelle et al., 2001) The identification of sites for FVIII synthesis in vivo, using immunostaining, has proven to be problematic due to the trace FVIII concentrations, and the lack of FVIII assays that are sensitive enough to detect the low levels of FVIII expressed by those cells has confounded in vitro studies. Nevertheless, several groups have shown that extrahepatic vascular endothelial cells constitute an important site for FVIII production.(Follenzi et al., 2008; Jacquemin et al., 2006; Shahani et al., 2010; Shi et al., 2010; Shovlin et al., 2010) Amongst those studies, Shahani et al. demonstrated that endothelial cells derived from different vascular beds varied widely in their ability to produce FVIII, in vitro, by using a functional assay with improved sensitivity for FVIII (Shahani et al., 2010). MSC within the body are believed to reside in and derive from the perivascular niche,(Crisan et al., 2008; Sacchetti et al., 2007; Shi and Gronthos, 2003; Zannettino et al., 2008) and therefore are ubiquitously present throughout the body in a privileged location that could allow these cells to secrete FVIII into circulation. Therefore, we investigated whether MSC derived from several different tissues (BM, liver, brain, and lung) were able to produce and secrete functional FVIII protein. These cells were isolated based on Stro-1 positivity and were previously shown to meet the criteria of MSC, both by phenotypical analysis, expressing CD105, CD90, CD44, and CD29, and by their ability to generate adipocytes, cartilage, and bone (Airey et al., 2004; Chamberlain et al., 2007; Colletti et al., 2009; Porada and Almeida-Porada, 2010). qRT-PCR analysis of RNA extracted from lung-, liver-, brain-, and bone marrow- derived MSC showed that these cells expressed FVIII mRNA, but lacked expression of von Willebrand Factor. In addition, none of the MSC populations synthetized message for Factor V, Factor VII, or Factor IX (data not shown). Using confocal microscopy, and immunostaining with an antibody specific for FVIII, we demonstrated that all of the MSC populations expressed the FVIII protein. Furthermore, results obtained by aPTT assays measuring the activity/function of the secreted FVIII, showed that on a cell/cell basis, MSC, other than BM-derived, produced roughly 7-14% the amount of FVIII, at the protein/activity level, of HHSEC, suggesting that they do contribute to the FVIII pool. Our results thus place MSC FVIII production in the same range as that reported by Shahani et al. for microvascular endothelial cells isolated from pulmonary, dermal, and intestinal beds. While our results show that the levels of FVIII produced and secreted by BM-derived MSC were lower than that of MSC from other tissues, it is possible that these levels are still of physiologic significance, since a recent study demonstrated that transplantation of BM-derived MSC could protect hemophilia A mice from bleeding challenge (Follenzi et al., 2012).
Here we also demonstrate that MSC have very low levels of intracellular FVIII and that FVIII in culture supernatants increase with the time in culture, suggesting that MSCs do not store FVIII protein and likely secrete most of the FVIII they produce.
Vascular endothelial cells have been demonstrated to accumulate FVIII in storage pools(Rosenberg et al., 2000; Shahani et al., 2010) that are released upon stimulation in vitro, and in vivo.(Corrall et al., 1980; Rizza, 1961) The FVIII pool is co-stored with von Willebrand factor (VWF) in Weibel-Palade-like bodies.(Rosenberg et al., 2000; Shahani et al., 2010; Van Den Biggelaar et al., 2007) Although MSC do not express VWF, we investigated whether these cells could store/export FVIII in vesicles or, rather, secreted the protein continuously into the supernatant. To directly address this issue we transduced MSC with a lentiviral vector encoding an expression/secretion-optimized porcine FVIII to ensure that the cells were producing high amounts of FVIII. Immunofluorescence microscopy on these transduced cells demonstrated that the FVIII being produced was homogeneously located in the cytoplasm and no granular pattern was observed. These results are in agreement with those published by Rosenberg et al., in which AtT-20, also negative for VWF, were used as a storage and secretory cell model of FVIII/VWF.(Rosenberg et al., 1998). Since it has been shown in other models that FVIII and VWF have to be synthesized in the same cell for VWF to serve as an intermolecular chaperone (Rosenberg et al., 1998), it is possible that FVIII produced by MSC within the body is continuously secreted, and is then transported through the fenestrated capillary endothelium into circulation where it associates with VWF.
While it could be argued that FVIII expression by MSCs is only the result of ex-vivo culture conditions and not truly a reflection of their innate physiological role, this does not seem to be the case, since MSC expressed similar amounts of FVIII regardless passage number (data not shown). In conclusion, our studies identify another population of cells ubiquitously distributed throughout major organs that constitutively produce and secrete FVIII, and can contribute at low levels to the extrahepatic production of this protein.
Supplementary Material
Acknowledgments
National Institutes of Health
HL73737
HL97623
REFERENCES
- Airey JA, Almeida-Porada G, Colletti EJ, Porada CD, Chamberlain J, Movsesian M, Sutko JL, Zanjani ED. Human Mesenchymal Stem Cells Form Purkinje Fibers in Fetal Sheep Heart. Circulation. 2004;109(11):1401–1407. doi: 10.1161/01.CIR.0000124222.16321.26. [DOI] [PubMed] [Google Scholar]
- Bianco P. Back to the future: moving beyond "mesenchymal stem cells". J Cell Biochem. 2011;112(7):1713–1721. doi: 10.1002/jcb.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempo FA, Lewis JH, Gorenc TJ, Spero JA, Ragni MV, Scott JP, Starzl TE. Liver transplantation in hemophilia A. Blood. 1987;69(6):1721–1724. [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J, Yamagami T, Colletti E, Theise ND, Desai J, Frias A, Pixley J, Zanjani ED, Porada CD, Almeida-Porada G. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46(6):1935–1945. doi: 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- Colletti E, Lindstedt S, Park PJ, Almeida-Porada G, Porada CD. Early fetal gene delivery utilizes both central and peripheral mechanisms of tolerance induction. Experimental Hematology. 2008;36(7):816–822. doi: 10.1016/j.exphem.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletti EJ, Airey JA, Liu W, Simmons PJ, Zanjani ED, Porada CD, Almeida-Porada G. Generation of tissue-specific cells from MSC does not require fusion or donor-to-host mitochondrial/membrane transfer. Stem Cell Res. 2009;2(2):125–138. doi: 10.1016/j.scr.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrall RJM, Webber RG, Frier BM. Increase in Coagulation Factor-Viii Activity in Man Following Acute Hypoglycemia - Mediation Via an Adrenergic Mechanism. British Journal of Haematology. 1980;44(2):301–305. doi: 10.1111/j.1365-2141.1980.tb01212.x. [DOI] [PubMed] [Google Scholar]
- Crisan M, Corselli M, Chen CW, Peault B. Multilineage stem cells in the adult: a perivascular legacy? Organogenesis. 2011;7(2):101–104. doi: 10.4161/org.7.2.16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Do H, Healey JF, Waller EK, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem. 1999;274(28):19587–19592. doi: 10.1074/jbc.274.28.19587. [DOI] [PubMed] [Google Scholar]
- Doering CB, Josephson CD, Craddock HN, Lollar P. Factor VIII expression in azoxymethane-induced murine fulminant hepatic failure. Blood. 2002;100(1):143–147. doi: 10.1182/blood.v100.1.143. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Benten D, Novikoff P, Faulkner L, Raut S, Gupta S. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. The Journal of Clinical Investigation. 2008;118(3):935–945. doi: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Raut S, Merlin S, Sarkar R, Gupta S. Role of bone marrow transplantation for correcting hemophilia A in mice. Blood. 2012;119(23):5532–5542. doi: 10.1182/blood-2011-07-367680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan B, Parker ET, Ide LM, Spencer HT, Doering CB. High-level expression of porcine factor VIII from genetically modified bone marrow-derived stem cells. Blood. 2006;107(10):3859–3864. doi: 10.1182/blood-2005-12-4961. [DOI] [PubMed] [Google Scholar]
- Hollestelle MJ, Thinnes T, Crain K, Stiko A, Kruijt JK, van Berkel TJC, Loskutoff DJ, van Mourik JA. Tissue distribution of factor VIII gene expression in vivo - A closer look. Thromb Haemost. 2001;86(3):855–861. [PubMed] [Google Scholar]
- Hollestellel MJ, Geertzen HGM, Straatsburg IH, van Gulik TM, van Mourik JA. Factor VIII expression in liver disease. Thromb Haemost. 2004;91(2):267–275. doi: 10.1160/TH03-05-0310. [DOI] [PubMed] [Google Scholar]
- Jacquemin M, Neyrinck A, Hermanns MI, Lavend'homme R, Rega F, Saint-Remy J-M, Peerlinck K, Van Raemdonck D, Kirkpatrick CJ. FVIII production by human lung microvascular endothelial cells. Blood. 2006;108(2):515–517. doi: 10.1182/blood-2005-11-4571. [DOI] [PubMed] [Google Scholar]
- Kelly DA, Summerfield JA, Tuddenham EGD. Localization of Factor-Viiic-Antigen in Guinea-Pig Tissues and Isolated Liver-Cell Fractions. British Journal of Haematology. 1984;56(4):535–543. doi: 10.1111/j.1365-2141.1984.tb02178.x. [DOI] [PubMed] [Google Scholar]
- Kumaran V, Benten D, Follenzi A, Joseph B, Sarkar R, Gupta S. Transplantation of endothelial cells corrects the phenotype in hemophilia A mice. J Thromb Haemost. 2005;3(9):2022–2031. doi: 10.1111/j.1538-7836.2005.01508.x. [DOI] [PubMed] [Google Scholar]
- Lamont PA, Ragni MV. Lack of desmopressin (DDAVP) response in men with hemophilia A following liver transplantation. J Thromb Haemost. 2005;3(10):2259–2263. doi: 10.1111/j.1538-7836.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- Lenting PJ, van Mourik JA, Mertens K. The Life Cycle of Coagulation Factor VIII in View of Its Structure and Function. Blood. 1998;92(11):3983–3996. [PubMed] [Google Scholar]
- Madeira CL, Layman ME, de Vera RE, Fontes PA, Ragni MV. Extrahepatic factor VIII production in transplant recipient of hemophilia donor liver. Blood. 2009;113(21):5364–5365. doi: 10.1182/blood-2009-02-206979. [DOI] [PubMed] [Google Scholar]
- Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62 doi: 10.1016/j.addr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport SI, Ames SB, Mikkelsen S, Goodman JR. Plasma Clotting Factors in Chronic Hepatocellular Disease. New England Journal of Medicine. 1960;263(6):278–282. doi: 10.1056/NEJM196008112630604. [DOI] [PubMed] [Google Scholar]
- Rizza CR. Effect of exercise on the level of anithaemophilic globulin in human blood. The Journal of Physiology. 1961;156(1):128–135. doi: 10.1113/jphysiol.1961.sp006663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JB, Foster PA, Kaufman RJ, Vokac EA, Moussalli M, Kroner PA, Montgomery RR. Intracellular trafficking of factor VIII to von Willebrand factor storage granules. J Clin Invest. 1998;101(3):613–624. doi: 10.1172/JCI1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JB, Greengard JS, Montgomery RR. Genetic Induction of a Releasable Pool of Factor VIII in Human Endothelial Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(12):2689–2695. doi: 10.1161/01.atv.20.12.2689. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-Renewing Osteoprogenitors in Bone Marrow Sinusoids Can Organize a Hematopoietic Microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Shahani T, Lavend'homme R, Luttun A, Saint-Remy J-M, Peerlinck K, Jacquemin M. Activation of human endothelial cells from specific vascular beds induces the release of a FVIII storage pool. Blood. 2010;115(23):4902–4909. doi: 10.1182/blood-2009-07-232546. [DOI] [PubMed] [Google Scholar]
- Shi Q, Fahs SA, Kuether EL, Cooley BC, Weiler H, Montgomery RR. Targeting FVIII expression to endothelial cells regenerates a releasable pool of FVIII and restores hemostasis in a mouse model of hemophilia A. Blood. 2010 doi: 10.1182/blood-2010-03-272419. blood-2010-2003-272419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular Niche of Postnatal Mesenchymal Stem Cells in Human Bone Marrow and Dental Pulp. Journal of Bone and Mineral Research. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Shovlin CL, Angus G, Manning RA, Okoli GN, Govani FS, Elderfield K, Birdsey GM, Mollet IsG, Laffan MA, Mauri FA. Endothelial Cell Processing and Alternatively Spliced Transcripts of Factor VIII: Potential Implications for Coagulation Cascades and Pulmonary Hypertension. PLoS ONE. 2010;5(2):e9154. doi: 10.1371/journal.pone.0009154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78(1):55–62. [PubMed] [Google Scholar]
- Van Damme A, Thorrez L, Ma L, Vandenburgh H, Eyckmans J, Dell'Accio F, De Bari C, Luyten F, Lillicrap D, Collen D, VandenDriessche T, Chuah MKL. Efficient Lentiviral Transduction and Improved Engraftment of Human Bone Marrow Mesenchymal Cells. STEM CELLS. 2006;24(4):896–907. doi: 10.1634/stemcells.2003-0106. [DOI] [PubMed] [Google Scholar]
- Van Den Biggelaar M, Bierings R, Storm G, Voorberg J, Mertens K. Requirements for cellular co-trafficking of factor VIII and von Willebrand factor to Weibel–Palade bodies. Journal of Thrombosis and Haemostasis. 2007;5(11):2235–2243. doi: 10.1111/j.1538-7836.2007.02737.x. [DOI] [PubMed] [Google Scholar]
- Veltkamp JJ, Asfaou E, Vandetor K, Vanderdo Ja, Vantilbu Nh, Pauwels EKJ. Extrahepatic Factor-Viii Synthesis - Lung Transplants in Hemophilic Dogs. Transplantation. 1974;18(1):56–62. doi: 10.1097/00007890-197407000-00009. [DOI] [PubMed] [Google Scholar]
- Webster WP, Penick GD, Peacock EE, Brinkhous KM. Allo-transplantation of spleen in hemophilia. NC Med J. 1967;28:505. [Google Scholar]
- Webster WP, Zukoski CF, Hutchin P, Reddick RL, Mandel SR, Penick GD. Plasma factor VIII synthesis and control as revealed by canine organ transplantation. AJP - Legacy. 1971;220(5):1147–1154. doi: 10.1152/ajplegacy.1971.220.5.1147. [DOI] [PubMed] [Google Scholar]
- Wion KL, Kelly D, Summerfield JA, Tuddenham EGD, Lawn RM. Distribution of factor VIII mRNA and antigen in human liver and other tissues. Nature. 1985;317(6039):726–729. doi: 10.1038/317726a0. [DOI] [PubMed] [Google Scholar]
- Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biology. 2009;10(11):8. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Nichols TC, Sarkar R, McCorquodale S, Bellinger DA, Ponder KP. Absence of a desmopressin response after therapeutic expression of factor VIII in hemophilia A dogs with liver-directed neonatal gene therapy. Proc Natl Acad Sci U S A. 2005;102(17):6080–6085. doi: 10.1073/pnas.0409249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannettino ACW, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214(2):413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.