Abstract
Anorexia nervosa (AN) is an eating disorder characterized by self-imposed severe starvation and often linked with excessive exercise. Activity-based anorexia (ABA) is an animal model that reproduces some of the behavioral phenotypes of AN, including the paradoxical increase in voluntary exercise following food restriction (FR). Although certain rodents have been used successfully in this animal model, C57BL/6 mice are reported to be less susceptible to ABA. We re-examined the possibility that female C57BL/6 mice might exhibit ABA vulnerability during adolescence, the developmental stage/sex among the human population with particularly high AN vulnerability. After introducing the running wheel to the cage for three days, ABA was induced by restricting food access to 1 hour per day (ABA1, N=13) or 2 hours per day (ABA2, N=10). All 23 exhibited increased voluntary wheel running (p<0.005) and perturbed circadian rhythm within two days. Only one out of five survived ABA1 for three days, while ten out of ten survived ABA2 for three days and could subsequently restore their body weight and circadian rhythm. Exposure of recovered animals to a second ABA2 induction revealed a large range of vulnerability, even within littermates. To look for the cellular substrate of differences in vulnerability, we began by examining synaptic patterns in the hippocampus, a brain region that regulates anxiety as well as plasticity throughout life. Quantitative EM analysis revealed that CA1 pyramidal cells of animals vulnerable to the second ABA2 exhibit less GABAergic innervation on cell bodies and dendrites, relative to the animals resilient to the second ABA (p<0.001) or controls (p<0.05). These findings reveal that C57BL/6J adolescent females can be used to capture brain changes underlying ABA vulnerability, and that GABAergic innervation of hippocampal pyramidal neurons is one important cellular substrate to consider for understanding the progression of and resilience to AN.
Keywords: anorexia nervosa, eating disorder, exercise, relapse, GABA, hippocampus
1. INTRODUCTION
Anorexia nervosa (AN) is an eating disorder characterized by self-imposed severe starvation. No less than 40% and as many as 80% of the patients with AN exhibit hyperactivity in the form of excessive exercise (Epling et al., 1983, Davis et al., 1997, Davis et al., 1999, Hebebrand et al., 2003). This combination of symptoms leads to severe malnutrition, causing osteoporosis, amenorrhea and a mortality rate that is the highest among all mental illnesses - 10–20% (Sullivan, 1995, Birmingham et al., 2005, Bulik et al., 2007). Currently, there exists no accepted pharmacological treatment for AN, due largely to the paucity of knowledge regarding the etiology, progression and relapse of this illness.
Analysis of the AN population provides some clues regarding the etiology of AN. The incidence of AN is ten times higher among females than males and has its first onset most commonly during adolescence. Although dieting is an almost universal phenomenon among adolescent females, only 0.5 – 1% of the female population are diagnosed with AN during their lifetime and the risk for AN is particularly high among those that have exhibited anxiety disorders starting in childhood (Kaye et al., 2004, Thornton et al., 2011). Results from twin studies underscore the genetic contribution to the etiology of AN (Bulik et al., 2007, Rask-Andersen et al., 2010). Moreover, this epidemiological pattern strongly suggests that there is a biological basis for the vulnerability to AN, beyond the socio-cultural factors (Kaye et al., 2009, Kaye et al., 2011). AN is also associated with relapses that are as high as 30–50% within a year of recovery from AN (Birmingham et al., 2005), suggesting that anorexic behavior during this pivotal, final stage of brain development may cause changes in brain connections that persist beyond weight restoration.
An animal model, called activity-based anorexia (ABA), has been useful for identifying some of the vulnerability factors and changes in brain connections evoked by AN. The condition of ABA is evoked in rats by limiting food access to 1 hour per day, while providing unlimited access to a running wheel. Under this condition, rats become hyperactive, voluntarily running even during the limited period of food access. This leads to a rapid weight loss and eventual death, unless the animal is removed from the ABA-inducing environment (Routtenberg and Kuznesof, 1967, Epling et al., 1983, Doerries et al., 1991, Dixon et al., 2003, Hillebrand et al., 2005a, Hillebrand et al., 2005b, Carrera et al., 2006, Makara et al., 2009, Aoki et al., 2012). ABA differs importantly from AN, in that food restriction (FR) is imposed by the experimenter. Animal models also cannot capture the psychological disturbances in body image or the individual’s fear of gaining weight. Nevertheless, this animal model has generated insight into the biological changes evoked within the body and brain following starvation that may lead to voluntary hyperactivity, one of the traits that is strongly linked to the pathogenesis, progression and relapse of AN.
Although ABA was first shown in rats, it has been observed in other rodents, including the mouse (Siegfried et al., 2003, Gelegen et al., 2006, Gelegen et al., 2007, Gelegen et al., 2008, Gelegen et al., 2010, Kas et al., 2010, Lewis and Brett, 2010, Klenotich and Dulawa, 2012). Using the mouse model of ABA, Klenotich and Dulawa (2012) demonstrated that females exhibit greater vulnerability to ABA than males, thereby demonstrating that the mouse model captures the sex-linked difference in AN vulnerability. Another trait linked to AN that is captured by the mouse model is anxiety: the DBA/2J, A/J (Gelegen, 2007; Gelegen, 2010) and Balb/cJ (Klenotich and Dulawa, 2012) strains of mice exhibit greater susceptibility to ABA as well as anxiety traits.
The availability of a wide array of genetically modified mice, in addition to the relative ease for generating new genetic modifications, make the mouse a particularly ideal species for analyzing the cellular, molecular and pathway–specific signatures associated with the development of and vulnerability to AN. However, the background used most commonly for genetic modifications, i.e., the C57BL/6 strain, has been reported to be relatively less susceptible to ABA: when put in the ABA-inducing environment of wheel access and food restriction (FR), these mice lose weight but reduce, rather than increase, their running wheel activity (Gelegen et al., 2006, Gelegen et al., 2007). Since the Gelegen studies used only adults, the possibility remained that these mice might exhibit ABA vulnerability during adolescence. Lewis and Brett (2010) used younger C57BL/6J mice but all were males and their ABA schedules evoked only modest or transient hyperactivity.
The current study sought to fill the gap in our knowledge by re-examining whether the C57BL/6J female mice might exhibit ABA vulnerability when FR is imposed closer to puberty onset, since this is the developmental stage/sex among the human population with higher AN vulnerability. The outcome of this study indicates that adolescent female C57BL/6J mice do, indeed, exhibit hyperactivity reliably following FR, but also that a second exposure to FR generates highly variable degrees of hyperactivity. This observation prompted us to conduct an ultrastructural study, testing the hypothesis that individual differences in ABA vulnerability might arise from differences in the inhibitory synaptic organization of the hippocampus.
Our reason for choosing to study the hippocampus was four-fold. First, the hippocampus has been recognized to undergo robust synaptic modifiability throughout life and especially during adolescence within the female brain (Smith and Woolley, 2004). Thus, we surmised that the hippocampus may be involved in the behavioral modification that followed the first exposure to ABA2. Second, our earlier study had shown increased expression of GABAA receptor subunits, and at the plasma membrane of CA1 pyramidal cells following just four days of ABA (Aoki et al., 2012), thereby suggesting that the GABAergic system is highly and rapidly responsive to ABA induction. Third, excitability and plasticity within the CA1 field of the adolescent female hippocampus is strongly influenced by acute and chronic stress, which in turn, affects anxiety traits (McEwen et al., 1993, Shen et al., 2007, Shen et al., 2010). Pyramidal cells of hippocampus have also been shown to undergo morphological changes following long durations of voluntary exercise (Stranahan et al., 2009), although the response of hippocampal inhibitory neurons to exercise remains unexplored. Fourth, an animal’s anxiety traits can be dampened strongly by infusing GABA receptor agonists into the hippocampus, and infusion of inverse agonists of the GABA-benzodiazepine receptors are anxiogenic (Huttunen and Myers, 1986, Kataoka et al., 1991, Talaenko, 1993). These findings point to the strong role played by GABAergic axons in the hippocampus for the regulation of anxiety. Therefore, it was reasoned that stress caused by FR and excessive voluntary activity might also prompt changes in the GABAergic input to pyramidal cells in the hippocampal CA1.
2. EXPERIMENTAL PROCEDURES
2.1 Animals
All procedures relating to the use of animals were according to the NIH Guide for the Care and Use of Laboratory Animals and also approved by the Institutional Animal Care and Use Committee of New York University (A3317-01).
All animals used in the study were bred at New York University’s animal facility. Breeding pairs were obtained from Jackson Laboratories (Bar Harbor, ME) and were of three different genotypes but all on a C57BL/6J background: CB6-Tg(Gad1-EGFP)G42Zjh/J (G42), which express green fluorescent protein (GFP) in parvalbumin-containing interneurons (Chattopadhyaya et al., 2004); FVB-Tg(GadGFP)45704Swn/J (GIN), which expresses GFP in somatostatin-containing interneurons (Chattopadhyaya et al., 2004), crossed at least 7 generations with C57BL/6J wildtype (WT) mice at NYU; and C57BL/6J wildtypes (WT).
The G42 mice have been used in 19 or more studies, indicating that parvalbumin-neurons of these animals exhibit normal dendritic branching and synaptic development (Brennaman and Maness, 2008, Guan and Maness, 2010), normal experience- and activity-dependent maturation (Chattopadhyaya et al., 2004), normal excitatory/inhibitory balance (Wallace et al., 2012), normal cognitive function and network activity (Iguchi et al., 2011, Verret et al., 2012), and normal short and long-term synaptic plasticity (Wallace et al., 2012).
The GIN mice have been used in 13 or more studies, indicating that hippocampal and cortical somatostatin-neurons of these animals exhibit normal physiological (Ma et al., 2006) and morphological (Di Cristo et al., 2004, Adesnik et al., 2008, Gibson et al., 2009) phenotypes through postnatal development (Oliva et al., 2000).
We chose to use these genetically modified mice, in preparation for future studies of in vitro whole cell recordings that analyze the physiological properties of inhibitory and excitatory synapses upon identified neurons following ABA induction.
Female mice of the colony showed no vaginal opening prior to P28 and showed vaginal opening at postnatal day (P) 30 to P32. In this study, puberty onset was considered the time of vaginal opening, and adolescence to be the subsequent ages, up to P60. Animals P60 – P70 were considered to be in the transition between adolescence and adulthood (Bath et al., 2012). Following weaning at P28 (pre-pubertal), female and male mice were separated and housed 2–4 animals per cage until P36 (adolescence), at which point they entered the ABA-inducing environment, described below. Control females of age P59 and P60 were weaned at P28, separated from male but housed 2–4 per cage until P38, from which day they were singly housed without FR and without access to the wheel until the day of euthanasia by transcardial perfusion. Additional adult females, group-housed 2–4 per cage, without FR and without wheel access, were also used for the ultrastructural study.
No difference in the basal wheel activity, FR-induced hyperactivity or body weights was detected across the three genotypes. Therefore, data presented in this report were pooled from the three genotypes. Altogether, 23 adolescent female mice underwent ABA-induction and 19 mice of ages older than P58 were used as controls.
2.2. ABA induction, recovery and second ABA induction
2.2.1. Running wheel acclimation and baseline
Starting P36, C57BL/6J mice were housed individually with unrestricted access to food via a food receptacle positioned in the corner, near the base of the cage. Water access was unlimited, via an automatic watering system. The cages were kept under a 12-hour light/dark cycle (lights on at 7 AM; lights off at 7 PM). The animals were given 24-hour access to a running wheel, which could fit comfortably within the standard mouse cage (Med Associates Low-Profile Wireless Running Wheel for Mouse Product #: ENV-044), together with enrichment paper products that they could use for nest-building. Wheel-running activity was recorded continuously using Med Associates’ “Wheel Manager” software (Product #: SOF-860). Animals’ body weight and food consumption were recorded daily, just prior to 7 PM. Running wheel acclimation occurred during the two to three days preceding FR. The daily measurements of animals body weight, food consumption and wheel running served as baseline data under the singly-housed condition, to be compared against the additional impact of FR that began on P40.
2.2.2. ABA schedule 1 (ABA1)
For the ABA1 schedule, thirteen animals were used, collected from one litter of the G42 genotype (3 females) and two litters of the GIN genotype (5 + 5 females). Food access was restricted to 1 hour per day. All food was removed at 8PM on the first day of FR. Once the ABA1 schedule began, mice were allowed free access to dry chow (PMI Mouse Diet 5001; 336 kcal per 100 g, 28.507% protein, 57.996% carbohydrates, 13.496% fat) for the first hour of the dark-cycle (7 PM to 8 PM). Wheel access continued to be unrestricted, as during the acclimation period. Body weight was recorded daily just prior to 7 PM. Food weight was measured before 7 PM and right after removal of food at 8 PM.
2.2.3. ABA2 schedule
The ABA2 schedule was tried for ten female mice, collected from three litters. One litter was of the GIN genotype (5 females), another of the G42 genotype (3 females) and the third litter was of the wildtype (WT) (2 females). Food access was restricted to 2 hours per day. These ten animals were separate from those that went on the ABA1 schedule. Food was removed at 12 PM on the first day of food restriction. Subsequently, mice were allowed free access to both dry chow (PMI Mouse Diet 5001) as well as a nutritionally complete soft food (Clear H2O DietGel® 76A; 99.8 kcal per 100 g, 4.7% protein, 17.9% carbohydrates, 1.5% fat, 73.4% moisture) for the first two hours of the dark-cycle (7 PM to 9 PM), beginning the same day that food was first removed at noon. Wheel access continued to be unrestricted, just as during the wheel acclimation period. Body weight was recorded daily, just prior to 7 PM. Food weight was measured just before 7 PM and right after food removal at 9 PM.
2.2.4. Recovery of the ABA2 group
After 72 hours of ABA2, starting at 12 PM, ad libitum food access was restored and the running wheel was removed from the cage. This corresponded to the end of the third day of FR. The ad libitum food on the first few days of recovery consisted of both the dry and wet foods. On subsequent days, just dry food was given, because the animals’ body weights restored to pre-ABA2 levels within 24 hours. Body weight and food consumption were measured over 6 – 7 days of the recovery period. At the end of the recovery period, animals were placed in a cage with a running wheel, so as to re-assess the running wheel activity in the absence of FR.
2.2.5. Second Exposure to ABA2
After 7 – 11 days of 24-hour access to food, all of the ABA2 animals were returned to the ABA2 schedule, whereby they were given food access for 2 hours, from 7 PM to 9 PM, in the presence of a running wheel. Their body weights, food consumption and wheel activity were measured as described above.
2.3. Quantitative ultrastructural analysis of contacts formed by GAD-immunoreactive axon terminals on CA1 pyramidal cells of the dorsal hippocampus
2.3.1. Animals used for electron microscopic immunocytochemistry
Five of the mice (two GIN and three G42) that received the second ABA2 were euthanized at the end of the third day of FR, at ages P59 or P60, by anesthetizing them with urethane (i.p. 0.34 g/g body weight) during the hours of 12 noon to 4 pm, then transcardially perfusing them with a buffer containing 4% paraformaldehyde. Glutaraldehyde-fixation was withheld until after immunocytochemistry, so as to optimize antigen-retention. Brains were also collected from control mice (CON) that were never FR and never given access to a running wheel. These consisted of ten female adults, group-housed throughout life, and nine C57BL/6J females of ages P59 or P60 that were singly-housed beginning on P38. Since FR adult mice are held in the diestrus phase, due to deficiency of estrogen and progesterone (Nelson et al., 1985, Riddle et al., 2013), only CON females (three group-housed and three singly-housed), verified through histology of vaginal smears (Caligioni, 2009) to be in the diestrus phase were included in this analysis.
2.3.2. The GAD immunocytochemistry procedure
Details of the GAD immunocytochemical procedure are similar to the steps described in a previous publication from this laboratory (Sarro et al., 2008).
The serum containing anti-glutamic acid decarboxylase (GAD) antibody (Lot 0607034641 of Chemicon’s catalog number AB1511; heretofore referred to as anti-GAD), was generated in a rabbit. This anti-GAD is extremely well characterized: it has been shown to recognize exactly two protein bands by Western blots, corresponding to the 65kD and 67 kD isoforms of GAD. Electron microscopy using this anti-GAD results in labeling of axon terminals forming exclusively symmetric synapses [(Sarro et al., 2008) and references therein]. The light microscopic distribution pattern is as described previously, revealing puncta throughout the neuropil and surrounding unlabeled cell bodies and also labeling of aspiny dendrites of cells spanning all layers of cortex and hippocampus (Swanson et al., 1987).
Multiple vibratome sections from each animal’s brain, prepared in the coronal plane at a thickness of 40 m and containing the dorsal hippocampus were incubated overnight, at room temperature, under constant agitation, in a buffer consisting of 0.01M phosphate buffer/0.9% sodium chloride (PBS), adjusted to pH 7.4, together with 1% bovine serum albumin (BSA, w/v, from Sigma Chem.), 0.05% sodium azide (w/v, from Sigma Chem.) and the GAD antibody at a dilution of 1:400. At the end of the incubation period, sections were rinsed in PBS over a 30 min period, then incubated for 1 hour at room temperature under constant agitation in the PBS/BSA/azide buffer containing biotinylated goat anti-rabbit IgG (Vector, Inc.) at a dilution of 1:200. At the end of this incubation period, sections were rinsed in PBS over a 30 min period, then incubated in PBS containing the avidin-biotinylated horseradish peroxidase complex (ABC, from Vector’s ABC Elite Kit) for 1 hour at room temperature, under constant agitation. These sections were then rinsed in PBS, and reacted with the peroxidase substrate, consisting of 3,3’-diaminobenzidine-hydrochloride HCl (DAB, 10 mg tablets from Sigma Chem) per 44 ml of PBS buffer and hydrogen peroxide. The peroxidase-substrate reaction was begun by adding hydrogen peroxidase (4 l of 30% hydrogen peroxide per 44 ml of DAB solution), and terminated at the end of 12 minutes by rinsing repeatedly in PBS. Vibratome sections were post-fixed using 2% glutaraldehyde in PBS.
Following this immunocytochemical procedure, the vibratome sections were processed by a conventional electron microscopic procedure, consisting of post-fixation by immersion in 1% osmium tetroxide/0.1M phosphate buffer for 1 hour, then dehydrated using graded concentrations of alcohol, up to 70%, then post-fixed overnight using 1% uranyl acetate, dissolved in 70% ethanol. On the following day, dehydration continued up to 100%, then was rinsed in acetone, and infiltrated in EPON 812 (EM Sciences), which was cured by heating the tissue at 60°C, while sandwiched between two sheets of Aclar plastic, with lead weights placed on top of the Aclar sheets, so as to ensure flatness of the EPON-embedded sections. These flat-embedded vibratome sections were re-embedded in Beem capsules (EM Sciences) filled with EPON 812, then ultrathin-sectioned at a plane tangential to the vibratome sections. The number of ultrathin sections collected from any one animal’s brain section depended on the natural curvature that the section took on, even while cured under lead weights. Usually, a minimum of ten ultrathin sections needed to be collected from each vibratome section, so as to ensure that the surface-most portions of the vibratome section, where immunoreactivity was expected to be maximal, were sampled for each anatomical layer of the CA1 of hippocampus (stratum oriens, stratum pyramidale, stratum radiatum and stratum lacunosum-moleculare). The ultrathin sections were collected onto formvar-coated, 400 mesh thin-bar nickel grids (EM Sciences). A subset of these ultrathin sections was counter-stained with Reynold’s lead citrate.
2.3.3. The procedure for quantifying the proportion of pyramidal cell plasma membranes contacted by GAD-immunoreactive terminals
Digital electron microscopic images were captured from ultrathin sections, using a 1.2 megapixel Hamamatsu CCD camera from AMT (Boston, MA) from the JEOL 1200XL electron microscope. The extent of contact formed by GAD-immunoreactive axon terminals upon pyramidal cells of the hippocampus was quantified using the segmented line tool of NIH’s software, Image J (version 1.46r), to measure plasma membrane lengths of the neuronal profiles within digital images of the ultrathin sections. The longitudinal lengths of sampled dendritic profiles were also recorded, so as to be able to assess the total lengths of dendritic tree trunks that underwent quantitative analysis. After determining the total membrane length of a profile identified to be belonging to a pyramidal neuron, the proportion of the plasma membrane contacted by GAD-immunoreactive axon terminals was determined by re-tracing those portions of the plasma membrane residing directly opposite to the HRP-DAB-labeled axon terminals. Portions of the plasma membrane associated with thick PSDs were excluded from measurements of the plasma membrane lengths, since these occurred directly opposite to GAD-negative, presumably excitatory (Peters et al., 1991) axon terminals and could not receive GAD-contacts. On the other hand, portions of spine heads lacking PSDs, including spine necks, were included in the measurement of plasma membrane lengths, since these often were target sites for GAD-immunoreactive axon terminals.
Axon terminals were identified to be GAD-immunoreactive based on the accumulation of the DAB reaction product in the cytoplasm, excluding the lumen of vesicles and mitochondria. These terminals were identified to be forming contacts, based on apposition of the axonal plasma membrane with that of the pyramidal cell body or dendrites (Peters et al., 1991). Sometimes, GAD-immunoreactive axon terminals were near the dendrites or somata of pyramidal cells but were interleaved by thin lamella of astrocytes. These GAD-terminals were not considered to be forming contacts. On the other hand, GAD-terminals residing immediately adjacent to the plasma membrane, without interleaving astrocytic processes, but lacking the parallel alignment of the two plasma membranes were considered to be forming contacts, since GABA regulates neuronal excitability through non-synaptic as well as synaptic release mechanisms (Liang et al., 2004).
Only those dendritic profiles that could be verified as arising from pyramidal cells were included in the analysis. The features used to identify dendritic profiles as those of pyramidal neurons were as follows: visualization of spine protrusions emanating from dendritic shafts; and further confirmation that GAD-immunoreactivity was absent. Somatic profiles were identified to be of pyramidal cells, and not GABAergic, based on the absence of indentations along the nuclear membranes, homogeneous distribution of chromatin within the nucleoplasm (Feldman, 1984, Schlander and Frotscher, 1986) and absence of GAD-immunoreactivity in the cytoplasm. Their identity as pyramidal cells of the CA1 (as opposed to granule cells of the dentate gyrus) was verified by their highly clustered arrangement within the neuropil, positioned immediately ventral to the white matter tract of the corpus callosum and dorsal to the series of blood vessels that coursed along the hippocampal fissure. Large dendritic profiles containing Nissl bodies and the Golgi apparatus were recognized to be the most proximal portions of dendrites that are in transition between somata and dendrites (Peters et al., 1991): the plasma membrane in these transition zones were excluded from analysis, so as to minimize ambiguity of the ‘somata’ versus ‘dendrite’ classifications. Otherwise, all of the profiles identifiable to be of dendrites and somata of pyramidal neurons were included in the analysis, strictly in the order that they were encountered along the vibratome-section surfaces, where penetration by immunoreagents would be maximal. This necessitated sampling of multiple grids, so as to ensure sampling from the surfaces of vibratome-sections for every layer of CA1 of the dorsal hippocampus.
In order to optimize detection of DAB-HRP labeling of GAD-immunoreactivity, lead citrate counterstaining of EM grids was omitted for half of the sets of analyzed grids. In spite of this precaution, some of the GAD-immuno-negative synapses appeared to be inhibitory (Peters et al., 1991), in that they lacked thick PSDs and were on non-spinous portions of dendritic and somatic plasma membranes of pyramidal cells. It is unlikely that these apparently symmetric synapses found along non-spinous portions of dendritic and somatic plasma membrane are actually profiles belonging to asymmetric excitatory synapses, because pyramidal cells receive excitatory input almost exclusively on dendritic spines (Megias et al., 2001). Nevertheless, for the purpose of this study, these symmetric synaptic junctions lacking detectable GAD-immunoreactivity were not included in the calculation of the proportion of the plasma membrane contacted by GAD-immunoreactive axon terminals. This is because our goal was to selectively quantify the contacts made by GAD-immunoreactive axon terminals, rather than the total coverage of plasma membranes by axon terminals forming symmetric synapses.
2.3.4. The sampling procedure
Sampling of the synaptic neuropil of the hippocampal CA1 was designed to optimize immunodetection, while also maintaining randomness. Four regions of interest were sampled and analyzed separately – stratum oriens (SO), stratum pyramidale (SP), stratum radiatum (SR), and stratum lacunosum-moleculare (SLM). For each region of interest, multiple ultrathin sections revealing the surface-most portions of vibratome sections for that layer were chosen. The electron microscopist sampling the ultrathin section was kept blind to the animal’s ante mortem behavioral characteristics. For each dendritic or somatic profile, the proportion contacted by GAD-immunoreactive axon terminals was determined, the sampling size of the neuronal profiles from each animal was equalized to within 10% difference, and these values were compared across the three behavioral (minimally hyperactive following exposure to the second ABA2, strongly hyperactive following exposure to the second ABA2, and controls; details of the rationale for this grouping can be found within the Results section) using statistical tests described below. The width of axon terminals contacting somata and dendrites was also quantified and compared across the three behavioral groups. Further details of the sampling parameters are described in Table 1.
Table 1.
Numerical Summary of the Sample Size for GAD Immunocytochemistry
| Behavioral Group |
Hippocampal Layer |
No. of profiles sampled per animal |
Sum of plasma membrane lengths |
Total Surveyed Area |
No. of GAD- terminals encountered |
|---|---|---|---|---|---|
| ABA2 | SO | 17 dendrites | 676,680 nm | 3,137 µm2 | 125 |
| CON | SO | 20–23 dendrites | 362,097 nm | 790 µm2 | 67 |
| ABA2 | SP | 8–9 somata | 1,180,160 nm | 10,589 µm2 | 248 |
| CON | SP | 7–9 somata | 651,077 nm | 3,544 µm2 | 232 |
| ABA2 | SR | 16–18 dendrites | 811,947 nm | 2,682 µm2 | 132 |
| CON | SR | 18–21 dendrites | 528,468 nm | 1,534 µm2 | 102 |
| ABA2 | SLM | 17–20 dendrites | 765,955 nm | 1,894 µm2 | 172 |
| CON | SLM | 19–21 dendrites | 695,942 nm | 1,195 µm2 | 71 |
2.4. Statistics
Statistical analyses used the software, Statistica (versions 6 and 10). Data were verified to be of normal distribution by using the Kolmogorov-Smirnov, the Lilliefors, and the Shapiro-Wilk’s W tests. For the data set that were not normally distributed, nonparametric tests, consisting of the Kruskal-Wallis ANOVA and median test were used to determine the level of significance of the differences in their distributions and multiple comparisons of mean ranks to compare p-values. For data that were normally distributed, comparisons of two groups used the Student’s t-test (unpaired) to determine significance of the differences. For comparison of three or more groups, one-way ANOVA was conducted, followed by the Fisher’s post hoc analysis. Differences of the distributions of the groups were accepted as significant for p-values less than 0.05.
3. RESULTS
3.1. ABA1 (1 hour of food access)
We began with a method that is standard for ABA induction in rats (Aoki et al., 2012), consisting of food access that is restricted to one hour per day, at the beginning of the dark phase of the dark-light cycle (7 PM to 8 PM), together with unrestricted access to a running wheel (ABA1). For adolescent female rats, this paradigm leads to hyperactivity within two days and a survival rate that is greater than 90% beyond the fourth day (Aoki et al., 2012).
3.1.1. Body weight decrease following ABA1
Altogether 13 female adolescent mice underwent the ABA1 schedule, three of which were G42, with GFP expressed in parvalbumin-positive interneurons and ten of which were GIN, with GFP expressed in somatostatin-positive interneurons. Except for the green fluorescence of a subset of GABAergic interneurons, these animals are physiologically and developmentally normal (further descriptions of these mice are provided under section 2.1).
Of these 13 mice, the body weight was measured consistently over the two days before and two days after FR for eight female adolescent mice. Two of these mice were of the G42 genotype and six were of the GIN genotype, acclimated to singly-housed cages with a wheel for at least two days before FR began. The average body weight of the group was 16.5 ± 0.8 g before FR began and dropped by 21% in two days to 13.0 ± 0.6 g (Figure 1A). This change was significant (p<0.001). The remaining animals that were excluded from this averaging, due to less complete record, but showed weight loss ranging from 13% to 27%
Figure 1. Body weight loss, hyperactivity and disruption of the circadian rhythm following ABA1.
The results show mean ± SEM of eight C57BL6/J adolescent mice that underwent the ABA1 schedule. Panel A: Body weight loss was dramatic and significant (p<0.05) within two days of FR (grey portion of the graph). The values represent averages of one day before (−1), the value just before FR began (0), and up to two days of FR, measured from two G42 and six GIN mice on a C57BL6/J background. Panel B: Activity, measured by the distance (in km per day) run on the wheel, increased three-fold during the two days following FR, relative to the average values measured during the two days that preceded FR. Values of this panel and Panel C represent the mean and SEM values from eight GIN mice on a C57BL6/J background. Panel C: Wheel activity is increased significantly in three out of the four 6-hour sectors of the day. The greatest increase was during the hours of 1 PM to 7 PM, the sector immediately preceding food access of 7 PM to 8 PM. The values represent the average of the two days before and the two days after FR. * p<0.01; ** p<0.001; *** p=0.001; **** p<0.00005.
3.1.2. Wheel activity increase following ABA1
Of the 13 animals that underwent ABA1, wheel activity was collected across the two days immediately preceding FR and during the first two days of FR from 8 animals (all of GIN genotype). The average activity (in km/day) increased three-fold after FR, from 6.3 ± 1.7 (mean ± SEM) to 18.0 ± 2.2. This change was significant (p<0.00005 by the unpaired t-test, t value=−9.08849; Figure 1B).
The five remaining animals (three G42 and two GIN) underwent the ABA1 schedule but were not included in the statistical analyses of the wheel activity, because their acclimation to singly-housed cage with a wheel was less than 2 days and/or because the wheel running activity was incomplete (due to malfunction of the wheel or computer). All of these five animals also exhibited increased activity during the FR period, compared to the pre-FR day.
3.1.3. Circadian rhythm, as revealed by wheel activity, following ABA1
FR has been shown to alter adult male mice’s circadian rhythm, particularly during the food anticipatory period that precedes feeding (Lewis and Brett, 2010). In order to determine whether the food anticipatory activity (FAA) (Mistlberger, 1994) of adolescent female C57BL6/J mice was increased by FR, wheel activity was analyzed for each of the four 6-hour bins of the two days preceding FR and the two days during FR. Analysis of the four, 6-hour bins per day was conducted for the eight mice for which the pre-FR baseline wheel activity was measured.
Before FR, all mice showed strong circadian cycles of activity, with very similar levels of dark-cycle activity and with the least activity occurring during the latter half of the light-cycle (1 PM – 7 PM, Figure 1C). During the first two days of FR, these animals exhibited significantly greater running wheel activity during both the dark and light cycles (Figure 1C). The greatest increase in running wheel activity occurred during the latter half of the light cycle, which previously were of the hours with the least activity (1 PM – 7 PM, Figure 1C). Notably, this 6-hr bin coincides with the period that would be characterized as FAA (Mistlberger, 1994). Specifically, the values (in km/6 hours) during the period from 1 PM to 7 PM increased from 0.2 ± 0.1 before FR to 4.5 ± 0.8 after FR (p<0.001; t-value = −5.3971).
3.1.4. Survival of animals on the ABA1 schedule
Of the five animals kept in the ABA environment for more than two days (one G42 and four GIN), only one survived to the third day (the GIN genotype). This high mortality rate would limit analysis of slower and longer-lasting changes in brain structures or changes associated with recovery from ABA. Thus, an alternative schedule, i.e., ABA2 was tried on separate cohorts, which allowed for animals to survive three full days of FR and restore their body weight afterwards.
3.2. ABA2 (2 hours of food access)
3.2.1. Food consumption of mice on the ABA2 schedule, compared to those on the ABA1 schedule
Ten C57BL6/J female adolescent mice from three litters were tested under the schedule of ABA2 (five G42, three GIN and two WT). In this paradigm, the food access period was increased to two hours, and mice were provided with the choice to consume wet or dry food. Food consumption increased over the level observed during the ABA1 schedule, although the caloric increase was not statistically significant (Figure 2).
Figure 2. Food consumption of animals undergoing ABA1 and ABA2.
Weights of food consumed per 24 hour were measured during the day before and at the end of the first day of FR, then converted to calories consumed. FR caused drastic reductions in food consumption of both ABA1 and ABA2. The food consumed during the two hours of food access for ABA2 was slightly more than during the one hour of food access for ABA1, but this increase was not statistically significant. The values represent mean ± SEM, obtained from three GIN mice for ABA1 and five G42 mice for ABA2.
3.2.2 Body weight decrease of mice on the ABA2 schedule
The mean starting body weight of the ten animals was 18.3 ± 0.5 g. During acclimation to the running wheel and single housing, their body weights decreased by a small amount, then reached a plateau at 17.7 ± 0.5 g. Within two days following FR, the average body weight decreased by 18%, to 14.4 ± 0.3 g (p<0.005, comparing the weights before FR to the weights after FR on Experimental Day 0 versus Day 3) (Figure 3A).
Figure 3. Body weight loss, hyperactivity and disruption of the circadian rhythm following the first and second exposures to the ABA2 schedule.
The results show mean ± SEM values of ten C57BL/6J adolescent mice that underwent the ABA2 schedule. Five of the mice were of G42 genotype, three of GIN genotype and two of WT, all on a C57BL/6J background. Panel A: ABA2 induced progressive body weight loss during the three days of FR (left grey zone, measured at 7 PM on Experimental days 1, 2 and 3), but the animals’ weight recovered to the pre-FR level within 24 hours of ad libitum food access and subsequently exceeded the pre-FR level. Following nine to eleven days of recovery, animals were returned to the ABA2 schedule (right grey zone, on Experimental days 1 through 4, indicated with red numbers and lines). Body weights declined but reached a plateau by the fourth day. Panel B: The wheel running activity per day increased significantly during the three days of FR of the first ABA2 schedule but not significantly during the second ABA2 schedule. The values represent the average wheel running activity across the two days before FR (“Baseline”) and the two days after FR (“ABA2”). ‘Recov’ indicates the wheel activity of five animals during the last two days of the recovery period that followed the first ABA2 schedule. “2nd ABA2” shows activity per day during the three days that the ten animals were returned to the ABA2 schedule, after their body weight had fully restored. Panel C: During both the first and second exposures to the ABA2 schedule, FR induced the greatest increase in wheel running during the latter half of the light phase, corresponding to the hours from 1 PM to 7 PM. Running increased during the other three six-hour sectors, as well. The values represent the average running across the two days before (‘Baseline” and the two days after FR (‘ABA2’). ‘Recovery’ refers to the wheel activity of the three animals during the last two days that followed recovery from ABA. ‘2nd ABA2’ represents the wheel activity during the three days of exposure to the second ABA2 schedule. Panel D: In order to determine whether wheel running was increased significantly during the hours other than the food-anticipatory period of 1 PM to 7 PM, the wheel running activity during the period of 7 PM to 1 PM of the next day was measured. This analysis revealed a statistically significant increase in non-food anticipatory activity. Panel E: Each of the ten ABA2 animals’ wheel running activity over the 24 hour period was averaged across the first two days of FR (‘ABA) and across two days of prior to FR (‘Baseline’), then plotted against each animal’s weight prior to acclimation to the wheel. This analysis indicated that the degree of increase in wheel running activity following FR did not correlate with the animal’s initial body weight. For all panels, * p≤0.05; ** p < 0.01; *** p<0.005.
3.2.3. Hyperactivity of mice on the ABA2 schedule
All ten of these mice exhibited hyperactivity after the onset of FR. The average of the ten animals’ activity (in km/day) increased significantly after FR, from 9.6 ± 1.3 km/day (mean ± SEM, with each animal’s value being the average across two days) during the days preceding FR, to 15.5 ± 1.4 km/day across the two days of FR (Fig. 3B; p<0.05 by one-way ANOVA, p <0.05, comparing first ABA2 to baseline). Eight out of the ten animals exhibited diminished activity by the third day of FR, and none perished.
3.2.4 Circadian rhythm, as measured by wheel activity, of ABA2 mice
Wheel activity was also used to measure circadian rhythms before and after ABA2 and to determine whether the hyperactivity was occurring during the FAA period of 1 PM to 7 PM, coinciding with the 6-hours that preceded feeding.
Following FR, all ten ABA2 mice exhibited increased wheel running activity throughout the day. Before FR began, mice were the least active during the latter six hours of the light-cycle (from 1PM – 7PM). During the first two days of FR, the mice were 20 times more active during this time period – increasing in the wheel running activity from 0.08 ± 0.03 km/6 hours before FR (mean ± SEM, with each animal’s value being the average across three days) to 2.0 ± 0.6 km/6 hours after FR (p<0.01 by Student’s t-test, comparing wheel activity during the hours of 1PM – 7PM before versus after FR; t-value = −3.007; df=18) (Figure 3C).
The remaining three six-hour sectors of the day also exhibited increased wheel activity (7 PM to 1 AM, 1 AM to 7 AM, 7 AM to 1 PM; Figure 3C), such that the increase in the total wheel activity across the remaining 18 hours reached statistical significance (p<0.05 by Student’s t-test; t-value = −2.13280; df=18; Figure 3D). Specifically, wheel activity during the 18 hours spanning from 7 PM to 1 PM of the next day was 9.6 m/18 hours before FR, and increased to 13.8 km/18 hours after FR.
3.2.5. Wheel activity before and after FR, relative to initial body weight
Although all animals exhibited hyperactivity following FR, the degree of hyperactivity varied among the animals. One possible source for the variability was physical maturity. All animals that entered the ABA2 schedule were visually verified to have entered puberty, based on vaginal opening, and within a narrow age range of P36 to P41, derived from three litters. Nevertheless, variability was noted, even within single litters. Since puberty onset has been shown to be correlated with body weight (Hansen et al., 1983), differences in body weight at the time of entry into the ABA2 schedule might be reflective of slight differences in maturity, even among animals of equal chronological age. With this idea in mind, we compared the initial body weight relative to the hyperactivity measured during FR (‘1st ABA2’ in graph, Fig. 3E) and during the days preceding FR (‘baseline’ in Fig. 3E). These analyses revealed no significant correlation between the two parameters (p=0.57 for regression analysis comparing body weight to activity). These results indicate that the initial body weight was not a strong predictor of ABA vulnerability, measured as the degree of hyperactivity evoked by FR.
3.2.6. Weight restoration and the return of the circadian rhythm during the ABA2 recovery period
All ten animals in the ABA2 schedule survived three full days of FR. This ABA schedule was followed by six to seven days of recovery, during which food access was ad libitum all day and the wheel was removed. Animals remained individually housed.
All animals recovered at least 95% of their pre-ABA baseline body weight within the first day in the recovery environment, and all exceeded their starting weight within three days of recovery and reached a plateau of 20.7 g (Experimental Day 6 in Figure 3A).
After 6 – 7 days of recovery from ABA2, the running wheel was replaced within the cage of a subset of animals. Measurement of five animals’ wheel activity over a two-day period following recovery revealed that the activity had returned to the low levels seen before FR (p=0.95 by Fisher’s post hoc analysis, comparing the values before FR to the Recovery values) (‘Baseline’ and ‘Recov’ values in Figure 3B). Analysis of their wheel running activity during each of the 6-hour bins indicated that the nocturnal activity as well as the diurnal inactivity were also restored completely (p=0.90 by Fisher’s post hoc analysis, comparing the Recovery value of the ‘1 PM – 7 PM’ sector to the baseline value before FR; Figure 3C).
3.3. Individual responses to ABA2
3.3.1. Variability in the wheel activity during the first ABA2
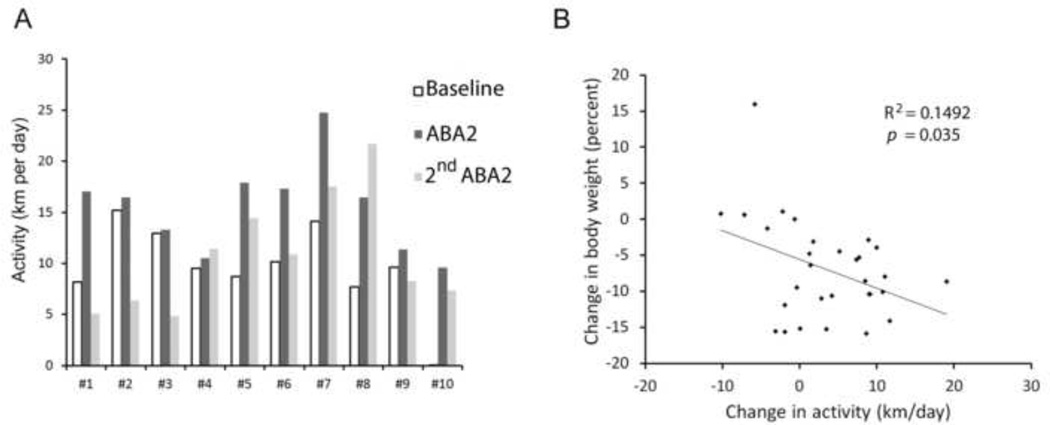
While all animals exhibited hyperactivity following FR, their degree of hyperactivity was variable, as was noted above. About half of the animals exhibited only a minimal increase in activity following the first FR [less than 1.7 km per day increase, Animals #2 (G42), #3 (G42), #4 (G42), #9 (WT); compare the white (Baseline) to dark grey (ABA2) bars in the Figure 4A], while the remaining half of the animals exhibited an increase in activity by at least 7 km per day [Animals #1(G42), #5 (G42), #6 (GIN), #7 (GIN), #8 (GIN), #10 (WT)].
Figure 4. Individual differences in vulnerability to ABA2.
Panel A. Data from the same ten animals, shown in Figure 3, are presented here to depict individual differences. Animals #1 through #5 were littermates of a G42 genotype, animals #6 through #8 were littermates of the GIN genotype and animals #9 and #10 were WT littermates. All were on a C57BL/6J background. Animals varied in their baseline wheel activity but all exhibited increased activity in response to the first ABA2. The degree of change in activity fell into two groups, with some exhibiting an increase that was greater than 7 km per day, and the remaining animals exhibiting an increase that was modest (less than 2 km per day). Differences in their responses were observed within single litters. The animals’ responses to the second ABA2 schedule were variable, with most animals exhibiting less hyperactivity than after the first ABA2, four of them less activity than of its baseline level, and Animal 8 being the only one to exhibit more hyperactivity following the second exposure to ABA2. Panel B. Mice that underwent the ABA2 schedule exhibited a range of hyperactivity responses. For all ten animals, the change in body weight, relative to the previous day’s weight was compared against the change in wheel running activity, relative to the level of activity on the day before FR. This analysis was run for data collected during the first exposure to ABA2. Thus, three data points are represented for each of ten animals, representing the changes in body weight and activity across the three days of exposure to the first FR. R2 and p-values were determined, based on a multiple regression analysis. This analysis revealed that increased wheel running activity correlates with a reduction of body weight on that day.
3.3.2. Variability in weight loss during the first ABA2
The degree of weight loss and hyperactivity also varied within the three days of FR, with most animals exhibiting reduction of activity by the third FR day. Analysis of their daily body weight loss and daily activity change within each of the three days of FR indicated that the two parameters are correlated. Specifically, on the days that animals lost more body weight, they had become more hyperactive. Conversely, on the days that animals were less active or unchanged in activity were the same days in which their body weights were maintained (Figure 4B; p<0.05; R=0.3862). This correlation indicated that losses of body weight are ascribable to increases in activity. On the other hand, the body weight loss was not correlated to the following day’s wheel activity (p=0.533; R=0.118), indicating that hyperactivity on any particular day was less directly related to physiological signals arising from negative energy levels on the previous day.
3.3.3. Variability in hyperactivity following the second ABA2
Following the weight and circadian rhythm restoration, animals were subjected to a second ABA2 exposure. The second ABA2 schedule was exactly as described for the first ABA, with food access limited to the two hours of 7 PM to 9 PM over a four-day period. Analysis of the animals during the second exposure to the ABA schedule revealed additional variability among the animals’ ABA vulnerability.
A subset of animals exhibited resistance to the second ABA exposure, in that their activity levels after FR were less than the levels measured during the initial acclimation to the wheel [comparing the white bars indicating ‘Baseline’ activity to the lighter grey bars, - second ABA2; in Figure 4A, Animals #1(G42), #2(G42), #3 (G42), #9(WT)]. Five of the ten animals exhibited the hyperactivity again, following FR, but to varying degrees [Animals #4(G42), #5(G42), #7(GIN), #8(GIN), #10(WT)]. Among those that became hyperactive to the second ABA2, Animal #8 responded with even greater degree to the second ABA2 schedule (+9 km/day to the first ABA2; +14 km/day for the second ABA2). For the other four animals that exhibited hyperactivity, the response to the second ABA2 was dampened relative to their responses to the first ABA2 (Figure 4A). Animal #4(G42) was unique among the ten, in that she responded to FR with minimal hyperactivity during both the first and second ABA2 exposures (+1.0 km/day to the first ABA2; +1.9 km/day to the second ABA). The mean response of all ten animals to the second ABA2 indicated no significant difference relative to their baseline activity (10.8 ± 1.8 km/day following the second ABA; 9.6 ± 1.3 km/day baseline activity; p = 0.61) and significantly less than was seen during the first ABA2 (p < 0.05) (Figure 3B).
3.4. GAD-immunoreactive axon terminals contacting hippocampal pyramidal neurons of animals following the second ABA2
What might be the cellular basis for the variability in the animals’ response to the second FR? A previous study had pointed to GABAA receptors expressed by pyramidal neurons in the dorsal hippocampal CA1 as one system and structure altered by ABA (Aoki et al., 2012). This observation led us to ask whether interanimal differences in the behavioral responses to the second FR might originate from differences in the excitability of hippocampal neurons. The excitability of hippocampal neurons is controlled by the balance of their excitatory and inhibitory inputs. In order to assess the extent of inhibitory inputs, we quantified the proportion of the plasma membrane of CA1 pyramidal cells contacted by axons containing GAD (glutamic acid decarboxylase, the rate-limiting enzyme for the synthesis of GABA). This ultrastructural analysis was conducted for five of the ABA2 animals that underwent three days of exposure to a second ABA2, in addition to adult and P59/60 control female mice that were never exposed to FR or the wheel.
Prior to the ultrastructural analysis, the five ABA2 animals were divided into two groups: those that exhibited strong hyperactivity to the second ABA (Animals #5, #7 and #8, showing a change in wheel running activity, relative to the pre-FR baselines of +5.7, +3.4 and +14 km/day, respectively) and those that did not respond strongly (Animals #1 and #4, showing changes of −3.1 and +1.9 km/day, respectively). For each animal, the degree of innervation by GAD-immunoreactive axon terminals was analyzed separately for stratum oriens (SO), stratum pyramidale (SP), stratum radiatum (SR) and stratum lacunosum-moleculare (SLM). Images used for the quantification were captured and analyzed, while the experimenter was kept blind to their degree of ante mortem hyperactivity.
3.4.1. Non-quantitative observations
Perikaryal plasma membranes and smooth portions of dendrites were preferential sites for contact by GAD-immunoreactive axon terminals, but the base of spine necks were also targeted by these terminals (Fig. 5). Moreover, for all groups of animals (strongly hyper, minimally hyper, and controls), some of the axon terminals appeared to be forming inhibitory synapses, based on their site of contact on smooth, non-spinous portions of dendritic shafts lacking thick PSDs, yet were GAD-immuno-negative, even at surface-most portions of vibratome sections and in the vicinity of other intensely GAD-immunoreactive axon terminals (double arrows in Fig. 5). Portions of the plasma membranes were also juxtaposed by astrocytes (asterisks in Fig. 5).
Figure 5. Electron microscopic identification of GAD-immunoreactive axon terminals contacting pyramidal cells following the second ABA2.
Electron micrographs were taken from CON animals in Panels A, C and D and from strongly hyperactive animals in Panels B, D and F. In all panels, arrows point to portions of the plasma membrane contacted by GAD-immunoreactive axon terminals, while the double arrows point to portions of the plasma membrane contacted by GAD-immuno-negative axon terminals. Asterisks indicate portions of the plasma membrane juxtaposed to glia. Examples of GAD-immunoreactive axon terminals contacting the perikaryal plasma membrane of SP are shown in panels A and B, terminals contacting apical dendrites (d) of SR are shown in panels C and D and terminals contacting distal spiny dendrites in SLM are shown in panels E and F. ‘s’ indicates the surfaces of vibratome sections that are included in the micrographs to demonstrate proximity of the surface to the analyzed portions of the neuropil. Calibration bars = 500 nm in all panels.
3.4.2. GAD-immunoreactive axon terminals contacting dendrites of CA1 pyramidal neurons in SR
Plasma membranes of apical dendrites in SR of strongly hyperactive animals (Animals #5, #7 and #8) were often contacted by the small caliber axon terminals (e.g., compare Fig. 5C versus Fig. 5D). The frequency of encounter with dendritic profiles lacking any GAD-immunoreactive terminal apposition was only 1 out of 34 for brains of the minimally hyperactive animals but was 14 out of 52 for the strongly hyperactive animals. Direct measurement of the plasma membrane lengths revealed that the proportion contacted by GAD-immunoreactive axon terminals was 17 ± 7% (mean ± SEM) for Animal #4, the one that exhibited only a small increase in response to the first and second FR. This value was similar to that of Animal #1 (16 ± 2 %), which exhibited no hyperactivity to the second ABA2 (−3.1 km/day), even though she had exhibited strong hyperactivity following the first ABA2 (Figure 4A).
The extent of GAD-immunoreactive axonal profiles’ innervation was less for the three strongly hyperactive animals. Specifically, the values were 9 ± 2% for Animal #5 and 7 ± 2 % for Animals #7 and #8. Finally, the values for the controls were in between the values observed for the strongly and the minimally hyperactive animals: 10 ± 3%, 12 ± 3% and 12 ± 2%.
Comparisons of the three pooled behavioral groups indicated a highly significant difference (p<0.0005, H(2,N=143)=15.65672 by the Kruskal-Wallis ANOVA and median test). Pair-wise multiple comparisons revealed a significant difference between the minimally hyper versus strongly hyper (p<0.0005) and control (p<0.05) groups. In contrast, there was no difference between the strongly hyper and control groups (p=0.52) (Figure 6B).
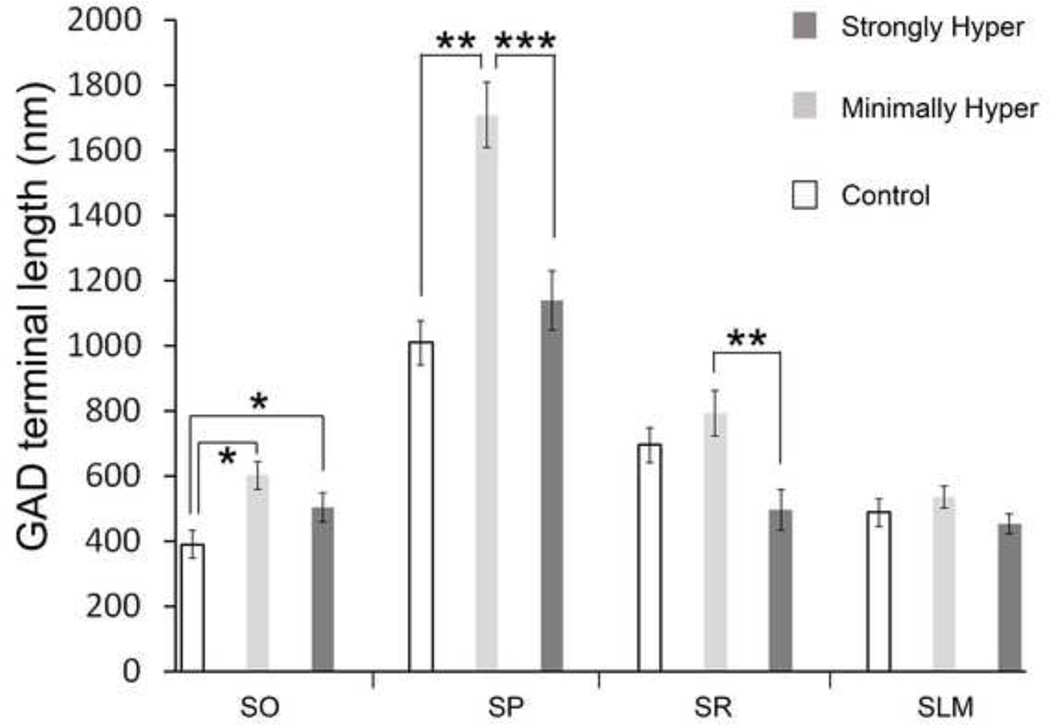
Figure 6. Quantification of the proportion of plasma membrane contacted by GAD-immunoreactive axon terminals.
All graphs represent mean ± SEM values. Five of the animals that underwent the second ABA2 were assigned to two groups – the minimally hyperactive (“hyper”) and strongly hyper. Panel A shows the percent of the dendritic plasma membrane in stratum radiatum (SR) contacted by GAD-immunoreactive axon terminals. Numbers along the x-axis indicate the values for the animals whose ante mortem behavior were shown in Figure 5. Animals A, B and C were controls, first two of which were group-housed and a third that was singly housed starting P38 but otherwise unexposed to FR or to the wheel. Values from brains of the two minimally hyperactive animals were not significantly different from one another, values of the three animals that became strongly hyperactive were not significantly different from one another, and values of the three controls were also not significantly different from one another. Panel B shows the proportion of the plasma membrane contacted by GAD-immunoreactive axon terminals across four layers of the CA1 of dorsal hippocampus – SO (stratum oriens), SP (stratum pyramidale), SR (stratum radiatum) and SLM (stratum lacunosum-moleculare). Comparisons of the dendritic and somatic profiles revealed that those from the minimally hyperactive animals were more extensively covered by GAD-immunoreactive axon terminals than were the profiles from brains of the strongly hyperactive animals. The difference across the two behavioral groups was the greatest in SR (p<0.0005), also evident in SP and SLM (p<0.05) and minimally different but still reached a statistically significant difference in SO (p<0.05). The values of the minimally hyperactive animals were significantly different from the corresponding values of the controls in SO, SR and SLM.
Analysis of the lengths of contacts formed by GAD-immunoreactive axon terminal profiles upon dendritic profiles in SR indicated that this difference across the behavioral groups is due, at least in part, to differences in the size of the axonal contacts (Figure 7) (p<0.01 by the Kruskal-Wallis ANOVA and median test, H(2,N=234)=9.525203), with p<0.001 comparing terminals of the minimally hyper versus strongly hyper groups and p=0.54 comparing CON to the minimally hyper groups). Specifically, the mean widths of GAD-contacts onto the dendrites were reduced for the strongly hyperactive group (497 ± 63 nm), relative to the contact sizes for the minimally hyperactive group (792 ± 72 nm), with the corresponding values for the controls being intermediate (695 ± 54 nm).
Figure 7. GAD terminal lengths at sites of contact with the plasma membrane of pyramidal neurons.
The same profiles analyzed for Figure 6 were re-analyzed to determine the mean widths of GAD-terminals forming contact with the plasma membrane of pyramidal cells in the layers SO, SP, SR and SLM. All graphs represent mean ± SEM values. Statistically significant enlargement of GAD-terminal contacts within the CA1 of the minimally hyper, relative to the strongly hyper tissue, was detected in SP and SR. The minimally hyper group exhibited significant enlargement, relative to the controls, in SO and SP.
3.4.3. GAD-immunoreactive axon terminals contacting cell bodies of pyramidal neurons in SP
The difference in the contact pattern by GAD-immunoreactive axon terminals in SR across the three behavioral groups prompted us to extend our analysis to other layers of the hippocampus. The cell body profiles of pyramidal neurons were much more extensively contacted by GAD-terminals than were the dendritic profiles in SR (Figure 6B). The percent values were 40 ± 3% for the minimally hyperactive group, 27 ± 3% for the strongly hyperactive group, and 36 ± 2% for the control group. Comparisons of the three behavioral groups revealed significantly less contact formed upon cell bodies from animals exhibiting strong hyperactivity (p<0.05 by one-way ANOVA, p<0.005 comparing the strongly hyperactive versus minimally hyperactive group and p<0.05, comparing the strongly hyperactive versus CON groups by post hoc LSD test) (“SP” of Figure 7B).
The mean widths of the axon terminals contacting somata of the minimally hyperactive animals was 1709 ± 100 nm, while the corresponding values were 1140 ± 90 nm for the strongly hyperactive animals and 1009 ± 69 nm for the controls (SP in Figure 7). The apparent increase in the widths of axon terminals within the minimally hyperactive group was statistically significant (p<0.00001, Kruskal-Wallis ANOVA and median test H(2,N=480)=23.50437; p<0.005 comparing the minimally hyperactive versus the strongly hyperactive groups; p<0.000005 comparing the minimally hyperactive versus the controls), contrasted by the lack of difference in the terminal sizes between the strongly hyperactive versus control groups (p=0.79). These differences in GAD-terminal widths at sites of contact indicate that the change in sizes of the GAD-immunoreactive axon terminals is likely to have contributed significantly to the differences in the proportion of the plasma membrane receiving GAD-immunoreactive axonal contact.
3.4.4. GAD-immunoreactive axon terminals contacting pyramidal neurons in SLM
In SLM, the protrusion of spines from dendritic shafts was used to positively identify those dendrites belonging to pyramidal cells. Portions of ultrathin sections capturing the surface-most portions of vibratome sections and spanning within 25 m from the blood vessels of the hippocampal fissure were analyzed to ensure that sampling was from SLM. The mean length of GAD-immunoreactive terminals in this layer was 535 ± 33 nm for the minimally hyperactive, 454 ± 30 nm for the strongly hyperactive and 480 ± 35 nm for the controls. These values were not statistically significantly different across the three behavioral groups (p=0.19 by the Kruskal-Wallis test: H(2,N=222)=3.322152; p=1.00 comparing controls to the strongly hyperactive group; p=0.34, comparing the minimally hyperactive versus the control; and p=0.31, comparing the minimally hyperactive to the strongly hyperactive) (Figure 7). Nevertheless, the percentage of the plasma membrane contacted by GAD-immunoreactive terminals was much higher for the tissue from the minimally hyperactive group (18 ± 1%), relative to the control (9 ± 1%) and strongly hyperactive groups (10 ± 1%) (p<0.0001 by the Kruskal-Wallis test H(2,N=157)=21.95593; p<0.00005 comparing the minimally hyperactive group, relative to the control and p<0.005, comparing the two ABA2 groups), while the corresponding values were indistinguishable between the strongly hyperactive and the control groups (p=0.80). Accordingly, as many as 17 out of 62 dendritic profiles of the controls and 10 out of the 54 dendritic profiles of the strongly hyperactive group exhibited no contact by GAD-terminals, while the corresponding value for the minimally hyperactive group was only 4 out of 41.
3.4.5. GAD-immunoreactive axon terminals contacting pyramidal neurons in SO
GAD-immunoreactive axon terminals contacted spiny dendrites of pyramidal neurons in SO as well. However, 17 out of 51 dendritic profiles from the strongly hyperactive animals lacked contact by GAD-immunoreactive axon terminals, while only 4 out of 34 dendritic profiles from the minimally hyperactive animals lacked contacts by GAD-immunoreactive axon terminals. This is similar to the pattern observed for the dendrites in SR, and quantitative analysis indicated that the extent of their innervation aligned with their behavioral responsiveness to the second ABA2. Specifically, the proportion of the dendritic plasma membranes contacted by GAD-immunoreactive axon terminals was 12 ± 2% for the minimally hyperactive group, 8 ± 2% for the strongly hyperactive group, and 7 ± 1% for the controls. The increased innervation by the minimally hyperactive group was statistically significant (p<0.01 by the Kruskal-Wallis test H(2,N=149)=9.291965; p<0.05 comparing the minimally hyper group to the strongly hyper group and controls; p=1.000 comparing the strongly hyper group to the controls).
Analysis of the lengths of contacts formed by GAD-immunoreactive axon terminals onto spiny dendrites in SO revealed an increase by both the minimally and strongly hyperactive group, relative to the control group (mean contact lengths 602 ± 47 nm and 503 ± 49 nm, respectively, for the minimally and strongly hyperactive groups and 390 ± 42 nm for the controls; p<0.0005 comparing control to the minimally hyperactive group; p<0.05 comparing the strongly hyperactive group to controls; and p=0.48, comparing the two groups that underwent ABA2). However, this increase in GAD-contact size by the strongly hyperactive group did not translate into increased proportion of the dendritic plasma membrane being contacted by GAD-immunoreactive terminals.
Thus, analyses of contacts formed by GAD-immunoreactive axon terminals in the hippocampal CA1 indicate that the suppression of FR-induced hyperactivity aligns well with increases in the extent of GAD-immunoreactive axon terminals’ contact at proximal and distal portions of the pyramidal neurons.
4. DISCUSSION
All 23 C57BL/6J adolescent female mice that we tested exhibited robust signatures of ABA, the animal model of AN. The salient features captured across the G42, GIN and WT genotypes of this mouse strain were as follows: (1) disruption of the circadian rhythm and hyperactivity throughout the day; (2) emergence of food anticipatory activity (FAA) during the hours preceding the restricted hours of feeding; (3) paradoxical, voluntary running, even during the limited hours of food access; (4) restoration of normal body weight and circadian rhythm, as soon as the animals were returned to ad libitum food access; (5) resistance to a second ABA that was seen in some animals and which were associated with greater contact of the CA1 pyramidal neurons by GAD-immunoreactive axon terminals.
4.1 Suitability of the animal model of AN for C57BL/6J mice
4.1.1. Comparisons with previous reports on mouse ABA using the C57BL/6J strain
Family and twin studies have suggested that genetic factors contribute to AN vulnerability (Bulik et al., 2007). The ability to induce ABA in adolescent female mice with genetic modifications, most of which are on the C57BL/6J background, will be useful for future studies that examine the genetic and biological factors contributing towards vulnerability to AN and to the progression of the disorder.
Previous studies reported that the C57BL/6J mouse strain is less susceptible to the ABA schedule, in that they lose weight but do not become hyperactive (Gelegen et al., 2006, Gelegen et al., 2007). One reason for the difference in our conclusions may be the manner in which the data were interpreted. While the Gelegen group focused on behavioral changes evoked only during the dark phase to conclude that these animals lacked FR-evoked hyperactivity (Gelegen et al., 2008), we calculated hyperactivity across the entire 24-hour period. Our data are in agreement with their previous report, namely that the greatest change in activity is during the food-anticipatory period, which happens during the light phase (Lewis and Brett, 2010). However, our cohort of animals also exhibited FR-evoked hyperactivity during the remaining, non-food anticipatory hours that included the dark phase. The increase during non-food anticipatory hours was significant for the ABA2 group and was even more dramatic for the ABA1 group, latter of which is an ABA schedule that Lewis and Gelegen’s groups did not test.
The difference in hyperactivity across the labs may also arise from differences in the ages of the animals. Previous studies examined ABA susceptibility in C57BL/6J mice that were 3 – 5 months old adults at the start of the experiment. In contrast, our animals were all adolescent, acclimated to the running wheel starting at an age of postnatal day P36 to P41 and put on the FR schedule within a few days later. Animals exposed to the second ABA were P51, still much younger than those tested in earlier studies. One possible explanation for the difference in ABA susceptibility observed across the studies is the difference in the animals’ weights at the time that FR began, rather than developmental stages. However, a regression analysis conducted among the mice of the present study indicated that the range of variations observed for the starting body weight (15.68 to 20.15 g) was not a factor contributing to the degree of hyperactivity of these adolescent mice (R=0.24; p=0.57). Therefore, we favor the interpretation that ABA vulnerability is linked to immaturity of the brain of adolescent mice, relative to that of adult mice. What factors differ between brains of adolescents and adults and contribute towards ABA vulnerability is an important topic that we hope to address in the future.
4.1.2. Comparisons of ABA1 to ABA2
Comparisons of the outcome of ABA1 and ABA2 revealed that both schedules elicited strong hyperactivity. Although the maximal hyperactivity attained was comparable (18 km/day for ABA1 and 15.5 km/day for ABA2), ABA1 led to a much higher mortality rate (4 out of 5 for ABA1, 0 out of 10 for ABA2). This difference in lethality makes ABA2 the more preferable schedule to use for research aiming to understand alterations in brain circuits that underlie recovery from ABA and increased vulnerability to ABA relapse. For studies that aim to compare the efficacy of pharmacological treatments to ameliorate hyperactivity and the ‘voluntary’ FR, ABA2 may, again, be more preferable than ABA1, because ABA2 provides a wider temporal window for drug actions.
4.1.3 Comparison of the rat and mouse ABA models
When compared to the rat ABA model, the mouse model provides greater opportunity for using genetic modifications with well-documented behavioral phenotypes, including anxiety traits. The one disadvantage of using mice is that this species is generally more active than rats, thereby making the detection of activity changes more difficult. In particular, we noted that FR without a running wheel does not serve as a good control for separating the effect of FR from that of increased exercise. This is because FR mice without wheels ‘found’ other ways to become hyperactive – such as to jump and climb up and down immobilized wheels and walls repeatedly. In contrast, rats that undergo FR without a wheel are not visibly hyperactive (unpublished observations).
4.2. Comparison of body weight changes to hyperactivity
We observed a correlation between changes in body weight on particular days and activity during the 24 hours that preceded body weight measurement (Figure 4). This indicates that the loss of body weight results from hyperactivity. In contrast, changes in body weight of any one day did not correlate with hyperactivity on the following day. This was contrary to our expectation, since we, and others, had hypothesized that hyperactivity is related to an adaptive foraging behavior which, in turn, is evoked by starvation (Spatz and Jones, 1971) or for counteracting the decrease in body temperature (Gutierrez et al., 2006). It appears that the negative energy balance has a bimodal effect, with it initially stimulating foraging behavior and wheel running but eventually suppressing activity. Earlier work on mouse ABA reported that those strains noted for their anxiety traits remain hyperactive, in face of increasingly negative energy levels (Gelegen et al., 2007, Gelegen et al., 2010). The tipping point for hyper/hypoactivity may interact with yet unknown genetically based factors that affect anxiety traits. The present study indicated that C57BL/6J mice exhibit large differences in FR-induced hyperactivity, even within a litter. Perhaps environmental factors contribute towards differences in anxiety traits that, together with age and sex, lead to differences in FR-induced hyperactivity and vulnerability to ABA.
4.3 Differences in the hyperactivity responses and the extent of contacts upon CA1 pyramidal neuron by GAD-immunoreactive axons
Hyperactivity was elicited in all mice following the first exposure to FR but only to half of the mice following the second exposure to FR. The uniformity in their response to the first FR suggests that FR-evoked hyperactivity may rely on a built-in mechanism. Within the confines of the experimental setting, the reduction of hyperactivity following the second ABA2 was more adaptive and may reflect learning from the first ABA2 exposure. If so, then the varying degrees of hyperactivity during the second ABA2 may reflect varying degrees of learning, with those suppressing FR-evoked activity being the good learners that attained resilience to ABA. If this working model is correct, then the cellular changes associated with learning would be expected to be found within brains of animals with minimally hyperactive behavior, and this change would be detectable as a difference from the controls and from those that remained hyperactive during the second ABA2.
4.3.1. Functional implications of the differences in GAD-immunoreactivity across the two behavioral groups
Resistance to the second exposure of ABA2, measured by the absence of hyperactivity following FR, aligned strongly with the extent of GAD-immunoreactive axon terminal contacts formed upon pyramidal neurons of the dorsal CA1. Our findings support the idea that the enhanced GABAergic inhibition in the dorsal hippocampus contributes to the animals’ reduced levels of hyperactivity following the second ABA2. The anatomical results suggest that the differences in behavior between the minimally and strongly hyperactive animals reflect both a gain of function by the minimally hyperactive group as well as a loss of function by the strongly hyperactive group. Anatomical findings supporting this view are summarized below.
In stratum pyramidale, GAD-terminals originate from the parvalbumin- and CCK-containing fast-spiking interneurons (Freund and Buzsaki, 1996, Maccaferri et al., 2000). In this layer, the mean width of GAD terminals was significantly greater for the minimally hyperactive animals than for the control and the strongly hyperactive animals. This pattern suggests that the minimally hyperactive animals may have responded to the first ABA2 exposure by enlarging their GAD-immunoreactive axon terminals (Figure 8). This anatomical change could reduce the firing rate of pyramidal neurons powerfully and also alter the synaptic properties and calcium signals at distal portions of the dendrites that are triggered by back-propagation of action potentials (Freund and Buzsaki, 1996, Magee et al., 1998, Frick et al., 2004, Spruston, 2008).
Figure 8. Figure Legend for the Graphic Art Abstract.
Food restriction (FR) evokes hyperactivity in some but not all of the adolescent female mice. Comparisons with control hippocampal tissue indicate that enlargement of GABAergic terminals onto the cell bodies of pyramidal neurons, together with the increased input throughout the dendritic tree, may contribute towards suppression of FR-evoked hyperactivity and resilience to food restriction. Conversely, the reduction of GABAergic inputs in SR may contribute towards the persistence of FR-evoked hyperactivity and severe weight loss. These findings indicate that the adolescent female mice can serve well as an animal model of anorexia nervosa (AN) and for exploring the cellular basis for the individual differences in vulnerability to AN.
In stratum lacunosum-moleculare, where GABAergic innervation arise from the somatostatin-, NPY and calbindin D-positive variety of GABAergic interneurons (Freund and Buzsaki, 1996, Maccaferri et al., 2000), there was no detectable difference in GAD terminal sizes across the three behavioral groups. However, the extent of coverage of spiny dendrites by GAD-terminals was greater for the minimally hyperactive animals than for the strongly hyperactive animals or the controls. This pattern suggests that the minimally hyperactive animals responded to the first ABA2 experience by increasing the number of boutons contacting dendrites in this layer (Figure 8).
The possible loss of function within the CA1 of the strongly hyperactive animals is suggested by decrease in the proportion of perikaryal membranes contacted by GAD-terminals, relative to CA1 of controls as well as the minimally hyperactive animals (Figure 8). Although HRP-DAB is the most sensitive immunolabel for detecting the presence of an antigen, the enzymatic signal amplification makes the label less suitable for quantifying an antigen’s local concentration, such as of GAD in the axonal cytoplasm. Nevertheless, the juxtaposition of intensely immunolabeled and unlabeled axon terminals, even within portions of the ultrathin sections that captured the portions of vibratome sections closest to the surface, suggests that some axon terminals of the inhibitory interneurons contain lower levels of GAD. Some of the GABAergic axon terminals of the strongly hyperactive animals may have undergone reductions of GAD levels and/or failed to maintain synaptic junctions.
In support of the idea that GABAergic inhibition may be strengthened within brains of animals with resilience to ABA, we have observed that, in rats, the membranous expression of the GABAA receptor subunits and in CA1 pyramidal cells increases by the 4th day of ABA (Aoki et al. 2012). Moreover, rats with behaviors indicative of ABA-resistance exhibit significantly higher levels of GABA receptor subunits in the cytoplasm by the 2nd day of FR (Aoki et al., unpublished observations). This, together with current observations suggest that pharmacological treatments aimed to boost inhibition of CA1 pyramidal cells may dampen the stress-induced anxiety evoked by FR, thereby dampening the wheel activity that exacerbates the negative energy state and progression of AN.
5. CONCLUSIONS
In conclusion, our results, combined with those of previous works, show that the C57BL/6J mice can be used as an animal model to examine environmental, genetic, sex and age-dependent influences upon ABA vulnerability. We also show that the C57BL/6J mice can be used to identify ABA-evoked changes in brain circuits that may underlie individual differences in vulnerability to AN relapse. Results from the first system studied so far - GABAergic axons in the dorsal CA1 – indicate that FR-evoked hyperactivity may be linked to low levels GABAergic inhibition and that drugs boosting this system could help to dampen the severity of weight loss associated with over-exercise. The C57BL/6J strain of mice can be used to study many other brain regions that are likely to undergo change, including the amygdala, which has been reported to be hyperactive among patients affected by AN (Joos et al., 2011) and within brains of mice exhibiting anxiety traits (Tasan et al., 2011). Other brain regions suspected to undergo change include the bed nucleus of stria terminalis (Waddell et al., 2008, Bangasser and Shors, 2010) and the hypothalamic paraventricular nucleus (Kinzig and Hargrave, 2010), which, together with the hippocampus (Shen et al., 2010) and the amygdala (Tasan et al., 2011), are structures known to mediate stress-dependent modulation of learning and are well worth the attention in future studies.
HIGHLIGHTS.
Designed an animal model of anorexia nervosa, ABA, that suits C57BL/6 female mice.
Food restriction (FR) elicits hyperactivity reliably in adolescent female C57BL/6s.
ABA vulnerability can be quantified by measuring hyperactivity following FR.
Hippocampal CA1 pyramidal cells of ABA-resilient mice receive enhanced GAD contacts.
ACKNOWLEDGEMENT
This study was supported by the following grants: The NEI Core grant EY13079; NYU’s Research Challenge Fund; The Klarman Family Foundation. The analysis of post mortem brain tissue was supported by these grants, in addition to the NIH grant R21MH091445-01. We thank Dr. Mark Klinger, DVM, DACLAM, Director of the Office of Veterinary Resources (OVR) and Andrea M. Grappone for their advice in developing and executing the ABA protocols.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5597–5602. doi: 10.1073/pnas.0800946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Sabaliauskas N, Chowdhury T, Min JY, Colacino AR, Laurino K, Barbarich-Marsteller NC. Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA(A) receptor alpha4 and delta subunits at the plasma membrane of hippocampal CA1 spines. Synapse. 2012;66:391–407. doi: 10.1002/syn.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. Critical brain circuits at the intersection between stress and learning. Neuroscience and biobehavioral reviews. 2010;34:1223–1233. doi: 10.1016/j.neubiorev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, Lee FS. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biological psychiatry. 2012;72:499–504. doi: 10.1016/j.biopsych.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham CL, Su J, Hlynsky JA, Goldner EM, Gao M. The mortality rate from anorexia nervosa. The International journal of eating disorders. 2005;38:143–146. doi: 10.1002/eat.20164. [DOI] [PubMed] [Google Scholar]
- Brennaman LH, Maness PF. Developmental regulation of GABAergic interneuron branching and synaptic development in the prefrontal cortex by soluble neural cell adhesion molecule. Molecular and cellular neurosciences. 2008;37:781–793. doi: 10.1016/j.mcn.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Slof-Op't Landt MC, van Furth EF, Sullivan PF. The genetics of anorexia nervosa. Annu Rev Nutr. 2007;27:263–275. doi: 10.1146/annurev.nutr.27.061406.093713. [DOI] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] Appendix. 2009;4 doi: 10.1002/0471142301.nsa04is48. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera O, Gutierrez E, Boakes RA. Early handling reduces vulnerability of rats to activity-based anorexia. Developmental psychobiology. 2006;48:520–527. doi: 10.1002/dev.20175. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. The Journal of neuroscience. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Katzman DK, Kaptein S, Kirsh C, Brewer H, Kalmbach K, Olmsted MP, Woodside DB, Kaplan AS. The prevalence of high-level exercise in the eating disorders: etiological implications. Comprehensive psychiatry. 1997;38:321–326. doi: 10.1016/s0010-440x(97)90927-5. [DOI] [PubMed] [Google Scholar]
- Davis C, Katzman DK, Kirsh C. Compulsive physical activity in adolescents with anorexia nervosa: a psychobehavioral spiral of pathology. J Nerv Ment Dis. 1999;187:336–342. doi: 10.1097/00005053-199906000-00002. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang ZJ. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nature neuroscience. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Dixon DP, Ackert AM, Eckel LA. Development of, and recovery from, activity-based anorexia in female rats. Physiology & behavior. 2003;80:273–279. doi: 10.1016/j.physbeh.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Doerries LE, Stanley EZ, Aravich PF. Activity-based anorexia: relationship to gender and activity-stress ulcers. Physiology & behavior. 1991;50:945–949. doi: 10.1016/0031-9384(91)90419-o. [DOI] [PubMed] [Google Scholar]
- Epling WF, Pierce D, Stefan L. A theory of activity-based anorexia. Int J Eat Disord. 1983;3:26–46. [Google Scholar]
- Feldman ML. Morphology of the neocortical pyramidal neuron. New York: Plenum Press; 1984. [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nature neuroscience. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Gelegen C, Collier DA, Campbell IC, Oppelaar H, Kas MJ. Behavioral, physiological, and molecular differences in response to dietary restriction in three inbred mouse strains. Am J Physiol Endocrinol Metab. 2006;291:E574–E581. doi: 10.1152/ajpendo.00068.2006. [DOI] [PubMed] [Google Scholar]
- Gelegen C, Collier DA, Campbell IC, Oppelaar H, van den Heuvel J, Adan RA, Kas MJ. Difference in susceptibility to activity-based anorexia in two inbred strains of mice. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007;17:199–205. doi: 10.1016/j.euroneuro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Gelegen C, Pjetri E, Campbell IC, Collier DA, Oppelaar H, Kas MJ. Chromosomal mapping of excessive physical activity in mice in response to a restricted feeding schedule. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2010;20:317–326. doi: 10.1016/j.euroneuro.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Gelegen C, van den Heuvel J, Collier DA, Campbell IC, Oppelaar H, Hessel E, Kas MJ. Dopaminergic and brain-derived neurotrophic factor signalling in inbred mice exposed to a restricted feeding schedule. Genes Brain Behav. 2008;7:552–559. doi: 10.1111/j.1601-183X.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Huber KM, Sudhof TC. Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13883–13897. doi: 10.1523/JNEUROSCI.2457-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Maness PF. Perisomatic GABAergic innervation in prefrontal cortex is regulated by ankyrin interaction with the L1 cell adhesion molecule. Cerebral cortex. 2010;20:2684–2693. doi: 10.1093/cercor/bhq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E, Baysari MT, Carrera O, Whitford TJ, Boakes RA. High ambient temperature reduces rate of body-weight loss produced by wheel running. Q J Exp Psychol (Colchester) 2006;59:1196–1211. doi: 10.1080/17470210500417688. [DOI] [PubMed] [Google Scholar]
- Hansen PJ, Schillo KK, Hinshelwood MM, Hauser ER. Body composition at vaginal opening in mice as influenced by food intake and photoperiod: tests of critical body weight and composition hypotheses for puberty onset. Biology of reproduction. 1983;29:924–931. doi: 10.1095/biolreprod29.4.924. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Exner C, Hebebrand K, Holtkamp C, Casper RC, Remschmidt H, Herpertz-Dahlmann B, Klingenspor M. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiology & behavior. 2003;79:25–37. doi: 10.1016/s0031-9384(03)00102-1. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJ, Koeners MP, de Rijke CE, Kas MJ, Adan RA. Leptin treatment in activity-based anorexia. Biological psychiatry. 2005a;58:165–171. doi: 10.1016/j.biopsych.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJ, van Elburg AA, Kas MJ, van Engeland H, Adan RA. Olanzapine reduces physical activity in rats exposed to activity-based anorexia: possible implications for treatment of anorexia nervosa? Biological psychiatry. 2005b;58:651–657. doi: 10.1016/j.biopsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Huttunen P, Myers RD. Tetrahydro-beta-carboline micro-injected into the hippocampus induces an anxiety-like state in the rat. Pharmacology, biochemistry, and behavior. 1986;24:1733–1738. doi: 10.1016/0091-3057(86)90513-7. [DOI] [PubMed] [Google Scholar]
- Iguchi N, Ohkuri T, Slack JP, Zhong P, Huang L. Sarco/Endoplasmic reticulum Ca2+-ATPases (SERCA) contribute to GPCR-mediated taste perception. PloS one. 2011;6:e23165. doi: 10.1371/journal.pone.0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos AA, Saum B, van Elst LT, Perlov E, Glauche V, Hartmann A, Freyer T, Tuscher O, Zeeck A. Amygdala hyperreactivity in restrictive anorexia nervosa. Psychiatry research. 2011;191:189–195. doi: 10.1016/j.pscychresns.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Kas MJ, Gelegen C, van Nieuwerburgh F, Westenberg HG, Deforce D, Denys D. Compulsivity in mouse strains homologous with chromosomes 7p and 15q linked to obsessive-compulsive disorder. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2010;153B:252–259. doi: 10.1002/ajmg.b.30994. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Shibata K, Miyazaki A, Inoue Y, Tominaga K, Koizumi S, Ueki S, Niwa M. Involvement of the dorsal hippocampus in mediation of the antianxiety action of tandospirone, a 5-hydroxytryptamine1A agonistic anxiolytic. Neuropharmacology. 1991;30:475–480. doi: 10.1016/0028-3908(91)90009-z. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature reviews Neuroscience. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wagner A, Fudge JL, Paulus M. Neurocircuity of eating disorders. Current topics in behavioral neurosciences. 2011;6:37–57. doi: 10.1007/7854_2010_85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL. Adolescent activity-based anorexia increases anxiety-like behavior in adulthood. Physiology & behavior. 2010;101:269–276. doi: 10.1016/j.physbeh.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenotich SJ, Dulawa SC. The activity-based anorexia mouse model. Methods Mol Biol. 2012;829:377–393. doi: 10.1007/978-1-61779-458-2_25. [DOI] [PubMed] [Google Scholar]
- Lewis DY, Brett RR. Activity-based anorexia in C57/BL6 mice: effects of the phytocannabinoid, Delta9-tetrahydrocannabinol (THC) and the anandamide analogue, OMDM-2. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2010;20:622–631. doi: 10.1016/j.euroneuro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. The Journal of pharmacology and experimental therapeutics. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. The Journal of physiology. 2000;524(Pt 1):91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J, Hoffman D, Colbert C, Johnston D. Electrical and calcium signaling in dendrites of hippocampal pyramidal neurons. Annual review of physiology. 1998;60:327–346. doi: 10.1146/annurev.physiol.60.1.327. [DOI] [PubMed] [Google Scholar]
- Makara JK, Losonczy A, Wen Q, Magee JC. Experience-dependent compartmentalized dendritic plasticity in rat hippocampal CA1 pyramidal neurons. Nature neuroscience. 2009;12:1485–1487. doi: 10.1038/nn.2428. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Cameron H, Chao HM, Gould E, Magarinos AM, Watanabe Y, Woolley CS. Adrenal steroids and plasticity of hippocampal neurons: toward an understanding of underlying cellular and molecular mechanisms. Cellular and molecular neurobiology. 1993;13:457–482. doi: 10.1007/BF00711583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neuroscience and biobehavioral reviews. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biology of reproduction. 1985;32:515–522. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. New York: Oxford University Press; 1991. [Google Scholar]
- Rask-Andersen M, Olszewski PK, Levine AS, Schioth HB. Molecular mechanisms underlying anorexia nervosa: focus on human gene association studies and systems controlling food intake. Brain Res Rev. 2010;62:147–164. doi: 10.1016/j.brainresrev.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Riddle MC, McKenna MC, Yoon YJ, Pattwell SS, Santos PM, Casey BJ, Glatt CE. Caloric Restriction Enhances Fear Extinction Learning in Mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. Journal of comparative and physiological psychology. 1967;64:414–421. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cerebral cortex. 2008;18:2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlander M, Frotscher M. Non-pyramidal neurons in the guinea pig hippocampus. A combined Golgi-electron microscope study. Anatomy and embryology. 1986;174:35–47. doi: 10.1007/BF00318334. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nature neuroscience. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried Z, Berry EM, Hao S, Avraham Y. Animal models in the investigation of anorexia. Physiology & behavior. 2003;79:39–45. doi: 10.1016/s0031-9384(03)00103-3. [DOI] [PubMed] [Google Scholar]
- Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med. 2004;71(Suppl 2):S4–S10. doi: 10.3949/ccjm.71.suppl_2.s4. [DOI] [PubMed] [Google Scholar]
- Spatz C, Jones SD. Starvation anorexia as an explanation of "self-starvation" of rats living in activity wheels. Journal of comparative and physiological psychology. 1971;77:313–317. doi: 10.1037/h0031656. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nature reviews Neuroscience. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152:1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kohler C, Bjorklund A. The Limbic Region. I: Septohippocampal system. In: Bjorklund A, et al., editors. Integrated Systems of the CNS, Part I Hypothalamus, Hippocampus, Amygdala, Retina. vol. 5. Amsterdam: Elsevier; 1987. pp. 125–277. [Google Scholar]
- Talaenko AN. The Neurochemical profiles of the dorsal hippocampus and the antiaversive effects of anxiolytics in different models of anxiety in rats. J Nerv Ment Dis. 1993;43:621–626. [PubMed] [Google Scholar]
- Tasan RO, Bukovac A, Peterschmitt YN, Sartori SB, Landgraf R, Singewald N, Sperk G. Altered GABA transmission in a mouse model of increased trait anxiety. Neuroscience. 2011;183:71–80. doi: 10.1016/j.neuroscience.2011.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LM, Dellava JE, Root TL, Lichtenstein P, Bulik CM. Anorexia nervosa and generalized anxiety disorder: further explorations of the relation between anxiety and body mass index. Journal of anxiety disorders. 2011;25:727–730. doi: 10.1016/j.janxdis.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Bangasser DA, Shors TJ. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. The Journal of neuroscience. 2008;28:5290–5294. doi: 10.1523/JNEUROSCI.1129-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ML, Burette AC, Weinberg RJ, Philpot BD. Maternal loss of Ube3a produces an excitatory/inhibitory imbalance through neuron type-specific synaptic defects. Neuron. 2012;74:793–800. doi: 10.1016/j.neuron.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]