Abstract
BACKGROUND
Monitoring brain tissue PO2 (PbtO2) is part of multimodality monitoring of patients with traumatic brain injury (TBI). However, PbtO2 measurement is a sampling of only a small area of tissue surrounding the sensor tip.
OBJECTIVE
To examine the effect of catheter location on the relationship between PbtO2 and neurological outcome.
METHODS
A total of 405 patients who had PbtO2 monitoring as part of standard management of severe traumatic brain injury were studied. The relationships between probe location and resulting PbtO2 and outcome were examined.
RESULTS
When the probe was located in normal brain, PbtO2 averaged 30.8 ± 18.2 compared with 25.6 ± 14.8 mm Hg when placed in abnormal brain (P< .001). Factors related to neurological outcome in the best-fit logistic regression model were age, PbtO2 probe position, postresuscitation motor Glasgow Coma Scale score, and PbtO2 trend pattern. Although average PbtO2 was significantly related to outcome in univariate analyses, it was not significant in the final logistic model. However, the interaction between PbtO2 and probe position was statistically significant. When the PbtO2 probe was placed in abnormal brain, the average PbtO2 was higher in those with a favorable outcome, 28.8 ± 12.0 mm Hg, compared with those with an unfavorable outcome, 19.5 ± 13.7 mm Hg (P = .01). PbtO2 and outcome were not related when the probe was placed in normal-appearing brain.
CONCLUSION
These results suggest that the location of the PbtO2 probe determines the PbtO2 values and the relationship of PbtO2 to neurological outcome.
Keywords: Brain tissue PO2, Head injury, PO2 monitoring, Traumatic brain injury
Progress in the management of severe traumatic brain injury (TBI) has largely been through improvements in prehospital care, development of imaging techniques, and improved neurocritical care management. No specific therapies have been shown to improve neurological recovery. The major goal of neurocritical care management is to prevent secondary brain injury.
Present strategies in the management of patients with brain injury revolve around maintaining and improving cerebral oxidative metabolism.1 Monitoring brain tissue PO2 (PbtO2) is part of multimodality monitoring of patients with TBI and is becoming more widely used in the management of patients with TBI, subarachnoid hemorrhage, and other acute neurological problems.2,3 PbtO2 monitoring in patients with TBI may help optimize cerebral perfusion pressure (CPP) by providing continuous data regarding regional or global brain oxygenation, and PbtO2-directed therapies may improve outcome.4,5
However, PbtO2 measurement is a localized sampling of only a small area of tissue immediately surrounding the sensor tip. Because of the heterogeneous nature of the brain injury in TBI and such localized measurement of the PbtO2 with this technique, probe position is a critical aspect in correctly interpreting the resulting data. In general, placement of the PbtO2 probe in normal brain is thought to give a measure of global brain tissue oxygenation, whereas the probe placed in or near injured brain reflects only local oxygenation in the surrounding brain tissue. Probe location (within normal brain vs injured brain) can therefore influence the detection of cerebral hypoxia and consequently patient management. There has been no general consensus on which position is the best strategy for brain tissue oxygen monitoring after severe TBI, and often the probe is placed on the basis of convenience (ie, at the site of the intracranial pressure [ICP] monitor) rather than as a result of a strategic clinical decision.
The purpose of this study was to examine the effect of catheter location on the relationship between PbtO2 and long-term neurological outcome.
PATIENTS AND METHODS
The study design was a database review of deidentified research data that had been collected prospectively as a part of Institutional Review Board–approved research studies.
Patient Characteristics
A total of 405 patients who were admitted to Ben Taub General Hospital between July 1995 and March 2009 and had PbtO2 monitoring as part of their standard monitoring of a severe TBI were studied. Inclusion criteria were the following: TBI, motor component of the Glasgow Coma Scale (GCS) score ≤5 (either after resuscitation or after subsequent deterioration), valid PbtO2 data collected as a part of an ongoing research protocol, and demographic and injury characteristics and neurological outcome collected as a part of an ongoing research protocol. Exclusion criteria included GCS score of 3 with fixed, dilated pupils and loss to follow-up before 3 months after injury.
Clinical Management
The patients were managed by a standard protocol that emphasized the prevention of secondary insults and the prompt evacuation of intracranial masses. General management goals were PaO2 > 100 mm Hg, PaCO2 of 35 to 40 mm Hg, systolic blood pressure > 120 mm Hg, central venous pressure of 5 to 10 mm Hg, and urine output > 0.5 to 1 mL·kg−1·h−1. Phenytoin was given for 7 days as prophylaxis for seizures.
Invasive multimodal continuous monitoring included an ICP monitor, usually a ventriculostomy, a PbtO2 probe, a jugular bulb catheter for jugular venous oxygen saturation (SjvO2) monitoring, an arterial line for blood sampling and blood pressure monitoring, and a central venous catheter.
When the PbtO2 probe was placed at the time of surgery for an intracranial mass lesion, it was positioned in the brain in what the neurosurgeon thought would be normal but vulnerable brain tissue based on the computed tomography (CT) scan appearance on admission. In diffuse injuries and with nonsurgical mass lesions, the PbtO2 probe was usually placed at the site of the ICP monitor. When there was a unilateral parenchymal lesion, the PbtO2 probe was placed on that side targeting perilesional tissue. When there was no parenchymal lesion, the PbtO2 probe was usually placed on the right side. Regardless of the location, all PbtO2 probes were positioned without the use of a bolt device. Confirmation of the location of the monitor was obtained on a follow-up CT scan usually within 24 hours after insertion.
The goals of management were ICP < 20 mm Hg and CPP > 60 mm Hg, unless SjvO2 was < 50% or PbtO2 was < 10 mm Hg, indicating the need for higher CPP. This threshold for PbtO2 treatment was chosen because alterations in metabolism have been observed only when PbtO2 drops < 10 mm Hg.6,7 Treatment of intracranial hypertension was managed with the principles in the Brain Trauma Foundation guidelines for management of severe TBI8 and involved surgical removal of mass lesions, use of cerebrospinal fluid drainage via ventriculostomy, sedation, neuromuscular paralysis, mannitol, and mild to moderate hyperventilation. Barbiturate coma, moderate hypothermia, and decompressive craniectomy were treatment options used for refractory intracranial hypertension. Forty-five patients (11.1%) underwent decompressive craniectomy. In 15 cases, the primary surgery was decompressive craniectomy for refractory intracranial hypertension with no evacuation of hematoma, and in 30 cases, the bone flap was left off at end of surgery for evacuation of a hematoma.
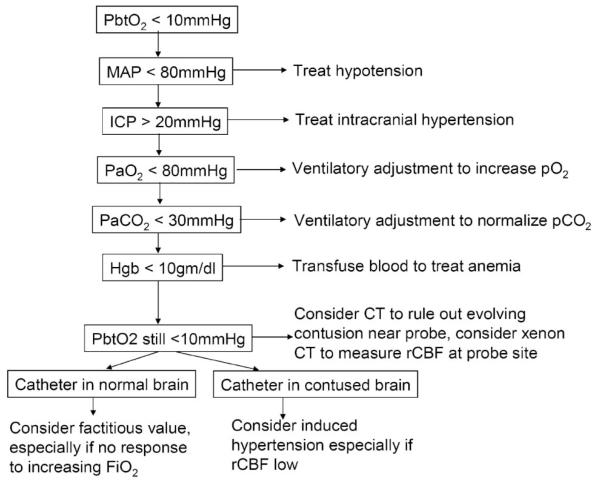
Low cerebral oxygenation (PbtO2 < 10 mm Hg) was treated with the algorithm shown in Figure 1. This management algorithm was intended first to normalize factors that might impair cerebral oxygen delivery, including intracranial hypertension, hypotension, hypoxia, hypocarbia, and anemia. If PbtO2 remained low after correction of these factors, normobaric hyperoxia or induced hypertension was used to try to improve oxygenation.
FIGURE 1.
Algorithm for treatment of a low brain tissue PO2 (PbtO2) used throughout the study period. CT, computed tomography; Hgb, hemoglobin; ICP, intracranial pressure; MAP, mean arterial pressure; rCBF, regional cerebral blood flow.
Monitoring of ICP, PbtO2, and SjvO2 was continued until both the ICP and brain oxygenation were normal for about 24 hours without treatment. At the end of the monitoring period, the PbtO2 probes were removed and calibration drift was determined by measuring a stable PO2 in room air, in blood gas standard calibration solutions, and in a no-oxygen “zero” solution.
Data Analysis
The demographic and clinical data collected included age, sex, mechanism of injury, type of intracranial injury, extent and severity of all injuries, and surgical procedures required. Trauma scores collected included the GCS score and pupil size/reactivity at the accident scene and in the emergency center and the Injury Severity Score. Cerebral oxygenation values and the other physiological parameters were recorded hourly within a few hours after intensive care unit admission and for the duration of the monitoring. The admission CT scan and a follow-up CT scan when the location of the PbtO2 probe could be confirmed were available for analysis.
The Marshall CT category9 was used to describe the admission CT scan findings, and the results were collapsed into the following 3 groups: mild diffuse injury (diffuse injury 1 and 2), severe diffuse injury (diffuse injury 3 and 4), and mass lesions (evacuated and unevacuated mass lesions). The GCS score on admission was also classified into the following 2 categories according to the motor GCS score: motor GCS score 4 to 6 and of 1 to 3. Pupil reactivity was classified as both pupils reactive, 1 unreactive pupil, or both pupils unreactive.
The patients had long-term Glasgow Outcome Scale (GOS) scores available (320 patients [79%] at 6 months and an additional 85 patients [21%] at 3 months). The analyses were performed for both the 320 patients with 6-month GOS scores and the 405 patients with the last known GOS score with similar results. In the data reported here, the last known GOS score was used for long-term outcome so that the data from all 405 patients could be used. The long-term GOS scores were dichotomized as favorable recovery (good recovery or moderate disability) and unfavorable recovery (severe disability, vegetative, or dead).
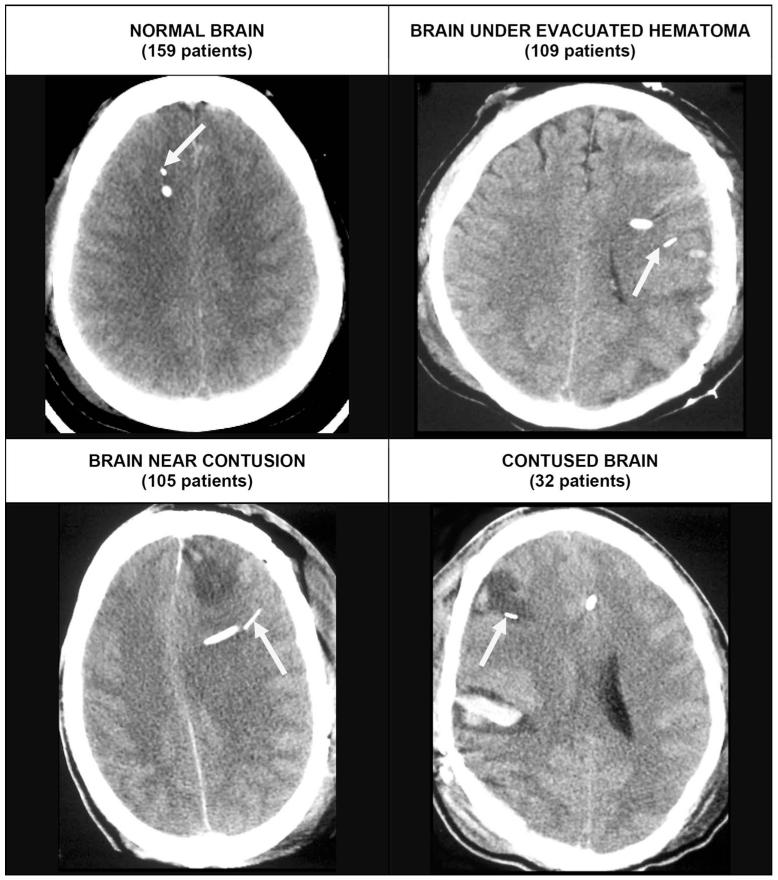
PbtO2 findings were characterized for analysis in several different ways. The trend pattern of PbtO2 over time was classified as a benign pattern when the values were always ≥ 10 mm Hg or only transiently, < 10 mm Hg at the onset of monitoring and as a tissue hypoxia pattern when the values were persistently < 10 mm Hg or decreased to < 10 mm Hg during the hospital course. In addition, the average PbtO2 values for the entire monitoring period and for the duration of time that PbtO2 was less than various thresholds (< 10, < 15, and < 20 mm Hg) were calculated for each patient. Follow-up CT scans were reviewed, and the position of the PbtO2 probe within the brain was classified into one of the following 4 positions: in normal-appearing brain, near contused brain, in brain under an evacuated hematoma, or within contused brain (Figure 2). The last 3 probe positions were collapsed for the analysis as being in vulnerable or abnormal brain.
FIGURE 2.
Computed tomography scans illustrate typical appearance of the different catheter positions. The arrow identifies the brain tissue PO2 (PbtO2) probe in each case. Top left, the PbtO2 probe in normal-appearing right frontal lobe in a patient with a diffuse brain injury. Top right, the PbtO2 probe in brain after evacuation of a subdural hematoma. The probe was placed at the time of surgery in brain tissue. The brain appears normal but has been compressed by the subdural hematoma. Bottom left, a PbtO2 probe in the left frontal lobe near a contusion. If the contusion expands, this tissue is likely to become involved or to be compressed. Bottom right, a PbtO2 within a contusion in the right frontal lobe.
The relationship of demographic/injury characteristics (including age, GCS score, injury type, and the relationship of the PbtO2 parameters described above) to GOS score was studied. For categorical data, the χ2 test was used. For numerical data, the t test was used when the data were normally distributed; otherwise, the rank-sum test was used. Factors found to be significantly related to outcome in the univariate analyses were further studied with logistic regression analysis. The final logistic regression model was fit by use of a backward stepwise procedure. The final model was used to generate graphic representations of the effects of different variables.
RESULTS
Patient Characteristics
PbtO2 data from 405 patients were available for analysis. Demographic and injury characteristics of all 405 patients, which are summarized in Table 1, were typical for a severe TBI population. Men predominated in the group, 327 (80.7%) compared with 78 women (19.3%). The mean age for the group was 34.2 ± 14.1 years, and the mean Injury Severity Score was 30.5 ± 8.0. The mechanism of injury was motor vehicle collision in 268 (66.2%), assault in 41 (10.1%), fall/jump in 58 (14.3%), and other in 21 (5.2%). In 17 patients (4.2%), the mechanism was unknown. Prehospital hypoxia and hypotension occurred in 29.4% and 12.8% of the patients, respectively.
TABLE 1. Demographic and Injury Severity Characteristics of 405 Patients With PbtO2 Monitoring and Long-term Neurological Outcomea.
| Variable | Mean ± SD or n (%) |
|---|---|
| Age, y | 34.2 ± 14.1 |
| Sex | |
| Male | 327 (80.7) |
| Female | 78 (19.3) |
| Race | |
| White | 112 (27.6) |
| Black | 98 (24.2) |
| Hispanic | 186 (45.9) |
| Asian | 9 (2.2) |
| Mechanism of injury | |
| Motor vehicle collision | 268 (66.2) |
| Fall/jump | 58 (14.3) |
| Assault | 41 (10.1) |
| Other | 21 (5.2) |
| Unknown | 17 (4.2) |
| Motor GCS | |
| 1-3 | 188 (46.4) |
| 4-6 | 215 (53.1) |
| Untestable | 2 (0.5) |
| Pupils | |
| Both reactive | 236 (58.3) |
| 1 Unreactive | 43 (10.6) |
| Both unreactive | 105 (25.9) |
| Untestable | 19 (4.7) |
| Injury Severity Score | 30.5 ± 8.0 |
| Apache II Score | 20.8 ± 6.6 |
| Prehospital hypotension | |
| Yes | 52 (12.8) |
| No | 353 (87.2) |
| Prehospital hypoxia | |
| Yes | 119 (29.4) |
| No | 286 (70.6) |
| Type of injury (Marshall CT category) | |
| Diffuse injury 1 or 2 | 135 (33.3) |
| Diffuse injury 3 or 4 | 87 (21.5) |
| Mass lesion | 183 (45.2) |
| Glasgow Outcome Scale | |
| Good recovery | 62 (15.3) |
| Moderate disability | 67 (16.5) |
| Severe disability | 162 (40.0) |
| Vegetative | 28 (6.9) |
| Dead | 86 (21.2) |
CT, computed tomography; GCS, Glasgow Coma Scale.
An admission GCS score was available for 403 of the patients. The motor component of the GCS score was 1 to 3 in 188 patients (46.4%) and 4 to ± in 215 patients (53.1%). In 2 patients (0.5%), an admission GCS score could not be obtained because of pharmacological paralysis. A small fraction of patients (3.6%) had a motor score of 6 on their postresuscitation examination but subsequently deteriorated to < 6. Pupils were reactive on admission in 236 patients (58.3%), 1 pupil was unreactive in 43 patients (10.6%), and both pupils were unreactive in 105 patients (25.9%). For 19 patients (4.7%), the pupils could not be assessed because of eye swelling or injury. The CT scan of the head on admission was classified as diffuse injury 1 or 2, diffuse injury 3 or 4, and mass lesion in 135 patients (33.3%), 87 patients (21.5%), and 183 patients (45.2%), respectively.
The GOS score was assessed at 3 and 6 months. A total of 129 patients (31.9%) had a favorable outcome, whereas 276 patients (68.1%) had an unfavorable outcome. Eighty-six of the 405 patients (21.2%) died.
PbtO2 Variables
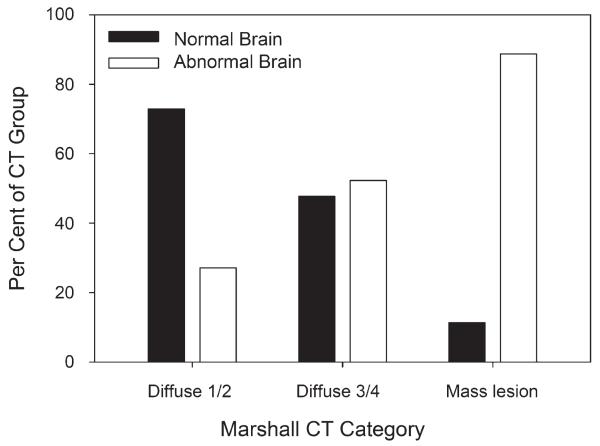
PbtO2 was measured with a Licox sensor in almost all of the patients (396 patients [97.7%]). Alternative catheters (either Paratrend 7 or Neurotrend catheters) were used in 9 patients (2.2%). The PbtO2 sensor was positioned in normal brain in 159 patients (39.3%) and in abnormal brain in 246 patients (60.7%). The average time for start of PbtO2 monitoring was 10.5 ± 0.6 hours in patients who required surgery on admission and 10.4 ± 0.5 hours in patients who were taken directly from the emergency center to the intensive care unit. As might be expected from the nature of traumatic injuries, the type of injury strongly influenced the position of the PbtO2 probe (Figure 3). The position of the probe was divided evenly between normal and abnormal brain only in patients with diffuse injury 3 or 4. In patients with mild diffuse injuries (Marshall CT category diffuse injury 1 or 2), 71.1% of the patients had the PbtO2 probe placed in normal-appearing brain. In contrast, in patients with mass lesions, the probe was placed in normal-appearing brain in only 11.5%.
FIGURE 3.
The position of the brain tissue PO2 (PbtO2) probe was significantly influenced by the type of computed tomography (CT) scan lesion (P < .001).
ICP and PbtO2 were monitored for an average of 163.5 ± 118.9 and 96.8 ± 48.2 hours, respectively. Data were analyzed from a total of 39 097 hours of continuous PbtO2 monitoring. Summary values for ICP, mean arterial pressure, CPP, SjvO2, ETCO2, and SaO2 are listed in Table 2.
TABLE 2. Brain Tissue Po2 Catheter Information and Physiology in 405 Patientsa.
| Variable | Mean ± SD, Median (Interquartile Range), or n (%) |
|---|---|
| Type of catheter | 236 (58.3) |
| Licox Po2 and Licox temperature probes | |
| Licox Po2/temperature combination probe | 160 (39.5) |
| Neurotrend Po2, Pco2, pH probe | 7(1.7) |
| Paratrend 7 Po2, Pco2, pH probe | 2 (0.5) |
| Location of catheter | |
| Normal brain | 159 (39.3) |
| Brain underlying evacuated hematoma | 109 (26.9) |
| Brain near contusion | 105 (25.9) |
| Contused brain | 32 (7.9) |
| Average Pbto2, mm Hg | |
| All patients | 27.6 ± 14.4 |
| Duration of Pbto2 monitoring, h | |
| Time < 10 mm Hg | 1 (0-18) |
| Time < 15 mm Hg | 11 (1-34) |
| Time < 20 mm Hg | 24 (5-50.25) |
| Pbto2 trend pattern | |
| Always ≥ 10 mm Hg | 214 (52.8) |
| Transiently < 10 mm Hg at start | 129 (31.9) |
| Persistently < 10 mm Hg | 15 (3.7) |
| Decreased to < 10 mm Hg after normal at start |
47 (11.6) |
| Average ICP, mm Hg | 19.0 ± 9.5 |
| Average MAP, mm Hg | 91.6 ± 9.2 |
| Average CPP, mm Hg | 72.6 ± 14.2 |
| Average Sjvo2, % | 73.9 ± 5.5 |
| Average ETco2, mm Hg | 31.3 ± 3.9 |
| Average Sao2, % | 99.0 ± 0.9 |
CPP, cerebral perfusion pressure; ICP, intracranial pressure; MAP, mean arterial pressure; Pbto2, brain tissue Po2; Sjvo2, jugular venous oxygen saturation.
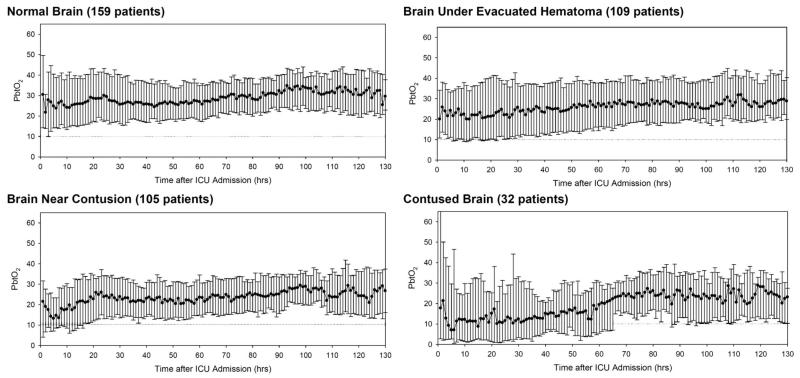
Trend graphs for PbtO2 over time (median ± interquartile ranges) for the different catheter positions are illustrated in Figure 4. When the PbtO2 probe was placed in normal-appearing brain (Figure 4, top left), the median values for PbtO2 were > 20 mm Hg throughout the monitoring period. Very few values were < 10 mm Hg. When the PbtO2 probe was placed in normal-appearing brain underlying an evacuated hematoma (Figure 4, top right), median PbtO2 values were slightly lower but remained above 20 mm Hg throughout the monitoring period. More PbtO2 values were around 10 mm Hg during the first day after injury. In patients in whom the PbtO2 probe was placed near a contusion (Figure 4, bottom left), the median PbtO2 values tended to decrease over the first few hours after injury, and a substantial portion of the PbtO2 values were < 10 mm Hg during the first 24 hours after injury. When the PbtO2 probe was placed in contused brain (Figure 4, bottom right), the median PbtO2 values decreased after admission and were near or < 10 mm Hg throughout the first 30 hours after injury.
FIGURE 4.
Trend graphs of brain tissue PO2 (PbtO2) over time (median ± interquartile range) for the different catheter positions. ICU, intensive care unit.
Several methods for summarizing the PbtO2 data were examined to try to capture the information observed in Figure 4. These variables are summarized in Tables 2 and 3 and are described below first for all patients and then by PbtO2 probe position.
TABLE 3. Brain Tissue Po2 Variables by Probe Position in 405 Patients With Long-term Neurological Outcomea.
| Variable | Probe in Normal Brain, Mean ± SD or Median (Interquartile Range) |
Probe in Abnormal Brain, Mean ± SD or Median (Interquartile Range) |
P |
|---|---|---|---|
| Patients, n | 159 | 246 | |
| Average Pbto2 | 30.8 ± 18.2 | 25.6 ± 14.8 | <.001 |
| Pbto2 duration below thresholds, h | |||
| Time < 10 mm Hg | 0 (0-6.0) | 5 (0-23.0) | <.001 |
| Time < 15 mm Hg | 4.0 (0-20.8) | 15 (3-43) | <.001 |
| Time < 20 mm Hg | 16 (2-36) | 30 (9-58) | <.001 |
| Pbto2 trend pattern | <.001 | ||
| Always ≥ 10 mm Hg | 108 (50.5) | 106 (49.5) | |
| Transiently < 10 mm Hg at start | 42 (32.6) | 87 (67.4) | |
| Persistently < 10 mm Hg | 2 (13.3) | 13 (86.7) | |
| Decreased to < 10 mm Hg after normal at start | 7 (14.9) | 40 (85.1) |
Pbto2, brain tissue Po2.
The average PbtO2 was 27.6 ± 14.4 mm Hg for the whole monitoring period in all patients. When the probe was located in normal brain, the average PbtO2 was 30.8 ± 18. compared with 25.6 ± 14.8 mm Hg when then probe was placed in abnormal brain (P < .001). The cumulative hours that PbtO2 stayed below the thresholds of 20, 15, and 10 mm Hg were a median of 24 (5-50.25), 11 (1-34), and 1 (0-18), respectively, in all patients. The time that PbtO2 was below each threshold was significantly longer in patients in whom the PbtO2 probe was placed in abnormal brain (Table 3).
Four characteristic patterns for the change in PbtO2 over time were observed in the individual patients. Trends in which PbtO2 was always ≥ 10 mm Hg or PbtO2 was only transiently decreased < 10 mm Hg at the beginning of the monitoring period were considered benign patterns. Most patients (343 [84.7%]) had such a pattern (Table 2). The remaining patients had a PbtO2 pattern in which values were persistently < 10 mm Hg (15 [3.7%]) or decreased to < 10 mm Hg during the monitoring period after initially being normal (47 [11.6%]). These trend patterns were also strongly related to the PbtO2 probe position (Table 3), with the probe being in abnormal brain in most of the patients (86%) having the abnormal PbtO2 trends over time.
Relationship of PbtO2 Variables to Outcome
In the univariate analyses, the factors that were significantly related to outcome included age, sum GCS score, the motor component of the GCS score from the neurological examination in the emergency center after resuscitation, pupil reactivity, type of injury, all of the PbtO2 variables, and the ICP and CPP summary variables (Table 4). Patients who had a favorable recovery were younger, had a higher GCS score on admission, and were less likely to have unreactive pupils. Patients with an admission Marshall CT scan category of diffuse injury 1 or 2 were also more likely to be in the favorable outcome group (P = .01) than those patients with diffuse injury 3 or 4 or a mass lesion.
TABLE 4. Relationship of Demographic, Injury Severity, Brain Tissue Po2 and Other Hemodynamic Variables to Neurological Outcome (405 Patients With Outcome Data)a.
| Variable | Favorable Outcome, Mean ± SD, Median (Interquartile Range), or n (%) |
Unfavorable Outcome, Mean ± SD, Median (Interquartile Range), or n (%) |
Univariate Analysis P |
|---|---|---|---|
| Patients, n | 129 | 276 | |
| Demographic and injury severity variables | |||
| Age | 29.4 ± 12.6 | 37.4 ± 14.7 | <.001 |
| Pbto2 catheter position | .03 | ||
| Normal brain | 61 (47.3) | 98 (35.5) | |
| Abnormal brain | 68 (52.7) | 178 (64.5) | |
| Sum GCS | .006 | ||
| 9-15 | 35 (27.1) | 39 (14.1) | |
| 3-8 | 93 (72.1) | 236 (85.5) | |
| Untestable | 1 (0.8) | 1 (0.4) | |
| Initial motor GCS | <.001 | ||
| 1-3 | 32 (24.8) | 156 (56.5) | |
| 4-6 | 96 (74.4) | 119 (43.1) | |
| Untestable | 1 (0.8) | 1 (0.4) | |
| Pupil reactivity | <.001 | ||
| Both pupils reactive | 98 (76.0) | 138 (50.0) | |
| 1 or Both pupils nonreactive | 27 (20.9) | 123 (44.6) | |
| Untestable | 4(3.1) | 15 (5.4) | |
| Injury Severity Score | 30.1 ± 7.4 | 30.7 ± 8.3 | .46 |
| Apache II Score | 18.2 ± 6.2 | 21.9 ± 6.4 | <.001 |
| Prehospital hypotension | .33 | ||
| Yes | 13 (10.1) | 39 (14.1) | |
| No | 116 (89.9) | 237 (85.9) | |
| Prehospital hypoxia | |||
| Yes | 30 (23.3) | 89 (32.3) | .008 |
| No | 99 (76.7) | 187 (67.7) | |
| Type of injury (Marshall CT category) | .01 | ||
| Diffuse injury 1 or 2 | 56 (43.4) | 79 (28.6) | |
| Diffuse injury 3 or 4 | 22 (17.1) | 65 (23.6) | |
| Mass lesion | 51 (39.5) | 132 (47.8) | |
| Pbto2 Variables | |||
| Average Pbto2, mm Hg | 32.2 ± 16.3 | 25.1 ± 13.5 31.4 ± 13.1 |
<.001 |
| Average Pbto2 × catheter position | |||
| Normal brain | 33.8 ± 19.4 | ||
| Abnormal brain | 28.8 ± 12.0 | 19.5 ± 13.7 | |
| Tme Pbto2 < 10 mm Hg, h | 0 (0-6.25) | 6 (0-25.5) | <.001 |
| Tme Pbto2 < 15 mm Hg, h | 3 (0-19.25) | 16 (3-42) | <.001 |
| Time Pbto2 < 20 mm Hg, h | 11 (1.75-39.25) | 31 (9.0-56.75) | <.001 |
| Pbto2 trend pattern | <.001 | ||
| Never < 10 mm Hg | 85 (65.9) | 129 (46.7) | |
| Transiently < 10 mm Hg at start | 39 (30.2) | 90 (32.6) | |
| Persistently < 10 mm Hg or decreasing | 5 (3.9) | 57 (20.7) | |
| Other hemodynamic variables | |||
| Average ICP (mm Hg) | 16.5 ± 4.6 | 20.0 ± 10.9 | <.001 |
| Tme ICP > 25 mm Hg, h | 6 (1-37.5) | 17 (2-45) | .004 |
| Tme ICP > 30 mm Hg, h | 1 (0-7) | 4 (0-17) | .002 |
| Tme ICP > 40 mm Hg, h | 0 (0-1) | 0 (0-3) | .006 |
| Highest ICP, mm Hg | 35.5 ± 12.7 | 44.0 ± 24.1 | <.001 |
| Average MAP, mm Hg | 91.8 ± 7.1 | 91.4 ± 10.0 | .70 |
| Tme MAP < 70 mm Hg, h | 1 (0-5) | 1 (0-5) | .17 |
| Tme MAP < 80 mm Hg, h | 14 (3.75-30) | 14 (5-33.5) | .51 |
| Tme MAP < 90 mm Hg, h | 49 (22-81.2) | 48.5 (20.5-88) | .60 |
| Average CPP, mm Hg | 75.3 ± 7.4 | 71.4 ± 16.3 | .009 |
| Time CPP < 50 mm Hg, h | 1 (0-4) | 2 (0-11) | .007 |
| Time CPP < 60 mm Hg, h | 11 (2-24.5) | 13 (3-42.5) | .08 |
| Time CPP < 70 mm Hg, h | 34 (10.75-74.5) | 40.5 (16-89) | .15 |
| Average Sjvo2, % | 72.9 + 5.6 | 74.4 + 5.5 | .02 |
| Time Sjvo2 < 50%, h | 0 (0-2) | 0 (0-2) | .37 |
| Time Sjvo2 < 40%, h | 0 (0-0) | 0 (0-0) | .86 |
| Time Sjvo2 < 30%, h | 0 (0-0) | 0 (0-0) | .21 |
CPP, cerebral perfusion pressure; CT, computed tomography; GCS, Glasgow Coma Scale; ICP, intracranial pressure; MAP, mean arterial pressure; Pbto2, brain tissue Po2.
For the PbtO2 variables (Table 4), the average PbtO2, the duration of time that PbtO2 was less than each of the 3 thresholds, and the PbtO2 trend pattern were all significantly related to neurological outcome. Patients with a favorable outcome had an average PbtO2 of 32.2 ± 16.3 compared with 25.1 ± 13.5 in the patients with an unfavorable outcome (P < .001). Patients with a favorable outcome were also more likely to have a PbtO2 trend pattern in which PbtO2 was never < 10 mm Hg (65.9% vs 46.7% for those with an unfavorable outcome) and less likely to have a pattern in which PbtO2 was persistently < 10 mm Hg or decreased to < 10 mm Hg after initially being normal (3.9% vs 20.7%). The median duration of time that PbtO2 was < 10 mm Hg was 0 hours for patients with a favorable recovery compared with 6 hours in patients with an unfavorable outcome (P< .001).
The position of the PbtO2 was also significantly related to neurological outcome (P = .03), with patients having an unfavorable outcome more likely to have the probe placed in abnormal brain. Because the probe position was not randomly assigned in this study but was dependent on the type of injury and because the probe position was not a therapeutic intervention per se, it is most likely that this association with outcome reflects prognostic information from the type of injury.
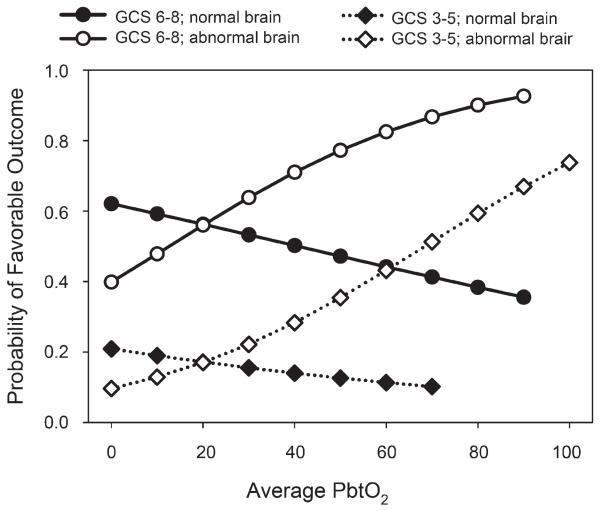
In the final best-fit logistic regression model (Table 5), the factors that were related to neurological outcome were age, PbtO2 probe position, initial motor GCS score, and the PbtO2 trend pattern. Although the type of injury was significantly related to outcome in univariate analyses, this factor fell out of the final logistic model. It is possible that the prognostic information from type of injury was contained in the probe position, which was closely related to type of injury. Although the average PbtO2 was significantly related to outcome in the univariate analyses, it was not significant in the final logistic model. However, the interaction between PbtO2 and probe position was statistically significant. In patients in whom the PbtO2 probe was placed in abnormal brain, the average PbtO2 was higher in those with a favorable outcome, 28.8 ± 12.0, compared with those with an unfavorable outcome, 19.5 ± 13.7 mm Hg (P = .01). There was no significant difference in PbtO2 with outcome when the probe was placed in normal-appearing brain: 33.8 ± 19.4 mm Hg for patients with favorable outcome vs 31.4 ± 13.1 mm Hg for patients with unfavorable outcome. The odds ratio for average PbtO2 to be associated with a favorable outcome was 0.988 when the PbtO2 probe was in normal brain but 1.033 when the PbtO2 probe was in abnormal brain. This odds ratio indicates that for every increase in average PbtO2 of 1 mm Hg, the chance of having a favorable outcome was 1.033 times greater. Figure 5 shows this interaction relationship for average PbtO2 and probe position in graphical form.
TABLE 5. Best-Fit Logistic Regression Modela.
| Variable | Best-Fit Logistic Model P |
|---|---|
| Age | <.001 |
| PbtO2 catheter position | .03 |
| Initial motor GCS | <.001 |
| Motor GCS × catheter position interaction | .14 |
| Average PbtO2 | .33 |
| Average PbtO2 × catheter position interaction |
.01 |
| PbtO2 trend pattern | .04 |
GCS, Glasgow Coma Scale; PbtO2, brain tissue PO2.
FIGURE 5.
Results of logistic regression model for the relationship of average brain tissue PO2 (PbtO2) and neurological outcome. The chances for a favorable outcome are generally less in patients with poor admission neurological status (diamonds) than in patients with better admission neurological status (circles). The open symbols show the chances of favorable outcome significantly improving with increasing PbtO2 when the probe is placed in abnormal tissue; the solid symbols show no significant relationship between outcome and PbtO2 when the probe is placed in normal brain. GCS, Glasgow Coma Scale.
DISCUSSION
The local nature of the PbtO2 values that are obtained with currently available probes is both a potential advantage and a problem. The advantage is that unlike global measurements of oxygenation or cerebral blood flow, the PbtO2 probe has the possibility of being able to monitor focal regions of the brain. The problem is that the probe has to be placed strategically in the brain tissue of interest to take advantage of this monitoring characteristic. The critical importance of probe location is underemphasized in most studies reporting PbtO2 data. In addition, the common practice of placing the PbtO2 probe at the same site as the ICP monitor limits the ability to choose the optimal location for the PbtO2 probe.
A number of previous studies have shown a relationship between PbtO2 values and neurological outcome. Most of these studies have reported that their monitoring strategy was to place the probe in normal-appearing brain. Not all of the studies adjusted the PbtO2 findings for other injury severity indicators. In 1998, Bardt et al10 reported that a PbtO2 < 10 mm Hg for > 30 minutes reduced the percent of favorable long-term outcomes from 73% to 22%, and Valadka et al11 found that increasing durations of PbtO2 < 15 mm Hg were associated with increasing risk of death. The Valadka et al model included age and duration of monitoring but not other important injury severity indicators. Van den Brink et al12 also saw that a PbtO2 < 10 mm Hg for > 30 minutes was associated with a greater chance of a poor outcome. PbtO2 remained a significant predictor of outcome even when adjusted for clinical characteristics and CT scan findings.
Likewise, several previous studies have examined differences in PbtO2 and other metabolic parameters in normal and pericontusional brain. A few have even compared different tissues in the same patient using 2 different probes simultaneously.13,14 Longhi et al15 compared PbtO2 in pericontusional tissue with values obtained in normal-appearing brain after diffuse brain injury. Like the present study, they observed significantly lower PbtO2 and longer durations of low PbtO2 in pericontusional tissue. They also found different trends over time, with PbtO2 recovering to normal over time in pericontusional tissue. One difference in methods was that the data collection started on day 2 after injury and the relationship to outcome was not studied. Extending these types of observations by adding measurements of extracellular biochemistry using microdialysis, Timofeev et al6 reported higher levels of lactate, glycerol, lactate/pyruvate ratio, and lactate/glucose ratio in pericontusional tissue, even when adjusted for other factors including the PbtO2.
The logical follow-up to these observational studies and others that relate PbtO2 findings to neurological outcome is to determine whether PbtO2 monitoring can direct therapy to maximize neurological outcome by preventing or treating early cerebral hypoxia. To date, such studies have been primarily comparisons with historical or concurrent controls that can reflect differences in injury severity and/or changes in other management practices over time. Compared with historical controls, several studies have found that PbtO2-directed management has resulted in improved neurological recovery and/or mortality rate.4,5,16 In contrast, Martini et al17 found that PbtO2-directed therapy performed at the discretion of the neurosurgeon resulted in no improvement in mortality rate but instead a worse neurological recovery and greater hospital resource use. The PbtO2-monitored group in this study was more severely injured, however, which may have affected the decision to monitor PbtO2. The probe placement strategy was described in these observational studies was normal-appearing brain5 or normal-appearing brain on the side of the most severe injury.4,16,17 A phase II randomized clinical trial examining the value of PbtO2-directed therapy to improve neurological outcome is planned (www.ClinicalTrials.gov; identifier NCT00974259). This may provide a more definite answer to these questions about whether PbtO2-directed therapy can alter outcome.
Although the strategy for placing the probes was described in most of these previous studies, the final location of the probe is not commonly characterized or analyzed. In the present study, the placement strategy was to target normal-appearing tissue on the side of the most severe injury, which was thought to be the most vulnerable brain tissue. However, this description was not found to adequately characterize the actual location of the probes. Therefore, the final placement of the probe was examined on a follow-up CT scan, characterized, and included in the analysis. Four different descriptions of the final location were used, and different PbtO2 trends over time were observed with each different location that described increasingly more severe injury of the brain.
Because of the retrospective nature of the study design, there are some limitations to the present study. The location of the PbtO2 probe is inherently related to the nature of injury. That is, in patients with a diffuse injury, there would not always be an abnormal area to monitor. In some patients with mass lesions, there may be very limited normal brain that can be selected for monitoring. In addition, the placement of the probe in this study was not randomized but rather directed by the nature of the injury in many cases. For these reasons, the conclusions that can be drawn are limited. However, the present study clearly demonstrates how critical the location of the PbtO2 probe is in determining the PO2 values that will be obtained.
In addition, the present study showed that additional prognostic information from the PbtO2 values was available only in patients in whom the probe was located in abnormal brain tissue, perhaps because there were fewer episodes of cerebral hypoxia in the patients in whom the probe was located in normal brain tissue. It might be also be that normal tissue is more resistant to cerebral hypoxia, and thresholds for hypoxic injury might be different in normal and abnormal brain tissue. This finding should not be interpreted to mean that monitoring PbtO2 in normal brain tissue is not useful because all tissue hypoxia events, regardless of location, were treated in this study. Because the probe location cannot be completely controlled by the strategy used for monitor placement, the present findings suggest that the final probe location will affect PbtO2 values and should be included in analyses.
The threshold for treatment of tissue hypoxia in this study was a PbtO2 of < 10 mm Hg. The optimal treatment threshold has not been clearly established for TBI patients, and it is even possible that thresholds for injury may differ in normal and injured brain tissue. Early studies observed a relationship between the duration of time PbtO2 was < 10 to 15 mm Hg and a poor neurological outcome.10-12 Others have recommended maintaining PbtO2 > 15 or even 20 mm Hg. Although the analysis included examination of time below several different thresholds, the results of this analysis might have been different if a higher treatment threshold had been used in managing the patients.
The PO2 catheter technology evolved significantly over the period of this study. There are small inherent differences in the performance of the various catheters used in the study.18 These differences are probably not clinically significant but could have introduced some variability into the analysis.
The GOS score at 6 months was not available in 85 patients and was imputed from the 3-month GOS score. Neurological recovery continues to improve over time, although the number of patients who would be expected to improve from unfavorable to favorable outcome between 3 and ± months is relatively small. It is possible that the results would have been different if the 6-month GOS score had been available in all patients. However, when the analyses were performed on only the 320 patients with 6-month GOS scores, the results were similar.
CONCLUSION
The purpose of this study was to examine the effect of catheter location on the relationship between PbtO2 and long-term neurological outcome. The results showed that the location of the probe determined both the PbtO2 values that were obtained and whether the PbtO2 values were related to long-term neurological outcome. The location of the PbtO2 probe must be kept in mind during the interpretation of data from individual patients and should be reported in publications that analyze PbtO2 data.
ABBREVIATIONS
- CPP
cerebral perfusion pressure
- GCS
Glasgow Coma Scale
- GOS
Glasgow Outcome Scale
- TBI
traumatic brain injury
Footnotes
Disclosures
This work was funded by National Institutes of Health grant P01-NS38660. The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury, X: rain oxygen monitoring and thresholds. J Neurotrauma. 2007;24(suppl 1):S65–S70. doi: 10.1089/neu.2007.9986. [DOI] [PubMed] [Google Scholar]
- 2.De Georgia MA, Deogaonkar A. Multimodal monitoring in the neurological intensive care unit. Neurologist. 2005;11(1):45–54. doi: 10.1097/01.nrl.0000149993.99956.09. [DOI] [PubMed] [Google Scholar]
- 3.Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007;23(3):507–538. doi: 10.1016/j.ccc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103(5):805–811. doi: 10.3171/jns.2005.103.5.0805. [DOI] [PubMed] [Google Scholar]
- 5.Narotam PK, Morrison JF, Nathoo N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J Neurosurg. 2009;111(4):672–682. doi: 10.3171/2009.4.JNS081150. [DOI] [PubMed] [Google Scholar]
- 6.Timofeev I, Czosnyka M, Carpenter KL, et al. Interaction between brain chemistry and physiology after traumatic brain injury: impact of autoregulation and microdialysis catheter location. J Neurotrauma. 2011;28(6):849–860. doi: 10.1089/neu.2010.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hlatky R, Valadka AB, Goodman JC, Contant CF, Robertson CS. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J Neurotrauma. 2004;21(7):894–906. doi: 10.1089/0897715041526195. [DOI] [PubMed] [Google Scholar]
- 8.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(suppl 1):S7–S95. doi: 10.1089/neu.2007.9995. [DOI] [PubMed] [Google Scholar]
- 9.Marshall LF, Marshall SB, Klauber MR, et al. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75(5 suppl):S14–S20. [Google Scholar]
- 10.Bardt TF, Unterberg AW, Härtl R, Kiening KL, Schneider GH, Lanksch WR. Monitoring of brain tissue PO2 in traumatic brain injury: effect of cerebral hypoxia on outcome. Acta Neurochir Suppl. 1998;71:153–156. doi: 10.1007/978-3-7091-6475-4_45. [DOI] [PubMed] [Google Scholar]
- 11.Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998;26(9):1576–1581. doi: 10.1097/00003246-199809000-00029. [DOI] [PubMed] [Google Scholar]
- 12.van den Brink WA, van Santbrink H, Steyerberg EW, et al. Brain oxygen tension in severe head injury. Neurosurgery. 2000;46(4):868–878. doi: 10.1097/00006123-200004000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Sarrafzadeh AS, Kiening KL, Bardt TF, Schneider GH, Unterberg AW, Lanksch WR. Cerebral oxygenation in contusioned vs nonlesioned brain tissue: monitoring of PtiO2 with Licox and Paratrend. Acta Neurochir Suppl. 1998;71:186–189. doi: 10.1007/978-3-7091-6475-4_54. [DOI] [PubMed] [Google Scholar]
- 14.Kiening KL, Schneider GH, Bardt TF, Unterberg AW, Lanksch WR. Bifrontal measurements of brain tissue-PO2 in comatose patients. Acta Neurochir Suppl. 1998;71:172–173. doi: 10.1007/978-3-7091-6475-4_50. [DOI] [PubMed] [Google Scholar]
- 15.Longhi L, Pagan F, Valeriani V, et al. Monitoring brain tissue oxygen tension in brain-injured patients reveals hypoxic episodes in normal-appearing and in perifocal tissue. Intensive Care Med. 2007;33(12):2136–2142. doi: 10.1007/s00134-007-0845-2. [DOI] [PubMed] [Google Scholar]
- 16.Spiotta AM, Stiefel MF, Gracias VH, et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg. 2010;113(3):571–580. doi: 10.3171/2010.1.JNS09506. [DOI] [PubMed] [Google Scholar]
- 17.Martini RP, Deem S, Yanez ND, et al. Management guided by brain tissue oxygen monitoring and outcome following severe traumatic brain injury. J Neurosurg. 2009;111(4):644–649. doi: 10.3171/2009.2.JNS08998. [DOI] [PubMed] [Google Scholar]
- 18.Haitsma I, Rosenthal G, Morabito D, Rollins M, Mass AI, Manley GT. In vitro comparison of two generations of Licox and Neurotrend catheters. Acta Neurochir Suppl. 2008;102:197–202. doi: 10.1007/978-3-211-85578-2_39. [DOI] [PubMed] [Google Scholar]