Abstract
Objectives
The PAREPET (Prediction of ARrhythmic Events with Positron Emission Tomography) study sought to test the hypothesis that quantifying inhomogeneity in myocardial sympathetic innervation could identify patients at highest risk for sudden cardiac arrest (SCA).
Background
Left ventricular ejection fraction (LVEF) is the only parameter identifying patients at risk of SCA who benefit from an implantable cardiac defibrillator (ICD).
Methods
We prospectively enrolled 204 subjects with ischemic cardiomyopathy (LVEF ≤35%) eligible for primary prevention ICDs. Positron emission tomography (PET) was used to quantify myocardial sympathetic denervation (11C-meta-hydroxyephedrine [11C-HED]), perfusion (13N-ammonia) and viability (insulin-stimulated 18F-2-deoxyglucose). The primary endpoint was SCA defined as arrhythmic death or ICD discharge for ventricular fibrillation or ventricular tachycardia >240 beats/min.
Results
After 4.1 years follow-up, cause-specific SCA was 16.2%. Infarct volume (22 ± 7% vs. 19 ± 9% of left ventricle [LV]) and LVEF (24 ± 8% vs. 28 ± 9%) were not predictors of SCA. In contrast, patients developing SCA had greater amounts of sympathetic denervation (33 ± 10% vs. 26 ± 11% of LV; p = 0.001) reflecting viable, denervated myocardium. The lower tertiles of sympathetic denervation had SCA rates of 1.2%/year and 2.2%/year, whereas the highest tertile had a rate of 6.7%/year. Multivariate predictors of SCA were PET sympathetic denervation, left ventricular end-diastolic volume index, creatinine, and no angiotensin inhibition. With optimized cut-points, the absence of all 4 risk factors identified low risk (44% of cohort; SCA <1%/year); whereas ≥2 factors identified high risk (20% of cohort; SCA ~12%/year).
Conclusions
In ischemic cardiomyopathy, sympathetic denervation assessed using 11C-HED PET predicts cause-specific mortality from SCA independently of LVEF and infarct volume. This may provide an improved approach for the identification of patients most likely to benefit from an ICD. (Prediction of ARrhythmic Events With Positron Emission Tomography [PAREPET]; NCT01400334)
Keywords: 11C-meta-hydroxyephedrine, myocardial viability, positron emission tomography, sudden cardiac arrest, sympathetic denervation
Numerous clinical and demographic variables have been associated with an increased risk of arrhythmic death, and many electrophysiological approaches have been proposed and evaluated in an attempt to better identify the patient population at highest risk of future arrhythmic death (1,2). However, despite multiple large clinical trials performed during the last 30 years, left ventricular ejection fraction (LVEF) remains the only parameter clinically used to distinguish high- and low-risk groups. Based on this approach, randomized trials have unequivocally established the benefit of prophylactic implantable cardiac defibrillator (ICD) placement to prevent arrhythmic death and improve survival in patients with a LVEF ≤35% (3–5). Nevertheless, the majority of patients never require device therapy to prevent a lethal ventricular arrhythmia. The ability to identify risk of arrhythmic death independently of LVEF could better target therapy among current ICD candidates, as well as identify patients with LVEF >35% who are at high risk of SCA (1,2). Although the latter population has a lower rate of arrhythmic death, it actually accounts for a larger number of arrhythmic events (6).
Previous basic and clinical studies have demonstrated an important role for sympathetic activation in the development of lethal ventricular arrhythmias, and inhomogeneity in myocardial sympathetic innervation may create a myocardial substrate particularly vulnerable to arrhythmic death (7). This inhomogeneity can reflect sympathetic denervation from infarction, as well as reversible ischemia. For example, in reperfused infarcts, the extent of sympathetic denervation exceeds infarct volume and approximates the entire area at risk of myocardial ischemia (8). In chronic coronary disease, reversible ischemia (from angina or silent ischemia) also creates inhomogeneity in myocardial sympathetic innervation that is independent of infarction, occurring in both stunned and hibernating myocardium (9). Pre-clinical models of hibernating myocardium have a high rate of arrhythmic death from spontaneous ventricular tachycardia (VT)/ventricular fibrillation (VF) that develops in the absence of infarction and heart failure (10), and 11C-meta-hydroxyephedrine (11C-HED) positron emission tomography (PET) demonstrates extensive sympathetic denervation (11).
On the basis of these observations, we tested the hypothesis that quantifying inhomogeneity in myocardial sympathetic innervation and/or hibernating myocardium increased the risk of arrhythmic death independently of LV function. The PAREPET (Prediction of ARrhythmic Events with Positron Emission Tomography) study was designed as an initial step toward this goal evaluating primary prevention ICD candidates with coronary artery disease (12).
Methods
The PAREPET trial, sponsored by the National Institutes of Health, is a prospective, observational cohort study designed to determine whether imaging hibernating and/or denervated myocardium can predict arrhythmic death in ischemic cardiomyopathy. This study was approved by the University at Buffalo and Veterans Affairs Western New York Healthcare System Institutional Review Boards. Methodological details of the study have been published and are provided in the Online Appendix (12).
Study design
The study population (n = 204) included patients with ischemic cardiomyopathy who were eligible to receive a primary prevention ICD (pre-enrollment LVEF ≤35% for Class ≥II and ≤30% for Class I). They had stable ischemic heart disease and heart failure on optimal medical therapy, and were not considered candidates for coronary revascularization. Exclusion criteria included a prior cardiac arrest or ICD discharge, recent infarction (<30 days), or revascularization (PCI <3 months; bypass grafting <1 year) (12).
Echocardiography and PET
Two-dimensional echocardiography (Sonos 7500, Philips Medical Inc., Andover, Massachusetts) was performed on the day of PET imaging as previously described (12,13). An echocardiographer blinded to events quantified cardiac volumes, LVEF, and mitral regurgitation, as recommended by the American Society of Echocardiography.
The PET imaging was performed on a ECAT EXACT HR+ (CTI, Knoxville, Tennessee) PET scanner (15.5 cm axial field-of-view; resolution ~5.4 mm3 full-width-at-half-maximum) (12,13). Sympathetic innervation was assessed with 11C-HED [740 MBq], resting perfusion with 13NH3 [740 MBq], and viability with 18F-2-deoxyglucose (18FDG) [241MBq] during a hyperinsulinemic-euglycemic clamp (13). Attenuation correction was performed using a 68Ge rod source (12,13). Of the planned 585 PET images, 96% were completed and quantifiable with 176 subjects having complete data. Results from imaging were not provided for patient management. Radiation exposure was <12 mSv.
Quantitative PET analysis
Blinded analysis used Flow-Quant (Ottawa Heart Institute, Ottawa, Ontario, Canada) (13,14) and decay corrected reconstructed images (zoom 2; Hann filter cutoff 0.3 cycles/pixel). Late uptake defined the left ventricle (LV) with bottle-brush sampling (15). Late myocardial uptake was averaged from 4 frames of data for each imaging set: 15 to 60 min after 11C-HED; 3 to 19 min after 13NH3; 15 to 40 min after 18FDG. We normalized myocardial activity to the highest 5% of sectors (496 sector model) (15). Normal 13NH3 and 18FDG uptake were ≥80% of peak (12,14). Normal 11C-HED uptake was considered ≥75% of peak, on the basis of the estimated ratio of reduced versus normal 11C-HED retention fraction among zones with normal resting flow (16). All PET parameters were expressed as % of LV. Infarcted and hibernating myocardium were quantified from a mismatch analysis between 13NH3 and 18FDG (14). To evaluate the potential influence of global downregulation in 11C-HED uptake, 11C-HED retention was determined for the segment with maximal 11C-HED uptake in each subject. 11C-HED retention (min−1) was calculated by dividing mean segment activity from the late uptake image by the integrated arterial activity (from 0 to 60 min) (11).
Classification of events
Subjects were contacted at 3-month intervals to review interval ICD therapy, hospitalizations, and symptoms. The primary endpoint was sudden cardiac arrest (SCA). This included arrhythmic death or ICD discharge for VF or VT >240 beats/min (12). The frequency of ICD discharge for these arrhythmias approximates the reduction in mortality with an ICD (12,17,18). Clinical notes and electrograms from ICD discharges were independently reviewed.
We classified deaths (i.e., SCA, cardiac nonsudden and noncardiac) using modified Hinkle-Thaler criteria (19,20). Available details (i.e., medical records, witnesses, family, death certificates), activity levels, and symptoms before death were reviewed by 2 cardiologists. In the event of disagreement, a consensus was reached with a third reviewer. Cardiac transplantation and arrhythmic events with end-stage heart failure or hospice care were classified as cardiac nonsudden deaths (19). Revascularization procedures were performed on 26 subjects after enrollment.
Statistical analyses
Data are presented as mean ± SD. Subjects with and without SCA were compared with unpaired Student t tests (continuous data) and chi-square test analysis (categorical data). The time to SCA was analyzed graphically using Kaplan-Meier plots and was tested using Cox proportional hazard models and the log-rank statistic. Optimal cut-points for continuous variables were determined retrospectively by values that maximized the log-rank statistic. Stepwise selection was used to generate the optimal multivariate model to predict time to SCA from PET, demographics, medications, echocardiographic, hemodynamic, and laboratory parameters. All statistical analyses were performed with commercial software (Microsoft Excel version 2010, [Microsoft, Redmond, Washington] and SAS version 9.1, [SAS Institute Inc., Cary, North Carolina]).
Results
Table 1 summarizes demographic variables. Average LVEF was 27 ± 9%, age was 67 ± 11 years, New York Heart Association functional class was 2.1 ± 0.8, and Canadian Cardiovascular Society angina class was 1.8 ± 0.7. There were 21 women (10%). Diabetes was present in 47%, 78% had prior coronary revascularization, and 81% had an ICD prior to an event. Medical therapy was very good, with almost all subjects treated with anti-platelet therapy or warfarin (99%), β-blockers (96%), and angiotensin inhibition therapy (angiotensin-converting-enyzme inhibitor or angiotensin receptor antagonist, 90%).
Table 1.
Clinical Parameters and SCA
| SCA (n = 33) |
No SCA (n = 171) |
p Value | |
|---|---|---|---|
| Demographic parameters | |||
| Age (yrs) | 66 ± 8 | 67 ± 12 | 0.57 |
| Female | 0 (0) | 21 (12) | 0.07 |
| Body mass index (kg/m2) | 28.3 ± 4.5 | 28.6 ± 5.1 | 0.76 |
| Body surface area (m2) | 2.1 ± 0.2 | 2.0 ± 0.2 | 0.27 |
| NYHA functional class | 2.3 ± 0.6 | 2.1 ± 0.8 | 0.33 |
| CCS angina class | 1.8 ± 0.7 | 1.8 ± 0.7 | 0.48 |
| Diabetes mellitus | 17 (52) | 78 (46) | 0.67 |
| Coronary revascularization | 21 (64) | 138 (81) | 0.053 |
| Cardiac rhythm (not sinus) | 5 (16) | 17 (10) | 0.56 |
| QRS duration (ms) | 137 ± 32 | 136 ± 35 | 0.79 |
| Medications | |||
| Beta-blockers | 32 (97) | 163 (95) | 0.97 |
| Amiodarone | 3 (9) | 16 (9) | 0.78 |
| Antiplatelet agents or warfarin | 32 (97) | 169 (99) | 0.98 |
| Angiotensin inhibition therapy | 26 (79) | 157 (92) | 0.052 |
| Aldosterone antagonists | 14 (42) | 67 (39) | 0.88 |
| Digoxin | 13 (39) | 66 (39) | 0.91 |
| Hemodynamic parameters | |||
| Systolic blood pressure (mm Hg) | 127 ± 17 | 126 ± 21 | 0.93 |
| Heart rate (beats/min) | 63 ± 11 | 66 ± 12 | 0.23 |
| Rate pressure product (beats/min · mm Hg) | 7,952 ± 1,389 | 8,398 ± 2,282 | 0.28 |
| Laboratory parameters | |||
| Hematocrit (%) | 39.3 ± 5.3 | 41.0 ± 5.8 | 0.11 |
| Potassium (mEq/l) | 4.3 ± 0.6 | 4.3 ± 0.4 | 0.83 |
| Sodium (mEq/l) | 139 ± 3 | 139 ± 3 | 0.26 |
| Creatinine (mg/dl) | 1.7 ± 1.2 | 1.3 ± 0.7 | 0.04 |
| B-type natriuretic peptide (ng/l) | 741 ± 694 | 412 ± 479 | 0.001 |
Values are mean ± SD or n (%).
CCS = Canadian Cardiovascular Society; NYHA = New York Heart Association; SCA = sudden cardiac arrest.
PET quantification of denervated, infarcted, and viable myocardium
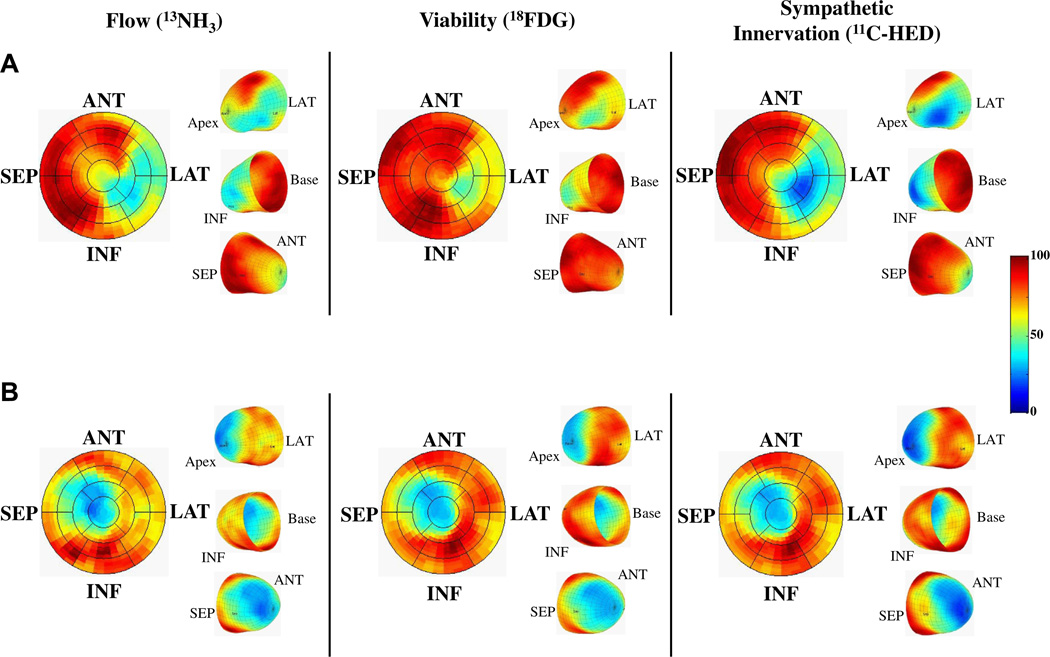
PET images from 2 representative subjects comparing resting flow (13NH3), viability (insulin-stimulated 18FDG), and myocardial sympathetic innervation (11C-HED) are shown in Figure 1. Quantitative image analysis determined that the average infarct volume was 20 ± 9% of the LV. In comparison, the average volume of denervated myocardium was considerably larger, averaging 27 ± 11% of the LV (p < 0.001 vs. infarcted), with 8 ± 6% of the LV denervated, but viable. Hibernating myocardium (viable myocardium with reduced resting perfusion) was uncommon, reflecting the high prevalence of prior revascularization, and averaged 3 ± 3% of the LV.
Figure 1. PET imaging of Flow, Viability, and Sympathetic Innervation.
(A) A subject experiencing sudden cardiac arrest (SCA). There is a mismatch in infarct size (reduced 18F-2-deoxyglucose [18FDG]), which was smaller than the volume of sympathetic denervation (reduced 11C-meta-hydroxyephedrine [11C-HED]). There was also reduced perfusion (13N-ammonia [13NH3]) with preserved 18FDG indicating hibernating myocardium. In contrast, B shows a subject with matched reductions in flow, infarct volume, and sympathetic denervation. ANT = anterior; INF = inferior; LAT = lateral; PET = positron emission tomography; SEP = septum.
Clinical and imaging correlates of SCA
During a median follow-up of 4.1 years (range: 2.5 to 7.2 years), there were 33 adjudicated sudden cardiac arrests. There were no differences between the 22 subjects with arrhythmic death versus the 11 with ICD discharges for VF or VT >240 beats/min. All SCA events occurred in men (p = 0.06). Selected imaging parameters associated with SCA are summarized in Table 2. Subjects developing SCA had significantly larger volumes of denervated and viable denervated myocardium, whereas infarct volume and hibernating myocardium were not different. Although the LVEF and LV mass were not significantly different, those who experienced SCA had larger LV volume indices and more mitral regurgitation. Among laboratory parameters, serum creatinine and B-type natriuretic peptide (BNP) were higher in patients developing SCA (Table 1).
Table 2.
Imaging Parameters in Subjects With SCA
| SCA (n = 33) |
No SCA (n = 171) |
p Value | |
|---|---|---|---|
| PET parameters (% LV) | |||
| Denervated myocardium | 33 ± 10 | 26 ± 11 | 0.001 |
| Infarcted myocardium | 22 ± 7 | 19 ± 9 | 0.18 |
| Viable, denervated myocardium | 10 ± 6 | 7 ± 5 | 0.02 |
| Hibernating myocardium | 3 ± 2 | 3 ± 3 | 0.50 |
| Echocardiography | |||
| LV ejection fraction (%) | 24 ± 8 | 28 ± 9 | 0.053 |
| LV end-diastolic volume index (ml/m2) | 107 ± 28 | 86 ± 30 | <0.001 |
| LV end-systolic volume index (ml/m2) | 81 ± 21 | 63 ± 26 | <0.001 |
| LV mass index (g/m2) | 167 ± 37 | 150 ± 48 | 0.07 |
| Mitral regurgitation | 2.0 ± 1.2 | 1.6 ± 1.1 | 0.04 |
Values are mean ± SD.
LV = left ventricle; SCA = sudden cardiac arrest.
Quantitative imaging and SCA
The primary analysis of the PAREPET study was the evaluation of PET imaging parameters as continuous variables to predict time to SCA. The cumulative event rate curves of tertiles for each PET parameter are summarized in Figure 2. The volume of denervated myocardium had the strongest correlation with SCA (p = 0.001) (Table 3). Patients in the highest tertile of sympathetic denervation had an SCA event rate of ~6.7%/year, whereas the intermediate and lowest tertiles had event rates of 2.2%/year and 1.2%/year, respectively. Each 1% increase in the volume of denervated myocardium was associated with a 5.7% increase in the risk of SCA. Similarly, the volume of viable denervated myocardium was significantly associated with the time to SCA (p = 0.025), with risk of SCA increasing by 6.7% for every 1% increase in viable, denervated myocardium. The ability of denervated myocardium to predict SCA was not influenced by any global downregulation in 11C-HED uptake. The 11C-HED retention in segments with maximal 11C-HED uptake was 0.136 ± 0.036 min−1, with no difference among those with and without subsequent SCA (0.134 ± 0.027 min−1 vs. 0.137 ± 0.037 min−1; p=0.69).There was no significant association between SCA and the volume of infarcted or hibernating myocardium (Table 3).
Figure 2. PET Parameters and SCA.
Kaplan-Meier curves show the incidence of SCA for tertiles of PET-defined myocardial substrates (median follow-up 4.1 years). As continuous variables, the total volume of denervated myocardium, as well as viable denervated myocardium, predicted SCA. Neither infarct volume nor hibernating myocardium was significant as continuous variables. Abbreviations as in Figure 1.
Table 3.
Significant Predictors of Time to SCA
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p Value | HR (95% CI) | p Value |
| PET parameters (per 1% of LV) | ||||
| Denervated myocardium | 1.057 (1.023–1.092) | 0.001 | 1.069 (1.023–1.117) | 0.003 |
| Viable, denervated myocardium | 1.067 (1.008–1.130) | 0.025 | ||
| Infarcted myocardium | 1.029 (0.990–1.069) | 0.15 | ||
| Hibernating myocardium | 0.950 (0.822–1.099) | 0.49 | ||
| Other significant parameters | ||||
| LVEDV index (per ml/m2) | 1.021 (1.011–1.032) | <0.0001 | 1.030 (1.010–1.050) | 0.003 |
| LVESV index (per ml/m2) | 1.022 (1.011–1.033) | <0.0001 | ||
| BNP (per ng/L) | 1.001 (1.000–1.001) | 0.0003 | ||
| Creatinine (per mg/dl) | 1.53 (1.21–1.95) | 0.0005 | 2.30 (1.12–4.71) | 0.023 |
| LA volume (per ml) | 1.011 (1.003–1.019) | 0.010 | ||
| No angiotensin inhibition therapy | 2.88 (1.25–6.67) | 0.014 | 4.31 (1.07–17.38) | 0.040 |
| LV ejection fraction (per 1%) | 0.951 (0.913–0.990) | 0.016 | ||
| LV mass index (per g/m2) | 1.007 (1.000–1.014) | 0.038 | ||
BNP = B-type natriuretic peptide; CI = confidence interval; HR = hazard ratio; LA = left atrial; LV = left ventricular; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; PET = positron emission tomography; SCA = sudden cardiac arrest.
Other parameters significantly associated with SCA, as continuous variables included larger LV end-diastolic and end-systolic volume indices, elevated BNP, elevated creatinine, larger left atrial volume, lower LVEF, and elevated LV mass index (p < 0.05 for each) (Table 3). In addition, SCA was associated with no angiotensin inhibition therapy (23% vs. 9%; p = 0.02). Multivariate analysis of these continuous and categorical variables revealed that denervated myocardium remained an independent predictor of time to SCA, in addition to LV end-diastolic volume index, creatinine, and no angiotensin inhibition therapy.
Predictors of risk using optimized cut-points
A post-hoc analysis was performed to determine the optimal cut-point for each of the continuous predictors (Fig. 3). Time to SCA was predicted by greater denervated myocardium (>37.6% of the LV, 19% of subjects), larger LV end-diastolic volume index (cut-point = 99 ml/m2, 34% of subjects), elevated creatinine (cut-point = 1.49 mg/dl, 26% of subjects) and no angiotensin inhibition therapy (10% of subjects). For example, less denervated myocardium (<37.6% LV) identified a large subgroup (81% of the cohort) at lower risk of SCA [SCA rate of 3.0%/year (95% CI: 1.9% to 4.7%) vs. 10.3%/year (95% CI: 6.2% to 16.1%); p = 0.001].
Figure 3. Predictors of SCA by Multivariate Analysis.
Kaplan-Meier curves illustrating the incidence of SCA greater than and less than the optimal cut-point for denervated myocardium (>37.6% of left ventricle [LV]), left ventricular end-diastolic volume index (LVEDVI >99 ml/m2), creatinine (>1.49 mg/dl), and no angiotensin inhibition therapy. The hazard ratio (HR) for each parameter was derived from the multivariate analysis. ACEI/ARA = angiotensin-converting-enzyme inhibitor/angiotensin receptor antagonist. Abbreviations as in Figure 1.
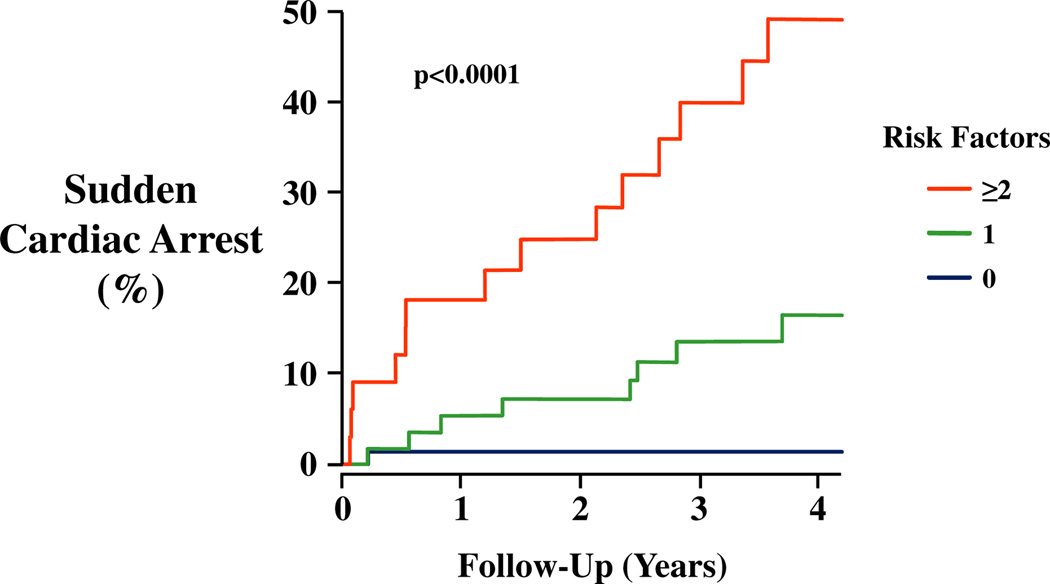
Then we tested how well these dichotomized factors could predict the risk of SCA. Figure 4 shows that the presence of 0, 1, or ≥2 of these risk factors could identify subgroups of subjects having significantly different risks of SCA (p < 0.001). Subjects having no high-risk predictor represented 44% of the cohort and had a very low risk of SCA (0.9%/year). In contrast, those with 1 risk factor (36% of the cohort) had an SCA rate of 3.9%/year, and those with 2 or more high risk factors (20% of the cohort) had an annual risk of SCA of 11.7%/year.
Figure 4. SCA Risk Factor Model.
Kaplan-Meier curves illustrating highly significant differences in the incidence of SCA in relation to the number of risk factors present (p < 0.0001). Subjects with no risk factor (blue, 44% of cohort) had an annual rate of SCA <1%. With 2 or more risk factors (red, 20% of cohort), the annual risk of SCA increased to ~12%. Patients with 1 risk factor (green, 36% of cohort) had an intermediate risk of SCA (~4%/year). Abbreviations as in Figure 1.
Discussion
Our results demonstrate that myocardial sympathetic denervation quantified using 11C-HED PET imaging identifies patients with ischemic cardiomyopathy who are at high risk of SCA. The risk of SCA in this primary prevention population was dependent on the total volume of denervated myocardium and was independent of other imaging parameters, including LVEF, infarct volume, and hibernating myocardium. Thus, quantifying the extent of myocardial sympathetic denervation may provide a novel approach to identify a subgroup of patients with ischemic cardiomyopathy who would derive the most benefit from a primary prevention ICD.
ICD therapy as a surrogate for aborted arrhythmic death
Currently, there is no consensus regarding which ICD therapies provide an appropriate surrogate for arrhythmic death in observational studies. The device therapies included in our primary endpoint were considerably more restrictive than the prevailing approach in contemporary observational imaging studies, which typically include all appropriate ICD therapies (anti-tachycardia pacing and ICD discharge) (21–23). Appropriate ICD therapies occur at least 3 times as frequently as fatal arrhythmic events (4,18), and self-terminating ventricular arrhythmias may have substrates and triggers that differ from those resulting in arrhythmic death (24). Our prospectively defined primary endpoint was SCA, which included adjudicated arrhythmic death and an ICD equivalent endpoint on the basis of device therapies most clearly associated with lethal ventricular arrhythmias (VF and VT >240 beats/min). In the MADIT II (Second Multicenter Automatic Defibrillator Implantation Trial), the frequency of these device therapies plus death in patients randomized to an ICD (18.3% at 2 years) was similar to the rate of death in the medically treated group (18% at 2 years) (17). Although this analysis supports using these events as surrogates of arrhythmic death in patients with an ICD (17,18), we cannot exclude the possibility that this conservative definition may have underestimated the rate at which arrhythmic death would have occurred in our population.
Myocardial sympathetic innervation and SCA
Recent studies have imaged global cardiac sympathetic innervation in heart failure using 123I-meta-iodobenzylguanidine (MIBG). They have consistently shown an association between reductions in MIBG uptake and endpoints reflecting a variety of composite cardiovascular outcomes in patients with heart failure (21,25–27). However, there is disagreement regarding the preferred imaging parameter (reduced heart-to-mediastinum [H/M] ratio vs. accelerated tracer washout rate) and the added benefit from regional tracer quantification. There is also a paucity of outcome data for cause-specific mortality from SCA.
In a small study of heart failure patients (LVEF <40%; n = 106, 52% ischemic cardiomyopathy), MIBG imaging was shown to predict arrhythmic death, as well as pump failure death and total cardiac mortality (26). Both the H/M ratio and the washout rate predicted arrhythmic death, but only the washout rate and LVEF remained independent after multivariate analysis. In contrast to our study, subjects taking beta-blockers, insulin, or those with significant renal dysfunction at entry were excluded. Thus, more than 97% of our study population would have been excluded, raising questions about generalizing these findings to risk stratification for SCA among contemporary heart failure patients.
A larger observational study (ADMIRE-HF [AdreView Myocardial Imaging for Risk Evaluation in Heart Failure]) imaged 961 subjects with an LVEF ≤35% (66% with ischemic cardiomyopathy) and New York Heart Association functional class II/III heart failure symptoms (27). The composite primary endpoint was heart failure progression (hospitalization or clinical assessment), potentially life-threatening arrhythmias (sustained VT, resuscitated cardiac arrest, or any appropriate ICD therapy including anti-tachycardia pacing), and cardiac death. A late H/M ratio <1.6 (reflecting both denervated myocardium and accelerated washout from increased sympathetic nerve activity) was present in ~80% of patients and predicted the composite endpoint (hazard ratio: 2.5). Evaluating regional sympathetic innervation and perfusion did not improve this in comparison with the global H/M ratio (27). In contrast, another study evaluating heart failure patients referred for an ICD (n = 116, 74% with ischemic cardiomyopathy) found neither the MIBG H/M ratio nor the washout rate to be predictors of appropriate ICD therapy (21). A semi-quantitative defect score predicted ICD therapy, which is consistent with our findings and supports the importance of quantifying regional sympathetic neuronal function for risk stratification of arrhythmic death (21).
Imaging of myocardial ischemia and infarct volume to predict SCA
Acute myocardial ischemia, fibrosis, and infarction have been demonstrated to be arrhythmogenic substrates. For example, in a large cohort with coronary disease referred for perfusion imaging, the extent of infarction plus ischemia (summed stress perfusion score) was the only independent imaging parameter to predict arrhythmic death (28). This is consistent with our study in that the volume of denervated myocardium approximates the area at risk for ischemia (8,11). Nevertheless, due to our study design and the stable nature of symptoms in our subjects, coronary anatomy was not evaluated, and we did not directly assess the presence or extent of stress-induced ischemia at enrollment. Therefore, the potential roles of inducible ischemia and coronary disease progression in SCA in this study are not defined.
Cardiac magnetic resonance imaging (MRI) has facilitated the quantification of infarct volume, as well as patchy fibrosis (“gray zone”) surrounding the infarct borders. Although cause-specific mortality from arrhythmic death has not been assessed, several studies using MRI have correlated scar volume with cardiac endpoints, including appropriate ICD therapy (22,23,29). Some studies (22), but not all (23,29), have shown that quantification of the “gray zone” improves risk prediction. Interestingly, the MRI “gray zone” is similar in size to viable, denervated myocardium assessed by PET (the difference between denervated and infarcted myocardium). Thus, they may ultimately prove to be assessing a similar substrate with increased risk of lethal arrhythmias. Further studies comparing infarct volume, “gray zone,” and denervated volume will be required to determine whether the 2 approaches provide similar risk stratification for SCA.
Predictive model to assess the risk of SCA
Our post-hoc multivariate analysis demonstrated that quantifying the volume of denervated myocardium along with 3 other variables (LV end-diastolic volume index, creatinine, and angiotensin inhibition therapy) independently predicted arrhythmic risk. Quantifying infarct size, LVEF, and BNP (and other variables) did not improve the predictive model. Although other parameters have been associated with cardiovascular mortality, they either preferentially predict progressive heart failure (as opposed to SCA), or their prognostic information was superseded by the 4 independent factors. Importantly, more than 40% of our study population had none of the risk factors and were at very low risk with an annual SCA rate of <1%. This is actually lower than the rate of arrhythmic death in patients with CAD and an LVEF between 35% and 50%, and these patients are currently not candidates for an ICD. At the other extreme, 20% of our subjects had ≥2 risk factors and a high annual SCA rate of ~12%. A limitation of this analysis relates to its post-hoc nature, inclusion of multiple parameters that could result in overfitting and the optimization of cut-points on a modest sample size. Nevertheless, identification of 4 independent predictors is reasonable in relation to the number of observed events. Further studies will be needed to prospectively validate the model, but because it is independent of ejection fraction among those with moderate-to-severe LV dysfunction, it could ultimately be useful to improve the risk stratification of a much larger number of patients at risk of SCA (6).
Study limitations
Our study specifically excluded patients with recent myocardial infarction, nonischemic cardiomyopathy, and those who had more preserved LV systolic function. Thus, future prospective studies will be required to identify whether PET imaging with 11C-HED can improve risk stratification for SCA in these groups.
Conclusions
Our results indicate that quantifying regional sympathetic denervation with 11C-HED PET may provide a novel approach to identify patients at high risk of arrhythmic death, independent of LVEF. Although PET/computed tomographic scanning is widely available, the use of 11C-HED would be limited to centers in close proximity to a cyclotron. Fortunately, 18fluorine-labeled norepinephrine analogs have a longer half-life that could facilitate broader application (30). With the use of these, molecular imaging of myocardial sympathetic denervation in those patients who have ischemic cardiomyopathy may afford a new approach to improve the targeting of ICDs to those who are at greatest risk of SCA.
Supplementary Material
Acknowledgments
The authors thank the PAREPET subjects and their families for the donation of their time and patience, without whom this study would not have been possible; Anne Coe and Marsha Barber for their assistance with clinical trial administration; Paul Galantowicz for PET imaging assistance, John Gon and Wendy Klein for echocardiography assistance, Ran Klein and Jennifer Renaud, FlowQuant assistance; and the recruitments efforts of our electrophysiology colleagues Arturo M. Valverde, MD, Donald Switzer, MD, and Chee Kim, MD, and the cardiology community of Western New York.
This research was supported by the U.S. National Institute of Health Heart, Lung, and Blood Institute (grant no. HL-76252). Dr. Haka is currently employed by Siemens Medical. Dr. Curtis has served as a consultant or received research grants or honoraria from Medtronic, Inc. and St. Jude Medical; and served on the advisory board of Bristol-Myers Squibb, Pfizer, Inc., Janssen Pharmaceuticals, Daiichi Sankyo, and Biosense Webster.
Abbreviations and Acronyms
- BNP
B-type natriuretic peptide
- 11C-HED
11C-meta-hydroxyephedrine
- 18FDG
18F-2-deoxyglucose
- ICD
implantable cardiac defibrillator
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- MIBG
123I-meta-iodobenzylguanidine
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- SCA
sudden cardiac arrest
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX
For a supplemental methods section, please see the online version of this article.
REFERENCES
- 1.Passman R, Goldberger JJ. Predicting the future: risk stratification for sudden cardiac death in patients with left ventricular dysfunction. Circulation. 2012;125:3031–3037. doi: 10.1161/CIRCULATIONAHA.111.023879. [DOI] [PubMed] [Google Scholar]
- 2.Lorvidhaya P, Addo K, Chodosh A, Iyer V, Lum J, Buxton AE. Sudden cardiac death risk stratification in patients with heart failure. Heart Fail Clin. 2011;7:157–174. vii. doi: 10.1016/j.hfc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 5.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 6.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 7.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 8.Matsunari I, Schricke U, Bengel FM, et al. Extent of cardiac sympathetic neuronal damage is determined by the area of ischemia in patients with acute coronary syndromes. Circulation. 2000;101:2579–2585. doi: 10.1161/01.cir.101.22.2579. [DOI] [PubMed] [Google Scholar]
- 9.Fallavollita JA, Canty JM., Jr Dysinnervated but viable myocardium in ischemic heart disease. J Nucl Cardiol. 2010;17:1107–1115. doi: 10.1007/s12350-010-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canty JM, Jr, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res. 2004;94:1142–1149. doi: 10.1161/01.RES.0000125628.57672.CF. [DOI] [PubMed] [Google Scholar]
- 11.Luisi AJ, Jr, Suzuki G, deKemp R, et al. Regional 11C-hydroxyephedrine retention in hibernating myocardium: Chronic inhomogeneity of sympathetic innervation in the absence of infarction. J Nucl Med. 2005;46:1368–1374. [PubMed] [Google Scholar]
- 12.Fallavollita JA, Luisi AJ, Jr, Michalek SM, et al. Prediction of arrhythmic events with positron emission tomography: PAREPET study design and methods. Contemp Clin Trials. 2006;27:374–388. doi: 10.1016/j.cct.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Fallavollita JA, Luisi AJ, Jr, Yun E, Dekemp RA, Canty JM., Jr An abbreviated hyperinsulinemic-euglycemic clamp results in similar myocardial glucose utilization in both diabetic and non-diabetic patients with ischemic cardiomyopathy. J Nucl Cardiol. 2010;17:637–645. doi: 10.1007/s12350-010-9228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beanlands RS, Ruddy TD, deKemp RA, et al. Positron emission tomography and recovery following revascularization (PARR-1): the importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J Am Coll Cardiol. 2002;40:1735–1743. doi: 10.1016/s0735-1097(02)02489-0. [DOI] [PubMed] [Google Scholar]
- 15.deKemp RA, Ruddy TD, Hewitt T, Dalipaj MM, Beanlands RS. Detection of serial changes in absolute myocardial perfusion with 82Rb PET. J Nucl Med. 2000;41:1426–1435. [PubMed] [Google Scholar]
- 16.Allman KC, Wieland DM, Muzik O, DeGrado TR, Wolfe ER, Jr, Schwaiger M. Carbon-11 hydroxyephedrine with positron emission tomography for serial assessment of cardiac adrenergic neuronal function after acute myocardial infarction in humans. J Am Coll Cardiol. 1993;22:368–375. doi: 10.1016/0735-1097(93)90039-4. [DOI] [PubMed] [Google Scholar]
- 17.Daubert JP, Wilber DJ, Lin A, et al. ICD therapy for fast ventricular tachycardia or ventricular fibrillation is a surrogate endpoint for mortality in MADIT II (abstr) Circulation. 2003;108:IV385. [Google Scholar]
- 18.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 19.Buxton AE, Lee KL, DiCarlo L, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 2000;342:1937–1945. doi: 10.1056/NEJM200006293422602. [DOI] [PubMed] [Google Scholar]
- 20.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 21.Boogers MJ, Borleffs CJ, Henneman MM, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55:2769–2777. doi: 10.1016/j.jacc.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 22.Roes SD, Borleffs CJ, van der Geest RJ, et al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009;2:183–190. doi: 10.1161/CIRCIMAGING.108.826529. [DOI] [PubMed] [Google Scholar]
- 23.Klem I, Weinsaft JW, Bahnson TD, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkiomaki JS, Bloch Thomsen PE, Kiviniemi AM, Messier MD, Huikuri HV. Risk factors of self-terminating and perpetuating ventricular tachyarrhythmias in post-infarction patients with moderately depressed left ventricular function, a CARISMA sub-analysis. Europace. 2011;13:1604–1611. doi: 10.1093/europace/eur166. [DOI] [PubMed] [Google Scholar]
- 25.Merlet P, Valette H, Dubois-Rande JL, et al. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med. 1992;33:471–477. [PubMed] [Google Scholar]
- 26.Tamaki S, Yamada T, Okuyama Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53:426–435. doi: 10.1016/j.jacc.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson AF, Senior R, Cerqueira MD, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. J Am Coll Cardiol. 2010;55:2212–2221. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Piccini JP, Horton JR, Shaw LK, et al. Single-photon emission computed tomography myocardial perfusion defects are associated with an increased risk of all-cause death, cardiovascular death, and sudden cardiac death. Circ Cardiovasc Imaging. 2008;1:180–188. doi: 10.1161/CIRCIMAGING.108.776484. [DOI] [PubMed] [Google Scholar]
- 29.de Haan S, Meijers TA, Knaapen P, Beek AM, van Rossum AC, Allaart CP. Scar size and characteristics assessed by CMR predict ventricular arrhythmias in ischaemic cardiomyopathy: comparison of previously validated models. Heart. 2011;97:1951–1956. doi: 10.1136/heartjnl-2011-300060. [DOI] [PubMed] [Google Scholar]
- 30.Bengel FM, Schwaiger M. Assessment of cardiac sympathetic neuronal function using PET imaging. J Nucl Cardiol. 2004;11:603–616. doi: 10.1016/j.nuclcard.2004.06.133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.