Summary
Regulators of mitosis have been successfully targeted to enhance response to taxane chemotherapy. Here, we show that the Salt Inducible Kinase 2 (SIK2) localizes at the centrosome, plays a key role in the initiation of mitosis and regulates the localization of the centrosome linker protein, C-Nap1, through S2392 phosphorylation. Interference with the known SIK2 inhibitor PKA induced SIK2-dependent centrosome splitting in interphase while SIK2 depletion blocked centrosome separation in mitosis, sensitizing ovarian cancers to paclitaxel in culture and in xenografts. Depletion of SIK2 also delayed G1/S transition and reduced AKT phosphorylation. Higher expression of SIK2 significantly correlated with poor survival in patients with high-grade serous ovarian cancers. These data identify SIK2 as a plausible target for therapy in ovarian cancers.
Introduction
Uncontrolled mitosis is a distinguishing feature of cancer cells that has been effectively exploited for cancer therapy using anti-tubulin drugs such as paclitaxel. The use of primary paclitaxel-based combination chemotherapy increases progression free survival and overall survival in hematological and solid malignancies including ovarian cancer (Martin et al., 2008; McGuire et al., 1996). While high grade serous ovarian cancers are known to be highly chemosensitive, a significant proportion of these cancers fail to respond to primary taxane therapy leading to the emergence of resistant disease. There is a pressing need for the discovery of synergistic therapies that may improve ovarian cancer response to taxane-based chemotherapy and overall prognosis. Currently available mechanisms for selective enhancement of taxane response, such as the use of kinesin motor inhibitors or the inhibition of mitotic exit, are focused on targeting cancer cells only in mitosis (Huang et al., 2009; Mayer et al., 1999). While such strategies have shown clinical promise, overall tumor response rates have been limited (Blagden et al., 2008; Gautschi et al., 2008; Lin et al., 2008; Strebhardt and Ullrich, 2006). Novel therapeutic targets that augment taxane effects in inhibiting mitosis while providing taxane- and mitosis-independent mechanisms of cancer cell death are needed to improve clinical chemotherapy response. In this work we conducted an siRNA-based screen to identify modulators of mitotic progression that may influence clinical paclitaxel response.
Results
A high content siRNA kinome screen identifies cell cycle regulators of ovarian cancer cells
Recent evidence suggests that delayed mitotic progression or the inhibition of mitotic exit are key predictors of cell death either with or without taxane treatment (Bekier et al., 2009; Huang et al., 2009). To identify regulators of mitotic progression in ovarian cancer cells that may modulate taxane response, we used a three-step approach: 1) we screened 779 pools of siRNAs (Dharmacon kinome library) targeting individual genes to identify potential regulators of the G2 or the M phases (G2/M) of the cell cycle, 2) several hits were selected for further validation using individual siRNAs that made up the pool used in the primary screen and finally, 3) time-lapse microscopy was used to identify specific regulators of mitotic progression that were then tested for synergistic interactions with paclitaxel.
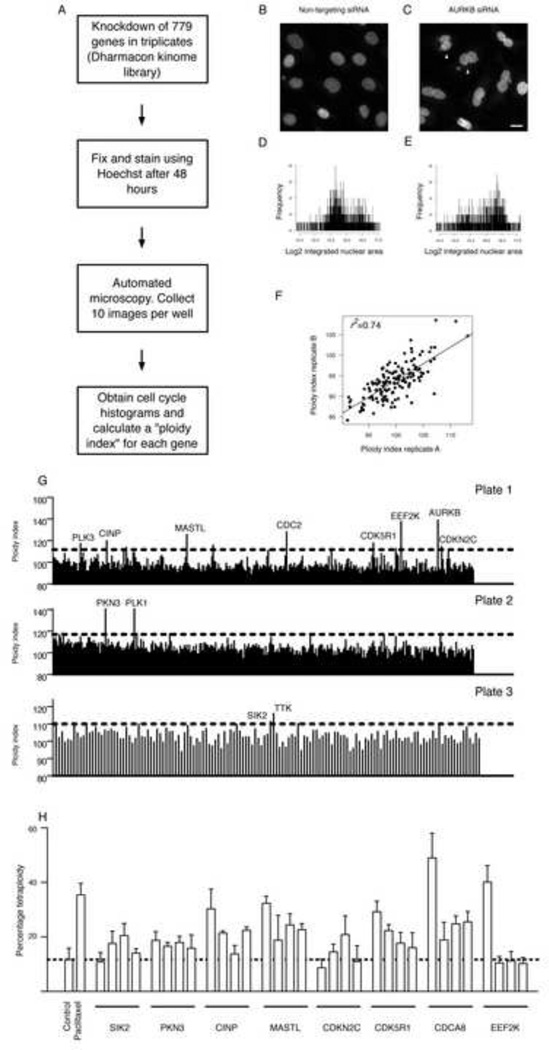
The primary screen utilized automated image acquisition and morphometric analysis of fixed cells in 384-well plates (high content analysis, Figure 1A) to measure single-cell DNA content following siRNA treatment. The integrated nuclear intensity values (defined as the nuclear area multiplied by its mean pixel intensity) were calculated as a measure for DNA content and used to generate cell cycle histograms following the knockdown (KD) of each of the 779 genes included in the screen. The percentage of shift of the median integrated nuclear intensity value following a gene KD compared to that following transfection using non-targeting siRNA was calculated and termed the ploidy index (Figure 1 B–E and Supplementary Table S1). Genes that upon KD induced a percentage shift (ploidy index) that was above the median and 2 standard deviations of all genes in a plate were identified as positive hits for further validation (Figure 1G and Supplementary Table S2). There was a high correlation between the ploidy indices across triplicate plates (median correlation coefficient =0.74) and a mean coefficient of repeatability for all genes tested of 0.1, indicating high data precision in the screen (Figure 1F, Supplementary Figure S1A and Supplementary Table S1). The identified hits included many genes that have been previously shown to regulate G2 or M phases of cell cycle progression such as Cyclin Dependent Kinase 1 (CDC2) and Polo Kinase 1 (PLK1) (Supplementary Table S2). The magnitude of ploidy shift varied according to the mechanism of G2/M regulation by a gene rather than the biological importance. For example, depletion of Aurora Kinase B (AURKB) resulted in cytokinesis failure and a profound increase in the ploidy index. In contrast, depletion of the centrosome kinase phosphotyrosine picked threonine kinase 1 (MPS1, also called TTK) resulted in a modest increase in ploidy that presumably reflects delayed mitotic transition (Supplementary Tables S1–S3).
Figure 1. A kinome siRNA screen identifies regulators of G2/M progression in ovarian cancer.
A) A schematic presentation of the siRNA kinome screen. B–H) Each of the 779 genes included in the screen was targeted using pools of 4 individual siRNAs. These pools were divided into three plates (320 pools for plates 1 and 2 and 139 pools in plate 3). Three replicates were used for each plate and ten images were obtained per well. Integrated nuclear intensity (INI) values for individual cells, defined as the nuclear area multiplied by its mean pixel intensity, were extracted from 32,400 images including a total of 1,946,532 cells. The calculation of the percentage shift of the median integrated nuclear intensity value (ploidy index) is explained in B–E. Images were obtained 48 hours following transfection with non-targeting siRNAs (B) or a pool of siRNAs targeting AURKB (C). Arrow heads in C point to examples of fused nuclei. Scale bar; 10 µm. The INI values were used to generate cell cycle histograms following non-targeting siRNA transfection (D) or AURKB siRNA transfection (E). Note the shift to the right of the log2 INI values in E as a consequence of tetraploidy. The ploidy index (PInd) was obtained as the percentage of the median INI following gene knockdown normalized to the median INI following control transfection. F) An example of the correlation between the ploidy indices of two replicate plates in the screen. In (G) the cutoff PInds for each plate and examples of the identified hits are shown. H) Shown is the percentage of the tetraploid cells in relation to the total number of cells following either paclitaxel treatment, as a positive control for tetraploidy, or knockdown of genes using four independent siRNAs. The mean ± standard deviations of triplicates is presented. See also Figure S1, Tables S1–3.
Among the identified hits, 7 were selected for further validation based on their potential novelty (Figure 1 H). To exclude off-target effects we used 4 individual siRNAs that made up the siRNA pool that was used in the primary screen to KD each gene and compared the percentage of tetraploid cells following genetic KD with the percentage following transfection with a non-targeting siRNA control. As a positive control for tetraploidy we used; 1) paclitaxel treated non-transfected cells and 2) KD of the chromosome passenger protein Borealin (CDCA8), which plays a critical role in cytokinesis. Six of the seven genes were validated with at least 2 independent siRNAs, which resulted in a significant increase in tetraploidy (p<0.05, Wilcoxon test, Figure 1H). Only one of the 4 siRNAs targeting the Eukaryotic Elongation Factor-2 Kinase (EEF2K) resulted in increased tetraploidy indicating an off-target effect. Interestingly, this siRNA had a perfect sequence match to EEF2K and also 70% sequence match to Borealin.
The 6 validated targets may be involved in the regulation of G2, mitosis or both. To determine specific regulators of mitosis we used bright field microscopy and time lapse imaging to identify targets that lead to delayed mitotic progression. To demonstrate the opposite effect, we used KD of the mitotic checkpoint protein BUBR1 and, as expected, this resulted in rapid mitotic progression (mean = 20 minutes following KD of BUBR1 compared to 58 minutes in controls, n= 10, p<0.01; Supplementary Movies Smov1 and Smov2). In contrast, depletion of SIK2, Cyclin Dependent Kinase 2 Interacting Protein (CINP) or CDK5 Regulatory Subunit 1 (CDK5R1) resulted in a significant delay in mitotic progression followed by mitotic exit without cell division (Supplementary Movie Smov3 and other data not shown).
The finding that SIK2 may play a role in regulating mitosis was intriguing since its established functional role has been in the regulation of cellular metabolism but not the cell cycle (Dentin et al., 2007; Katoh et al., 2004; Screaton et al., 2004). We have previously shown that KD of the Drosophila orthologues of Serine/Threonine Kinase 11 (STK11, also called LKB1), a known SIK2 kinase, and SIK3, an immediate family member of SIK2 results in severe mitotic abnormalities (Bettencourt-Dias et al., 2004). We, therefore, selected SIK2 for further validation. Time-lapse photography showed that loss of SIK2 resulted in a dramatic increase in the mean mitotic progression time (62 minutes in control cells transfected with non-targeting siRNA v 133, 209 and 172 minutes following SIK2 KD using three siRNAs, p<0.001, One-Way ANOVA and Tukey’s multiple comparisons test [Figure 2A–D]). Importantly many of the SIK2 depleted cells either did not exit mitosis during the time of recording, ruptured or failed to undergo cytokinesis (Supplementary Movie Smov3 and additional data not shown).
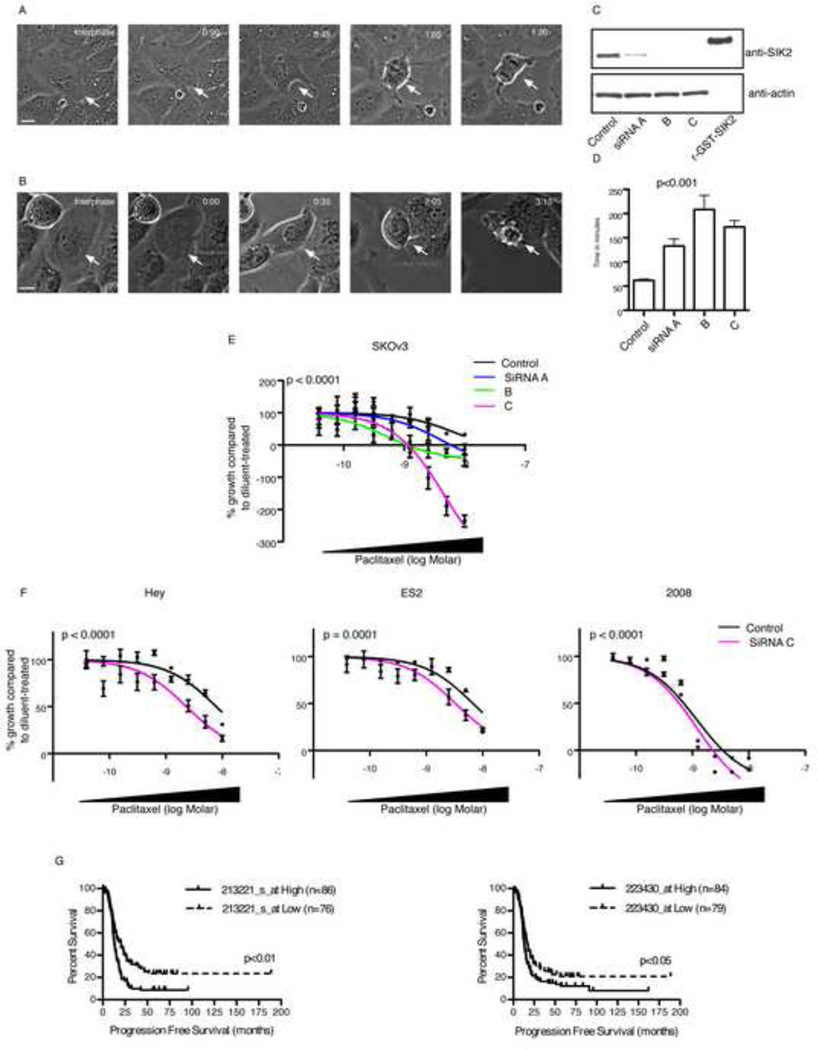
Figure 2. Depletion of SIK2 delays mitotic progression and sensitizes ovarian cancer cells to paclitaxel.
A–D) SKOv3 cells were transfected with either non-targeting siRNA (A) or SIK2 siRNA (B) for 48 hr then monitored for 16 hr (one image every 5 minutes) using bright field phase-contrast microscopy. The time interval from nuclear envelope breakdown (0:00 in A and B) to anaphase onset (01:20 in control cells) was estimated. Arrows point to the cell under study. Time is shown as hours:minutes. Scale bar, 10 µm. In C Western blot confirmation of knockdown of SIK2 using 3 independent siRNAs compared to non-targeting siRNA control is shown. r-GST-SIK2; recombinant GST-tagged SIK2 was used on the same Western blot to confirm the antibody specificity. D) The mean ± s.e.m of the mitotic transition time (obtained as in A and B) is presented following control and SIK2 siRNA transfections. In E, SKOv3 cells (5000 cells per well) were reverse transfected in 96 well plates using either non-targeting controls or SIK2 siRNAs A, B and C for 24 hr and cells were then either a) fixed and stained using crystal violet to estimate the number of cells on the day of paclitaxel treatment (Day 0) or b) treated with either diluent or paclitaxel at 9 different concentrations. The latter group of cells was fixed and stained 72 hr following drug treatment. Growth of cells from day 0 to day 3 was estimated and the percentage paclitaxel-induced growth inhibition for each siRNA in relation to diluent treated cells transfected with the same siRNA was calculated and a least-squares fit was obtained to estimate the GI 50 as described in methods (Monks et al., 1991). Shown is the mean ± s.e.m from three replicates per concentration. In F the effect of depletion of SIK2 using siRNA C on paclitaxel response in three cell lines is shown. The experiment was conducted as in E. Shown is the mean ± s.e.m from six replicates per concentration. Also shown is the p value for the comparison between the GI 50 in cells transfected with non-targeting siRNA control v cells transfected with SIK2 siRNA. G) Microarray expression data for two probe sets representing SIK2 were used to generate Kaplan Meier survival curves for high expressing (defined as cancers with values above the median + (0.5 × median absolute deviation [MAD]) or low expressing (below the median − (0.5 × MAD) for 229 high-grade serous (HGS) ovarian cancers. See also Figure S2 and Movies Smov1–3.
Depletion of SIK2 sensitizes ovarian cancers to paclitaxel
As depletion of SIK2 resulted in protracted mitotic progression time, we tested the hypothesis that this may sensitize ovarian cancer cells to paclitaxel. Transfection with SIK2 siRNA decreased the dose of paclitaxel required to reduce cancer cell growth by 50 % (GI 50) from 4.8 nM (95% confidence interval [CI]; 2.7–8.4 nM) in control-transfected cells to 1.4 nM (95% CI; 0.6–3.2 nM), 0.17 nM (95% CI; 0.05–0.6 nM) or 0.36 nM (95% CI; 0.01–0.1 nM) for SIK2 siRNAs A, B and C, respectively (Figure 2E). Importantly, this effect was not cell-specific as SIK2 KD significantly decreased paclitaxel GI 50 in Hey cells (from 7.8 nM for control siRNA to 2.3 nM for SIK2 siRNA), ES2 cells (from 6.7 nM for control siRNA to 3.3 nM for SIK2 siRNA) and 2008 cells (from 0.67 nM for control siRNA to 0.47 nM for SIK2 siRNA) ([Figure 2F]), indicating that selective depletion of SIK2 is sufficient to sensitize ovarian cancer cells to paclitaxel in vitro.
To test the biological significance of SIK2 in ovarian cancer we first examined its expression pattern in 59 ovarian cancers and 9 pools of normal ovarian surface epithelial cells using quantitative PCR. The range of expression of SIK2 was narrow suggesting that a large number of cases was required to identify a correlation between SIK2 expression and clinical outcome (data not shown). The importance of SIK2 in regulating mitosis and paclitaxel response was supported by the analysis of SIK2 expression in 229 patients with high-grade serous (HGS) ovarian cancers in a population-based study (Tothill et al., 2008). Higher expression of SIK2 significantly increased the hazard of cancer progression following taxane-based chemotherapy using the two independent affymetrix microarray probe sets that were found to accurately represent SIK2 expression (Figure 2G and Supplementary Figure S2A). These results were validated using quantitative PCR (Supplementary Figure S2B). In addition, the level of SIK2 microarray expression in paclitaxel or carboplatin resistant ovarian cancers was compared to that in paclitaxel or carboplatin responders in a prospective randomized clinical trial in which patients with primary ovarian cancer were treated initially with either single agent paclitaxel or single agent carboplatin for three cycles (Ahmed et al., 2007). SIK2 expression was significantly higher in paclitaxel resistant compared to paclitaxel-sensitive cancers (p=0.028, one-sided t-test, n=20, Supplementary Figure S2C). In contrast, no significant difference was found in SIK2 expression between carboplatin resistant or carboplatin sensitive cancers (p=0.7, one-sided t-test, n=15, Supplementary Figure S2C). All together these data support an important role of SIK2 in the regulation of mitosis and taxane response in cell culture and in ovarian cancer patients.
SIK2 localizes at the centrosome
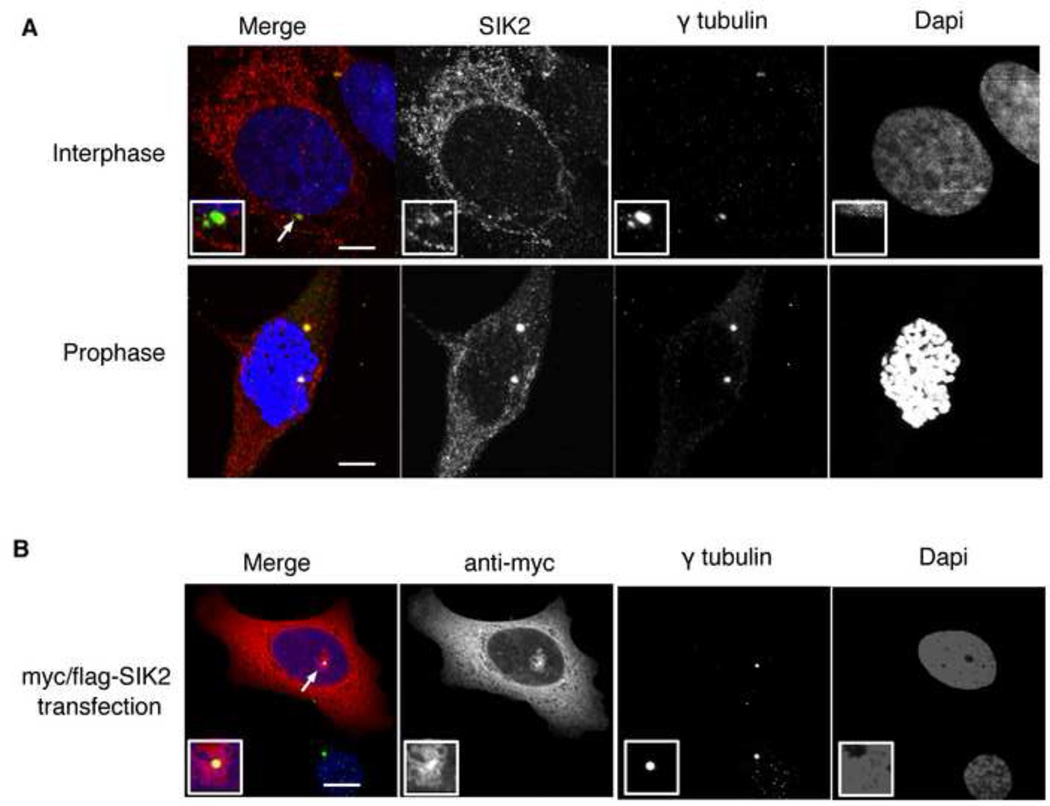
To investigate a possible physical association between SIK2 and the mitotic spindle, we examined SIK2’s subcellular localization using immunofluorescence (IF). As previously described, endogenous SIK2 was observed in the cytoplasm (Katoh et al., 2004). In addition, SIK2 co-localized with γ-tubulin in the centrosomes of 70 % of cells in interphase and in all cells in mitosis and co-immunoprecipitated with γ-tubulin (Figure 3A and Supplementary Figure S3A). Preincubation of the SIK2 antibody with recombinant SIK2 protein, but not with control proteins (BSA or recombinant histone H1), abolished the centrosome signal of SIK2 on IF (Supplementary Figure S3B). Further confirmation of specificity was achieved by showing that KD of SIK2 resulted in a significant decrease in the percentage of cells with detectable SIK2 in the centrosome using immunofluorescence (p<0.001, t test; Supplementary Figure S3C). The expression of SIK2 was predominantly pericentriolar as evidenced by immunostaining of Hela cells that stably expressed GFP-tagged centrin (Supplementary Figure S3D) and was independent of microtubules since it colocalized with γ-tubulin following nocodazole treatment (data not shown). The observation that endogenous SIK2 localized at centrosomes was further supported by showing that exogenous myc/flag-tagged SIK2 localized to the centrosomes of 50% of the cells examined (Figure 3B). In addition, differential centrosome localization was also observed following transfection using the untagged version of the SIK2 plasmid and detection using anti-SIK2 antibody (p<0.001, paired t test [Supplementary Figure S3E–H]). All together these results confirm that a pool of SIK2 localizes at the centrosome.
Figure 3. SIK2 localizes at the centrosome.
A) SKOv3 cells were fixed and stained for immunofluorescence (IF) using the indicated antibodies to show the co-localization between SIK2 and γ-tubulin. B) The co-localization of ectopically expressed myc-tagged SIK2 with γ-tubulin in SKOv3 cells is shown. Also note the peri-centrosomal expression of SIK2. Scale bar, 5 µm. See also Figure S3.
SIK2 induces centrosome splitting
We next tested the functional role of SIK2 in the centrosomes. Examination of the centrosomes in interphase cells transfected with a myc/flag-SIK2 expression construct revealed a significant increase in the percentage of myc-positive cells with centrosome splitting (CS) compared to the percentage in myc-negative cells or cells transfected with empty vectors (21.3, 5 and 3.9, respectively; p<0.001, one-way ANOVA, Figure 4A–C). This effect was not due to failure of reengagement following disengagement which is known to occur in late mitosis to license for centriole duplication (Tsou and Stearns, 2006) as in SKOv3 cells disengaging centrioles only separate by submicrometre distances (Supplementary Figure S4A–G). SIK2-induced CS was dependent on its kinase activity since overexpression of a kinase-inactive SIK2 mutant (SIK2_mt; K49M) (Katoh et al., 2006) did not result in an increase in the percentage of cells with CS (Figure 4B–C). The effect of wild type SIK2 in inducing CS was similar to the reported phenotype that is observed following the overexpression of NEK2 but less in magnitude (3.9% in control cells v 21.3% in SIK2-positive cells and 50.9% in NEK2-positive cells, p<0.001, Figure 4B–C). Similar results were observed in the osteosarcoma cell line U2OS, a cell line that is commonly used to study this phenotype (4, 29 and 55 for cells transfected with empty vector, SIK2-myc/flag and NEK2-myc/flag, respectively; p<0.001, one-way ANOVA; Figure 4C). As the metabolic function of SIK2 is activated following LKB1-induced phosphorylation, we tested the effect of co-expression of SIK2 and LKB1 on CS. LKB1 did not increase the percentage of cells with CS above what is already achieved by SIK2 alone (Supplementary Figure S4H). This implies that the activity of SIK2 in inducing centrosome splitting is independent of LKB1.
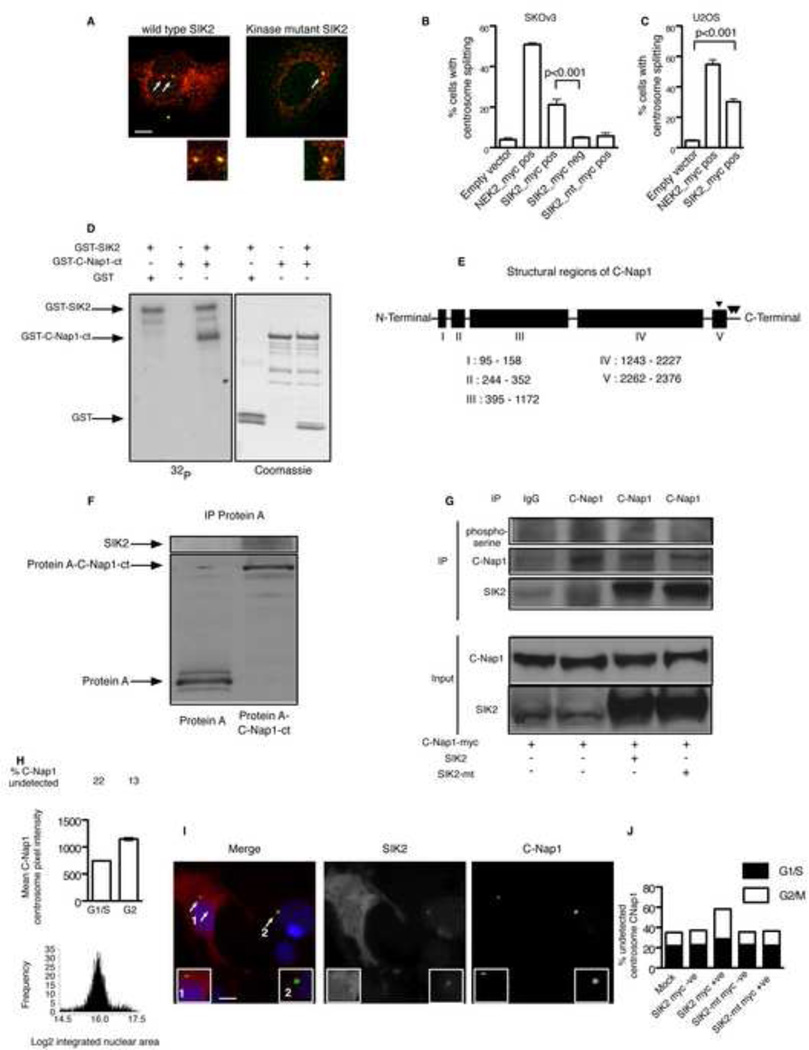
Figure 4. Overexpression of SIK2 induces centrosome splitting through phosphorylation of C-Nap1.
A) SKOv3 cells were transfected with myc/flag-tagged wild type SIK2 or myc/flag-tagged kinase mutant SIK2 (SIK2_mt) plasmid for 24 hr then cells were fixed and stained using anti-myc antibody (red), and anti-γ-tubulin antibody (green). Arrow heads show an example of CS (inter-centrosome distance > 2µm) following SIK2 transfection. B–C) Mean ± s.e.m of the percentage of cells with CS 24 hr following transfection using the indicated plasmids. Overexpression of NEK2, which is known to induce CS, was used as a positive control in this experiment. Experiments were performed at least in triplicates. Scale bar, 5 µm.
D) In vitro kinase assays were performed in the presence of either γ-32P-ATP followed by autoradiography, left, or ATP alone followed by Coomassie staining, right. The bands representing GST-C-Nap1-ct were cut and subjected to LC-MS analysis for protein identification and to detect sites of phosphorylation. E) A diagram showing the potential five coiled coil domains of C-Nap1 as predicted in the Uniport data base and the serine phosphohrylation sites detected by LC-MS at amino acids S2234, S2392 and S2394. F) Either Protein A alone or Protein A-tagged C-Nap1-ct were transiently expressed in 293-T cells then immunoprecipitated using Rabbit IgG coated beads followed by elution and separation of the precipitates using SDS-PAGE and immunoblotting using anti SIK2 antibody. G) 293T cells were transfected with the indicated plasmids for 24 hours, then lysates were collected and immunoprecipitation (IP) performed using the indicated antibodies. Immunoprecipitates were resolved by electrophoresis as described in methods then probed with pan anti-phosphoserine antibody initially then anti-C-Nap1 antibody using the same membrane. Also, shown is the immunoblot result for SIK2 performed on the same membrane. H) Cells were cultured in 384 well plates overnight then fixed and stained using Hoechst nuclear stain and anti-C-Nap1 antibody. Shown is the automated analysis of 4000 cells over 10 replicate wells (as described in Supplementary methods) to identify cell cycle histograms, mean centrosome intensity value for C-Nap1 ± s.e.m and the percentage of cells with undetectable C-Nap1 in the centrosomes. Mitotic cells were manually excluded from the analysis. I) Cells were transfected with SIK2 expressing construct for 24 hr then fixed and stained using the indicated antibodies. Note the lower C-Nap1 intensity in the centrosomes of SIK2-transfected cell (1) compared to untransfected cell (2) in the same field. Scale bar, 5 µm. Arrows point to magnified centrosome in the inset. J) Shown is the percentage of cells with undetectable C-Nap1 centrosome signal per cell cycle stage following transfection using the indicated plasmids. See also Figure S4.
We next took an unbiased approach to identify possible targets for SIK2 phosphorylation in the centrosome. Searching the Swiss-PROT database for human proteins that contains the previously identified putative SIK2 phosphorylation consensus sequence LX[HKR]S/TXSXXXL (Screaton et al., 2004), identified the peptide LHHSLSHSLL in the carboxy-terminal domain (amino acids: 2387–2396) of the centrosome protein CEP250 (also called C-Nap1) as a putative phosphorylation site. C-Nap1 is thought to be one of the key proteins that are required for centrosome cohesion in interphase and its centrosome localization is predominantly regulated by phosphorylation (Fry et al., 1998a; Mayor et al., 2002). Importantly, phosphorylation of the carboxy-terminal part of C-Nap1 by ectopically expressed NEK2 results in premature exit of C-Nap1 from the centrosome and CS (Fry et al., 1998a; Fry et al., 1998b; Mayor et al., 2002). Recombinant SIK2 directly phosphorylated a 15 amino-acid peptide derived from C-Nap1 that contained the putative SIK2 phosphorylation site at the underlined serine amino acids (LAGLHHSLSHSLLAV) in vitro as shown by liquid chromatography-mass spectrometry (LC-MS), data not shown. In addition, SIK2 catalyzed the incorporation of radioactive γ-32p-ATP into recombinant GST-tagged carboxy-terminal C-Nap1 (GST-C-Nap1-ct, aa; 1982–2442; 461 amino acids), but not GST alone (Figure 4D). Remarkably, LC-MS confirmed that the predominant serine phosphorylation site of the recombinant carboxy-terminal domain of C-Nap1 is S2392 at the predicted consensus phosphorylation sequence and to a lesser extent S2394 (LHHSLSHSLL). Importantly, SIK2 phosphoylation of carboxy-terminal C-Nap1 appeared highly specific as the peptide LHHSLSHSLL was the predominant site of serine phosphorylation in addition to a second site (S2234) at the fifth potential coiled coil region of C-Nap1 (Figure 4E). We next expressed a protein A-tagged version of the carboxyterminal domain of C-Nap1 in 293-T cells, which are known for their high transfection efficiency. Immunoprecipitation using rabbit IgG-coated beads of protein A-tagged carboxy-terminal C-Nap1 but not protein A alone co-precipitated endogenous SIK2 (Figure 4F) indicating that direct binding between SIK2 and exogenously expressed carboxy-terminal part of C-Nap1 occurs in cells. In addition, endogenous SIK2 partially co-localized with ectopically expressed C-Nap1 in the centrosome of SKOv3 cells (Supplementary Figure S4I). Co-expression of full length C-Nap1 with the kinase active SIK2 in 293-T cells resulted in the phosphorylation of C-Nap1 as detected by C-Nap1 immunoprecipitation followed by probing using anti-phosphoserine antibody. In contrast expression of the kinase mutant SIK2 did not result in phosphorylation of the co-expressed C-Nap1 (Figure 4G). We next investigated whether SIK2 displaces C-Nap1 from the centrosomes. IF confirmed the localization of endogenous C-Nap1 at the centrosomes of ovarian cancer cells and, in addition, showed that there was variability in the centrosome expression level (Supplementary Figure S4J). Knockdown of C-Nap1 rsulted in a 10 fold increase in SKOv3 cells with centrosome splitting (Supplementary Figure S4K). High content analysis of C-Nap1 in untransfected cells showed that its centrosome localization significantly increases in G2 compared to G1/S (p<0.001, t test, Figure 4H). In addition, the percentage of cells in which the centrosome expression of C-Nap1 was below the automated threshold of detection decreased from 22% in G1/S to 13% in G2. We next examined the effect of SIK2 transfection on the centrosome localization of C-Nap1 (Figure 4I). SIK2 transfection resulted in a modest but significant increase of the percentage of cells with undetectable C-Nap1 signal in G1 (22% in SIK2 negative, 27.2% in SIK2 positive, p<0.001, Chi square test, n=3376, Figure 4J). However, in G2, SIK2 expression resulted in a greater magnitude of C-Nap1 displacement (14.5% in SIK2 negative cells to 29.4% in SIK2 positive cells; p=0.004; Chi square test, n= 340, Figure 4J). In contrast, expression of a kinase inactive SIK2 did not increase the percentage of cells with undetectable C-Nap1 signal in G1/S or in G2 (Figure 4J). These results implicate C-Nap1 as a putative downstream target for SIK2 at the centrosome.
SIK2 is required for centrosome separation in mitosis As ectopic expression of SIK2 resulted in CS, we tested the hypothesis that endogenous SIK2 was required for centrosome separation in mitosis. Interestingly, in interphase, depletion of SIK2 resulted in uncoupling of the centrosomes from the nucleus (Supplementary Figure S5A–B). Importantly, co-transfection of SKOv3 cells with siRNA targeting the 3’UTR of SIK2 and a construct expressing the open reading frame of SIK2 completely rescued this phenotype (Supplementary Figure S5C). Inducing a wound in a confluent monolayer of SKOv3 cells following control siRNA transfection oriented the centrosomes towards the leading edge of the cell. In contrast, cells depleted from SIK2 failed to orient their centrosomes towards the leading edge in spite of the formation of lamellipodia (Supplementary Figure S5D–E). These results further support the notion of a centrosome-specific function of SIK2. In mitosis, depletion of SIK2 resulted in the accumulation of cells in prometaphase within 48 hr of transfection (p<0.01, t-test; Figure 5A–B). Importantly, this mitotic defect was not because of depletion of energy reserves as knockdown of SIK2 did not decrease cellular ATP content (Supplementary Figure S5F). There was a significant increase in the ratio of prometaphase to metaphase cells following SIK2 KD (2.1 in controls and 8.3 following SIK2 KD; Figure 5C). These results were confirmed in the U2OS osteosarcoma cell line (Figure 5D). The block in prometaphase was due to failure of SIK2-depleted cells to form bipolar mitotic cells (p<0.001, Oneway ANOVA; Figure 5A and Figure 5E). Importantly, the role of SIK2 in regulating centrosome separation in mitosis was evolutionarily conserved, as depletion of the orthologue of SIK2 in Drosophila (CG4290) in DMEL cells resulted in a significant increase in mitotic cells with defects in centrosome separation (p<0.01, t-test; Figure 5F–G). Thus, SIK2 is required for appropriate centrosome positioning in interphase and for centrosome separation in mitosis.
Figure 5. SIK2 is required for centrosome separation in mitosis.
A–E)Cancer cells were transfected with siRNAs for 48 hr as indicated then fixed and stained. In A and B SKOv3 cells were stained using anti-γ-tubulin (red) and anti-phosphohistone H3 (green) to reveal the centosome position in mitosis in relation to chromosomes. Note the presence of examples from different mitosis phases in control cells (1–4; prophase, prometaphase, metaphase and anaphase, respectively) compared to the predominance of prometaphase cells following SIK2 KD. Also shown are examples of multipolar (I) and monopolar (II) centrosome positioning following SIK2 KD. Bar plots in B and D represent the mean ± s.d. of the percentages of cells at different stages of mitosis. Bar plots in C represent the mean prometaphase/metaphase ratio ± standard deviation. F–G) DMEL Drosophila cells were transfected using non-targeting control double stranded RNA (dsRNA) or dsRNAs targeting the Drosophila orthologue of SIK2. Examples of mitotic cells with failed centrosome separation are presented in F. In G, the mean ± s.d. of mitotic cells with failed centrosome separation from three independent experiments is shown. Scale bars, 10 µm. See also Figure S5.
PRKAR2A inhibits SIK2 in the centrosome
We next investigated the mechanisms regulating SIK2 activity in the centrosome. The metabolic function of SIK2 in the cytoplasm is under inhibitory control by PKA (Katoh et al., 2004). Interestingly, the regulatory subunit of PKA, PRKAR2A, is tethered at the centrosome and the pericentriolar region of interphase cells by the anchoring protein AKAP450 (Carlson et al., 2001; Landsverk et al., 2001). In mitosis, CDC2 phosphorylates PRKAR2A resulting in its displacement from the centrosome (Carlson et al., 2001). Consistent with previous observations, we found that PRKAR2A localized to the centrosomes of SKOv3 cells in interphase. In addition, PRKAR2A disappeared from the centrosome early in prophase and this coincided with centrosome separation and a significant increase in the centrosome level of SIK2 and γ-tubulin (p<0.001, One-way ANOVA, Supplementary Figure S6A–F).
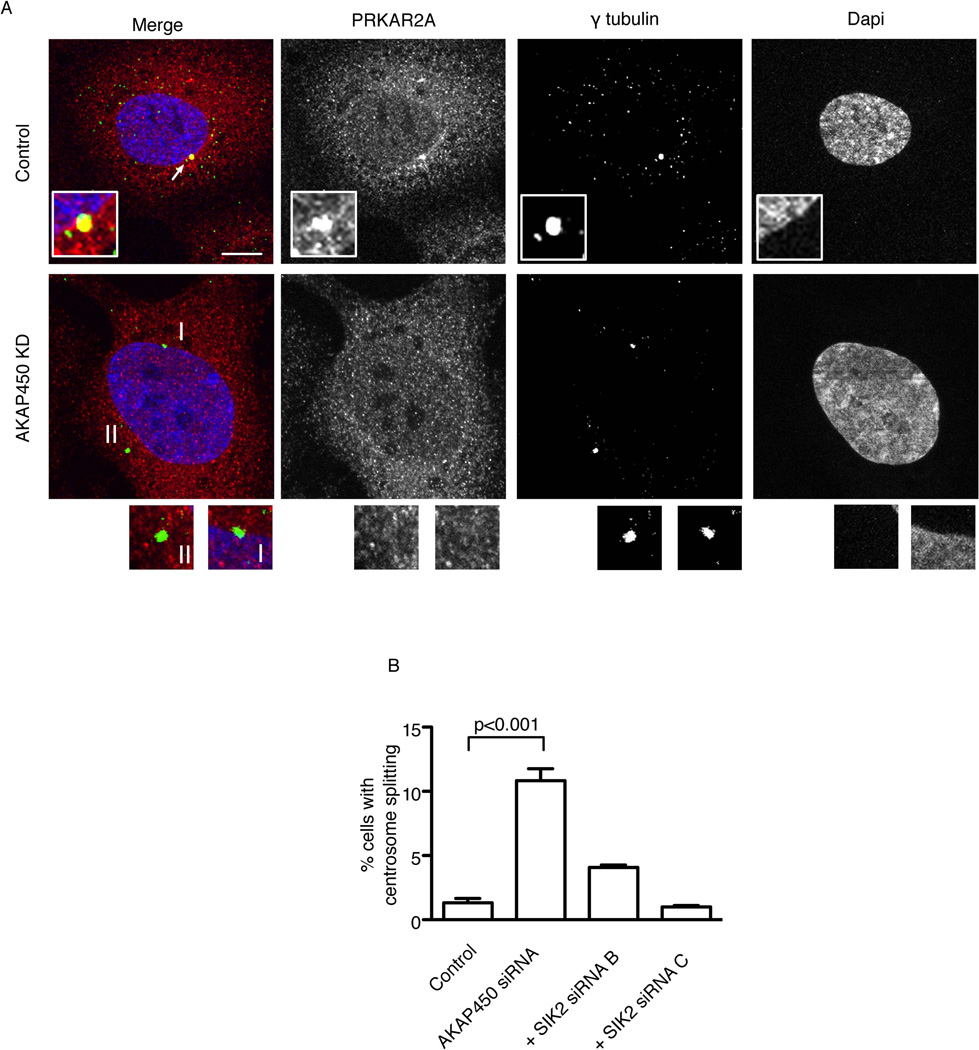
The centrosome localization of PRKAR2A was required for maintaining centrosome pairing since its depletion in SKOv3 cells resulted in 11.6 fold increase in interphase cells with centrosome splitting compared to control siRNA transfected cells (9.3% and 0.8% respectively; p<0.001, t-test [Supplementary Figure S6G]). To target the centrosome localization of PRKAR2A without interfering with its cytoplasmic level we depleted AKAP450 in SKOv3 cells (Figure 6A). This depletion abolished the centrosome localization of PRKAR2A but not that of SIK2 (Supplementary Figure S6H) and resulted in a significant increase in cells with centrosome splitting (CS) (10.8% in AKAP450 depleted cells v 1.3% in controls; p<0.001, One-way ANOVA [Figure 6B]). Similar results were obtained using 2 other AKAP450 siRNAs, not shown, Importantly, the increase in CS was dependent on SIK2 since co-transfection of SKOv3 cells with siRNAs targeting SIK2 and AKAP450 rescued the phenotype (Figure 6B). Thus PRKAR2A is required for preventing SIK2 from inducing CS in interphase and may play a key role in regulating the appropriate timing of centrosome separation early in mitosis
Figure 6. The centrosome localization of PRKAR2A is required for regulating the appropriate timing of centrosome separation by SIK2.
A-SKOv3 cells were transfected with the indicated siRNAs for 72 hours then fixed and stained. The centrosome expression of PRKAR2A is present in control cells (arrow) but absent following KD of AKAP450 (I and II). B) Bar plots represent the mean ± s.d. of triplicate percentages of cells with CS following transfection with the indicated siRNAs. Depletion of AKAP450 resulted in a significant increase in CS while co-depletion of AKAP450 and SIK2 rescued this phenotype. Scale bar, 5 µm. See also Figure S6.
SIK2 is required for AKT phosphorylation and growth of ovarian cancer cells
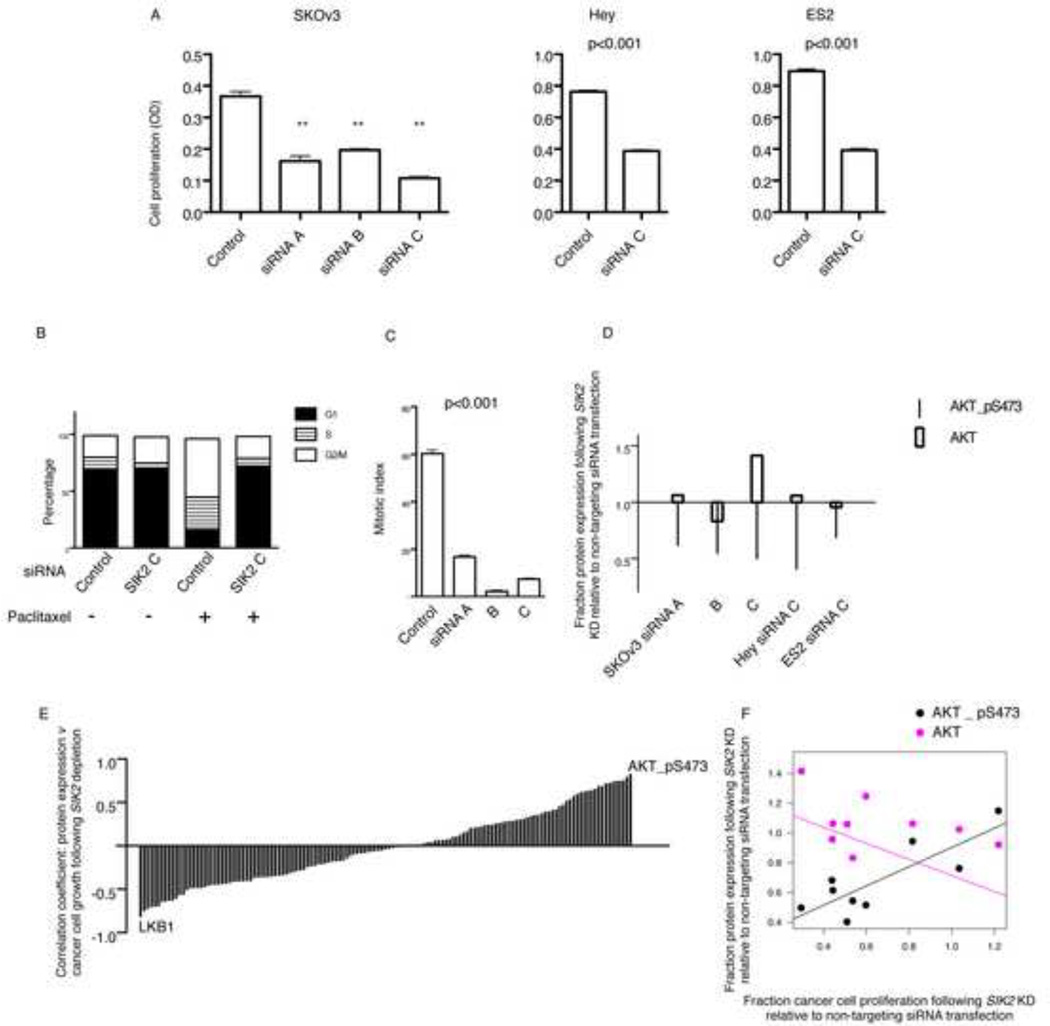
We next investigated the effect of SIK2 depletion on cancer cell growth independent from paclitaxel. Depletion of SIK2 in the ovarian carcinoma cell lines SKOv3, ES2 and Hey resulted in a significant decrease in cancer cell proliferation (Figure 7A, p<0.001, t-test). To investigate the mechanism of suppression of proliferation we tested the effect of depletion of SIK2 on the cell cycle with or without synchronization. Cell cycle analysis using flow cytometry of non-synchronized population of SKOv3 cells following depletion of SIK2 confirmed the accumulation of tetraploid cells (Figure 7B). However, synchronization of cells in G2/M using paclitaxel treatment following SIK2 depletion showed that a significant proportion of cells were delayed in G1 (Figure 7B) and failed to enter mitosis as indicated by the significant decrease in mitotic index following paclitaxel treatment (p<0.001, One-Way ANOVA; Figure 7C). This data indicated that loss of SIK2 results in defective G1/S transition in addition to delayed mitotic progression and that both may contribute to suppression of ovarian cancer cell proliferation. To investigate the molecular profile underlying delayed G1/S transition we investigated the effect of SIK2 depletion on the expression of 139 proteins using reverse phase protein arrays. Depletion of SIK2 resulted in a significant decrease in AKT phosphorylation at amino acid 473 without significantly influencing total AKT expression (Figure 7D). We next examined the effect of SIK2 depletion using 3 independent siRNAs in 3 ovarian cancer cell lines on the of expression of 139 proteins and correlated this with cancer cell growth for the cell lines tested, 9 expression values for each protein correlated with 9 growth proliferation values (Figure 7E and Supplementary Table S4). Decreased phosphorylation of AKT following SIK2 depletion resulted in the highest direct correlation with suppression of ovarian cancer cell growth (Pearson’s correlation coefficient = 0.83, p=0.006, Figure 7F). In contrast, depletion of SIK2 resulted in increased LKB1 expression and this significantly correlated with suppression of ovarian cancer cell growth, i.e. a strong negative correlation (Pearson’s correlation coefficient = − 0.82, p=0.007).
Figure 7. SIK2 is required for AKT phosphorylation and growth of ovarian cancer cells.
A) SKOv3, ES2 and Hey cells were either transfected using non-targeting control siRNA or the indicated SIK2 siRNAs for 5 days. Cells were fixed and stained using crystal violet. Shown is the mean ± s.e.m. of 12 replicate values per siRNA transfection type from two independent experiments in SKOv3 cells. ** indicates p<0.001. Error bars for Hey and ES2 cells represent the s.e.m. of 6 replicate values per siRNA transfection type from two independent experiments. B) Typical cell cycle distributions are presented following transfection of SKOv3 cells with the indicated siRNAs. C) Cells were transfected using the indicated siRNA for 48 hr then treated with paclitaxel 100 nM for 24 hr, fixed and stained using anti-phosphohistone H3 antibody to calculate the mitotic index (MI) using flow cytometry. Shown is the mean MI (± s.d.) of two independent experiments. D) Cell lines were transfected with the indicated SIK2 siRNAs and control non-targeting siRNAs for 48 hr then harvested for analysis of protein expression using reverse phase protein arrays. Shown is the fraction of expression of AKT-pS437 and total AKT following SIK2 siRNA transfection relative to protein expression following non-targeting siRNAs. E) The expression of 139 proteins (Supplementary Table S4) was estimated using reverse phase protein arrays as in D following transfection of cancer cells using three SIK2 siRNAs and one non-targeting control in 3 cell lines; SKOv3, Hey and ES2. The expression of each of the 139 proteins was correlated with cancer cell growth that was measured in A. Each bar represents the coefficient of the correlation between the expression of each of the proteins in 9 cell lines (3 SIK2 siRNAs × 3 cell lines) and the growth of the cancer cell line from which the protein was measured. Positive values indicate that higher expression of a protein correlates with more cancer cell growth. Negative values indicate that higher expression correlate with poor growth. F) Shown is the regression lines for the correlation between the fraction of ovarian cancer cell proliferation following SIK2 depletion relative to non-targeting control and the fraction expression of AKT-pS437 or total AKT following SIK2 depletion relative to non-targeting controls. See also Figure S7 and Table S4.
Loss of SIK2 sensitizes ovarian cancer to paclitaxel in vivo
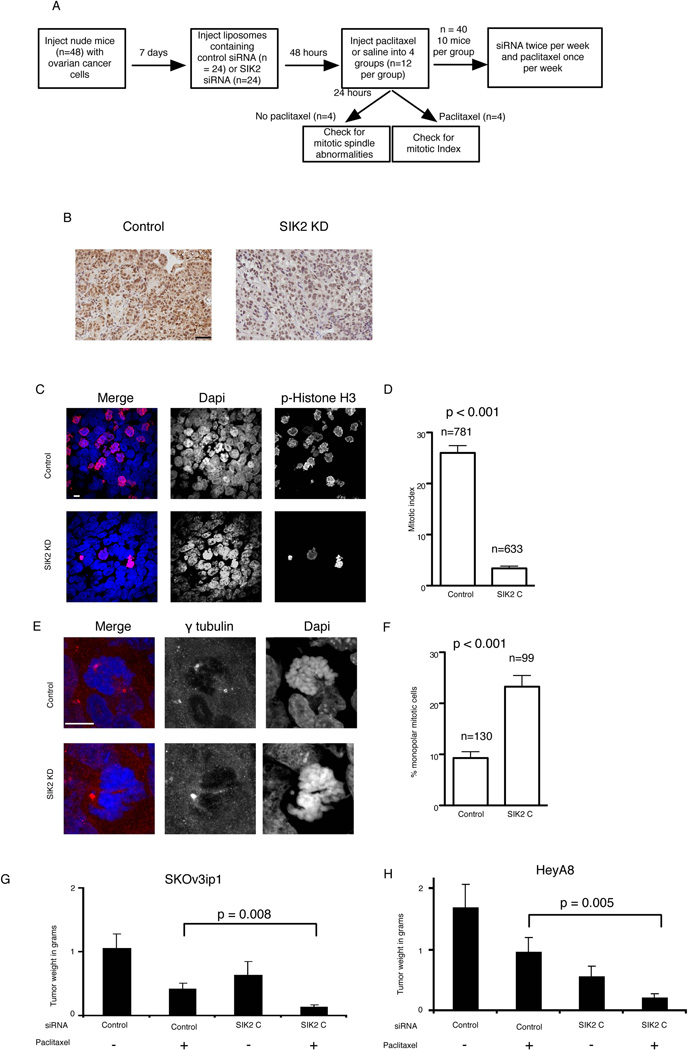
Based on the above findings we proposed that depletion of SIK2 interferes with G1/S progression leading to apparent early resistance to paclitaxel-induced apoptosis due to decreased paclitaxel-induced mitotic arrest (Figure 7C and Supplementary Figure S7). We hypothesized that cells escaping from G1/S following SIK2 depletion fail to undergo centrosome separation and that this leads to sensitization to paclitaxel-induced cytotoxicity. To test this hypothesis we utilized two orthotopic models of ovarian cancer metastasis (SKOv3ip and HeyA8 cancer cells) and in vivo SIK2 KD using neutral nanoliposomal (DOPC) delivery of siRNA (Figure 8A–B). As expected, depletion of SIK2 resulted in a significant decrease in the mitotic index 24 hours following paclitaxel treatment (p<0.001, t-test, Figure 8C–D). However, SIK2 KD resulted in a significant increase in monopolar mitotic cells (p<0.001, Figure 8E–F) and a significant decrease in tumor weight following paclitaxel treatment compared to non-targeting control siRNA-DOPC treated with paclitaxel (p=0.008 and 0.005 for SKOv3ip and HeyA8 models, respectively; t test, Figure 8G–H). Depletion of SIK2, independent from paclitaxel, resulted in a modest decrease in tumor weight in the SKOv3ip model (p=0.2) and a modest but significant decrease of tumor weights in the HeyA8 model (p=0.02). Taken together, these results confirm the effect of loss of SIK2 in inducing synergy to paclitaxel in vitro and in vivo.
Figure 8. Loss of SIK2 sensitizes ovarian cancer cells to paclitaxel in vivo.
A) A flow diagram showing the design of the in vivo experiment. B) Immunohistochemical confirmation of SIK2 depletion in SKOv3ip1 tissue sections using anti-SIK2 antibody. Scale bar, 50 µm. C–F) 5 µm sections from tumors were stained using the indicated antibodies. C–D) sections were stained using the indicated antibodies to calculate the mitotic index per field by manually counting phospho-Histone H3 positive nuclei and total number of nuclei. Shown in C is the mean ± s.e.m. of the MIs from 7 fields from two mice. In E an example of monopolar centrosome orientation following SIK2 KD in mice is shown. F) Bar plot representation of the mean ± s.e.m. of the percentage of mitotics with monopolar centrosome orientation from two mice (two slides per mouse). G–H) Mean tumor weights (± s.d.) obtained from 10 mice per siRNA type in SKOv3 cells and Hey cells. Scale bar, 10 µm.
Discussion
We have previously shown that LKB1, a master kinase for all members of the AMPK superfamily, plays an important role in regulating mitosis through mechanisms that remained unclear (Bettencourt-Dias et al., 2004). In this work we have identified a previously unrecognized role for SIK2, a member of the AMPK family in regulating centrosome separation during mitosis. The effect of depletion of SIK2 on mitosis and centrosome function is unlikely to be secondary to depletion of energy reserves. Previous work has shown that the key role that SIK2 plays in metabolism is in regulating energy balance in refeeding from starvation (Dentin et al., 2007; Ryu et al., 2009; Wang et al., 2008). During fasting ATP depletion leads to PKA activation which inhibits SIK2 resulting in dephosphorylation of its cytoplasmic target TORC2 and its accumulation in the nucleus. Nuclear TORC2 enhances CREB-mediated gluconeogenesis in an effort to increase ATP production to maintain its intracellular level. Upon re-feeding from starvation, rising ATP levels lead to inactivation of PKA and this results in SIK2 activation leading to phosphorylation of TORC2 and its cytoplasmic retention and degradation. This results in inhibition of gluconeogenesis and decreased ATP production through this pathway. Thus, based on this evidence depletion of SIK2 would not be expected to lead to loss of intracellular ATP under normal feeding conditions. Indeed, work in Drosophila has shown that depletion of the orthologue of SIK2 results in enhanced resistance to metabolic stress in neurons (Wang et al., 2008). We have also shown here that depletion of SIK2 did not result in decreased ATP levels in SKOv3 cells. Therefore, loss of ATP cannot explain the mitotic phenotypes that we have observed following SIK2 depletion.
We propose that displacement of PRKAR2A from the centrosome in prophase relieves SIK2 from inhibition. This molecular event coupled with higher centrosome expression of SIK2 at the onset of mitosis may initiate centrosome separation by inducing additional phosphorylation of centrosome linker proteins such as C-Nap1. We speculate that NEK2 may induce sub-threshold phosphorylation of C-Nap1 and that optimal phosphorylation is achieved at the onset of mitosis by the activity of SIK2. These molecular events could complement the known decrease in phosphatases, such as PP1α, that oppose NEK2 activity prior to the onset of mitosis (Meraldi and Nigg, 2001). Our findings add further proof to the emerging evidence of coupling cancer cell metabolism and mitosis functions of the AMPK family members.
It is important to note that SIK2 is a rare example of a therapeutic target that upon depletion results in mitosis-dependent synergy with paclitaxel as well as G1/S arrest and decreased cancer growth independent from taxanes. It is becoming clearer that regimens involving combination therapies targeting different phases of the cell cycle are desirable for optimal cancer response. Several microtubule-independent mitotic inhibitors have been tested in early clinical trials with or without taxanes. The results from these trials are still emerging but such approaches have so far resulted in disease stabilization or partial responses (Blagden et al., 2008; Gautschi et al., 2008; Perez de Castro et al., 2008; Strebhardt and Ullrich, 2006). Targeting SIK2 may offer inhibition of cancer cell growth by targeting multiple phases of the cell cycle. Using reverse phase protein arrays we showed that SIK2 is required for phosphorylation of AKT at S473 and this may contribute to the delayed cell cycle progression. Another possible contributing factor is the centrosome abnormalities following SIK2 depletion in interphase. It is well documented that the centrosome functions as a docking site for several proteins involved in cell cycle progression (Matsumoto and Maller, 2004). Centrosome dysfunction following loss of key centrosome proteins or centrosome displacement results in severe G1 arrest (Hinchcliffe et al., 2001; Keryer et al., 2003; Khodjakov and Rieder, 2001; Matsumoto and Maller, 2004). We have shown that loss of SIK2 results in profound abnormalities in centrosome position, and function that may be the underlying cause for the observed G1 arrest.
An important consideration for examining the potential therapeutic role of SIK2 will be to test the toxicity profile following its inhibition. This could be achieved in mouse models of ovarian cancer metastasis using SIK2 inhibitors. To our knowledge, no specific inhibitors for SIK2 are currently available. Future work focusing on resolving the structure of SIK2 to inform the development of inhibitors in parallel with running small-molecule inhibitor compound screens will be important to identify prototype inhibitors of SIK2. The work shown here provides extensive phenotypic and biochemical data to support the development of SIK2 inhibitors. Such inhibitors will be highly valuable for further testing of the biological and therapeutic potential of SIK2 and for examining its toxicity profile in vivo. It is important to consider that in mammals SIK2 plays a role in regulating energy balance in subcutaneous fat upon re-feeding from starvation. However, its role in maintaining energy reserves under normal feeding conditions remains unclear. Therefore, whether its inhibition under normal feeding conditions will result in energy imbalance and consequent toxicities remains to be tested in animal models.
In this work we showed that the expression level of SIK2 correlated with prognosis in patients with ovarian high grade serous cancers and with paclitaxel response. In addition we showed that the expression level of SIK2 did not correlate with carboplatin response in the context of a prospective randomized study. Furthermore, our data in vitro and in xenograft models show a clear relationship between the intracellular levels of SIK2 and response to paclitaxel. However, this work does not exclude the possibility of an effect of SIK2 on response to non anti-tubulin agents. It will be interesting to investigate the effect of SIK2 on response to other chemotherapeutic agents in vitro and in clinical samples in future studies. Regulation of SIK2 might also potentiate targeted therapies. Knockdown of SIK2 was shown to inhibit phosphorylation and activation of AKT which might be put to use in overcoming paradoxical activation of AKT by partial mTOR inhibitors such as rapamycin (O'Reilly et al., 2006).
In summary, this work identifies a previously unrecognized role of SIK2 in regulating cell cycle progression and suggests SIK2 as an attractive target for treatment in a proportion of ovarian cancers that overexpress this protein.
Experimental procedures
Automated image acquisition, segmentation and analysis (high content analysis)
Automated image acquisition and analysis were performed using the In Cell Analyzer 1000 and the In Cell investigator software, respectively (GE Healthcare).
The Australian Ovarian Cancer Study (AOCS)
This was a population-based, multicentre translational study that comprised prospective collection of bio-specimens, clinical and epidemiological data from patients with primary epithelial ovarian, primary peritoneal and fallopian tube cancer diagnosed between 2001 and 2005. All patients were prospectively consented using a protocol approved by human research ethics committees at multiple participating clinical and research centres. Further details of the AOCS cohort can be found at http://www.aocstudy.org and in the supplementary experimental procedures.
In vivo experiments in mice
All mouse experiments were approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center. Mice were cared for in accordance with guidelines set forth by the American Association for Accreditation of Laboratory Animal Care and the US Public Health Service Policy on Human Care and Use of Laboratory Animals. Female nude mice were inoculated with ovarian cancer cell lines (HeyA8 or SKOV3ip1) into the peritoneal cavity. After 1 week, SiRNA was incorporated in DOPC-nanoliposomes and administered intraperitoneally (IP) at a dose of 150 µg/kg per mouse, biweekly. Paclitaxel was administered IP once a week at a dose of 100 µg per mouse for HeyA8 and 75 µg per mouse for SKOV3ip1. Mice were treated for 3–4 weeks and all treatment groups were sacrificed and necropsied when any single group became moribund and assayed for tumor weight.
Supplementary Material
Significance.
Taxanes stabilize microtubules, inhibit mitosis, induce apoptosis and produce regression in a fraction of cancers that arise at many sites including the ovary. The anti-tumor activity of taxanes might be increased by concurrently regulating kinases that affect both cell cycle and mitosis. Here we show that SIK2 localizes at the centrosome, phosphorylates the centrosome linker protein C-Nap1 and plays a key role in regulating the the onset of mitosis. SIK2 depletion resulted in profound synergy with paclitaxel in inducing cytotoxicity, while higher expression in ovarian cancers correlated with poor prognosis. Independent of its antimitotic role, SIK2 targeting resulted in decreased G1/S transition and low AKT phosphorylation. Thus, SIK2 provides a multimodal therapeutic target in a subset of ovarian cancers.
Highlights.
A kinome screen identifies SIK2 as a centrosome kinase required for mitosis.
SIK2 phosphorylates C-Nap1 and is required for centrosome separation in mitosis.
Targeted SIK2 depletion results in synergy with taxanes in ovarian cancers.
Depletion of SIK2 results in delayed G1/S transition and low AKT phosphorylation.
Acknowledgements
We would like to thank Dr E Nigg and Dr Michel Bornens for giving valuable reagents. We also thank Dr R Laskey, Dr B Hassan and Dr G Smith for helpful comments on the manuscript. We thank Dr G Tzolovsky for technical help. This work was funded by Cancer Research UK, the University of Cambridge, the Zarrow Foundation, the Ovarian Cancer Research Fund Program Project Development Grant, and the University of Texas M.D. Anderson Cancer Center Ovarian Cancer Specialized Program of Research Excellence (P50 CA083639) and the Addenbrooke’s Charitable Trust. AA is a Cancer Research UK Clinician Scientist. The AOCS was supported by The Cancer Council Victoria, Queensland Cancer Fund, The Cancer Council New South Wales, The Cancer Council South Australia, The Cancer Foundation of Western Australia, the Cancer Council Tasmania and the National Health and Medical Research Council of Australia (NHMRC). The authors would like to thank Dr M Deery and members of the Cambridge Centre for Proteomics, Department of Biochemistry, University of Cambridge, members of the time-lapse microscopy facility (supported by NCI core grant 5p30ca016672-29), and members of the siRNA screening facility at the University of Texas M.D. Anderson Cancer Center for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Detailed additional methods are available as supplementary information.
References
- Ahmed AA, Mills AD, Ibrahim AE, Temple J, Blenkiron C, Vias M, Massie CE, Iyer NG, McGeoch A, Crawford R, et al. The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell. 2007;12:514–527. doi: 10.1016/j.ccr.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekier ME, Fischbach R, Lee J, Taylor WR. Length of mitotic arrest induced by microtubule-stabilizing drugs determines cell death after mitotic exit. Mol Cancer Ther. 2009;8:1646–1654. doi: 10.1158/1535-7163.MCT-08-1084. [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Giet R, Sinka R, Mazumdar A, Lock WG, Balloux F, Zafiropoulos PJ, Yamaguchi S, Winter S, Carthew RW, et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 2004;432:980–987. doi: 10.1038/nature03160. [DOI] [PubMed] [Google Scholar]
- Blagden SP, Molife LR, Seebaran A, Payne M, Reid AH, Protheroe AS, Vasist LS, Williams DD, Bowen C, Kathman SJ, et al. A phase I trial of ispinesib, a kinesin spindle protein inhibitor, with docetaxel in patients with advanced solid tumours. Br J Cancer. 2008;98:894–899. doi: 10.1038/sj.bjc.6604264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CR, Witczak O, Vossebein L, Labbe JC, Skalhegg BS, Keryer G, Herberg FW, Collas P, Tasken K. CDK1-mediated phosphorylation of the RIIalpha regulatory subunit of PKA works as a molecular switch that promotes dissociation of RIIalpha from centrosomes at mitosis. J Cell Sci. 2001;114:3243–3254. doi: 10.1242/jcs.114.18.3243. [DOI] [PubMed] [Google Scholar]
- Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, Yates J, 3rd, Montminy M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol. 1998a;141:1563–1574. doi: 10.1083/jcb.141.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. Embo J. 1998b;17:470–481. doi: 10.1093/emboj/17.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN, Jr, Gandara DR. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14:1639–1648. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 2001;291:1547–1550. doi: 10.1126/science.1056866. [DOI] [PubMed] [Google Scholar]
- Huang HC, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16:347–358. doi: 10.1016/j.ccr.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh Y, Takemori H, Horike N, Doi J, Muraoka M, Min L, Okamoto M. Salt-inducible kinase (SIK) isoforms: their involvement in steroidogenesis and adipogenesis. Mol Cell Endocrinol. 2004;217:109–112. doi: 10.1016/j.mce.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Takemori H, Lin XZ, Tamura M, Muraoka M, Satoh T, Tsuchiya Y, Min L, Doi J, Miyauchi A, et al. Silencing the constitutive active transcription factor CREB by the LKB1-SIK signaling cascade. Febs J. 2006;273:2730–2748. doi: 10.1111/j.1742-4658.2006.05291.x. [DOI] [PubMed] [Google Scholar]
- Keryer G, Witczak O, Delouvee A, Kemmner WA, Rouillard D, Tasken K, Bornens M. Dissociating the centrosomal matrix protein AKAP450 from centrioles impairs centriole duplication and cell cycle progression. Mol Biol Cell. 2003;14:2436–2446. doi: 10.1091/mbc.E02-09-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J Cell Biol. 2001;153:237–242. doi: 10.1083/jcb.153.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsverk HB, Carlson CR, Steen RL, Vossebein L, Herberg FW, Tasken K, Collas P. Regulation of anchoring of the RIIalpha regulatory subunit of PKA to AKAP95 by threonine phosphorylation of RIIalpha: implications for chromosome dynamics at mitosis. J Cell Sci. 2001;114:3255–3264. doi: 10.1242/jcs.114.18.3255. [DOI] [PubMed] [Google Scholar]
- Lin YG, Immaneni A, Merritt WM, Mangala LS, Kim SW, Shahzad MM, Tsang YT, Armaiz-Pena GN, Lu C, Kamat AA, et al. Targeting aurora kinase with MK-0457 inhibits ovarian cancer growth. Clin Cancer Res. 2008;14:5437–5446. doi: 10.1158/1078-0432.CCR-07-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Rodriguez-Lescure A, Ruiz A, Alba E, Calvo L, Ruiz-Borrego M, Munarriz B, Rodriguez CA, Crespo C, de Alava E, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Maller JL. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science. 2004;306:885–888. doi: 10.1126/science.1103544. [DOI] [PubMed] [Google Scholar]
- Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- Mayor T, Hacker U, Stierhof YD, Nigg EA. The mechanism regulating the dissociation of the centrosomal protein C-Nap1 from mitotic spindle poles. J Cell Sci. 2002;115:3275–3284. doi: 10.1242/jcs.115.16.3275. [DOI] [PubMed] [Google Scholar]
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- Meraldi P, Nigg EA. Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J Cell Sci. 2001;114:3749–3757. doi: 10.1242/jcs.114.20.3749. [DOI] [PubMed] [Google Scholar]
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de Castro I, de Carcer G, Montoya G, Malumbres M. Emerging cancer therapeutic opportunities by inhibiting mitotic kinases. Curr Opin Pharmacol. 2008;8:375–383. doi: 10.1016/j.coph.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Ryu D, Oh KJ, Jo HY, Hedrick S, Kim YN, Hwang YJ, Park TS, Han JS, Choi CS, Montminy M, Koo SH. TORC2 regulates hepatic insulin signaling via a mammalian phosphatidic acid phosphatase, LIPIN1. Cell Metab. 2009;9:240–251. doi: 10.1016/j.cmet.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro B, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–951. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- Wang B, Goode J, Best J, Meltzer J, Schilman PE, Chen J, Garza D, Thomas JB, Montminy M. The insulin-regulated CREB coactivator TORC promotes stress resistance in Drosophila. Cell Metab. 2008;7:434–444. doi: 10.1016/j.cmet.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.